A New Method for Abrin Detection Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. QSAR Model of Abrin Aptamers and Virtual Screening of Abrin Aptamer
2.3. Study on the Fluorescence Quenching Effect between Magnetic Microspheres and Fluorescent Molecules
2.4. Detection of Abrin Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres
3. Results
3.1. QSAR Model of Abrin Aptamers and Virtual Screening of Abrin Aptamer
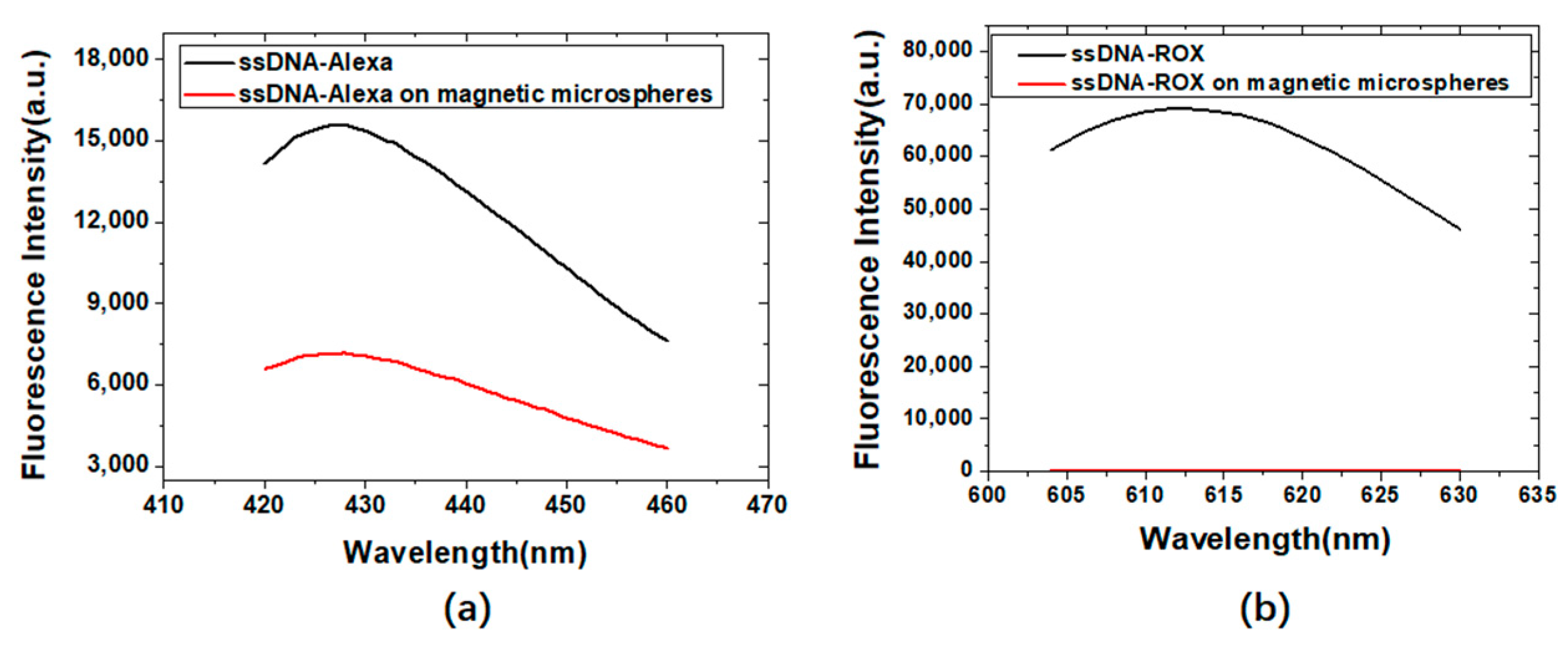
3.2. Study on the Fluorescence Quenching Effect between Magnetic Microspheres and Fluorescent Molecules
3.3. Detection of Abrin Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robb, C.S. The analysis of abrin in food and beverages. Trends Anal. Chem. 2015, 67, 100–106. [Google Scholar] [CrossRef]
- Tang, J.; Yu, T.; Guo, L.; Xie, J.; Shao, N.; He, Z. In vitro selection of DNA aptamer against abrin toxin and aptamer-based abrin direct detection. Biosens. Bioelectron. 2007, 22, 2456–2463. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Wang, Z. Real-time and in-situ monitoring of Abrin induced cell apoptosis by using SERS spectroscopy. Talanta 2019, 195, 8–16. [Google Scholar] [CrossRef]
- Dickers, K.J.; Bradberry, S.M.; Rice, P.; Griffiths, G.D.; Vale, J.A. Abrin poisoning. Toxicol. Rev. 2003, 22, 137–142. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Sun, C.Y.; Wang, X.C.; Wang, X.R.; Zhang, P.P.; Qiu, J.F.; Yang, R.F.; Zhou, L. Rapid detection of abrin in foods with an up-converting phosphor technology-based lateral flow assay. Sci. Rep. 2016, 6, 34926. [Google Scholar] [CrossRef] [PubMed]
- Garber, E.A.E.; Walker, J.L.; O’Brien, T.W. Detection of abrin in food using enzyme-linked immunosorbent assay and electrochemiluminescence technologies. J. Food Prot. 2008, 71, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ma, W.; Wen, Y.Z.; Yang, J.C.; Yu, X.M. Trace level detections of abrin with high SNR piezoresistive cantilever biosensor. Sens. Actuators B-Chem. 2015, 212, 112–119. [Google Scholar] [CrossRef]
- Liu, S.; Gao, C.; Tong, Z.Y.; Mu, X.H.; Liu, B.; Xu, J.J.; Du, B.; Wang, J.; Liu, Z.W. A highly sensitive electrochemiluminescence method for abrin detection by a portable biosensor based on a screen-printed electrode with a phage display affibody as specific labeled probe. Anal. Bioanal. Chem. 2022, 414, 1095–1104. [Google Scholar] [CrossRef]
- Liu, S.; Tong, Z.Y.; Mu, X.H.; Liu, B.; Du, B.; Liu, Z.W.; Gao, C. Detection of Abrin by Electrochemiluminescence Biosensor Based on Screen Printed Electrode. Sensors 2018, 18, 357. [Google Scholar] [CrossRef]
- Yang, H.; Deng, M.; Ga, S.; Chen, S.; Kang, L.; Wang, J.; Xin, W.; Zhang, T.; You, Z.; An, Y.; et al. Capillary-driven surface-enhanced Raman scattering (SERS)-based microfluidic chip for abrin detection. Nanoscale Res. Lett. 2014, 9, 138. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, H.; Niu, X.; Wang, Q.; Liu, Y.; Wu, Y. Aptamer-mediated carbon dots as fluorescent signal for ultrasensitive detection of carbendazim in vegetables and fruits. J. Food Compos. Anal. 2022, 114, 104730. [Google Scholar] [CrossRef]
