Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler
Abstract
1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
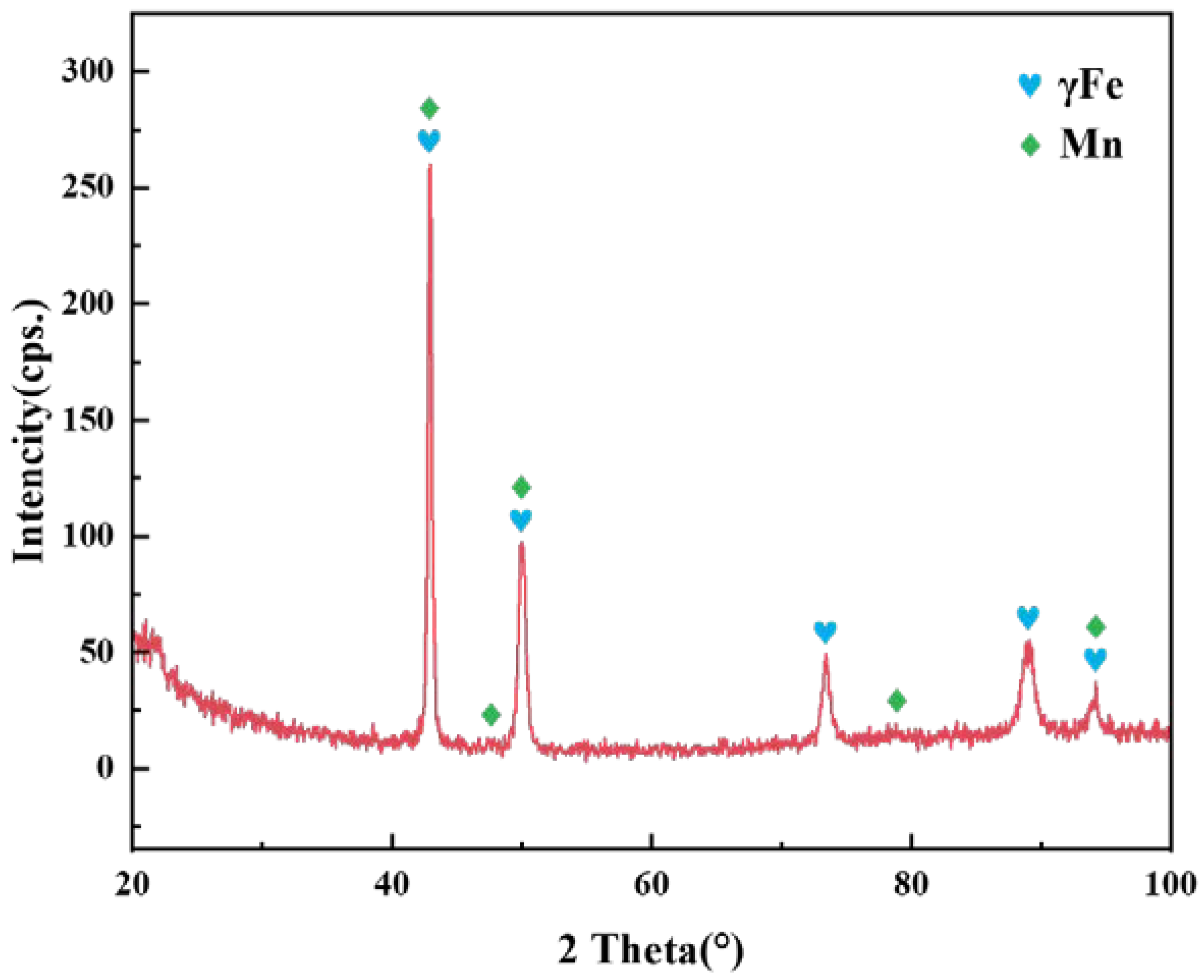
3.1. Typical Microstructure of the 1Cr21Ni5Ti/1Cr18Ni9Ti Joint Brazed Using BMn70NiCr Filler
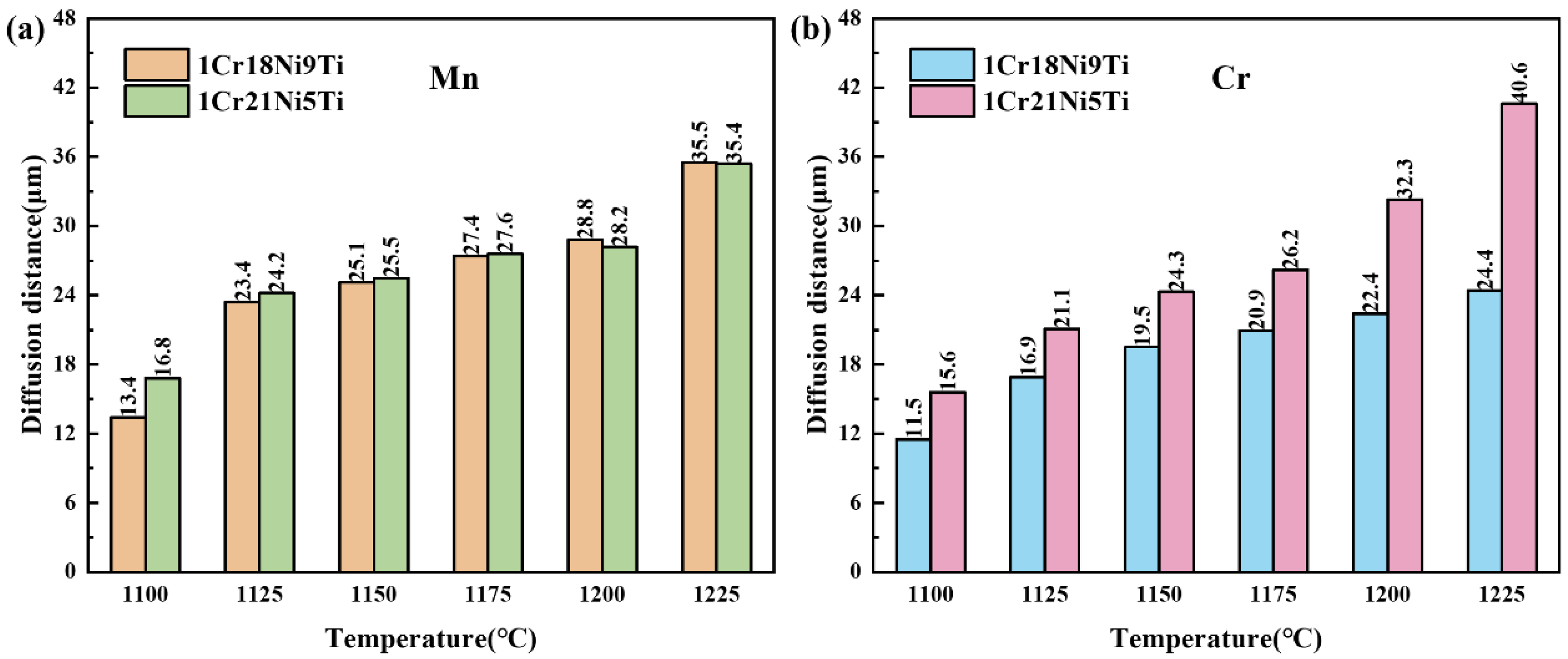
