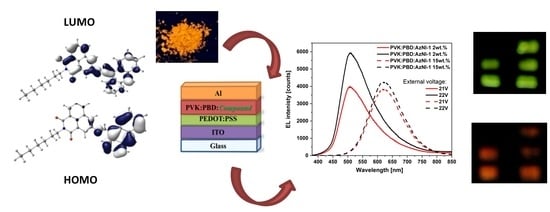
Six New Unsymmetrical Imino-1,8-naphthalimide Derivatives Substituted at 3-C Position—Photophysical Investigations
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterization Methods
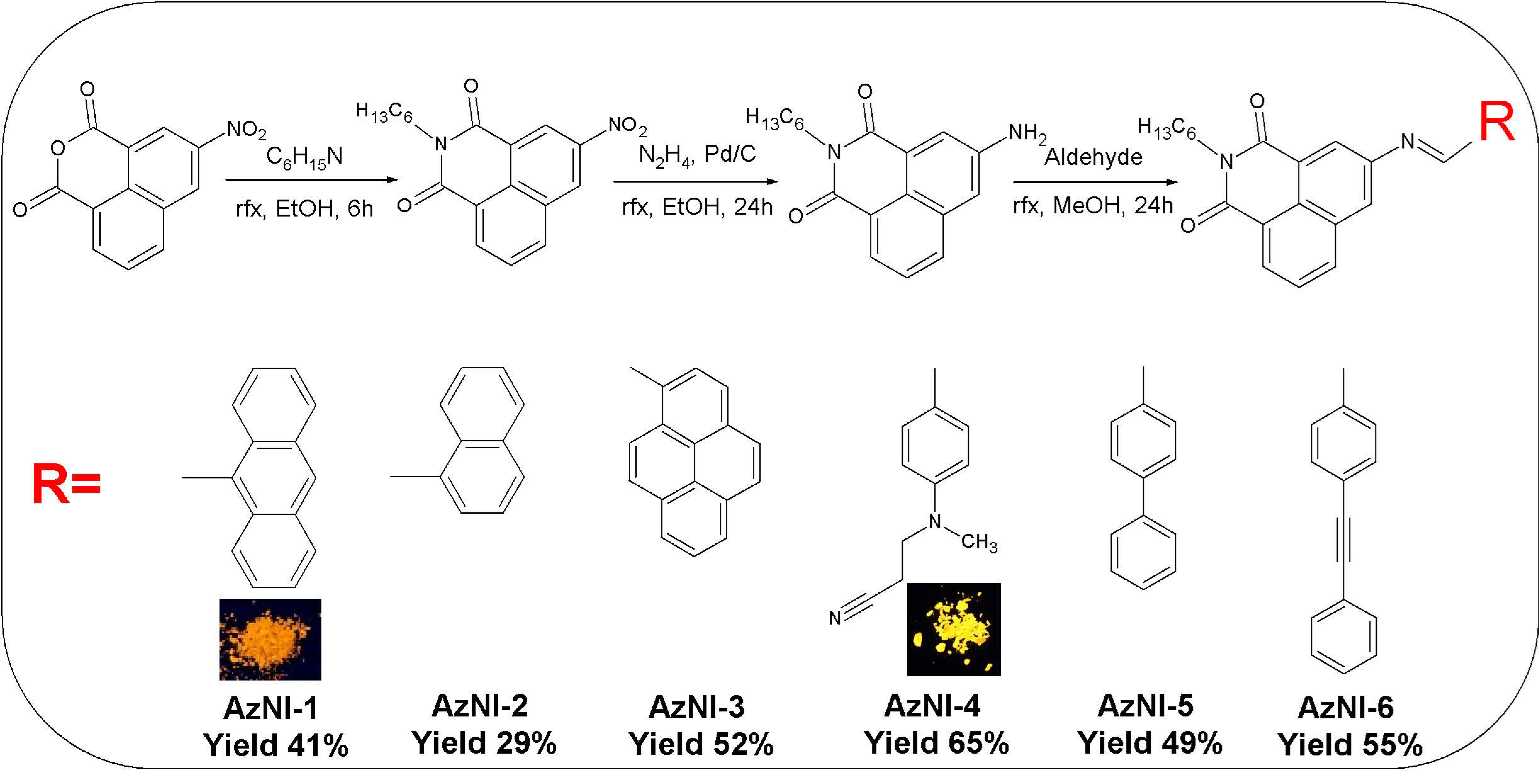
2.2. Synthesis Description
3. Result and Discussion
3.1. Structural Characterization
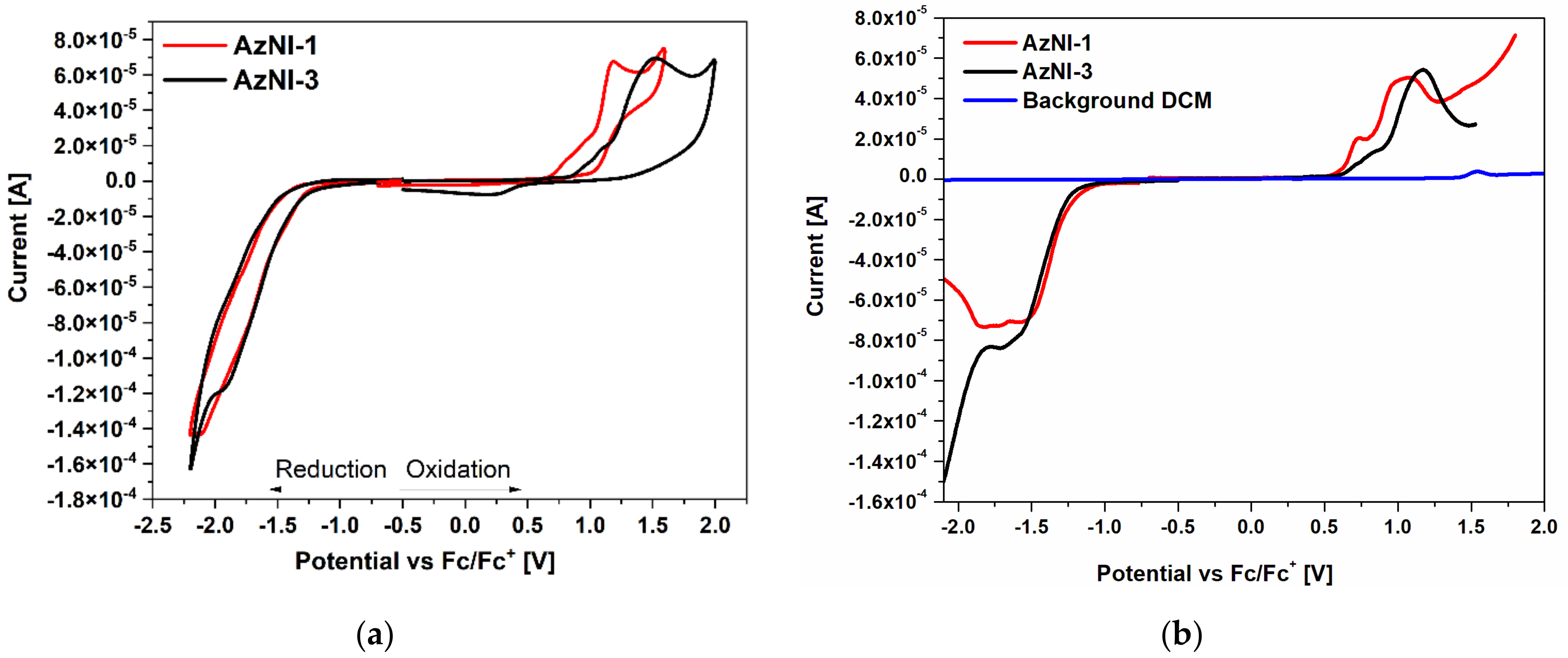
3.2. Redox Properties
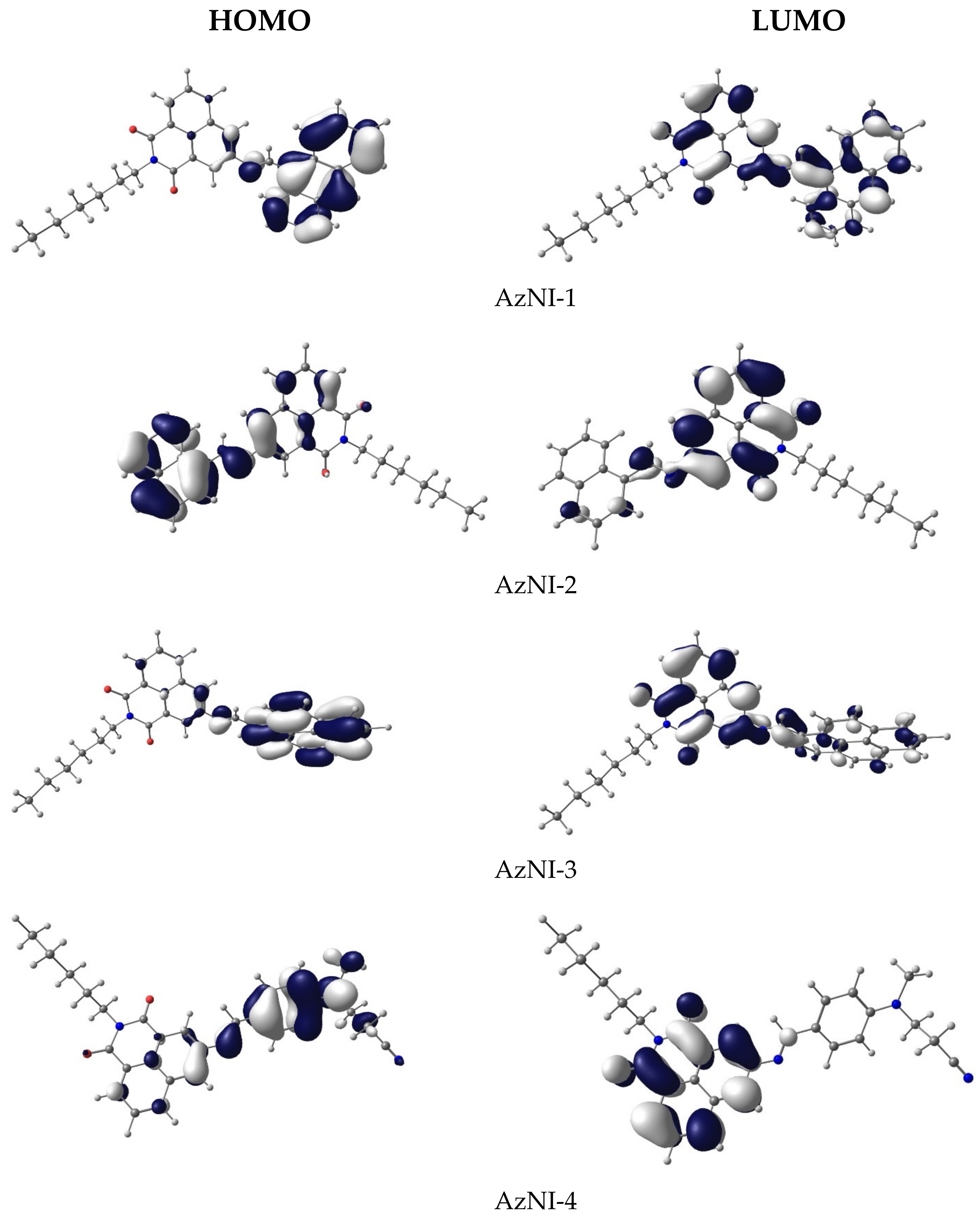
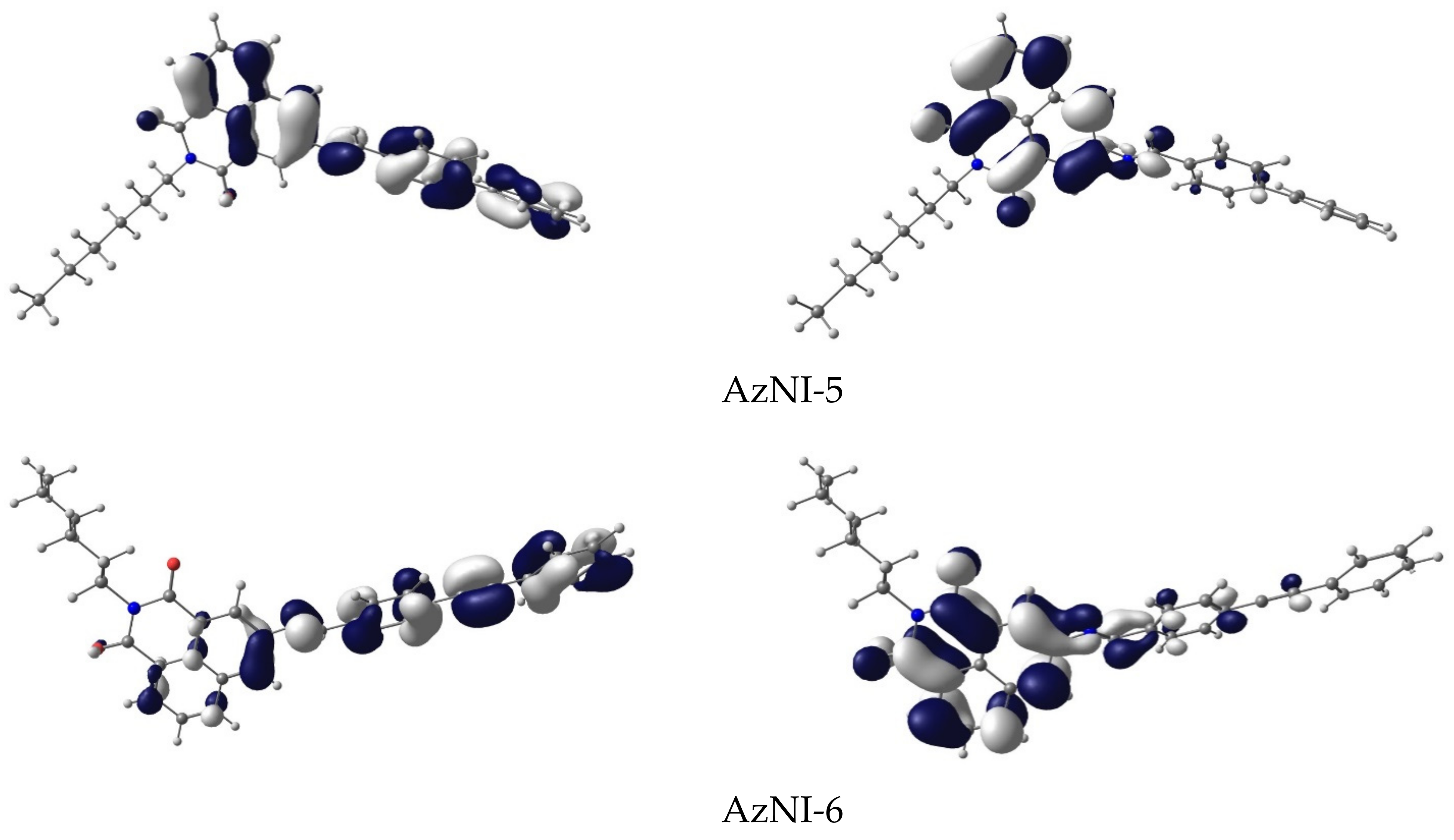
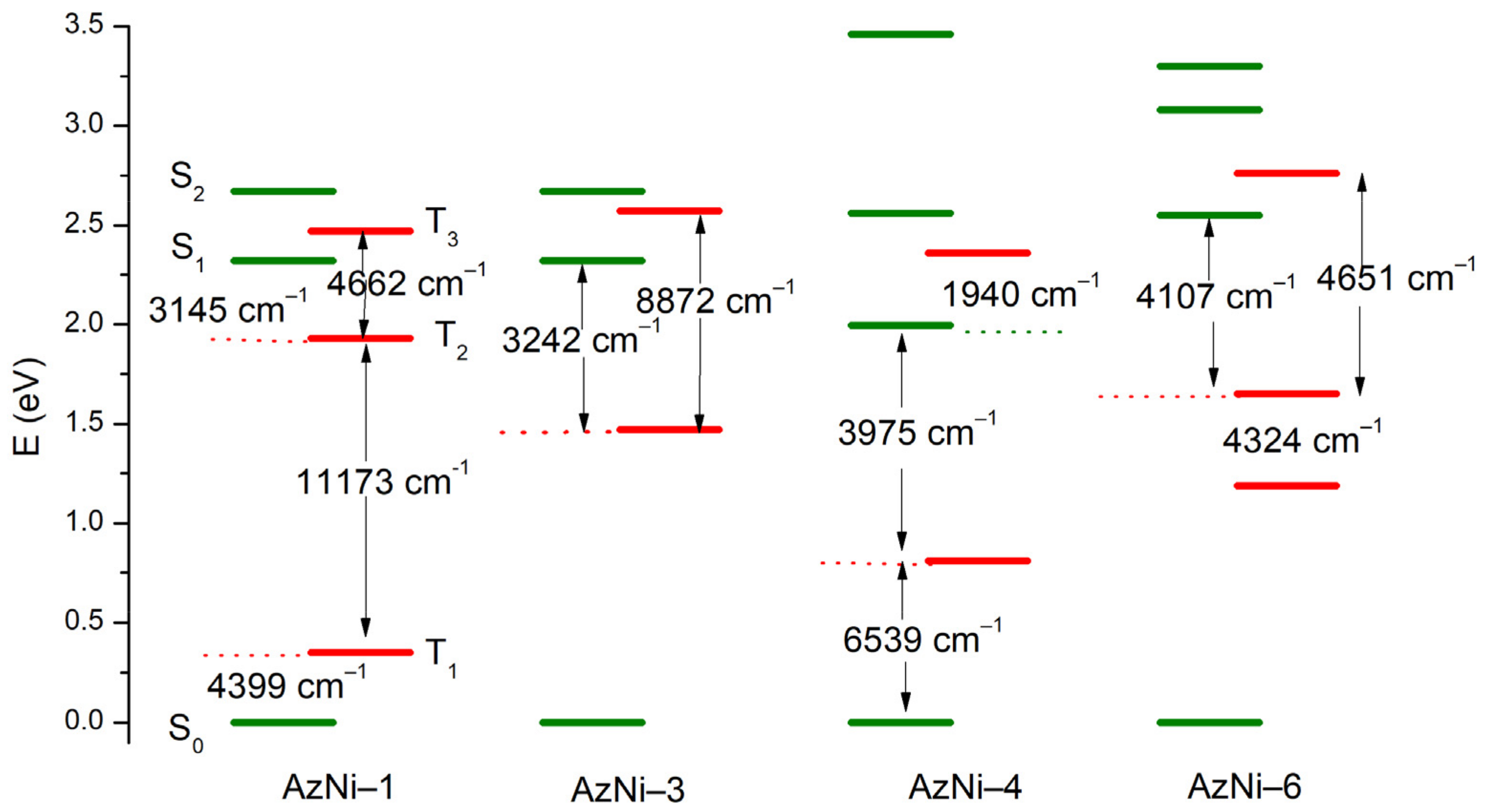
3.3. DFT Calculations
3.4. Optical Investigations
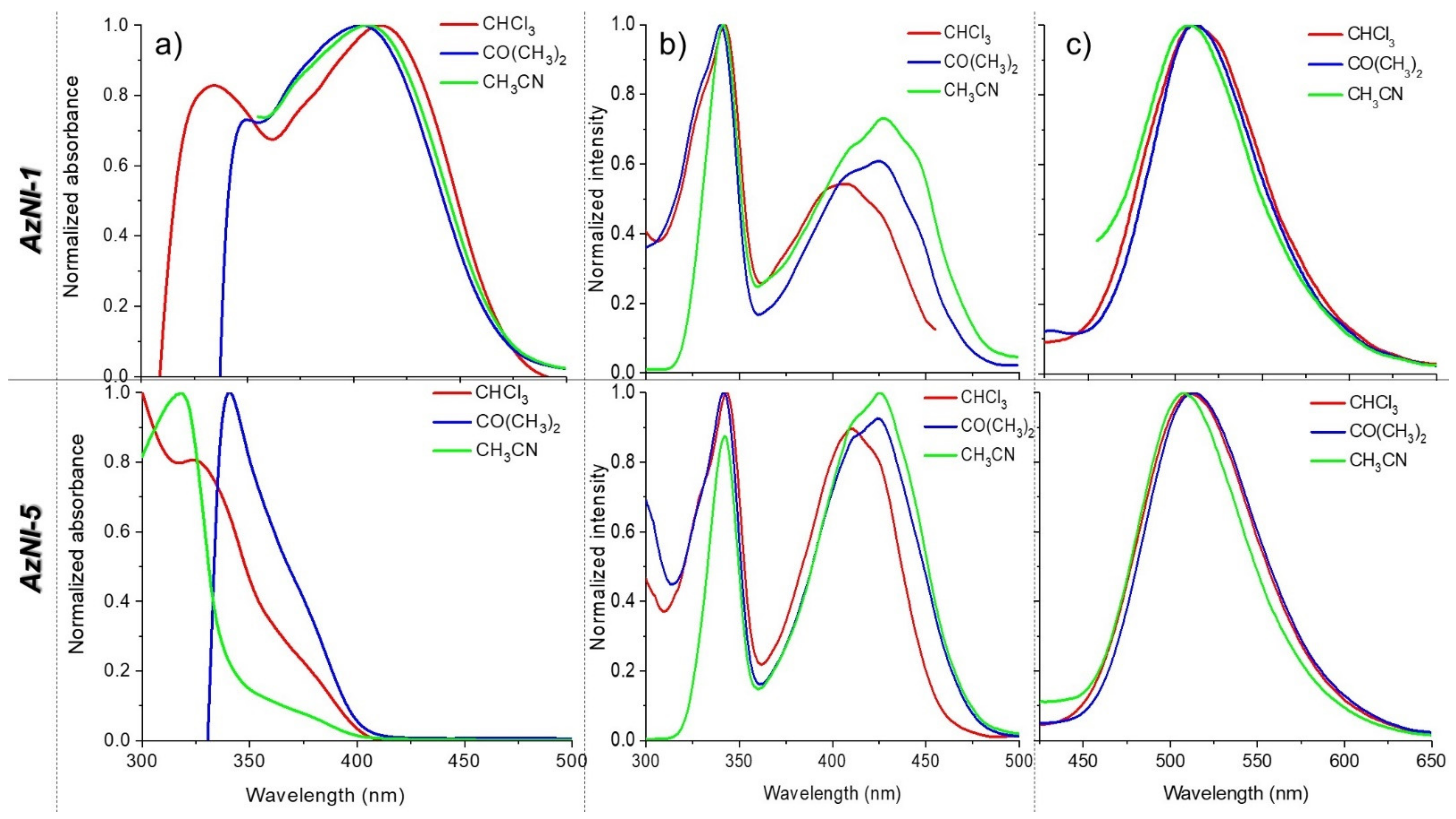
3.4.1. UV-Vis Absorption and Emission
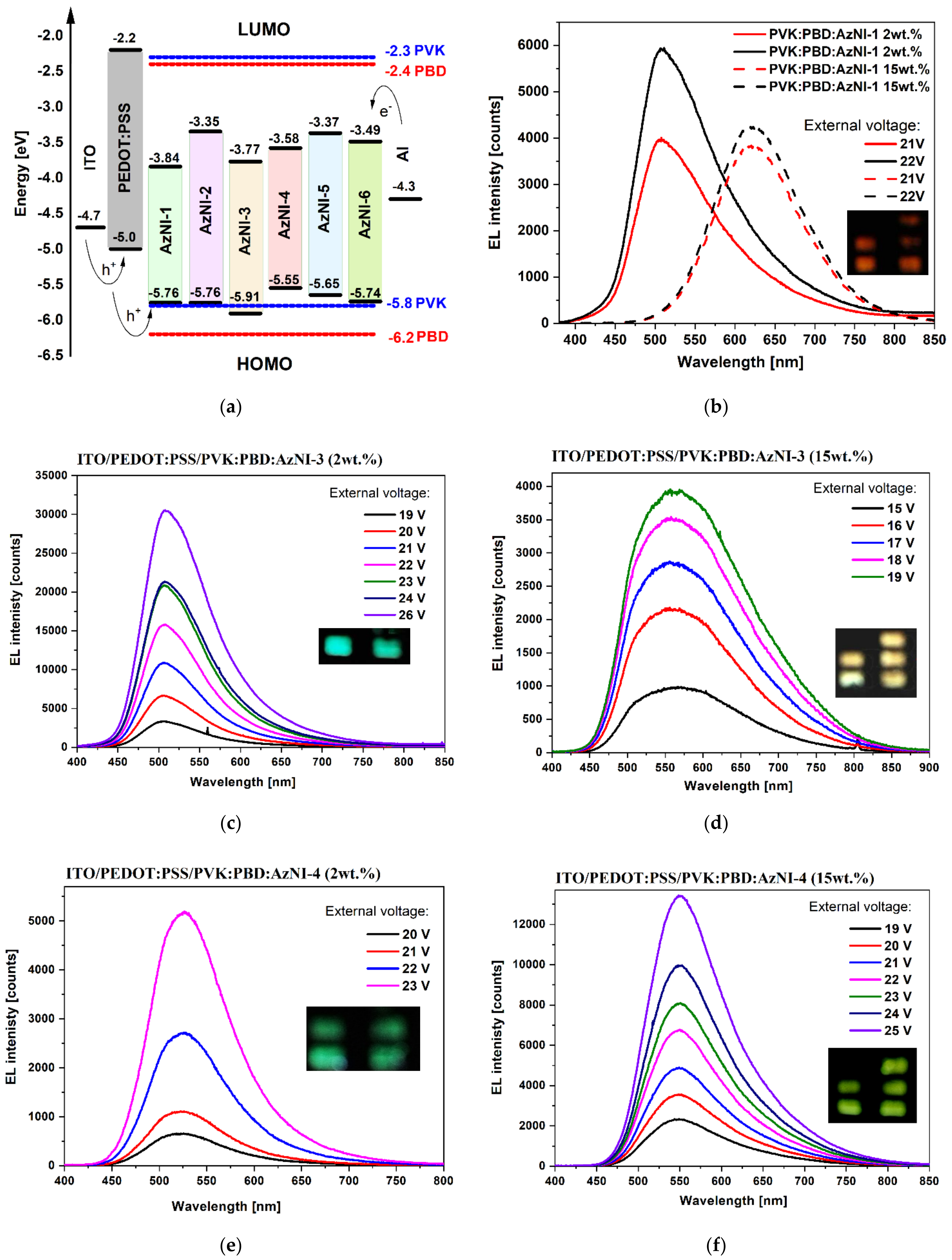
3.4.2. Electroluminescence
4. Conclusions
- crystalline compounds with Tm in the range of 132–182 °C with the possibility of their amorphization were obtained,
- azomethinoimides were electrochemically active with the low energy band gap (below 2.41 eV),
- the molecules showed a low PL quantum yield, due to probably the photoinduced electron transfer process and the prevailing non-radiation processes,
- an energy transfer from the PVK:PBD matrix to AzNI was observed in the blends, unfortunately slightly affecting the quantum yield values. However, the use of the matrix allowed to induction the emission of light under external voltage and the maximum of electroluminescence band from the blue to red spectral region dependent on the compounds content was seen.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.W.; Kim, O.Y.; Lee, J.Y. Organic materials for organic electronic devices. J. Ind. Eng. Chem. 2014, 20, 1198–1208. [Google Scholar] [CrossRef]
- Das, S.; Dhara, S. Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-819718-9. [Google Scholar]
- Yuan, Q.; Wang, T.; Yu, P.; Zhang, H.; Zhang, H.; Ji, W. A review on the electroluminescence properties of quantum-dot light-emitting diodes. Org. Electron. 2021, 90, 106086. [Google Scholar] [CrossRef]
- Hu, Y.-X.; Xia, X.; He, W.-Z.; Tang, Z.-J.; Lv, Y.-L.; Li, X.; Zhang, D.-Y. Recent developments in benzothiazole-based iridium(Ⅲ) complexes for application in OLEDs as electrophosphorescent emitters. Org. Electron. 2019, 66, 126–135. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, H.; Liu, Z.; Zhao, B.; Li, W. The application of charge transfer host based exciplex and thermally activated delayed fluorescence materials in organic light-emitting diodes. Org. Electron. 2019, 66, 227–241. [Google Scholar] [CrossRef]