- Chang, C.-C.; Li, C.-F.; Yang, Z.-H.; Lin, P.-Y.; Chang, H.-C.; Yang, C.-W. Target-induced recycling assembly of split aptamer fragments by DNA toehold-mediated displacement for the amplified colorimetric detection of estradiol. Sens. Actuators B Chem. 2022, 364, 131823. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, S.; Zhang, J.; Liang, N.; Zhao, L. A facile aptamer-based sensing strategy for dopamine detection through the fluorescence energy transfer between dye and single-wall carbon nanohorns. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121415. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Ye, H.; Guo, H.; Ma, X.; Yue, L.; Wang, Z. Aptamer truncation strategy assisted by molecular docking and sensitive detection of T-2 toxin using SYBR Green I as a signal amplifier. Food Chem. 2022, 381, 132171. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.C.; Feng, J.; Zhang, J.M.; Hu, K.; Wang, D.; Zha, L.; Hu, X.J.; Li, R.S. Aptamer/antibody sandwich method for digital detection of SARS-CoV2 nucleocapsid protein. Talanta 2022, 236, 122847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Li, S.; Yuan, R.; Xiang, Y. Target-triggered tertiary amplifications for sensitive and label-free protein detection based on lighting-up RNA aptamer transcriptions. Anal. Chim. Acta 2022, 1217, 340028. [Google Scholar] [CrossRef]
- Khoris, I.M.; Nasrin, F.; Chowdhury, A.D.; Park, E.Y. Advancement of dengue virus NS1 protein detection by 3D-nanoassembly complex gold nanoparticles utilizing competitive sandwich aptamer on disposable electrode. Anal. Chim. Acta 2022, 1207, 339817. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.; Lee, B.H.; Song, C.-S.; Gu, M.B. Specific detection of avian influenza H5N2 whole virus particles on lateral flow strips using a pair of sandwich-type aptamers. Biosens. Bioelectron. 2019, 134, 123–129. [Google Scholar] [CrossRef]
- Etedali, P.; Behbahani, M.; Mohabatkar, H.; Dini, G. Field-usable aptamer-gold nanoparticles-based colorimetric sensor for rapid detection of white spot syndrome virus in shrimp. Aquaculture 2022, 548, 737628. [Google Scholar] [CrossRef]
- Nguyen, A.T.V.; Duong, B.T.; Park, H.; Yeo, S.-J. Development of a peptide aptamer pair-linked rapid fluorescent diagnostic system for Zika virus detection. Biosens. Bioelectron. 2022, 197, 113768. [Google Scholar] [CrossRef]
- Basso, C.R.; Crulhas, B.P.; Magro, M.; Vianello, F.; Pedrosa, V.A. A new immunoassay of hybrid nanomater conjugated to aptamers for the detection of dengue virus. Talanta 2019, 197, 482–490. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Shahidi, M.; Moshtaghioun, S.M.; Haghiralsadat, F.; Ebadi, A.; Amini, A. MXene-based cytosensor for the detection of HER2-positive cancer cells using CoFe2O4@Ag magnetic nanohybrids conjugated to the HB5 aptamer. Biosens. Bioelectron. 2022, 195, 113626. [Google Scholar] [CrossRef]
