Abstract
Aluminum dross (AD) is a waste product produced during aluminum processing and can be used to prepare mullite ceramic materials. However, the research on the preparation of mullite porous ceramics entirely from solid waste is still in the development stage. In this paper, porous mullite ceramics were successfully fabricated using a solid-phase sintering process with AD and different silicon sources (fly ash, silica dust, and gangue) as raw materials. The bulk density, apparent porosity, and compressive strength of the specimens were obtained, and the phase compositions and microstructures of the sintered specimens were measured using XRD and SEM, respectively. The average activation energy of the phase transition of fly ash, silica dust, and gangue as silicon sources were 984 kJ/mol, 1113 kJ/mol, and 741 kJ/mol, respectively. The microstructures of the mullite in the specimens were prisms, random aggregates, and needle-shaped, respectively. The formation of needle-shaped mullite combined with the substrate enhanced the mechanical strength of the porous mullite ceramics. The apparent porosity, density, and compressive strength of the specimens with gangue as the silicon source were 33.13%, 1.98 g/cm3, and 147.84 MPa, respectively, when sintered at 1300 °C for 2 h.
1. Introduction
Aluminum dross (AD) is an industrial solid waste produced in the process of Al metal smelting, when the molten metal surface is in contact with the air [1,2,3]. With the expansion of aluminum production scale, the output of AD is increasing rapidly. The accumulated AD not only occupies land but also cause economic and environmental problems [4,5]. AlN in AD will hydrolyze with water, even at room temperature, producing NH3 and polluting the environment, and the accumulation of AD will cause the leaching of salts (KCl, NaCl, NaF, et al.) and gradually destroy the soil environment [6,7,8]. In our previous works [9,10], AD was treated using a hydrometallurgy process, and the AlN and soluble salts in AD were removed. The main component of the AD was rich in alumina, which is a renewable resource for the preparation of mullite materials.
Mullite porous ceramics is a widely studied ceramic [11], because it exhibits useful properties such as high temperature resistance, oxidation resistance, low thermal conductivity, small expansion coefficient, good thermal shock resistance, and high creep resistance [12,13,14]. It has been widely used in metallurgy, glass, ceramics, chemistry, electricity, national defense, gas, and cement, as well as other industrial fields, because of its distinctive properties [14,15].
Mullite is a stable binary compound in Al2O3-SiO2 system, which is a solid solution composed of 3Al2O3-2SiO2 and 2Al2O3-SiO2. However, natural mullite ore is rare, owing to its strict formation conditions. The main raw materials for synthesizing mullite are industrial alumina [16,17,18], bauxite [14], and kaolin [19,20]. However, these materials are obtained from non-renewable resources or have a high cost, and do not conform to the national energy conservation, emission reduction, and sustainable development concept. Therefore, in recent years, the preparation of mullite ceramics from solid wastes, such as AD [21,22], fly ash [23], silica dust [22], and gangue [24], and replacing industrial raw materials completely, has become a new research hotspot, which is beneficial for the reuse of solid waste resources and the reduction of production costs. The main components of these solid wastes are Al2O3 or SiO2, and mullite ceramics can be successfully sintered at 1300–1500 °C. For example, Ibarra, C.M.N. [25] prepared mullite-zirconia composites at 1500 °C using AD and zirconia as raw materials. Ma, B.Y. [26] reported that the porous mullite ceramics were successfully fabricated with a fly ash and bauxite via reaction synthesis process at 1450–1550 °C.
Apparently, it is feasible and advisable to synthesize mullite porous ceramics from solid wastes [27,28,29,30,31]. However, there are still some problems with the preparation of mullite from AD. In the process of sintering, AlN and salts in AD will seriously affect the properties of mullite ceramics, and there has been little research on the preparation of mullite ceramics completely from solid wastes. In addition, the sintering temperature is usually higher than 1400 °C, which causes a high energy consumption.
In the present work, in order to make better use of AD and realize the comprehensive utilization of solid waste, three different silicon sources (fly ash, silica dust, and gangue) were added in the process of forming mullite at 900–1300 °C, and a range of porous mullite ceramics were prepared using a solid-phase sintering process. The work was mainly focused on the effects of the silicon source and sintering temperature on the properties of the fabricated ceramics, especially the phase transition and kinetics calculation.