- Matsui, H.; Takeda, Y.; Tokito, S. Flexible and printed organic transistors: From materials to integrated circuits. Org. Electron. 2019, 75, 105432. [Google Scholar] [CrossRef]
- Wang, J.; Yao, H.; Xu, Y.; Ma, L.; Hou, J. Recent progress in reducing voltage loss in organic photovoltaic cells. Mater. Chem. Front. 2021, 5, 709–722. [Google Scholar] [CrossRef]
- Zheng, H.; Li, D.; Ran, C.; Zhang, Q.; Song, L.; Chen, Y.; Muller-Buschbaum, P.; Huang, W. Emerging Organic/Hybrid Photovoltaic Cells for Indoor Applications: Recent Advances and Perspectives. RRL Sol. 2021, 5, 2100042. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, F.; Cao, Y.; Li, D.; Liu, Q.; Liu, H.; Li, S. Progress on the stability and encapsulation techniques of perovskite solar cells. Org. Electron. 2022, 106, 106515. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Chapter 11-Imide Dyes. In Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–176. [Google Scholar]
- Gan, J.-A.; Song, Q.L.; Hou, X.Y.; Chen, K.; Tian, H. 1,8-Naphthalimides for non-doping OLEDs: The tunable emission color from blue, green to red. J. Photochem. Photobiol. A Chem. 2004, 162, 399–406. [Google Scholar] [CrossRef][Green Version]
- Ulla, H.; Kiran, M.R.; Garudachari, B.; Satyanarayan, M.N.; Umesh, G.; Isloor, A.M. Blue emitting halogen–phenoxy substituted 1,8-naphthalimides for potential organic light emitting diode applications. Opt. Mater. 2014, 37, 311–321. [Google Scholar] [CrossRef]
- Yordanova, S.; Grobchev, I.; Stoyanov, S.; Milusheva, V.; Petkov, I. Synthesis and functional characteristics of two new yellow-green fluorescent PAMAM dendrimers periphery modified with 1,8-naphthalimides. Inorg. Chim. Acta 2014, 409, 89–95. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Simeonov, D.B. Synthesis of highly photostable blue-emitting 1,8-naphthalimides and their acrylonitrile copolymers. Polym. Degrad. Stab. 2010, 95, 43–52. [Google Scholar] [CrossRef]
- Prezhdo, O.V.; Uspenskii, B.V.; Prezhdo, V.V.; Boszczyk, W.; Distanov, V.B. Synthesis and spectral-luminescent characteristics of N-substituted 1,8-naphthalimides. Dye. Pigment. 2007, 72, 42–46. [Google Scholar] [CrossRef]
- Kagatikar, S.; Sunil, D. A systematic review on 1,8-naphthalimide derivatives as emissive materials in organic light-emitting diodes. J.Mater. Sci. 2022, 57, 105–139. [Google Scholar] [CrossRef]
- Sonalin, S.; Sakthivel, K.; Nagarajan, S. Functionalization of 1, 8-Naphthalimides—An approach towards air-stable n- type organic semiconductors. Mater. Today Proc. 2018, 5, 16592–16597. [Google Scholar] [CrossRef]