3.2. Effect of Brazing Temperature on the Microstructure and Mechanical Properties of 1Cr21Ni5Ti/BMn70NiCr/1Cr18Ni9Ti Joints
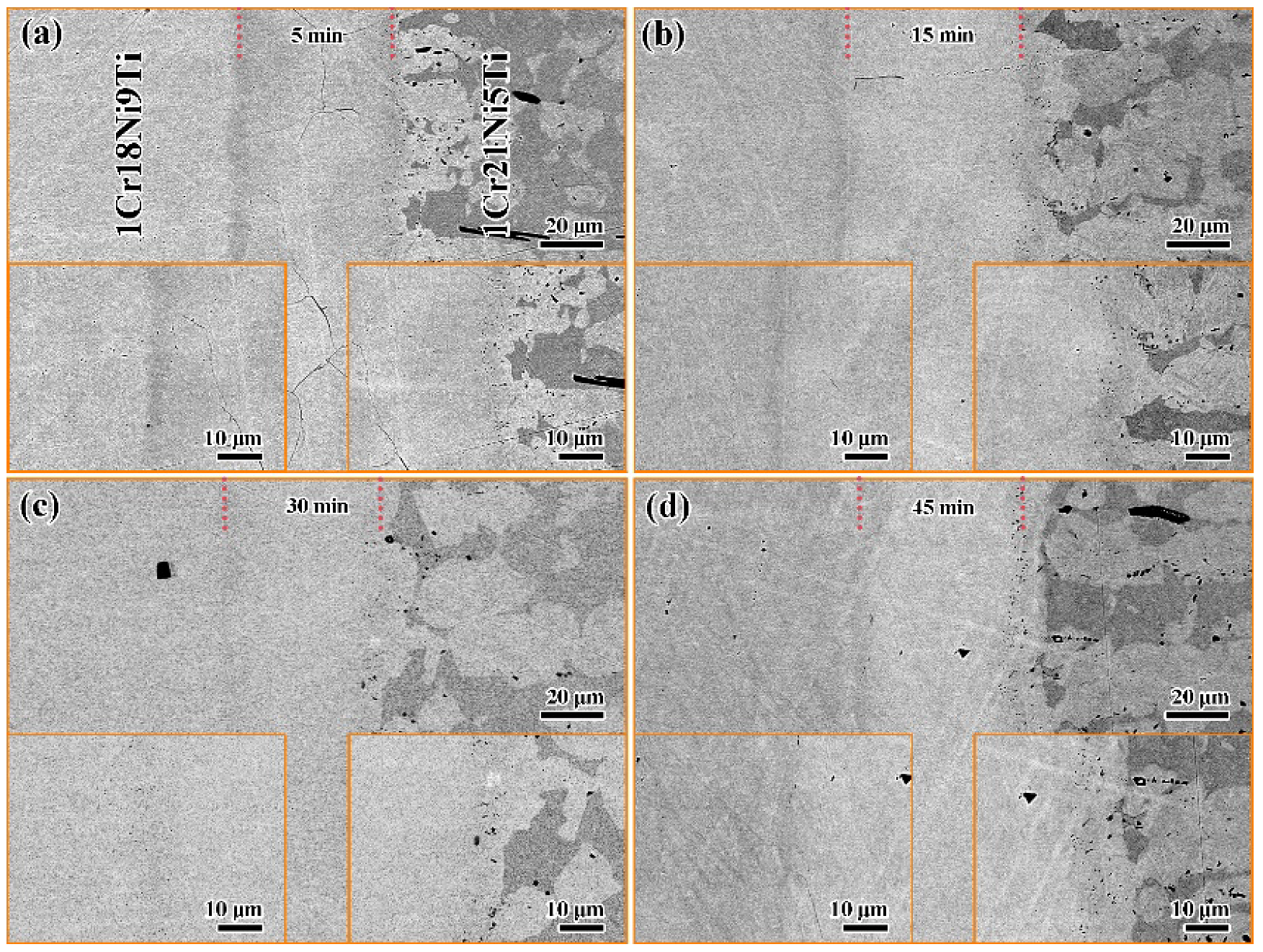
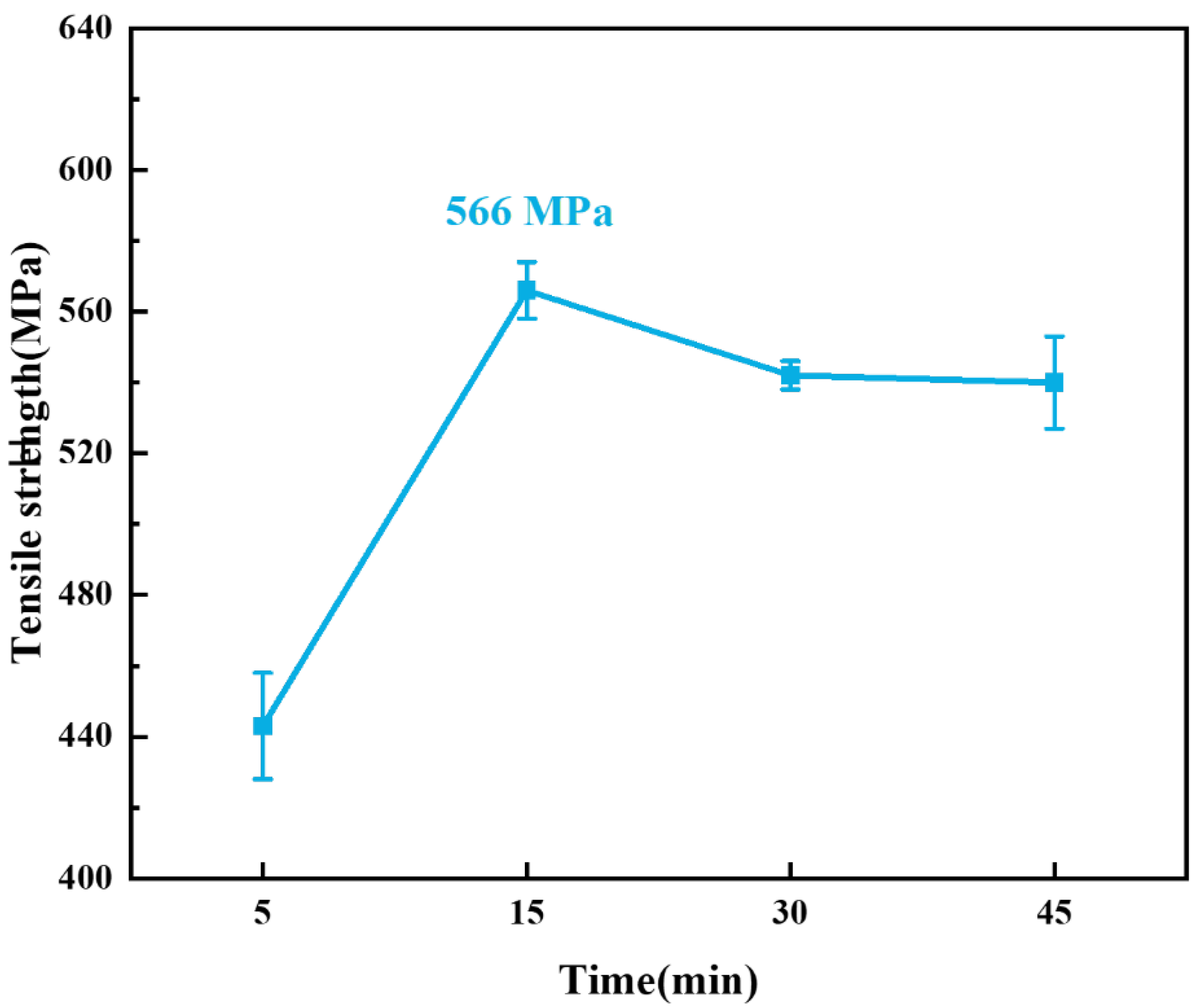
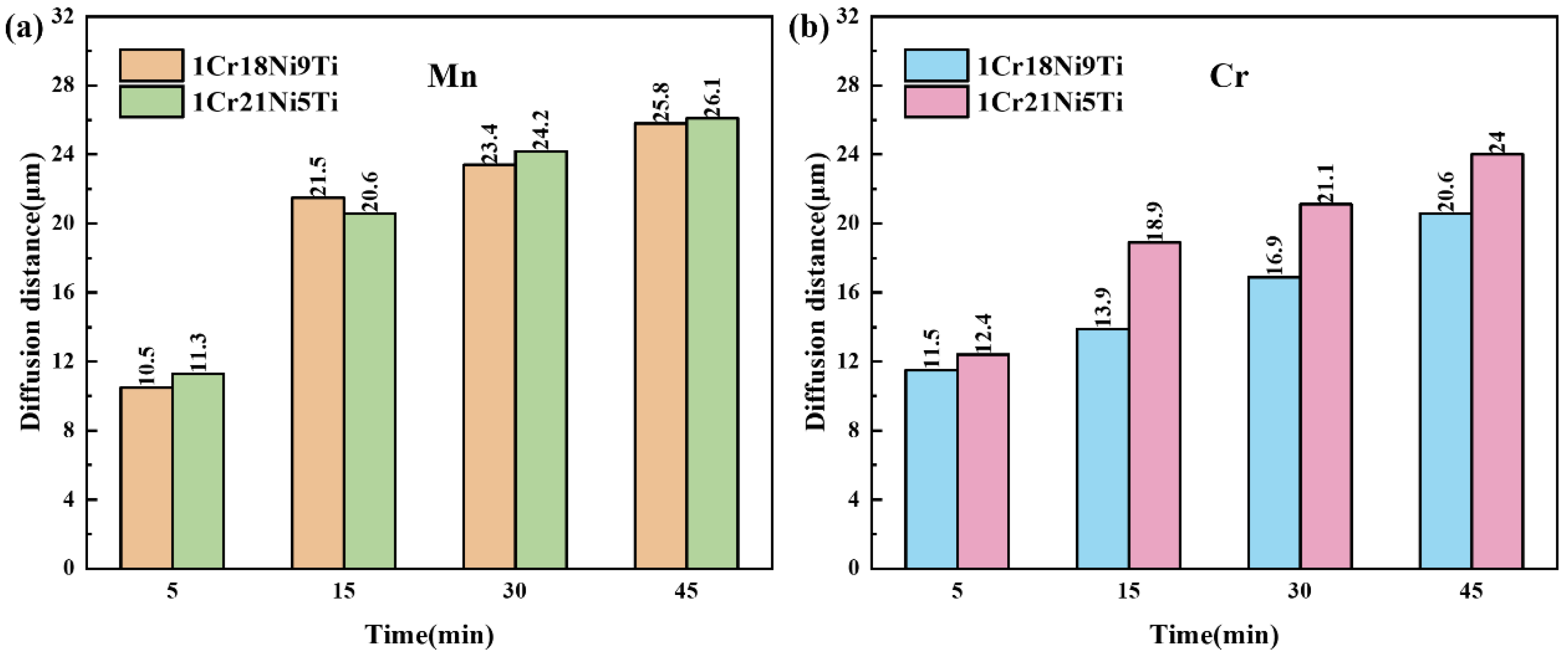