2. Experimental
2.1. Materials
The chemical compositions of the AD (from Inner Mongolia Heng-sheng Environmental Protection Technology Co., Ltd. Tongliao, China. D50 = 22.74 μm), fly ash (from Boulder Mining Co., Ltd., Shijiazhuang, China. D50 = 25.73 μm), silica dust (from Aotai Mineral Products Co., Ltd., Shijiazhuang, China. D50 = 25.86 μm), and gangue (from Yunshi Building Materials Co., Ltd., Shijiazhuang, China. D50 = 8.05 μm) are shown in Table 1. An X-ray diffraction (XRD) analysis of the raw materials is shown in Figure 1 and Figure 2 shows the microstructure of the raw materials.

Table 1.
Chemical composition of the raw materials (wt%).
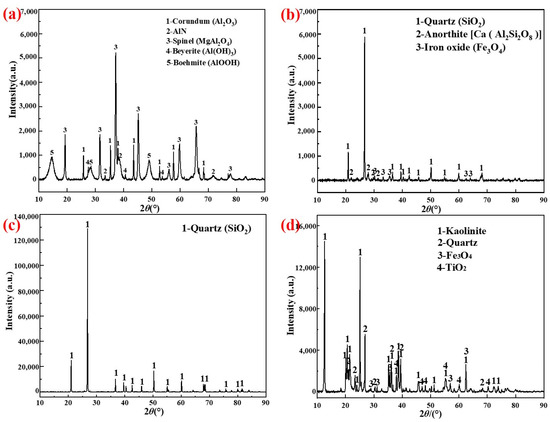
Figure 1.
XRD of raw materials: (a) AD; (b) Fly ash; (c) Silica dust; (d) Gangue.
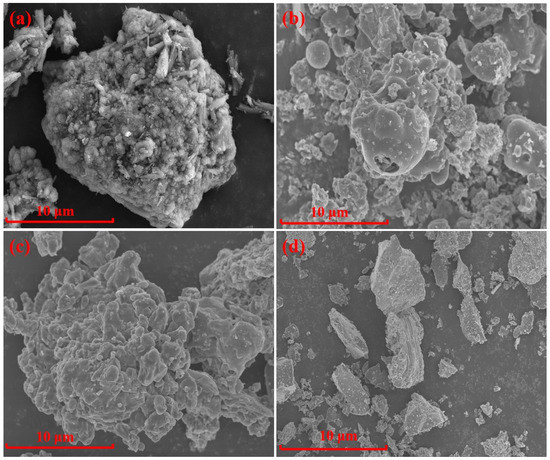
Figure 2.
SEM of raw materials: (a) AD; (b) Fly ash; (c) Silica dust; (d) Gangue.
2.2. Experimental Procedure
According to the theoretical molar ratio of mullite (Al2O3:SiO2 = 3:2), the corresponding weight of raw materials (in Table 2) was weighed, put in a high-energy planetary ball mixer for 2 h, and then put into a sample bag for 6 h. After that, the powders were pressed into several batches of cylinder green bodies (φ25 mm × 10 mm) under a pressure of 5 MPa. All green bodies were then dried at 105 °C and sintered in air atmosphere at 900–1300 °C for 2 h in a resistance furnace, with a heating rate of 5 K/min. Finally, the specimens were cooled to room temperature inside the furnace.

Table 2.
The ratio of different samples (mass, g).
2.3. Analytical and Characterization Methods
The XRD patterns of the raw materials and sintered specimens were analyzed using an X-ray diffractometer (D8 ADVANCE, Bruker AXS Co., Ltd., Karlsruhe, Germany) with Cu-Kα (40 kV, 80 mA); the scanning range was 10~90°, and the scanning speed was 10°/min. The XRF analysis of the raw materials was conducted using an X-ray fluorescence spectrometer (ZSX Primus II, Neo-Confucianism Co., Ltd., Tokyo, Japan). The optical tube voltage was 60 kV, and the current was 150 mA. Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were performed with a field emission scanning electron microscope (Quanta 250FEG, FEI Co., Ltd., Brno, Czech Republic). The acceleration voltage was 30 kV. The particle size distribution of the raw materials was determined using a laser particle size analyzer (Bettersize 2000, Bettersize Instruments Co., Ltd., Dandong, China). Thermogravimetric (TG) and differential thermal analysis (DTA) were processed using an HQT-4 integrated thermal analyzer (Beijing HENVEN Company in China). The TG-DTA were conducted at heating rates of 10, 20, 30, and 40 K/min in nitrogen atmosphere, with a heating range from room temperature to 1475 K. A universal material testing machine (CMTH-1, Meitesi Testing Machine Factory, Tianjin, China) was used to measure the compressive strength of the porous ceramics, and the loading rate was 0.5 mm/min. The apparent porosity and bulk density of the porous ceramics were measured according to the Chinese standard GB/T 1966–1996.