- Poddar, M.; Sivakumar, G.; Misra, R. Donor–acceptor substituted 1,8-naphthalimides: Design, synthesis, and structure–property relationship. J. Mater. Chem. C 2019, 7, 14798–14815. [Google Scholar] [CrossRef]
- Saini, A.; Thomas, K.R.J.; Sachdev, A.; Gopinath, P. Photophysics, Electrochemistry, Morphology, and Bioimaging Applications of New 1,8-Naphthalimide Derivatives Containing Different Chromophores. Chem. Asian J. 2017, 12, 2612–2622. [Google Scholar] [CrossRef]
- Izawa, H.; Yasufuku, F.; Nokami, T.; Ifuku, S.; Saimoto, H.; Matsui, T.; Morihashi, K.; Sumita, M. Unique Photophysical Properties of 1,8-Naphthalimide Derivatives: Generation of Semi-stable Radical Anion Species by Photo-Induced Electron Transfer from a Carboxy Group. ACS Omega 2021, 6, 13456–13465. [Google Scholar] [CrossRef]
- Masimukku, N.; Gudeika, D.; Volyniuk, D.Y.; Bezvikonnyi, O.; Simokaitiene, J.; Matulis, V.; Lyakhov, D.; Azovskyi, V.; Gražulevičus, J.V. Bipolar 1,8-naphthalimides showing high electron mobility and red AIE-active TADF for OLED applications. Phys. Chem. Chem. Phys. 2022, 24, 5070–5082. [Google Scholar] [CrossRef]
- Rout, Y.; Misra, R. Design and synthesis of 1,8-naphthalimide functionalized benzothiadiazoles. New J. Chem. 2021, 45, 9838–9845. [Google Scholar] [CrossRef]
- Kelly, L.A.; Roll, M.; Joseph, J.; Seenisamy, J.; Barrett, J.; Kauser, K.; Warner, K.S. Solvent-Dependent Photophysics and Reactivity of Monomeric and Dimeric 4-Amino-1,8-Naphthalimides. J. Phys. Chem. A 2021, 125, 2294–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yang, Q. Tetraphenylethenyl-Modified 1,8-Naphthalimide Dye with Efficient Aggregation-Enhanced Emisssion, Solvatochromism and Intramolecular Charge Transfer Characteristics. J. Phys. Conf. Ser. 2021, 2109, 012025. [Google Scholar] [CrossRef]
- Adair, L.D.; Trinh, N.; Verite, P.M.; Jacquwmin, D.; Jolliffe, K.A.; New, E.J. Synthesis of Nitro-Aryl Functionalised 4-Amino-1,8-Naphthalimides and Their Evaluation as Fluorescent Hypoxia Sensors. Chem. A Eur. J. 2020, 26, 10064–10071. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, Z.; Fan, C.; Liu, G.; Pu, S. 1,8-Naphthalimide-Based Highly Emissive Luminophors with Various Mechanofluorochromism and Aggregation-Induced Characteristics. ACS Omega 2019, 4, 14324–14332. [Google Scholar] [CrossRef]
- Pablos, J.L.; Hernández, E.; Catalina, F.; Corrales, T. Solid Fluorescence pH Sensors Based on 1,8-Naphthalimide Copolymers Synthesized by UV Curing. Chemosensors 2022, 10, 73. [Google Scholar] [CrossRef]