- Li, L.; Jiang, H.; Meng, X.; Wen, X.; Guo, Q.; Li, Z.; Wang, J.; Ren, Y.; Wang, K. Highly sensitive detection of cancer cells via split aptamer mediated proximity-induced hybridization chain reaction. Talanta 2021, 223, 121724. [Google Scholar] [CrossRef]
- Fukuyama, S.; Kumamoto, S.; Nagano, S.; Hitotsuya, S.; Yasuda, K.; Kitamura, Y.; Iwatsuki, M.; Baba, H.; Ihara, T.; Nakanishi, Y.; et al. Detection of cancer cells in whole blood using a dynamic deformable microfilter and a nucleic acid aptamer. Talanta 2021, 228, 122239. [Google Scholar] [CrossRef]
- Sun, D.; Lu, J.; Zhang, L.; Chen, Z. Aptamer-based electrochemical cytosensors for tumor cell detection in cancer diagnosis: A review. Anal. Chim. Acta 2019, 1082, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, C.M.; Fu, Z.F.; Zou, H.Y.; Huang, C.Z. Dual-aptamer-based enzyme linked plasmonic assay for pathogenic bacteria detection. Colloids Surf. B Biointerfaces 2022, 214, 112471. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Yang, H.; Wang, P.; Miao, X.; Feng, Q. An in situ quenching electrochemiluminescence biosensor amplified with aptamer recognition-induced multi-DNA release for sensitive detection of pathogenic bacteria. Biosens. Bioelectron. 2022, 196, 113744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, R.; Jia, L. Enhancement of the peroxidase-like activity of aptamers modified gold nanoclusters by bacteria for colorimetric detection of Salmonella typhimurium. Talanta 2021, 221, 121476. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Xie, H.; Xu, D. A novel method combining aptamer-Ag10NPs based microfluidic biochip with bright field imaging for detection of KPC-2-expressing bacteria. Anal. Chim. Acta 2020, 1132, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ni, P.; Dai, H.; Sun, Y.; Wang, Y.; Jiang, S.; Li, Z. Aptamer-based colorimetric biosensing of abrin using catalytic gold nanoparticles. Analyst 2015, 140, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Xiao, C.B.; Ying, G.Y.; Liu, X.F.; Zhao, X.H.; Wang, R.L.; Wan, L.; Yang, M.H. Magnetic microspheres-based cytometric bead array assay for highly sensitive detection of ochratoxin A. Biosens. Bioelectron. 2017, 94, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, D.J.; Liu, H.Y.; Wu, J. An automated immunoassay based on magnetic microspheres for the determination of clenbuterol in food. Instrum. Sci. Technol. 2015, 43, 524–535. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, Z.; Liu, B.; Wu, Y.; Du, B.; Wang, J.; Xu, J.; Gao, C.; Liu, S.; Ma, W. Ricin aptamer screening based on the QSAR model and construction of piezoresistive micro-cantilever aptasensor. Talanta 2022, 252, 123840. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.-H.; Liu, H.-F.; Tong, Z.-Y.; Du, B.; Liu, S.; Liu, B.; Liu, Z.-W.; Gao, C.; Wang, J.; Dong, H. A new rapid detection method for ricin based on tunneling magnetoresistance biosensor. Sens. Actuators B Chem. 2019, 284, 638–649. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, X.; Wang, Y.; Chen, Q.; Hammock, B.D.; Xu, Y. Nanobody-based fluorescence resonance energy transfer immunoassay for noncompetitive and simultaneous detection of ochratoxin a and ochratoxin B. Environ. Pollut. 2019, 251, 238–245. [Google Scholar] [CrossRef]
- Heiat, M.; Najafi, A.; Ranjbar, R.; Latifi, A.M.; Rasaee, M.J. Computational approach to analyze isolated ssDNA aptamers against angiotensin II. J. Biotechnol. 2016, 230, 34–39. [Google Scholar] [CrossRef]
- Reineck, P.; Gómez, D.; Ng, S.H.; Karg, M.; Bell, T.; Mulvaney, P.; Bach, U. Distance and Wavelength Dependent Quenching of Molecular Fluorescence by Au@SiO2 Core–Shell Nanoparticles. ACS Nano 2013, 7, 6636–6648. [Google Scholar] [CrossRef]



| Name | Sequence |
|---|---|
| Abrin-2 | 5′-TCGCAAGACGGACAGAAGGCTTGTGGTCTTCTTTTGGTG ATGTACTGCCGTTAATGGAGTGTTGGTGGAGCGATTTGT-3′ |
| Abrin-M1 | 5′-TCGCAAGACGGACAGAAGGCTTGTGGTCCTCTTTTGGTG ATGTACTGCCGTTAATGGAGTGTTGGTGGAGCGATTTGT-3′ |
| Abrin-M2 | 5′-TCGCAAGACGGACAGAAGGCTTGTGGTCCTCTTTTGGTG ATGTGCTGCCGTTAATGGAGTGTTGGTGGAGCGATTTGT-3′ |
| Abrin-M3 | 5′-TCGCAAGACGGACAGAAGGCTTGTGGTCCTCTTTTTGTG ATGTGCTGCCGTTAATGGAGTGTTGGTGGAGCGATTTGT-3′ |
| Sample | Added (ng mL−1) | Found (ng mL−1) | Recovery Ratio (%) | Relative Standard Deviation (%) |
|---|---|---|---|---|
| River water | 20 | 21.3 ± 1.1 | 106.5 | 5.16 |
| Fertilized soil | 20 | 20.2 ± 2.0 | 101.0 | 9.90 |
| Butter biscuit | 20 | 16.9 ± 1.2 | 84.5 | 7.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tong, Z.; Wu, Y.; Liu, B.; Feng, S.; Mu, X.; Wang, J.; Du, B.; Xu, J.; Liu, S. A New Method for Abrin Detection Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres. Materials 2022, 15, 6977. https://doi.org/10.3390/ma15196977
Liu Z, Tong Z, Wu Y, Liu B, Feng S, Mu X, Wang J, Du B, Xu J, Liu S. A New Method for Abrin Detection Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres. Materials. 2022; 15(19):6977. https://doi.org/10.3390/ma15196977
Chicago/Turabian StyleLiu, Zhiwei, Zhaoyang Tong, Yuting Wu, Bing Liu, Shasha Feng, Xihui Mu, Jiang Wang, Bin Du, Jianjie Xu, and Shuai Liu. 2022. "A New Method for Abrin Detection Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres" Materials 15, no. 19: 6977. https://doi.org/10.3390/ma15196977
APA StyleLiu, Z., Tong, Z., Wu, Y., Liu, B., Feng, S., Mu, X., Wang, J., Du, B., Xu, J., & Liu, S. (2022). A New Method for Abrin Detection Based on the Interaction between Target Molecules and Fluorescently Labeled Aptamers on Magnetic Microspheres. Materials, 15(19), 6977. https://doi.org/10.3390/ma15196977