3. Results and Discussion
3.1. Effects of Silicon Source on Phase Compositions
The XRD patterns of specimens with different silicon sources after sintering at 900–1300 °C for 2 h are presented in Figure 3.
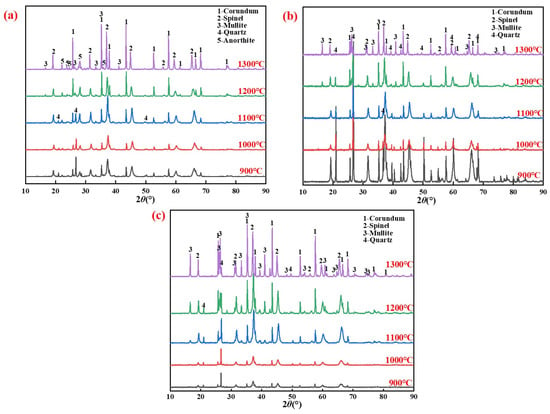
Figure 3.
XRD patterns of specimens prepared from different silicon sources after sintering at 900–1300 °C: (a) Fly ash; (b) Silica dust; (c) Gangue.
The major crystalline phases detected were Al2O3(corundum), 3Al2O3·2SiO2(mullite), and MgAl2O4(spinel).
Compositional differences in silicon sources can cause differences in phase composition, such as the anorthite (Ca(Al2Si2O8), in Figure 1b) contained in fly ash. The formation temperatures of the mullite phase in the porous mullite ceramic prepared with fly ash, silica dust, and gangue were 1200 °C, 1200 °C, and 1100 °C, respectively. In particular, kaolinite, which is mainly contained in gangue, will first form mullite nuclei after being calcined to remove crystal water [32]. The reaction [25,33,34,35] is shown in Equations (1)–(4).
When the temperature is 450~800 °C
When the temperature is 800~980 °C
or
When the temperature >1100 °C
In addition, the formation temperature of the mullite phase is lower than the synthesis temperature required by the solid-phase sintering process reported in the literature [32], which is mainly due to the existence of AlOOH and Al(OH)3 in the AD promoting the high temperature crystallization of mullite [36,37].
3.2. Effects of Silicon Source on Morphologies
Figure 4 and Figure 5 show SEM images and the EDS spectrums of the specimens with different silicon sources sintered at 1200 °C and 1300 °C for 2 h, respectively. The crystal structures of mullite formed in the specimens prepared with fly ash, silica dust, and gangue as silicon sources are prisms (Figure 4a), random aggregates (Figure 4b), and needle-shaped (Figure 4c) when sintering at 1200 °C for 2 h, respectively. Impurities in gangue led to the formation of silicate liquid phase; and according to the L-S mechanism [20,38,39], the existence of liquid phase can promote the development of needle-shaped mullite. When the sintering temperature reached 1300 °C, the specimens with different silicon sources all showed different degrees of melting state, the glass phase increased significantly, and the mullite phase and the substrate became fused together.
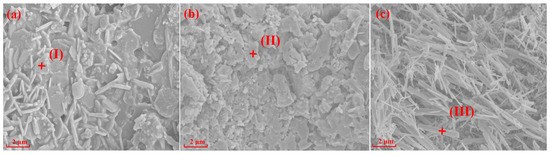
Figure 4.
SEM and EDS of specimens with different silicon sources after sintering at 1200 °C: (a) Fly ash; (b) Silica dust; (c) Gangue.
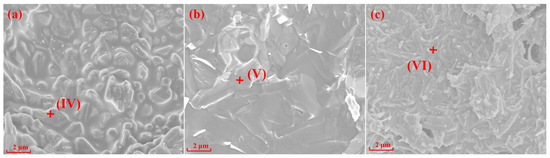
Figure 5.
SEM and EDS of specimens with different silicon sources after sintering at 1300 °C: (a) Fly ash; (b) Silica dust; (c) Gangue.