- Betancourt, F.; Valente, A.; Yan, H. 1,8-Naphthalimide derivatives as probes for protein surface hydrophobicity. J. Photochem. Photobiol. A Chem. 2021, 418, 113386. [Google Scholar] [CrossRef]
- Saito, G.; Velluto, D.; Resmini, M. Synthesis of 1,8-naphthalimide-based probes with fluorescent switch triggered by flufenamic acid. R. Soc. Open Sci. 2018, 5, 172137. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Wei, T.-B.; Ma, X.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Sun, Y.-J.; Shi, B.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. 1,8-Naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Triboni, E.R.; Fernandes, M.R.; Garcia, J.R.; Carreira, M.C.; Berlinck, R.G.S.; Filho, P.B.; Roman, L.S.; Hümmelgen, I.A.; Reyes, R.; Cremona, M. Naphthalimide-derivative with blue electroluminescence for OLED applications. J. Taibah Univ. Sci. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Arunchai, R.; Sudyoadsuk, T.; Prachumrak, N.; Namuangruk, S.; Promarak, V.; Sukwattanasinitt, M.; Rashatasakhon, P. Synthesis and characterization of new triphenylamino-1,8-naphthalimides for organic light-emitting diode applications. New J. Chem. 2015, 39, 2807–2814. [Google Scholar] [CrossRef]
- Luo, S.; Lin, J.; Zhou, J.; Wang, Y.; Liu, X.; Huang, Y.; Lu, Z.; Hu, C. Novel 1,8-naphthalimide derivatives for standard-red organic light-emitting device applications. J. Mater. Chem. C 2015, 3, 5259–5267. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Siwy, M.; Filapek, M.; Kula, S.; Malecki, G.; Laba, K.; Lapkowski, M.; Janeczek, H.; Domanski, M. New core-substituted with electron-donating group 1,8-naphthalimides towards optoelectronic applications. J. Lumin. 2015, 166, 22–39. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Shanghui, Y.; Liu, Y. Electroluminesent divinylene- and trivinylene-molecules with terminal naphthalimide or phthalimide segments. Synth. Met. 2009, 159, 492–500. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Pająk, A.K.; Golba, S.; Małecki, J.G.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E. New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide). Materials 2021, 14, 2714. [Google Scholar] [CrossRef] [PubMed]