3.3. Effect of Holding Time on the Microstructure and Mechanical Properties of 1Cr21Ni5Ti/BMn70NiCr/1Cr18Ni9Ti Joints
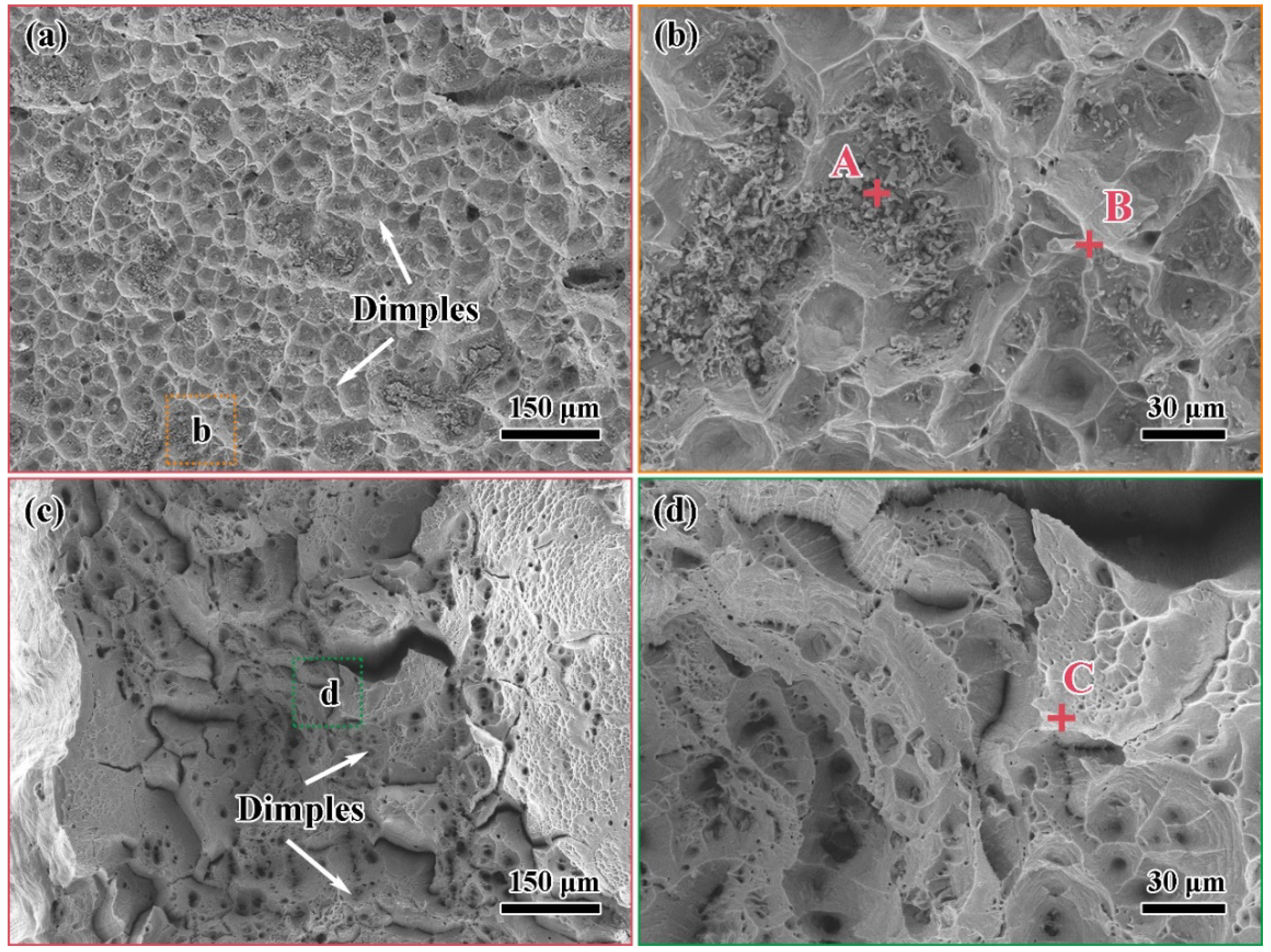
3.4. Fracture Analysis
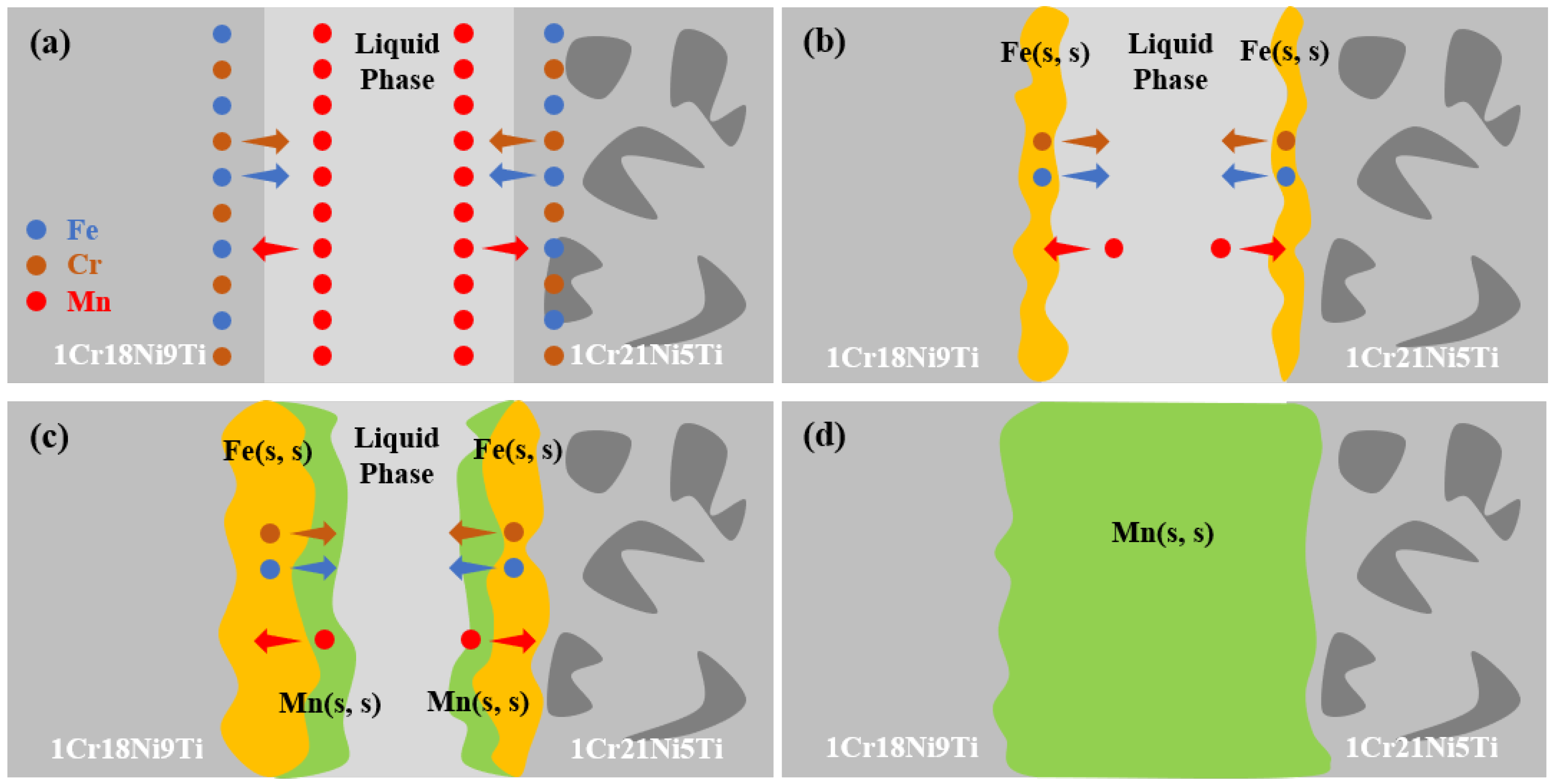
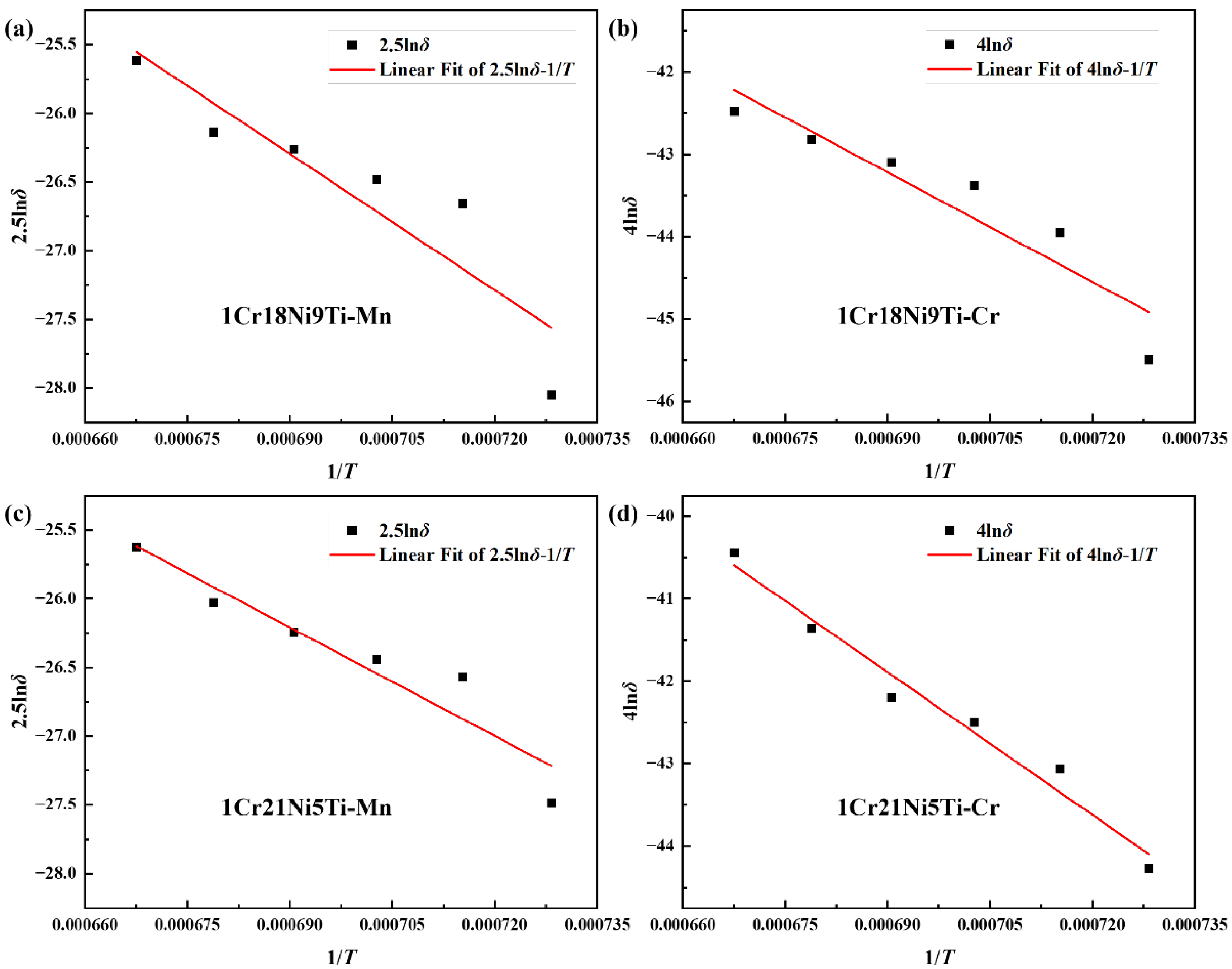
3.5. Diffusion Behavior of Mn and Cr in 1Cr21Ni5Ti/BMn70NiCr/1Cr18Ni9Ti Joints
4. Conclusions
- (1)
- The 1Cr18Ni9Ti/1Cr21Ni5Ti joints were successfully brazed using BMn70NiCr filler at 1175 °C/30 min without voids and cracks. The typical microstructure in the joint was 1Cr18Ni9Ti/Mn(s, s)/1Cr21Ni5Ti.
