High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials
Abstract
1. Introduction
2. Materials and Methods
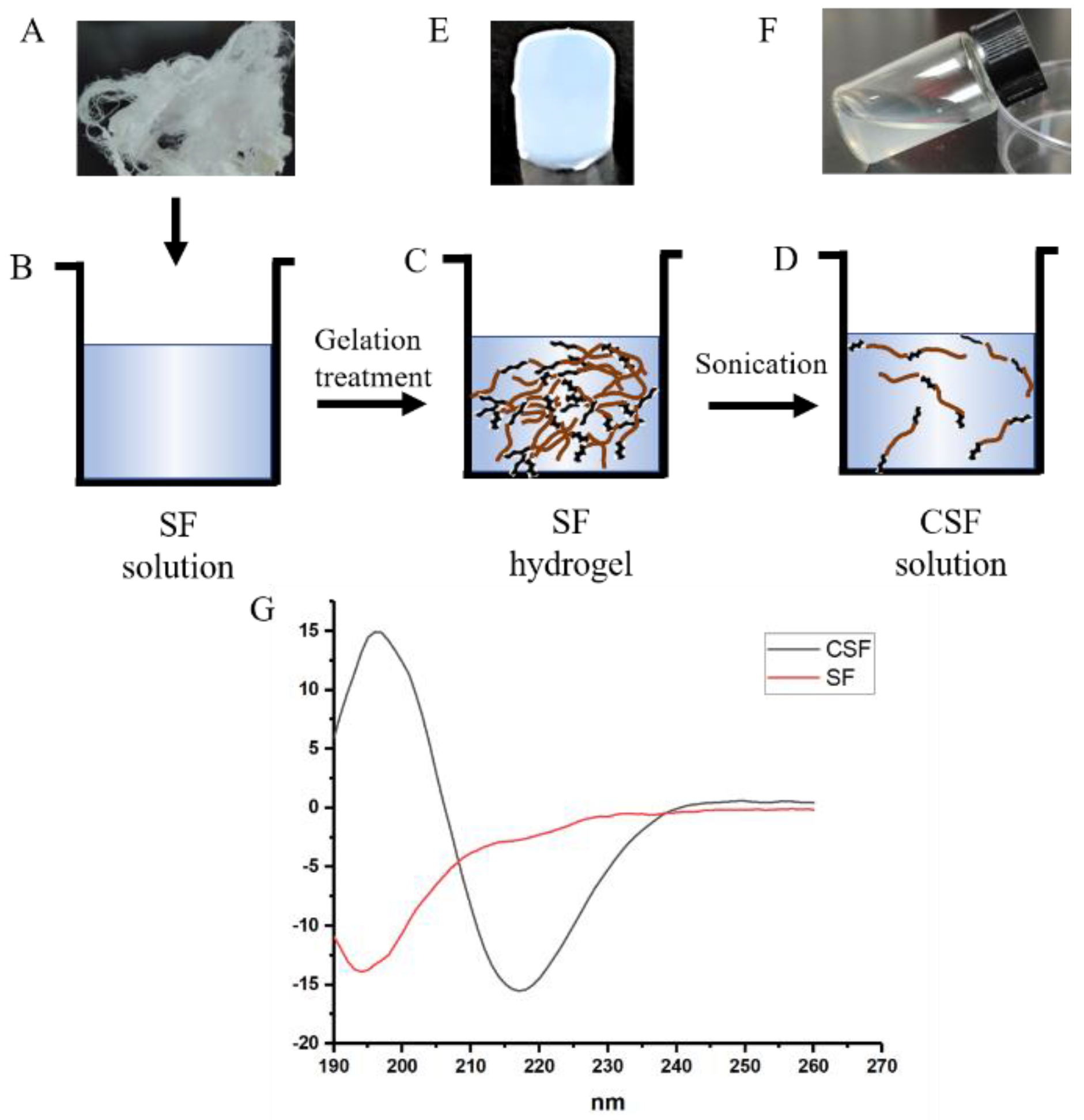
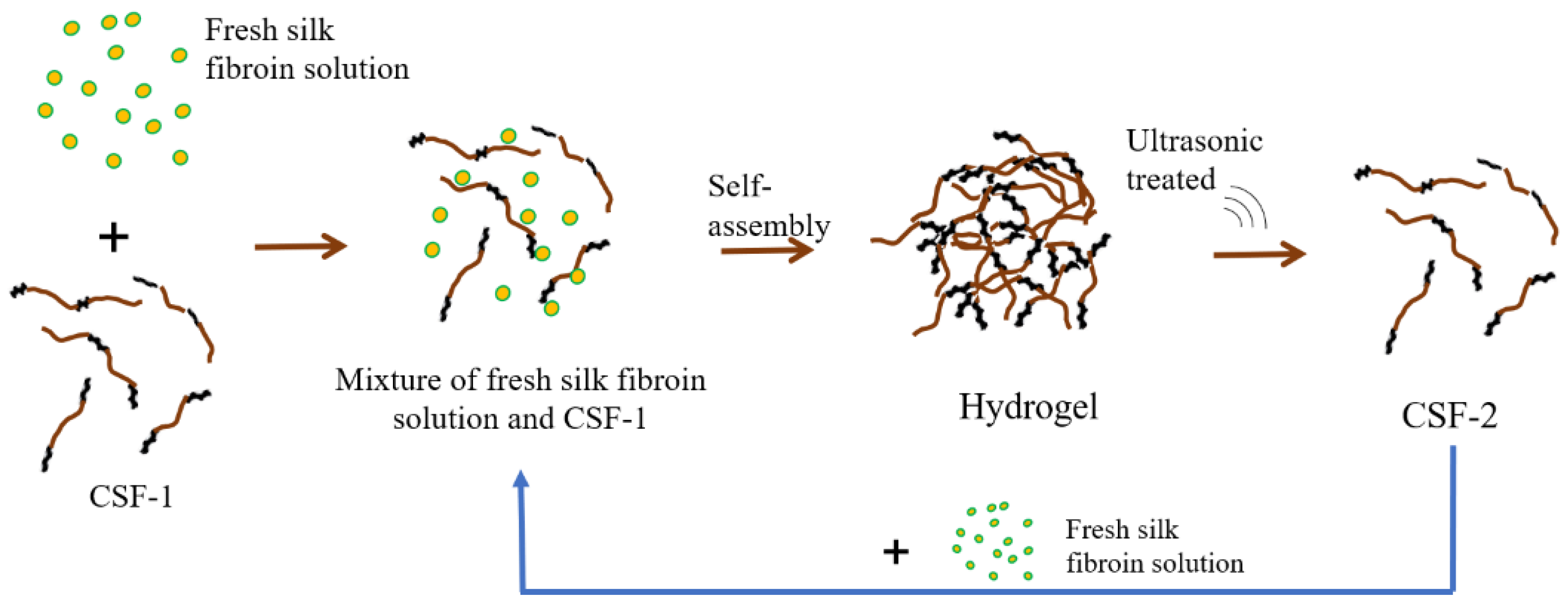
2.1. Preparation of Crystal Silk Fibroin (CSF) Solution and CSF-Based Biomaterials
2.1.1. Fabrication of Fresh Silk Fibroin Solution
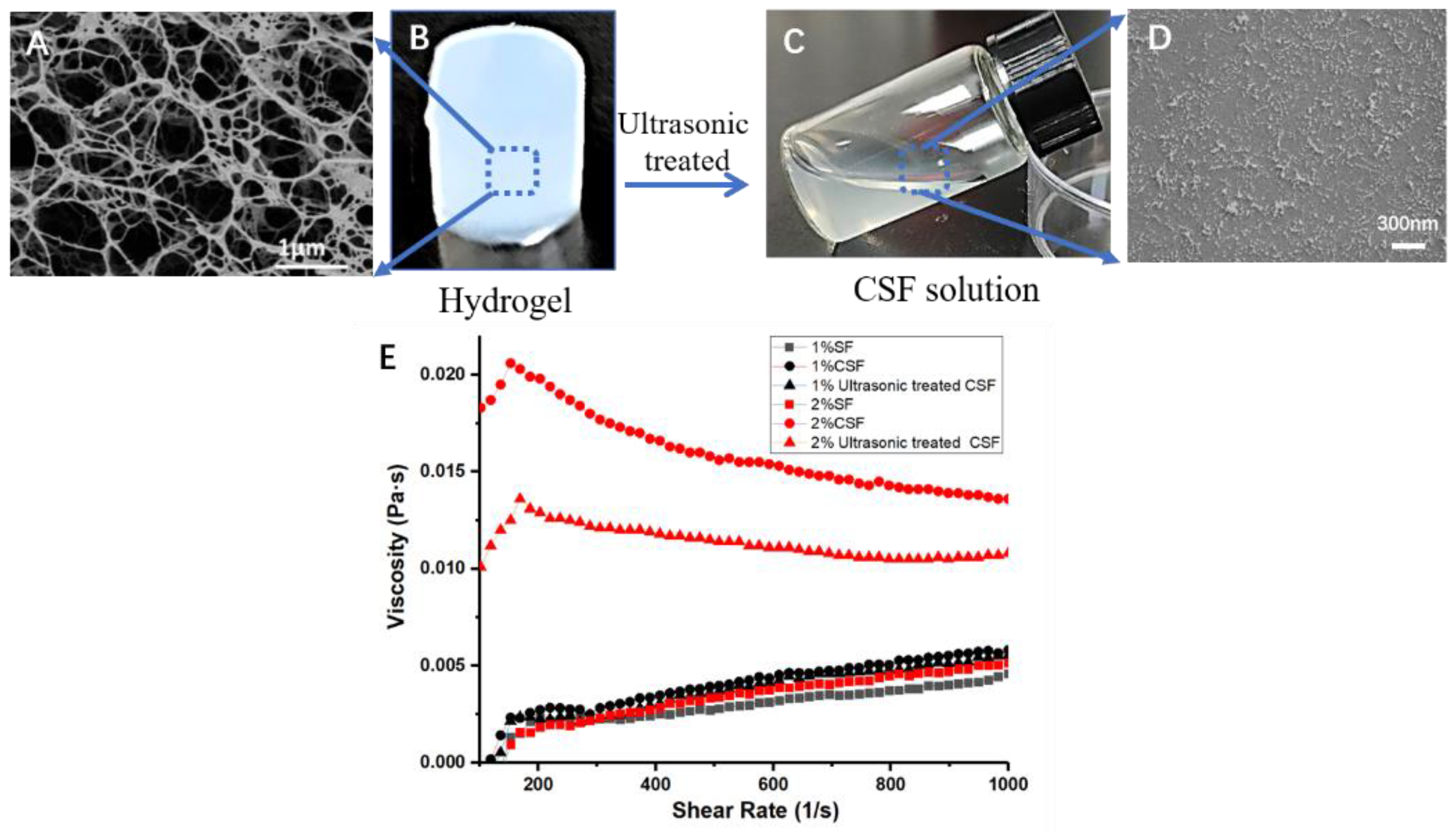
2.1.2. Preparation of CSF Solution
2.1.3. Preparation of CSF-Based Hydrogel
2.1.4. Preparation of CSF-Based Films
2.1.5. Preparation of CSF-Based Porous Scaffolds
2.1.6. Cell Culture
2.2. Characterization
2.2.1. Scanning Electron Microscopy (SEM)
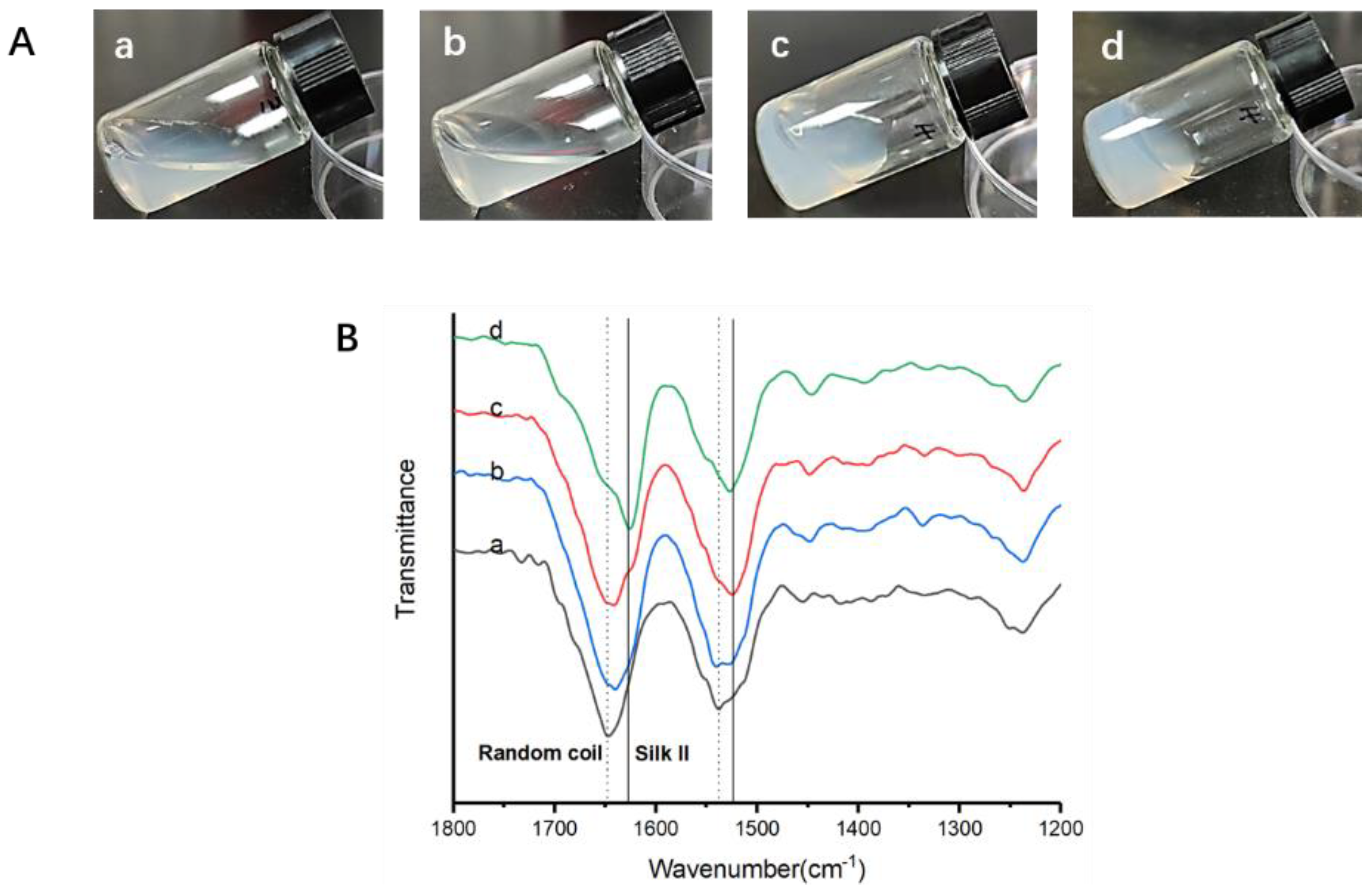
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.3. Circular Dichroism (CD)
2.2.4. Viscosity
2.2.5. Live/Dead Viability
2.3. Statistical Methods
3. Results and Discussion
3.1. Microstructure of Crystal Silk Fibroin
3.2. Structural Analysis
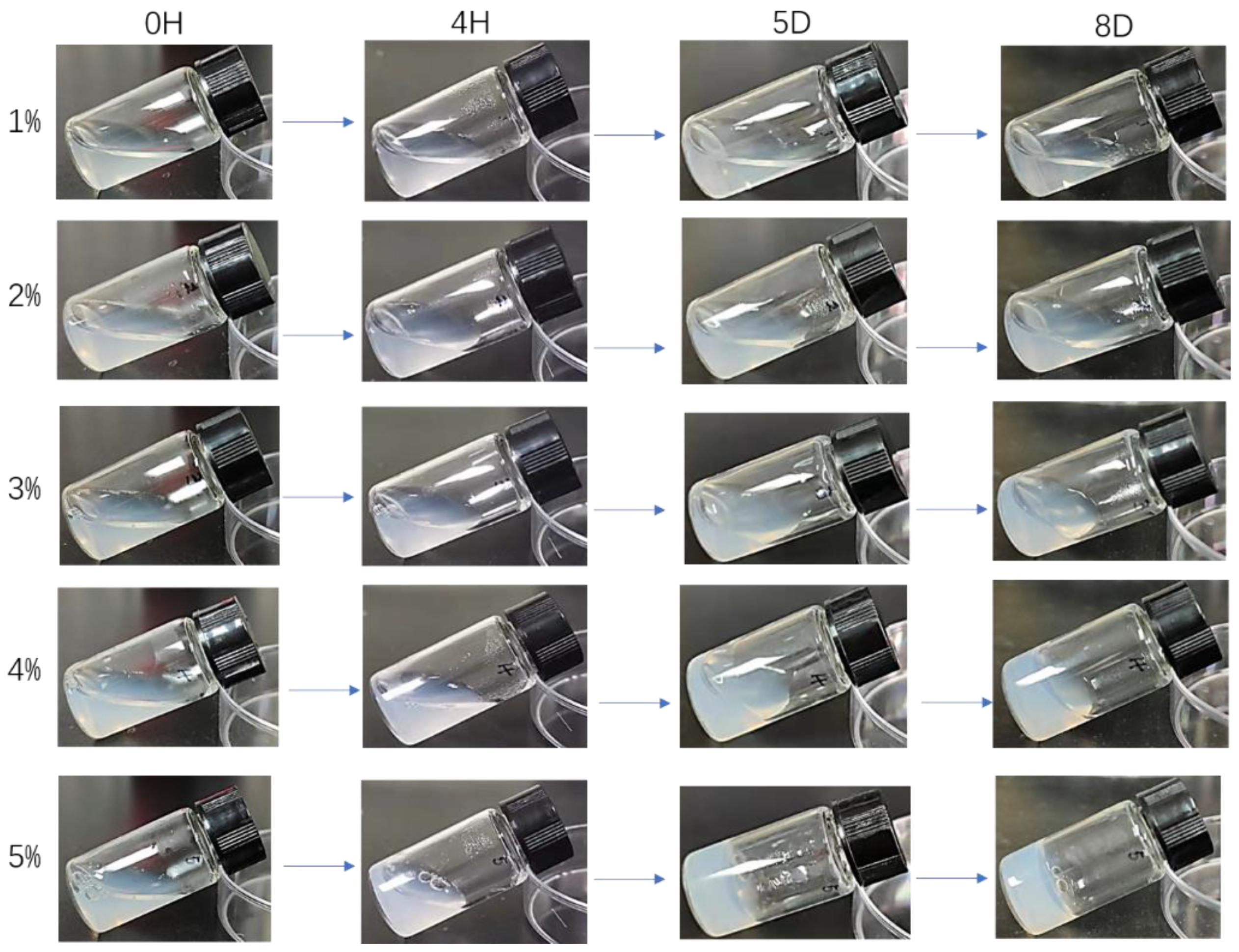