- Sęk, D.; Lapkowski, M.; Dudek, H.; Karoń, K.; Janeczek, H.; Jarząbek, B. Optical and electrochemical properties of three-dimensional conjugated triphenylamine-azomethine molecules. Synth. Met. 2012, 162, 1046–1051. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Malarz, K.; Krystkowska, A.; Mrozek-Wilczkiewicz, A.; Golba, S.; Siwy, M.; Maćkowski, S.; Schab-Balcerzak, E. Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives. Materials 2021, 14, 5504. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef] [PubMed]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Gaudin, O.P.M.; Samuel, I.D.W.; Amriou, S.; Burn, P.L. Thickness dependent absorption spectra in conjugated polymers: Morphology or interference? Appl. Phys. Lett. 2010, 96, 053305. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Janssen, R.A.J. The Effect of H- and J-Aggregation on the Photophysical and Photovoltaic Properties of Small Thiophene–Pyridine–DPP Molecules for Bulk-Heterojunction Solar Cells. Adv. Funct. Mater. 2017, 27, 1605779. [Google Scholar] [CrossRef]
- Glowacki, I.; Szamel, Z. The nature of trapping sites and recombination centres in PVK and PVK–PBD electroluminescent matrices seen by spectrally resolved thermoluminescence. J. Phys. D Appl. Phys. 2010, 43, 295101. [Google Scholar] [CrossRef]
- Korzec, M.; Kotowicz, S.; Gawecki, R.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Siwy, M.; Schab-Balcerzak, E.; Grzelak, J.; Maćkowski, S. 1,8-Naphthalimides 3-substituted with imine or β-ketoenamine unit evaluated as compounds for organic electronics and cell imaging. Dye. Pigment. 2021, 193, 109508. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Siwy, M.; Golba, S.; Malecki, J.G.; Janeczek, H.; Mackowski, S.; Bednarczyk, K.; Libera, M.; Schab-Balcerzak, E. Novel 1,8-naphthalimides substituted at 3-C position: Synthesis and evaluation of thermal, electrochemical and luminescent properties. Dye. Pigment. 2018, 158, 65–78. [Google Scholar] [CrossRef]
- Skórka, Ł.; Kurzep, P.; Wiosna-Sałyga, G.; Łuszczyńska, B.; Wielgus, I.; Wróbel, Z.; Ulański, J.; Kulszewicz-Bajer, I. New diarylaminophenyl derivatives of carbazole: Effect of substituent position on their redox, spectroscopic and electroluminescent properties. Synth. Met. 2017, 228, 1–8. [Google Scholar] [CrossRef]
- Kotwica, K.; Bujak, P.; Wamil, D.; Pieczonka, A.; Wiosna-Salyga, G.; Gunka, P.A.; Jaroch, T.; Nowakowski, R.; Luszczynska, B.; Witkowska, E.; et al. Structural, Spectroscopic, Electrochemical, and Electroluminescent Properties of Tetraalkoxydinaphthophenazines: New Solution-Processable Nonlinear Azaacenes. J. Phys. Chem. C 2015, 119, 10700–10708. [Google Scholar] [CrossRef]
- Hussain, S.A. An introduction to fluorescence resonance energy transfer (FRET). arXiv 2009. [Google Scholar] [CrossRef]