- (2)
- The brazing temperature, holding time, and brazing seam width affected the microstructure of the 1Cr18Ni9Ti/BMn70NiCr/1Cr21Ni5Ti joint. When the brazing temperature is low and the holding time was short, there were voids and cracks in the brazed seam due to insufficient elements’ diffusion.
- (3)
- The tensile strengths of the brazed joint first increased and then decreased with the rising of the brazing temperature and holding time. The maximum tensile strength of the joint was 566 MPa under the parameter of 1125 °C/15 min. When the temperature was too high or the holding time was too long, the tensile strength of the joint decreased due to the grains growth in 1Cr18Ni9Ti.
- (4)
- With the increase of the brazing temperature and the holding time, the diffusion rate and quantity of Mn or Cr increased, which led to a larger diffusion distance in the stainless steels. The formation of the liquid phase during the brazing process made the diffusion of Mn and Cr easier.
- (5)
- The difference in atomic concentration was the main driving force for the diffusion of Mn or Cr. The diffusion activation energy of the Mn element in 1Cr18Ni9Ti was 1.10 × 105 J/mol, and in 1Cr21Ni5Ti was 8.76 × 104 J/mol. The diffusion activation energy of Cr element in 1Cr18Ni9Ti was 9.22 × 104 J/mol, and in 1Cr21Ni5Ti was 1.20 × 105 J/mol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, F.; Hu, S.; Xu, R.; Han, X.; Qian, D.; Wei, W.; Hua, L.; Zhao, K. Strain rate sensitivity of the ultrastrong gradient nanocrystalline 316L stainless steel and its rate-dependent modeling at nanoscale. Int. J. Plast. 2020, 129, 102696. [Google Scholar] [CrossRef]
- An, L.; Cao, J.; Zhang, T.; Yang, Y. Cr diffusion and continuous repairing behavior during high-temperature oxidation of duplex stainless steel. Mater. Corros. 2017, 68, 1116–1122. [Google Scholar] [CrossRef]
- Wonneberger, R.; Seyring, M.; Freiberg, K.; Carlsson, A.; Rensberg, J.; Abendroth, B.; Stöcker, H.; Rettenmayr, M.; Undisz, A. Oxidation of stainless steel 316L–Oxide grains with pronounced inhomogeneous composition. Corros. Sci. 2018, 149, 178–184. [Google Scholar] [CrossRef]
- Oke, S.R.; Ige, O.O.; Falodun, O.E.; Okoro, A.M.; Mphahlele, M.R.; Olubambi, P.A. Powder metallurgy of stainless steels and composites: A review of mechanical alloying and spark plasma sintering. Int. J. Adv. Manuf. Technol. 2019, 102, 3271–3290. [Google Scholar] [CrossRef]
- Xie, B.; Guo, C.; Zhang, S.; Wang, Q.; Xu, Z.; Tan, Y.; Wang, M. The impact of brazing process on mechanical properties of S30408 stainless steel by experimental investigation. J. Constr. Steel Res. 2020, 175, 106326. [Google Scholar] [CrossRef]
- Manladan, S.; Zhang, Y.; Ramesh, S.; Cai, Y.; Ao, S.; Luo, Z. Resistance element welding of magnesium alloy and austenitic stainless steel in three-sheet configurations. J. Mater. Process. Technol. 2019, 274, 116292. [Google Scholar] [CrossRef]
- Soltani, H.M.; Tayebi, M. Comparative study of AISI 304L to AISI 316L stainless steels joints by TIG and Nd:YAG laser welding. J. Alloys Compd. 2018, 767, 112–121. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, Q.; Wang, F.; Zan, S.; Zhang, W. Numerical and experimental investigation on joining CFRTP and stainless steel using fiber lasers. J. Mater. Process. Technol. 2017, 240, 362–369. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Qu, Z.; Zhu, C.; Wang, X. Nondestructive testing of local incomplete brazing defect in stainless steel core panel using pulsed eddy current. Materials 2022, 15, 5689. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, H.; Awais, A.A.; Kim, S.; Kim, K.C. Experimental and numerical study on the thermal and hydraulic characteristics of porous-media heat exchangers in cryogenic conditions. Appl. Therm. Eng. 2022, 216, 119095. [Google Scholar] [CrossRef]