EDS analyses of the points I-VI in Figure 4 and Figure 5 are shown in Table 3. The results in Table 3 show that Mg elements (points I and IV) were always present in the mullite structure of the specimens prepared with fly ash as the silicon source, indicating that the specimens were composed of a mullite–magnesium aluminum spinel complex. When silica dust was the silicon source, the Si element content at 1200 °C was 20.55% (point II), which is much larger than the Si element content in the theoretical value of mullite, indicating a silicon-rich state, which is the main reason why it is difficult to form crystals. In the samples prepared with gangue as the silicon source, only Al, Si, and O elements were in the needle-shaped mullite (point III).

Table 3.
EDS analysis.
3.3. Effects of Silicon Source on Phase Transition Kinetics
Through kinetic calculation of the thermal analysis data, the process and mechanism of chemical reaction of substances could be understood, and kinetic parameters such as reaction rate could be determined. Commonly used non-isothermal kinetic analysis methods include the Kissinger method [37] (Equation (5)) and Ozawa method [40] (Equation (6)). The activation energy of the reaction could be calculated according to the slope of the linear fitting curve of each equation.
where Tp was the absolute temperature at the exothermic peak of the DTA curve in K; α was the heating rate in °C·min−1; was the activation energy in KJ·mol−1; R was the pervasive gas constant, and ν was the frequency factor; C was constant.
The results of TG-DTA analysis of different silicon sources (20 K/min) are shown in Figure 6. The DTA curve in Figure 6 shows that the exothermic peak positions of the samples prepared with fly ash, silica fume and coal gangue as silicon sources were 1380 K, 1430 K and 1270 K, respectively. TG curves showed that the weight loss rates of the samples prepared with fly ash, silica dust, and gangue as silicon sources were 11.8%, 4.6%, and 14.1% at 1400 K, respectively. The main reasons for this difference were the different compositions and amounts of the different silicon sources added.
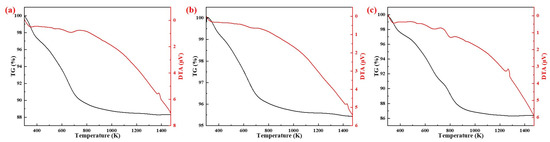
Figure 6.
TG-DTA curves of samples from different silicon sources: (a) Fly ash; (b) Silica dust; (c) Gangue.
Figure 7 shows the exothermic peak of DTA curves of AD mixed with three silicon sources at different heating rates. With the increase of heating rate, the exothermic peaks were shifted to the high temperature region, due to thermal hysteresis.
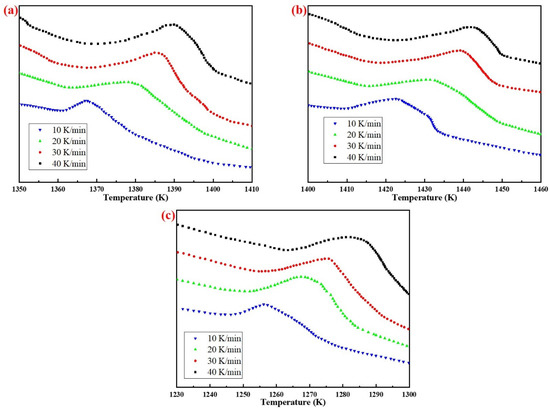
Figure 7.
Differential thermal analysis of specimens from different silicon sources: (a) Fly ash; (b) Silica dust; (c) Gangue.
According to the analysis results in Figure 7, the phase transition kinetics fitting results of AD mixed with the three silicon sources based on Kissinger (Equation (5)) and Ozawa (Equation (6)) method are shown in Figure 8 and Figure 9, respectively. It can be seen from the figures that the correlation coefficients R2 of the three samples based on different methods were all above 0.97, and the results of the relevant kinetic parameters are shown in Table 4.
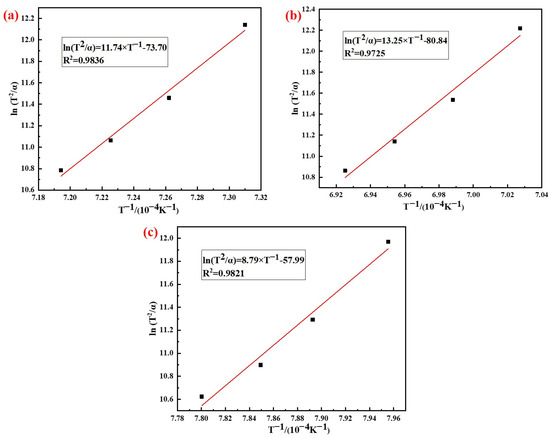
Figure 8.