- Yersin, H. Highly Efficient OLEDs with Phosphorescent Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 978-527-40594-7. [Google Scholar]
- Chen, T.; Lu, C.-H.; Huang, C.-W.; Zeng, X.; Gao, J.; Chen, Z.; Xiang, Y.; Zeng, W.; Huang, Z.; Gong, S.; et al. Tuning the emissive characteristics of TADF emitters by fusing heterocycles with acridine as donors: Highly efficient orange to red organic light-emitting diodes with EQE over 20%. J. Mater. Chem. C 2019, 7, 9087–9094. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron den sity. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Casida, M.E. Recent Developments and Applications of Modern Density Functional Theory, Theoretical and Computational Chemistry; Seminario, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 4, p. 391. [Google Scholar]
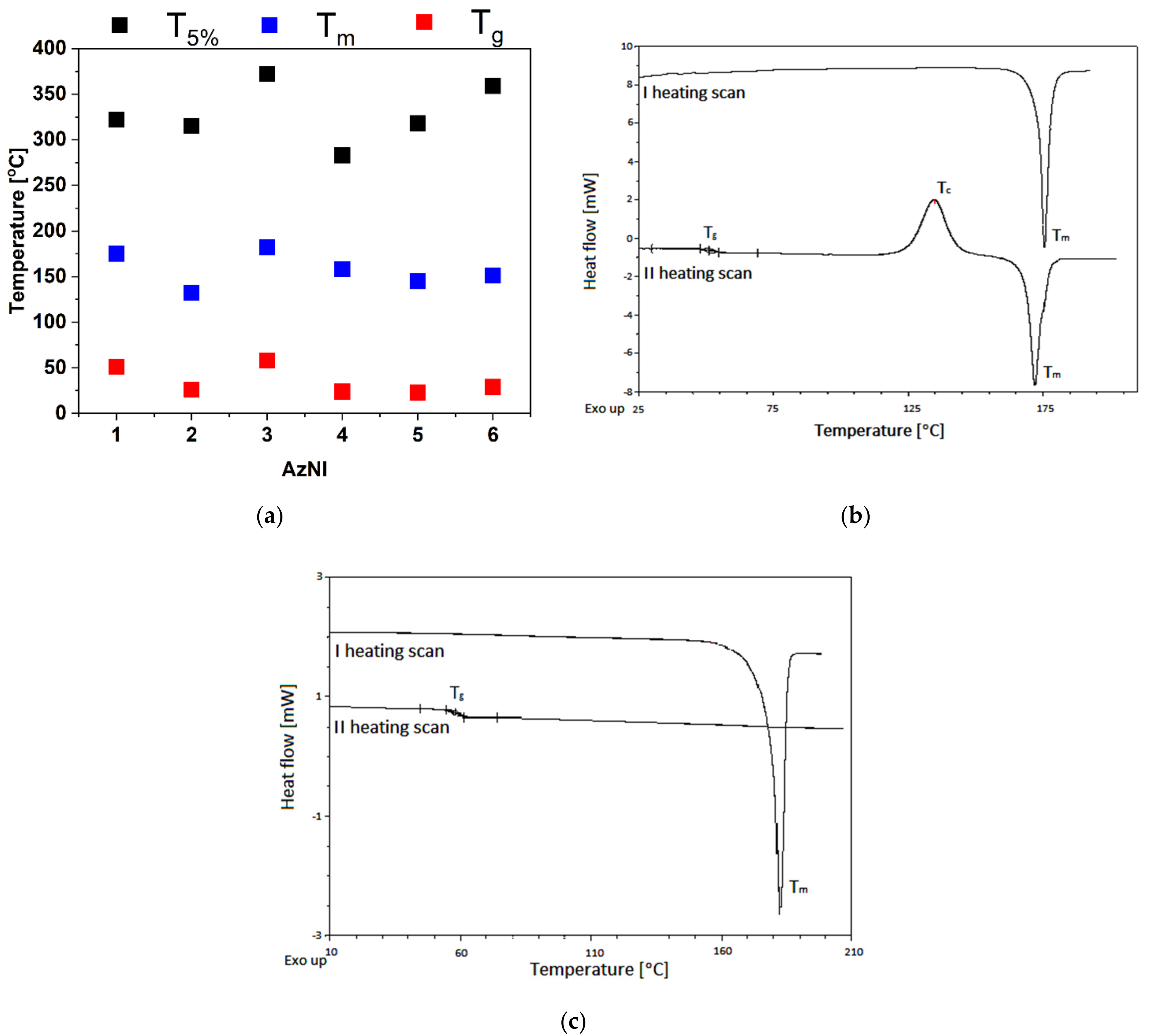

| Molecule | TGA | DSC | |||||
|---|---|---|---|---|---|---|---|
| I Heating Scan | II Heating Scan | ||||||
| T5 a (°C) | Tmax b (°C) | Residue | Tm c | Tg e | Tc d | Tm c | |
| at 600 °C (%) | (°C) | (°C) | (°C) | (°C) | |||
| AzNI-1 | 322 | 411 | 21 | 175 | 51 | 135 | 172 |
| AzNI-2 | 315 | 380 | 4 | 132 | 26 | 120 | 131 |
| AzNI-3 | 372 | 460 | 10 | 182 | 58 | nd | nd |
| AzNI-4 | 283 | 301,407 | 8 | 158 | 24 | 127 | 160 |
| AzNI-5 | 318 | 387 | 2 | 145 | 23 | nd | nd |
| AzNI-6 | 359 | 527 | 50 | 151 | 29 | 90 | 151 |
| Molecule | Method | Ered1 | Ered1(onset) | Eox1 | Eox1(onset) | ELUMO | LUMO a | EHOMO | HOMO a | Eg |
|---|---|---|---|---|---|---|---|---|---|---|
| [V] | [V] | [V] | [V] | [eV] | [eV] | [eV] | [eV] | [eV] | ||
| AzNI–1 | CV | −1.69 a | −1.26 | 0.82 a | 0.66 | −3.84 | −2.50 | −5.76 | −5.48 | 1.92 |
| DPV | −1.58 | −1.34 | 0.73 | 0.62 | −3.76 | −5.72 | 1.96 | |||
| AzNI–2 | CV | −1.88 b | −1.75 | 0.87 a | 0.66 | −3.35 | −2.46 | −5.76 | −5.89 | 2.41 |
| DPV | −1.92 | −1.65 | 0.78 | 0.56 | −3.45 | −5.66 | 2.21 | |||
| AzNI–3 | CV | −1.92 a | −1.33 | 0.95 a | 0.81 | −3.77 | −2.48 | −5.91 | −5.54 | 2.14 |
| DPV | −1.71 | −1.39 | 0.72 | 0.62 | −3.71 | −5.72 | 2.01 | |||
| AzNI–4 | CV | −1.98 a | −1.52 | 0.69 a | 0.45 | −3.58 | −2.40 | −5.55 | −5.46 | 1.97 |
| DPV | −1.73 | −1.41 | 0.49 | 0.31 | −3.69 | −5.41 | 1.72 | |||
| AzNI–5 | CV | −1.86 b | −1.73 | 0.75 a | 0.55 | −3.37 | −2.45 | −5.65 | −6.01 | 2.28 |
| DPV | −1.85 | −1.67 | 0.73 | 0.59 | −3.43 | −5.69 | 2.26 | |||
| AzNI–6 | CV | −1.85 a | −1.61 | 0.74 a | 0.64 | −3.49 | −2.48 | −5.74 | −5.86 | 2.25 |
| DPV | −1.82 | −1.60 | 0.64 | 0.49 | −3.50 | −5.59 | 2.09 |
| Molecule | CHCl3 | CO(CH3)2 | CH3CN | Film | Blend PVK:PBD | Blend PVK:PBD |