- Shi, X.; Li, Y.; Bai, Y.; Zheng, X.; Yao, S.; Zhu, Z.; Chen, H. Effect of Ni in pure Cu/304 stainless steel induction brazing joints. Mater. Charact. 2021, 182, 111562. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Si, X.; Wang, X.; Wang, Z.; Cao, J. A novel Ag–CuAlO2 sealant for reactive air brazing of 3YSZ and AISI 310S. Ceram. Int. 2021, 47, 31413–31422. [Google Scholar] [CrossRef]
- Luu, V.T.; Dinh, T.K.A.; Das, H.; Kim, J.-R.; Hong, S.-T.; Sung, H.-M.; Han, H.N. Diffusion enhancement during electrically assisted brazing of ferritic stainless steel alloys. Int. J. Precis. Eng. Manuf. Technol. 2018, 5, 613–621. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, H.; Lu, B.; Lu, F. The microstructural evolution of vacuum brazed 1Cr18Ni9Ti using various filler metals. Materials 2017, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Duan, P.-Y.; Liao, P.-P.; Zhou, G.-Y.; Tu, S.-T. Effect of temperature on metallurgical reactions and microstructure evolution of 316L/BNi-2 brazed joints. J. Mater. Eng. Perform. 2021, 31, 1631–1641. [Google Scholar] [CrossRef]
- Paidar, M.; Ravikumar, M.; Ojo, O.; Mehrez, S.; Mohanavel, V.; Ravichandran, M. Diffusion brazing of 321 stainless steel to IN738 using 54Ag-40Cu-5.0Zn-1.0Ni powder-mixture interlayer. Mater. Lett. 2021, 297, 129919. [Google Scholar] [CrossRef]
- Beura, V.K.; Xavier, V.; Venkateswaran, T.; Kulkarni, K.N. Interdiffusion and microstructure evolution during brazing of austenitic martensitic stainless steel and aluminum-bronze with Ag-Cu-Zn based brazing filler material. J. Alloys Compd. 2018, 740, 852–862. [Google Scholar] [CrossRef]
- Venkateswaran, T.; Xavier, V.; Sivakumar, D.; Pant, B.; GD, J.R. Brazing of stainless steels using Cu-Ag-Mn-Zn braze filler: Studies on wettability, mechanical properties, and microstructural aspects. Mater. Des. 2017, 121, 213–228. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, H.; Hao, X.; Li, P.; Li, S. Vacuum brazing of Ti6Al4V alloy to 316L stainless steel using a Ti-Cu-based amorphous filler metal. J. Mater. Process. Technol. 2019, 269, 35–44. [Google Scholar] [CrossRef]
- Luca, M.A.; Tierean, M.H.; Pisu, T.M.; Rodriguez, J.; Croitoru, C. The influence of concentrated solar energy flux on the structure and properties of stainless steel brazed joints. J. Therm. Anal. 2019, 141, 1291–1304. [Google Scholar] [CrossRef]
- Vodă, M.; Codrean, C.; Chicot, D.; Şerban, V.-A.; Uţu, D.; Linul, E.; Buzdugan, D. Characterization of brazed joints by electrical resistance spot brazing with Ni-based amorphous self-flux alloys. J. Manuf. Process. 2019, 37, 617–627. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, N.; Yan, J.; Cao, Y. The microstructure and mechanical properties of 1Cr17Ni2/QAl7 brazed joints using Cu-Mn-Ni-Ag brazing alloy. Mater. Sci. Eng. A 2016, 661, 25–31. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Li, Y.; Zhang, M.; Xu, Y. Boron-containing intermetallic compounds in Mn-Cu-Al/430 stainless steel brazed joints formed under a borate brazing flux. Mater. Lett. 2020, 260, 126977. [Google Scholar] [CrossRef]
- Mou, G.; Zheng, K.; Shen, C.; Xiang, H.; Hua, X.; He, L. Microstructure investigation and fracture mechanism of TC4−304L dissimilar joints fabricated by the cold metal transfer arc-brazing method. J. Mater. Res. Technol. 2021, 15, 6758–6768. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, P.; Zhou, Y.N. Laser welding-brazing of NiTi/304 stainless steel wires with beam defocus and large offset. Mater. Sci. Eng. A 2022, 835, 142660. [Google Scholar] [CrossRef]