Analysis results of the Kissinger method: (a) Fly ash; (b) Silica dust; (c) Gangue.
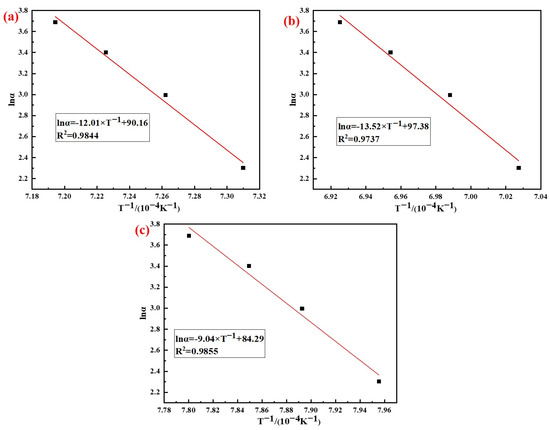
Figure 9.
Analysis results of Ozawa method: (a) Fly ash; (b) Silica dust; (c) Gangue.

Table 4.
Phase transition activation energies of specimens from different silicon sources.
The results in Table 4 showed that the average activation energy of the phase transition process of AD mixed with fly ash, silica dust, and gangue calculated using the Kissinger and Ozawa methods were 984 kJ/mol, 1113 kJ/mol, and 741 kJ/mol, respectively. The silicon sources had different effects on the activation energy of the phase transition process of mullite synthesis with AD; among which, the activation energy of AD mixed with gangue was the smallest. The reason for this difference was that the composition and impurity content of the silicon sources were different, and the thermal decomposition of kaolinite in gangue produced primary mullite, which could reduce the activation energy when forming the needle-shaped mullite.
3.4. Effects of Silicon Sources on Physical Properties
The physical properties of specimens with different silicon sources sintered at 900–1300 °C for 2 h are shown in Figure 10. The results showed that with the increase of temperature, the apparent porosity of the specimens prepared with different silicon sources gradually decreased, while the density and compressive strength increased significantly. When the temperature was 1300 °C, the apparent porosity of porous mullite ceramic specimens prepared from fly ash, silica dust, and gangue were 8.74%, 34.83%, and 33.13%, respectively. The densities were 1.95 g/cm3, 1.85 g/cm3, and 1.91 g/cm3, respectively. The compressive strengths were 123.28 MPa, 107.88 MPa, and 147.84 MPa, respectively. The apparent porosity of specimens with fly ash as the silicon source was the smallest, but the compressive strength did not grow expectedly. This was because the glass phase in the substrate mainly formed closed pores, while promoting firing and crystallization. The formation of needle-shaped mullite in the specimens with gangue as the silicon source reduced the apparent porosity and enhanced the mechanical strength of the porous mullite ceramic.
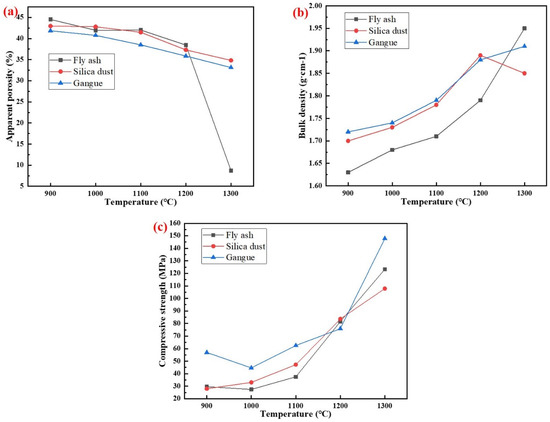
Figure 10.
Physical properties of specimens with different silicon sources after sintering at 900–1300 °C for 2 h: (a) Fly ash; (b) Silica dust; (c) Gangue.