|---|---|---|---|---|---|---|
| λmax (ε × 104) a (nm) | 2 wt.%b | 15 wt.%b | ||||
| AzNI-1 | - | - | - | - | 310 sh | 310 sh |
| 331(2.1) | 347 sh | 344 sh | 344 sh | |||
| 411(2.5) | 403(1.7) | 406(1.9) | 424 | - | 425 | |
| - | - | - | 310 sh | 310 sh | ||
| AzNI–2 | 338(7.5) | 337(3.6) | 334(3.6) | 344 sh | 344 sh | |
| 378 sh | 375 sh | 375 sh | - | - | ||
| AzNI–3 | - | - | - | - | 310 sh | 310 sh |
| 346 sh | 337(4.6) | - | - | 344 sh | 344 sh | |
| 381(3.2) | - | 378(4.9) | - | - | 383 | |
| 405(3.0) | 400 sh | 400 sh | 420 | - | 405 | |
| AzNI–4 | 349(4.3) | 348(5.7) | 348 sh | 344 | 310 sh | 310 sh |
| 344 sh | 344 sh | |||||
| AzNI–5 | 323(5.5) | 340(3.9) | 318(24.4) | - | 310 sh | 310 sh |
| 344 sh | 344 sh | |||||
| AzNI–6 | 335(6.3) | 340(6.1) | 319(7.1) | 356 | 310 sh | 310 sh |
| 344 sh | 344 sh | |||||
| Molecule | Medium | λmax | λem | Stokes Shift c | Φ | τeff | X2 | kr·106 d | knr·106 d |
|---|---|---|---|---|---|---|---|---|---|
| (nm) | (nm) | (cm−1) | (%) | (ns) | (s−1) | (s−1) | |||
| AzNI-1 | CHCl3 | 331 | 511 | 10,642 | 2.00 | 14.01 | 0.986 | 1.43 | 69.95 |
| 411 | 511 | 4761 | 0.42 | - | - | - | - | ||
| CO(CH3)2 | 403 | 511 | 5244 | - | - | - | - | - | |
| CH3CN | 406 | 508 | 4946 | 0.27 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 380;470 | 5942 | 3.42;1.56 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 380;494 | 5942 | 3.26;2.06 | - | - | - | - | |
| AzNI-2 | CHCl3 | 338 | 511 | 10,016 | 2.45 | 9.81 | 1.029 | 2.50 | 99.44 |
| CO(CH3)2 | 337 | 506 | 9911 | - | - | - | - | - | |
| CH3CN | 334 | 510 | 10,332 | 0.22 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 379;493 | 5873 | 4.02;1.41 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 379;493 sh | 55,873 | 3.58 | - | - | - | - | |
| AzNI-3 | CHCl3 | 381 | 499 | 6207 | 1.40 | 9.48 | 1.159 | 1.48 | 104.01 |
| 405 | 509 | 5045 | 0.20 | - | - | - | - | ||
| CO(CH3)2 | 337 | 513 | 10,180 | - | - | - | - | - | |
| CH3CN | 378 | 508 | 6770 | 0.11 | - | - | - | - | |
| Film | 420 | 547 | 5528 | 3.52 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 392;478 | 6748 | 2.50;2.10 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 377;494 | 5733 | 4.50;1.95 | - | - | - | - | |
| AzNI-4 | CHCl3 | 349 | 523 | 9533 | 4.42 | 8.25 | 1.028 | 5.36 | 115.85 |
| CO(CH3)2 | 348 | 511 | 9166 | - | - | - | - | - | |
| CH3CN | 348 | 511 | 9166 | 0.69 | - | - | - | - | |
| Film | 344 | 566 | 11,402 | 2.74 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 384;495 | 6216 | 2.40;1.70 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 380;513 | 5942 | 2.60;4.60 | - | - | - | - | |