3.3. Application of Crystalline Silk Fibroin Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, e1800465. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; D’Amone, L.; Kim, T.; Matzeu, G.; Mogas-Soldevila, L.; Napier, B.; Ostrovsky-Snider, N.; Roshko, J.; Ruggeri, E.; Omenetto, F.G. Silk materials at the convergence of science, sustainability, healthcare, and technology. Appl. Phys. Rev. 2022, 9, 011302. [Google Scholar] [CrossRef]
- Zhang, X.; Hang, Y.; Ding, Z.; Xiao, L.; Cheng, W.; Lu, Q. Macroporous Silk Nanofiber Cryogels with Tunable Properties. Biomacromolecules 2022, 23, 2160–2169. [Google Scholar] [CrossRef]
- Msa, B.; Qi, L.A.; Hy, A.; Jin, C.A.; Nw, B.; Ws, A.; Fz, A.; Zs, A.; Qm, A.; Hc, B. Cryo-self-assembled silk fibroin sponge as a biodegradable platform for enzyme-responsive delivery of exosomes. Bioact. Mater. 2021, 8, 505–514. [Google Scholar]
- Neubauer, V.J.; Scheibel, T. Silk-Based Materials for Hard Tissue Engineering. Materials 2021, 14, 674. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Carvalho, N.; Monteiro, F.J. Biomaterials with Potential Use in Bone Tissue Regeneration—Collagen/Chitosan/Silk Fibroin Scaffolds Cross-Linked by