4. Conclusions
In this study, AD after hydrometallurgical treatment was used as an aluminum source, and the three silicon sources were introduced into the mullitization reaction, to study the effects of different silicon sources on the preparation of porous mullite from the AD. The main findings were as follows:
When the sintering temperature reached 1200 °C, all of the specimens with fly ash, silica dust, and gangue as silicon sources could form mullite phases, and the structures of mullite were prisms, random aggregates, and needle-shaped, respectively. Among them, the formation of needle-shaped mullite in the specimens with gangue as a silicon source reduced the apparent porosity and enhanced the mechanical strength of the porous mullite ceramics; when the temperature was 1300 °C, the apparent porosity, density, and compressive strength were 33.13%, 1.98 g/cm3 and 147.84 MPa, respectively. The average activation energies of the phase transition process of the AD mixed with fly ash, silica dust, and gangue were 984 kJ/mol, 1113 kJ/mol, and 741 kJ/mol, respectively.
Author Contributions
Writing—review and editing and Formal analysis, H.-L.Y.; Investigation and Software, Z.-S.L.; Methodology and Validation, Q.-Q.G.; Project administration and Resources, Y.-D.D.; Methodology and Software, Y.-J.S.; Supervision and Project administration, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
Project (U20A20239) supported by the National Natural Science Foundation of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, due to industrial applications and confidentiality.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Shinzato, M.C.; Hypolito, R. Solid waste from aluminum recycling process: Characterization and reuse of its economically valuable constituents. Waste Manag. 2005, 25, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Huang, X.; Xu, Z. Recovery of Metals from Aluminum Dross and Saltcake. J. Miner. Mater. Charact. 2006, 5, 47–62. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Res. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Song, S.J.; Lee, M.S. Development of a hydrometallurgical process for the recovery of pure alumina from black dross and synthesis of magnesium spinel. J. Mater. Res. Technol. 2020, 9, 2568–2577. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, H.; Zuo, Z. A thermodynamic and kinetic study of catalyzed hydrolysis of aluminum nitride in secondary aluminum dross. J. Mater. Res. Technol. 2020, 9, 9735–9745. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Z.; Li, C. Hazardous characteristics and transformation mechanism in hydrometallurgical disposing strategy of secondary aluminum dross. J. Environ. Chem. Eng. 2021, 9, 2213–2223. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef]
- Su, N.; Li, Z.S.; Ding, Y.D. Waste to Wealth Strategy: Preparation and Properties of Lightweight Al2O3-SiO2-Rich Castables Using Aluminum Dross Waste. Materials 2021, 14, 13. [Google Scholar] [CrossRef]
- Yang, H.L.; Li, Z.S.; Ding, Y.D. Hydrolysis Behavior and Kinetics of AlN in Aluminum Dross during the Hydrometallurgical Process. Materials 2022, 15, 5499. [Google Scholar] [CrossRef]
- Nath, S.; Biswas, K.; Basu, B. Phase stability and microstructure development in hydroxyapatite–mullite system. Scr. Mater. 2008, 58, 1054–1057. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]
- Dong, Y.; Hampshire, S.; Zhou, J. Sintering and characterization of flyash-based mullite with MgO addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Kong, L.B.; Chen, Y.Z.; Zhang, T.S. Effect of alkaline-earth oxides on phase formation and morphology development of mullite ceramics. Ceram. Int. 2004, 30, 1319–1323. [Google Scholar] [CrossRef]
- Medvedovski, E. Alumina–mullite ceramics for structural applications. Ceram. Int. 2006, 32, 369–375. [Google Scholar] [CrossRef]
- Meng, B.; Peng, J. Effects of in situ synthesized mullite whiskers on flexural strength and fracture toughness of corundum-mullite refractory materials. Ceram. Int. 2013, 39, 1525–1531. [Google Scholar] [CrossRef]
- Shin, C.; Oh, S.H.; Choi, J.H. Synthesis of porous ceramic with well-developed mullite whiskers in system of Al2O3-Kaolin-MoO3. J. Mater. Res. Technol. 2021, 15, 1457–1466. [Google Scholar] [CrossRef]