| AzNI-5 | CHCl3 | 323 | 511 | 11,390 | 2.65 | 10.89 | 1.069 | 2.43 | 89.39 |
| CO(CH3)2 | 340 | 512 | 9881 | - | - | - | - | - | |
| CH3CN | 318 | 507 | 11,723 | 0.32 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 378;492 | 5803 | 3.70;1.87 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 378;494 sh | 5803 | 4.82 | - | - | - | - | |
| AzNI-6 | CHCl3 | 335 | 508 | 10,166 | 0.46 | 15.68 | 1.069 | 0.29 | 63.48 |
| CO(CH3)2 | 340 | 513 | 9919 | - | - | - | - | - | |
| CH3CN | 319 | 504 | 11,507 | 0.51 | - | - | - | - | |
| Film | 356 | 411 | 3759 | 2.13 | - | - | - | - | |
| Blend PVK:PBD a | 310 | 395;472 | 6942 | 2.80;2.50 | - | - | - | - | |
| Blend PVK:PBD b | 310 | 383;496 | 6148 | 3.50;2.10 | - | - | - | - |
| Devices Parameters | |||
|---|---|---|---|
| The Active Layer | λEL a | ELMax b | UELMax c |
| (nm) | (counts) | (V) | |
| PVK:PBD:AzNI-1 2 wt.% | 513 | 7580 | 23 |
| PVK:PBD:AzNI-1 15 wt.% | 620 | 4233 | 22 |
| PVK:PBD:AzNI-3 2 wt.% | 506 | 30,430 | 26 |
| PVK:PBD:AzNI-3 15 wt.% | 560 | 3931 | 19 |
| PVK:PBD:AzNI-4 2 wt.% | 526 | 5187 | 23 |
| PVK:PBD:AzNI-4 15 wt.% | 551 | 13,419 | 25 |
| PVK:PBD:AzNI-6 2 wt.% | 506 | 41,681 | 21 |
| PVK:PBD:AzNI-6 15 wt.% | 519 | 20,781 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotowicz, S.; Korzec, M.; Małecki, J.G.; Golba, S.; Siwy, M.; Maćkowski, S.; Schab-Balcerzak, E. Six New Unsymmetrical Imino-1,8-naphthalimide Derivatives Substituted at 3-C Position—Photophysical Investigations. Materials 2022, 15, 7043. https://doi.org/10.3390/ma15197043
Kotowicz S, Korzec M, Małecki JG, Golba S, Siwy M, Maćkowski S, Schab-Balcerzak E. Six New Unsymmetrical Imino-1,8-naphthalimide Derivatives Substituted at 3-C Position—Photophysical Investigations. Materials. 2022; 15(19):7043. https://doi.org/10.3390/ma15197043
Chicago/Turabian StyleKotowicz, Sonia, Mateusz Korzec, Jan Grzegorz Małecki, Sylwia Golba, Mariola Siwy, Sebastian Maćkowski, and Ewa Schab-Balcerzak. 2022. "Six New Unsymmetrical Imino-1,8-naphthalimide Derivatives Substituted at 3-C Position—Photophysical Investigations" Materials 15, no. 19: 7043. https://doi.org/10.3390/ma15197043
APA StyleKotowicz, S., Korzec, M., Małecki, J. G., Golba, S., Siwy, M., Maćkowski, S., & Schab-Balcerzak, E. (2022). Six New Unsymmetrical Imino-1,8-naphthalimide Derivatives Substituted at 3-C Position—Photophysical Investigations. Materials, 15(19), 7043. https://doi.org/10.3390/ma15197043