EDC/NHS. Materials 2021, 14, 1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, X.; Yu, H.; Ma, C.; Yao, J. Biomimicking the structure of silk fibers via cellulose nanocrystal as β-sheet crystallite. RSC Adv. 2014, 4, 14304–14313. [Google Scholar] [CrossRef]
- Gupta, S.; Prasad, P.; Roy, A.; Alam, M.M.; Ahmad, I.; Bit, A. Metallic ion-based graphene oxide functionalized silk fibroin-based dressing promotes wound healing via improved bactericidal outcomes and faster re-epithelization. Bioact. Mater. 2022, 17, 035010. [Google Scholar] [CrossRef]
- Yao, D.; Liu, H.; Fan, Y. Fabrication of water-stable silk fibroin scaffolds through self-assembly of proteins. RSC Adv. 2016, 6, 61402–61409. [Google Scholar] [CrossRef]
- Szymkowiak, S.; Sandler, N.; Kaplan, D.L. Aligned Silk Sponge Fabrication and Perfusion Culture for Scalable Proximal Tubule Tissue Engineering. ACS Appl. Mater. Inter. 2021, 13, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhao, Q.; Xiao, L.; Lu, Q.; Kaplan, D.L. Amorphous Silk Nanofiber Solutions for Fabricating Silk-Based Functional Materials. Biomacromolecules 2016, 17, 3000–3006. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.K.; Sengupta, A.; Das, M.; Bindhani, B.K.; Das, D.; Parhi, P.K. Extraction of Silk Fibroin with Several Sericin Removal Processes and its Importance in Tissue Engineering: A Review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- Roberts, S.; Harmon, T.S.; Schaal, J.L.; Miao, V.; Li, K.; Hunt, A.; Wen, Y.; Oas, T.G.; Collier, J.H.; Pappu, R.V. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 2018, 17, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Qian, Z.; Zhou, J.; Ge, P.; Gang, Z.; Liu, H.; Fan, Y. Facile incorporation of REDV into porous silk fibroin scaffolds for enhancing vascularization of thick tissues. Mat. Sci. Eng. C—Mater. 2018, 93, 96–105. [Google Scholar] [CrossRef]
- Ling, S.; Qiang, Z.; Kaplan, D.L.; Omenetto, F.; Buehler, M.J.; Zhao, Q. Printing of stretchable silk membranes for strain measurements. Lab Chip 2016, 16, 2459–2466. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Chen, X.; Shao, Z. Investigation of rheological properties and conformation of silk fibroin in the solution of AmimCl. Biomacromolecules 2012, 13, 1875–1881. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, S.; Wang, S.; Chen, X.; Shao, Z.J.B.S. Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure. Biomater. Sci. 2014, 2, 1338–1342. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, X.; Lu, Q.; Sheng, W.; Liu, L.; Dong, B.; Kaplan, D.L.; Zhu, H. Reversible Hydrogel—Solution System of Silk with High Beta-Sheet Content. Biomacromolecules 2014, 15, 3044–3051. [Google Scholar] [CrossRef]
- Yao, D.; Li, M.; Wang, T.; Sun, F.; Shi, T. Viscoelastic Silk Fibroin Hydrogels with Tunable Strength. ACS Biomater. Sci. Eng. 2021, 7, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.; Afrand, M.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason.Sonochem. 2019, 58, 104701. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, S.; Lu, Q.; Hu, X.; Kaplan, D.L.; Zhang, B.; Zhu, H. Salt-leached silk scaffolds with tunable mechanical properties. Biomacromolecules 2012, 13, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhu, H.; Zhang, C.; Zhang, F.; Zhang, B.; Kaplan, D.L. Silk self-assembly mechanisms and control from thermodynamics to kinetics. Biomacromolecules 2012, 13, 826–832. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef]
- Paulusse, J.M.; Sijbesma, R.P. Ultrasound in polymer chemistry: Revival of an established technique. J. Polym. Sci. Pol. Chem. 2006, 44, 5445–5453. [Google Scholar] [CrossRef]
- Kemmere, M.F.; Kuijpers, M.W.A.; Prickaerts, R.M.H.; Keurentjes, J.T.F. A Novel process for ultrasound-induced radical polymerization in CO2-expanded fluids. Macromol. Mater. Eng. 2005, 290, 302–310. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Li, C.; Jin, H.J.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.; Karageorgiou, V.; Kim, U.; Valluzzi, R.; Kaplan, D. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]

| Assignment | SF Solution | Mixed SF Solution | Flowable Hydrogel | Hydrogel |
|---|---|---|---|---|
| Side chain (%) | 2.0 ± 0.1 | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.3 |
| β-sheet (%) | 21.9 ± 0.5 | 33.9 ± 0.2 | 33.9 ± 0.3 | 48.4 ± 0.3 |
| random coil (%) | 33.0 ± 0.5 | 30.2 ± 0.6 | 29.5 ± 0.6 | 24.6 ± 1.0 |
| α-helix (%) | 14.8 ± 1.0 | 13.1 ± 0.6 | 13.4 ± 0.2 | 10.3 ± 0.5 |
| β-turn (%) | 28.4 ± 2.1 | 21.3 ± 1.4 | 21.6 ± 0.8 | 15.2 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, D.; Wang, T.; Zhang, X.; Wang, Y. High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials. Materials 2022, 15, 6930. https://doi.org/10.3390/ma15196930
Yao D, Wang T, Zhang X, Wang Y. High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials. Materials. 2022; 15(19):6930. https://doi.org/10.3390/ma15196930
Chicago/Turabian StyleYao, Danyu, Ting Wang, Xiaoli Zhang, and Yuqing Wang. 2022. "High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials" Materials 15, no. 19: 6930. https://doi.org/10.3390/ma15196930
APA StyleYao, D., Wang, T., Zhang, X., & Wang, Y. (2022). High Concentration Crystalline Silk Fibroin Solution for Silk-Based Materials. Materials, 15(19), 6930. https://doi.org/10.3390/ma15196930