- Sahraoui, T.; Belhouchet, H.; Heraiz, M. The effects of mechanical activation on the sintering of mullite produced from kaolin and aluminum powder. Ceram. Int. 2016, 42, 12185–12193. [Google Scholar] [CrossRef]
- Rashad, M.; Balasubramanian, M. Characteristics of porous mullite developed from clay and AlF3·3H2O. J. Eur. Ceram. Soc. 2018, 38, 3673–3680. [Google Scholar] [CrossRef]
- Kim, B.M.; Cho, Y.K.; Yoon, S.Y. Mullite whiskers derived from kaolin. Ceram. Int. 2009, 35, 579–583. [Google Scholar] [CrossRef]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Wassel, A.R.; Youness, R.A. Recycling of aluminum dross and silica fume wastes for production of mullite-containing ceramics: Powder preparation, sinterability and properties. Ceram. Int. 2022, 48, 31661–31676. [Google Scholar] [CrossRef]
- Valášková, M.; Blahůšková, V.; Vlček, J. Effects of kaolin additives in fly ash on sintering and properties of mullite ceramics. Minerals 2021, 11, 887. [Google Scholar] [CrossRef]
- Lian, W.; Liu, Y.; Wang, W.J. Preparation of environmentally friendly low-cost mullite porous Ceramics and the effect of waste glass powder on structure and mechanical properties. Int. J. Precis. Eng. Manuf.-Green Technol. 2022, 9, 577–585. [Google Scholar] [CrossRef]
- Ibarra, C.M.N.; Almanza, R.J.M.; Cortés, H.D.A. Development of mullite/zirconia composites from a mixture of aluminum dross and zircon. Ceram. Int. 2009, 35, 921–924. [Google Scholar] [CrossRef]
- Ma, B.Y.; Su, C.; Ren, X.M. Preparation and properties of porous mullite ceramics with high-closed porosity and high strength from fly ash via reaction synthesis process. J. Alloys Compd. 2019, 803, 981–991. [Google Scholar] [CrossRef]
- Xia, B.; Wang, Z.; Gou, L. Porous mullite ceramics with enhanced compressive strength from fly ash-based ceramic microspheres: Facile synthesis, structure, and performance. Ceram. Int. 2021, 48, 10472–10479. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chang, Y.H.; Wang, M.C. Effects of Al2O3 addition on the phases, flow characteristics and morphology of the porous kaolin ceramics. Mater. Sci. Eng. A 2004, 373, 221–228. [Google Scholar] [CrossRef]
- Li, J.H.; Ma, H.W.; Huang, W.H. Effect of V2O5 on the properties of mullite ceramics synthesized from high-aluminum fly ash and bauxite. J. Hazard. Mater. 2009, 166, 1535–1539. [Google Scholar] [CrossRef]
- Guo, A.; Liu, J.; Xu, R. Preparation of mullite from desilication fly ash. Fuel 2010, 89, 3630–3636. [Google Scholar] [CrossRef]
- Alves, H.P.A.; Silva, J.B.; Campos, L.F.A. Preparation of mullite based ceramics from clay–kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Chargui, F.; Hamidouche, M.; Belhouchet, H. Mullite fabrication from natural kaolin and aluminium slag. Bol. Soc. Esp. Cerám. Vidr. 2018, 57, 169–177. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, M.C.; Hon, M.H. Phase transformation and growth of mullite in kaolin ceramics. J. Eur. Ceram. Soc. 2004, 24, 2389–2397. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Microstructural evolution of mullite during the sintering of kaolin powder compacts. Ceram. Int. 2000, 26, 715–720. [Google Scholar] [CrossRef]
- Li, S.; Du, H.; Guo, A. Preparation of self-reinforcement of porous mullite ceramics through in situ synthesis of mullite whisker in flyash body. Ceram. Int. 2012, 38, 1027–1032. [Google Scholar] [CrossRef]
- Dong, Y.; Diwu, J.; Feng, X. Phase evolution and sintering characteristics of porous mullite ceramics produced from the flyash-Al(OH)3 coating powders. J. Alloys Compd. 2008, 460, 651–657. [Google Scholar] [CrossRef]
- Yuan, W.; Kuang, J.; Huang, Z. Effect of aluminum source on the kinetics and mechanism of mullite preparation from kaolinite. Chem. Phys. Lett. 2022, 787, 139242. [Google Scholar] [CrossRef]
- Chen, Y. Kinetics of secondary mullite formation in kaolin-Al2O3 ceramics. Scr. Mater. 2004, 51, 231–235. [Google Scholar] [CrossRef]
- Ondro, T.; Al-Shantir, O.; Csáki, Š. Kinetic analysis of sinter-crystallization of mullite and cristobalite from kaolinite. Thermochim. Acta 2019, 678, 178312. [Google Scholar] [CrossRef]
- Kuang, J.; Li, L.; Liu, P. Effect of Er2O3 and Pr6O11 on non-isothermal kinetics of mullite formation from kaolinite. J. Rare Earths 2017, 35, 831–836. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).