- Vetrivendan, E.; Varghese, P.; Krishnan, R.; Mathews, T.; Ningshen, S. Vacuum brazing of copper and SS316L using Ni–P eutectic alloy filler for nuclear applications. Weld. World 2022, 66, 2041–2052. [Google Scholar] [CrossRef]
- Kemmenoe, D.J.; Theisen, E.A.; Baker, S.P. Strength of 444 stainless steel single-lap joints brazed with Ni-based metallic glass foils for corrosive environments. Met. Mater. Trans. A 2022, 53, 1407–1418. [Google Scholar] [CrossRef]
- Niyaraki, M.N.; Nouri, M.D. Investigation of microstructure and mechanical behavior of AISI420/ BNi-2 brazed joints under impact loading at high strain rates with the split Hopkinson bar. Weld. World 2022, 66, 1957–1973. [Google Scholar] [CrossRef]
- Shi, H.; Yan, J.; Li, N.; Zhu, X.; Chen, K.; Yu, L. Microstructure and properties of FeCrMo damping alloy brazing joint using Mn-based brazing filler. Vacuum 2018, 159, 209–217. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Liu, Z.-P.; Zhou, G.-Y.; Tu, S.-T. Study on element diffusion behaviour of vacuum-furnace brazing 316 L/BNi-2 joints based on Boltzmann-Matano model. Weld. World 2021, 65, 2239–2246. [Google Scholar] [CrossRef]
- Xin, C.; Yan, J.; Wang, Q.; Feng, W.; Xin, C. The microstructural evolution and formation mechanism in Si3N4/AgCuTi/Kovar braze joints. J. Alloys Compd. 2019, 820, 153189. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J.; Duan, T.; Liu, C. Microstructure evolution and brazing mechanisms of the Ti2AlC/Ni joints using nickel based filler alloy. J. Eur. Ceram. Soc. 2016, 36, 3319–3327. [Google Scholar] [CrossRef]
- Bridges, D.; Nielsen, B.; Zhang, L.; Zhang, S.; Xu, R.; Hu, A. Wettability, diffusion behaviors, and modeling of Ni nanoparticles and nanowires in brazing inconel 718. Adv. Eng. Mater. 2020, 23, 2001053. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.; Xue, F.; Chen, X. Interfacial structure and growth kinetics of intermetallic compounds between Sn-3.5Ag solder and Al substrate during solder process. J. Alloys Compd. 2016, 682, 627–633. [Google Scholar] [CrossRef]
- Du, Y.; Chang, Y.A.; Huang, B.; Gong, W.; Jin, Z.; Xu, H.; Yuan, Z.; Liu, Y.; He, Y.; Xie, F.-Y. Diffusion coefficients of some solutes in fcc and liquid Al: Critical evaluation and correlation. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2003, 363, 140–151. [Google Scholar] [CrossRef]
- Shakiba, M.; Parson, N.; Chen, X.-G. Hot deformation behavior and rate-controlling mechanism in dilute Al–Fe–Si alloys with minor additions of Mn and Cu. Mater. Sci. Eng. A 2015, 636, 572–581. [Google Scholar] [CrossRef]
- Sprengel, W.; Lograsso, T.A.; Nakajima, H. Mn self-diffusion in single grain icosahedralAl70Pd21.5Mn8.5 quasicrystals. Phys. Rev. Lett. 1996, 77, 5233–5236. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Zhang, S.; Zhang, M. Brazing of Mn–Cu alloy and 430 stainless steel with Cu–34Mn–6Ni–10Sn filler metal. Mater. Trans. 2019, 60, 1674–1679. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Zhang, S.; Zhang, M. Joining of Mn-Cu alloy and 430 stainless steel using Cu-based filler by SIMA-imitated brazing process. Mater. Lett. 2019, 253, 401–404. [Google Scholar] [CrossRef]
- Liang, Q.; Xia, C.; Xu, X.; Zou, J. Research on vacuum brazing of W-Cu composite to stainless steel with Cu-Mn-Co brazing metal. Sci. Eng. Compos. Mater. 2015, 22, 245–250. [Google Scholar] [CrossRef]
- Dai, J.-H.; Jiang, B.; Peng, C.; Pan, F.-S. Effect of Mn additions on diffusion behavior of Fe in molten magnesium alloys by solid-liquid diffusion couples. J. Alloys Compd. 2017, 710, 260–266. [Google Scholar] [CrossRef]
- Huang, W. An assessment of the Fe-Mn system. Calphad 1989, 13, 243–252. [Google Scholar] [CrossRef]
- Zhang, W.; Cong, S.; Huang, Y.; Tian, Y.H. Micro structure and mechanical properties of vacuum brazed martensitic stainless steel/tin bronze by Ag-based alloy. J. Mater. Process. Technol. 2017, 248, 64–71. [Google Scholar] [CrossRef]
- Fujita, N.; Sahara, R.; Narushima, T.; Ouchi, C. Austenitic Grain Growth behavior Immediately after Dynamic Recrystallization in HSLA Steels and Austenitic Stainless Steel. ISIJ Int. 2008, 48, 1419–1428. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; He, Y.; Wang, Y.; Wang, Y.; Wang, T. Influence of annealing temperature on microstructure, tensile properties and tensile deformation mechanism of metastable austenitic stainless steel repetitively cold-rolled and annealed. Materialia 2019, 8, 100455. [Google Scholar] [CrossRef]
- Okayasu, M.; Ishida, D. Effect of Microstructural Characteristics on Mechanical Properties of Austenitic, Ferritic, and γ-α Duplex Stainless Steels. Metall. Mater. Trans. A 2019, 50, 1380–1388. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Iwasawa, H.; Inoue, T.; Nagae, M.; Takada, J. Application of fractography to the study of carbon diffusion in molybdenum. J. Alloys Compd. 2004, 377, 127–132. [Google Scholar] [CrossRef]
- Tsai, K.-Y.; Tsai, M.-H.; Yeh, J.-W. Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
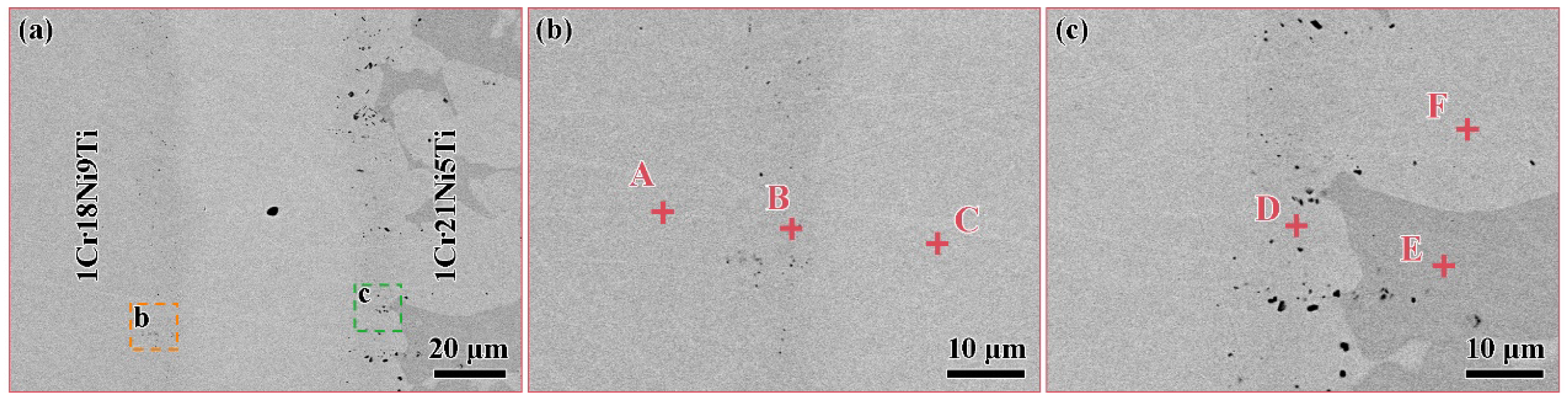
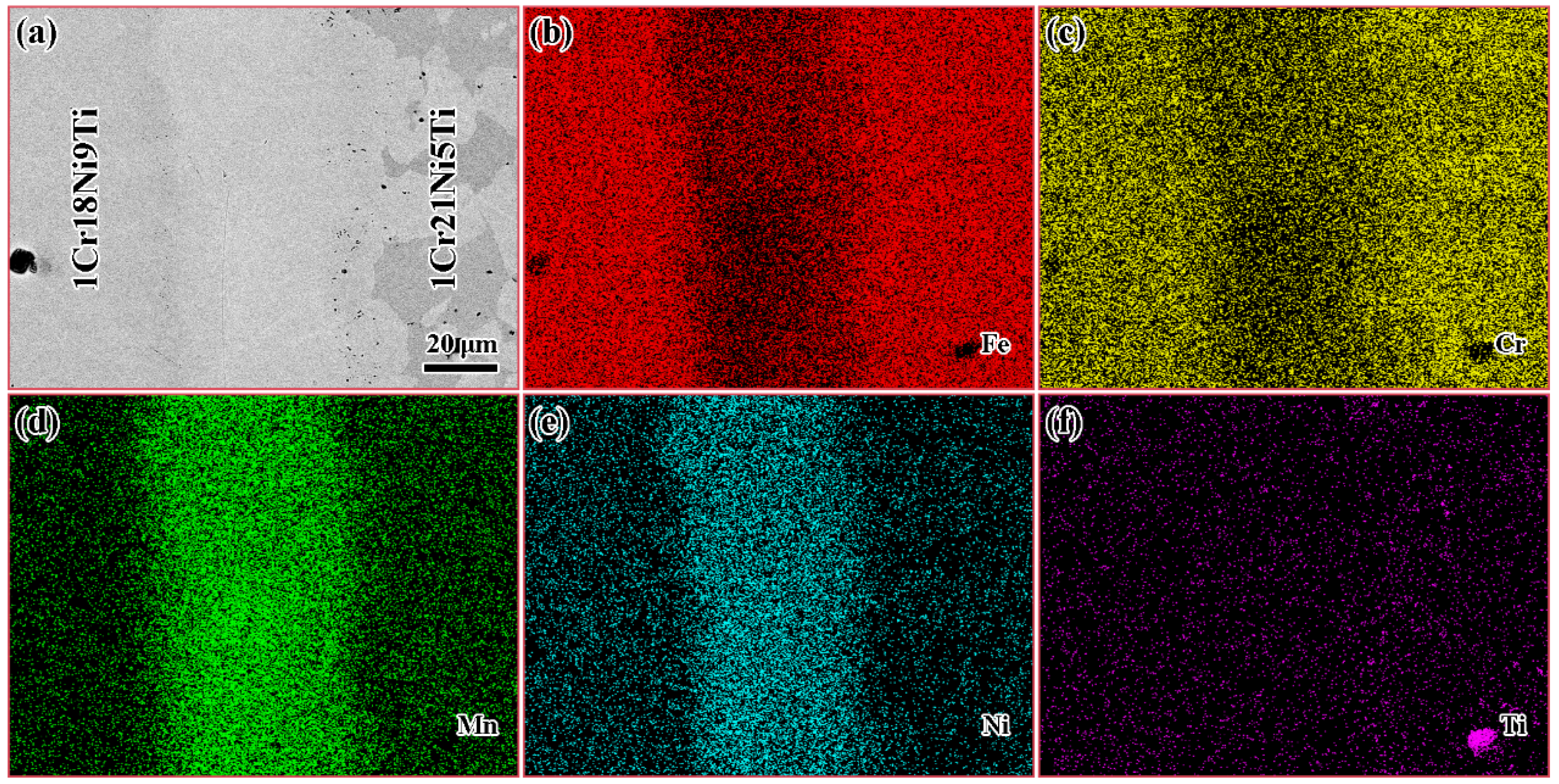
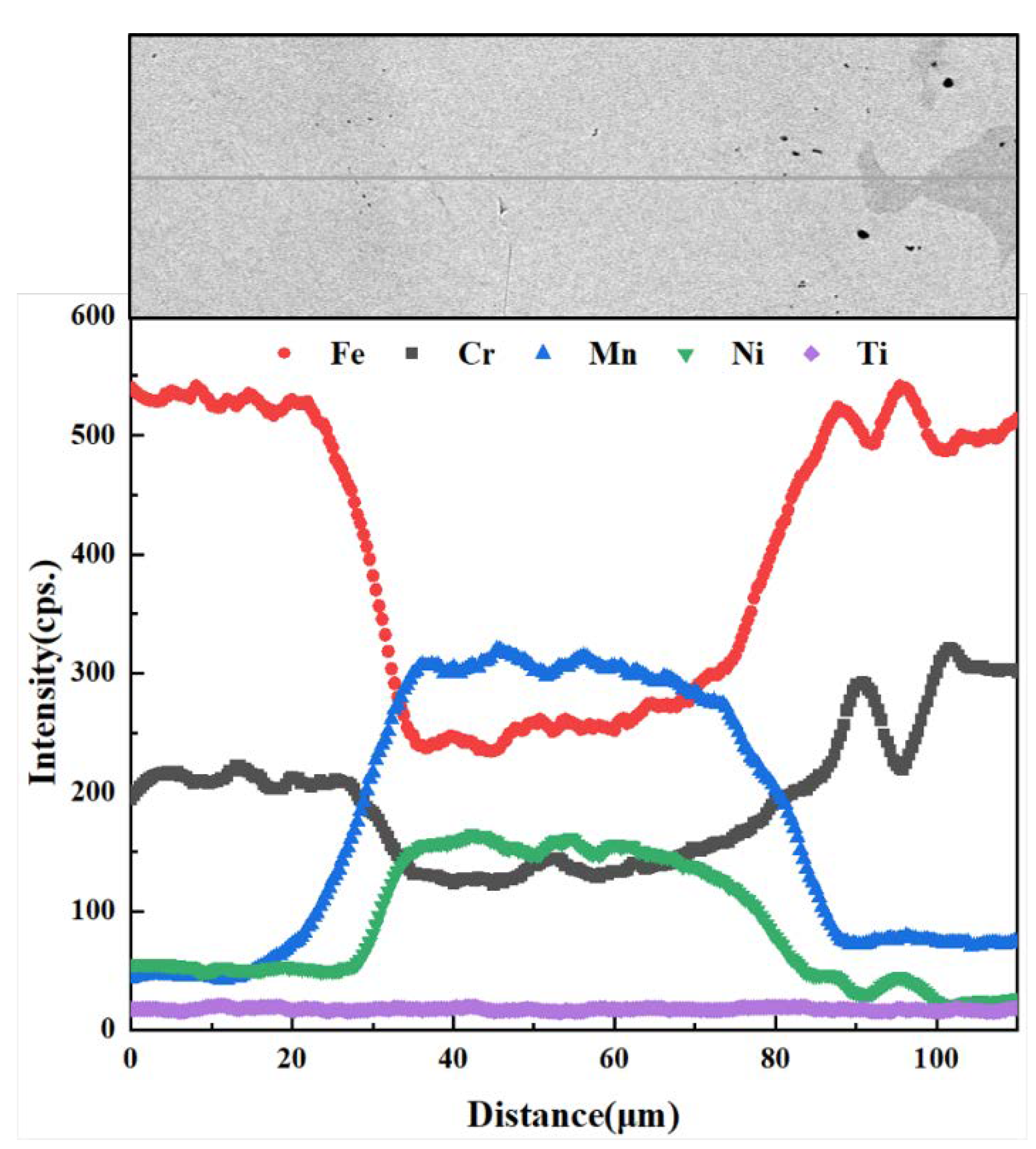



| Element | C | Si | Mn | Cr | Ni | Ti | Fe |
|---|---|---|---|---|---|---|---|
| 1Cr21Ni5Ti | 0.09~0.14 | ≤0.8 | ≤0.8 | 20~22 | 4.8~5.8 | ≤0.8 | Bal. |
| 1Cr18Ni9Ti | ≤0.12 | ≤1.0 | ≤2.0 | 17~19 | 8~11 | ≤0.8 | Bal. |
| BMn70NiCr | - | - | Bal. | 4.5~5.5 | 24.0~26.0 | - | - |
| Element/Points | Fe | Cr | Ni | Mn | Ti |
|---|---|---|---|---|---|
| A | 70.9 | 19.1 | 10.0 | - | - |
| B | 55.1 | 20.8 | 9.0 | 14.8 | 1.3 |
| C | 9.4 | 3.9 | 10.1 | 63.5 | - |
| D | 50.1 | 18.7 | 10.3 | 19.7 | 1.2 |
| E | 66.4 | 26.2 | 3.4 | 4.0 | - |
| F | 69.2 | 19.5 | 6.6 | 4.7 | - |
| Element/Points | Fe | Cr | Ni | Mn | Ti |
|---|---|---|---|---|---|
| A | 17.7 | 8.0 | 18.8 | 55.0 | 0.5 |
| B | 9.4 | 3.9 | 10.1 | 63.5 | 13.1 |
| C | 67.2 | 22.7 | 7.3 | 2.3 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Chen, H.; Yang, W.; Zhang, Q.; Yang, B.; Hu, Y.; Si, X.; Lin, T.; Cao, J.; Qi, J.; et al. Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler. Materials 2022, 15, 7021. https://doi.org/10.3390/ma15197021
Chen L, Chen H, Yang W, Zhang Q, Yang B, Hu Y, Si X, Lin T, Cao J, Qi J, et al. Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler. Materials. 2022; 15(19):7021. https://doi.org/10.3390/ma15197021
Chicago/Turabian StyleChen, Lei, Huize Chen, Weipeng Yang, Qinlian Zhang, Bo Yang, Yazhen Hu, Xiaoqing Si, Tong Lin, Jian Cao, Junlei Qi, and et al. 2022. "Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler" Materials 15, no. 19: 7021. https://doi.org/10.3390/ma15197021
APA StyleChen, L., Chen, H., Yang, W., Zhang, Q., Yang, B., Hu, Y., Si, X., Lin, T., Cao, J., Qi, J., & Li, C. (2022). Interfacial Microstructure and Mechanical Properties of 1Cr18Ni9Ti/1Cr21Ni5Ti Stainless Steel Joints Brazed with Mn-Based Brazing Filler. Materials, 15(19), 7021. https://doi.org/10.3390/ma15197021










