ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents for Sorbent Preparation
2.2. LDH Synthesis
2.3. Allophane Synthesis
2.4. Characterization Techniques
3. Results and Discussion
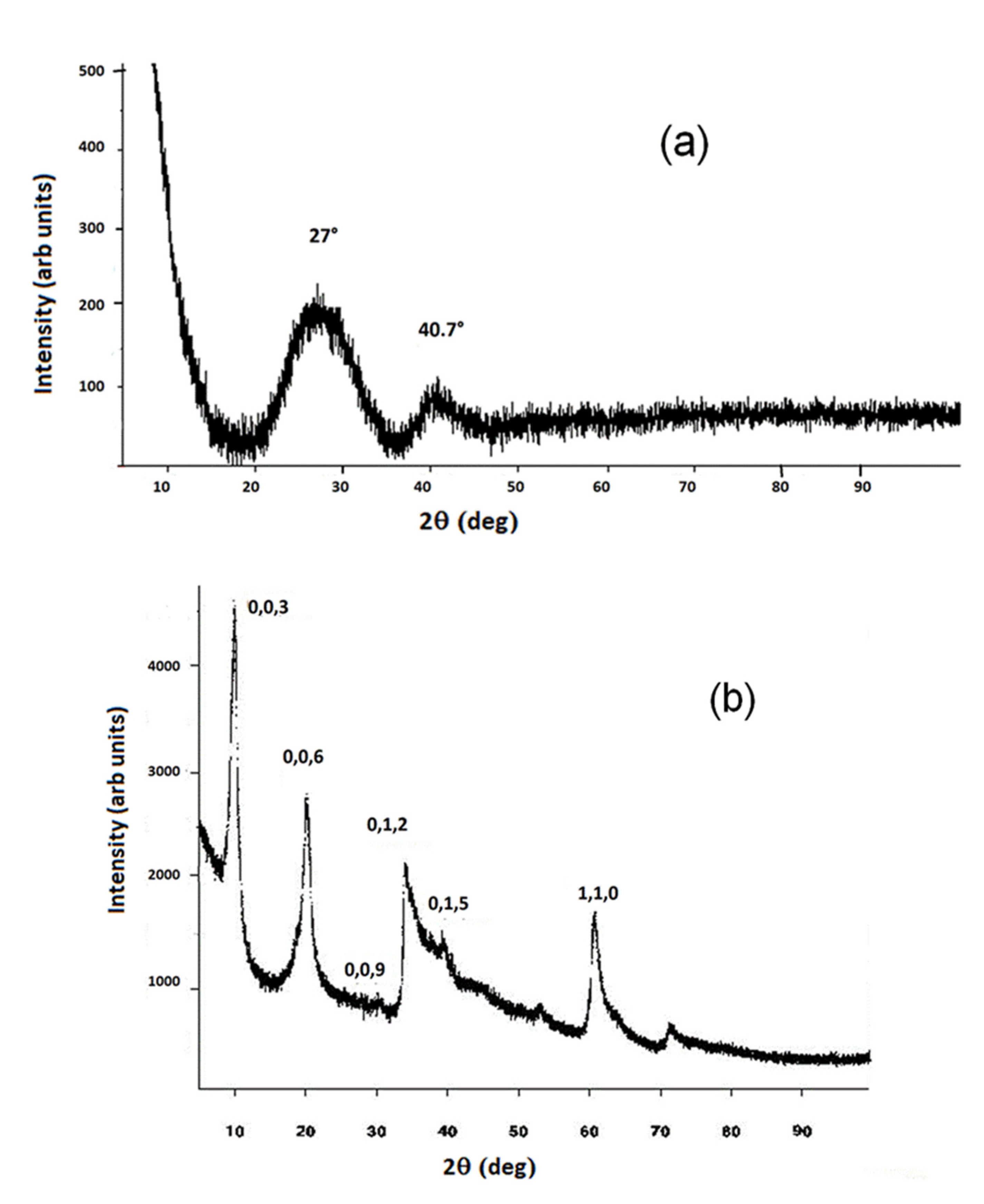
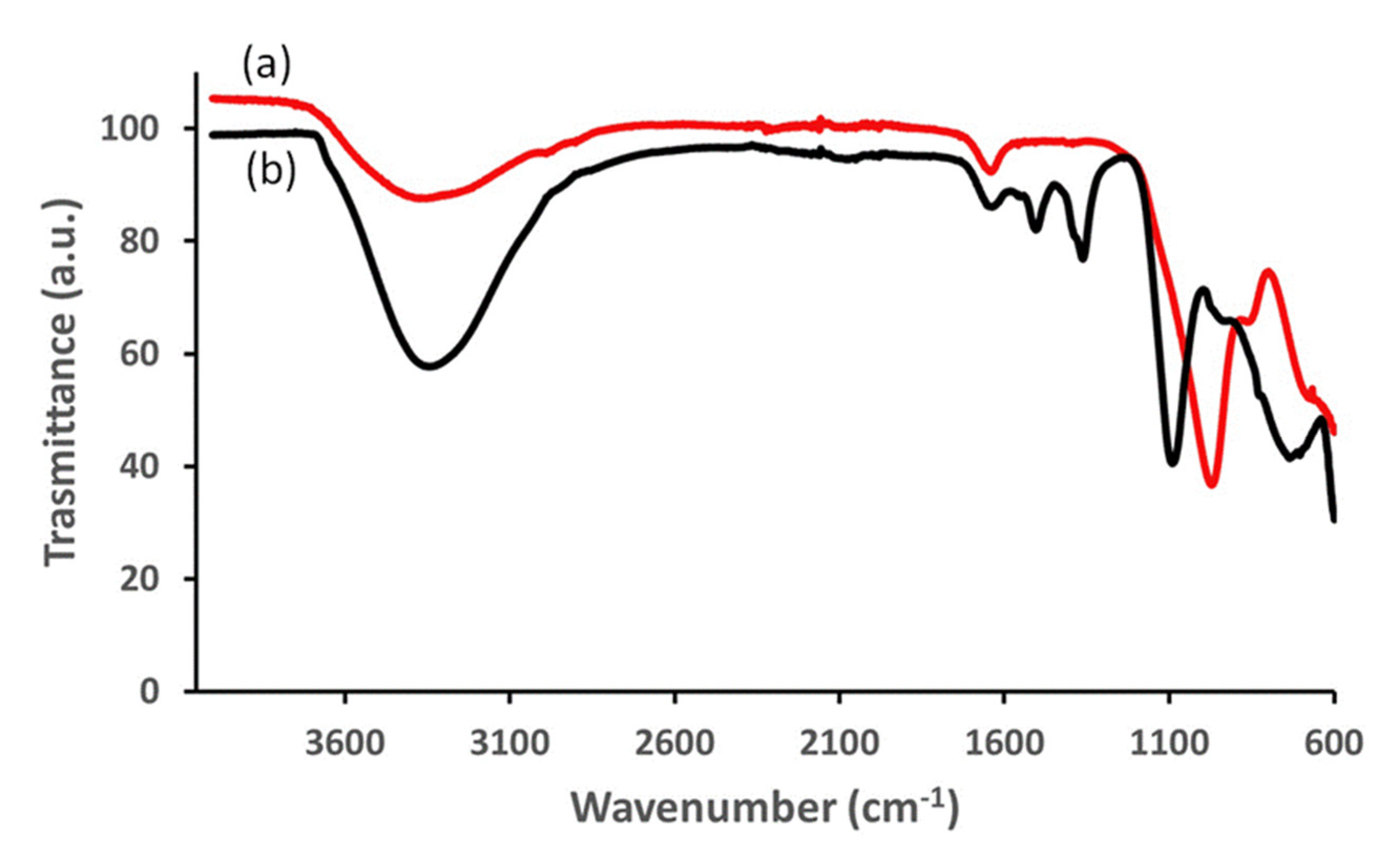
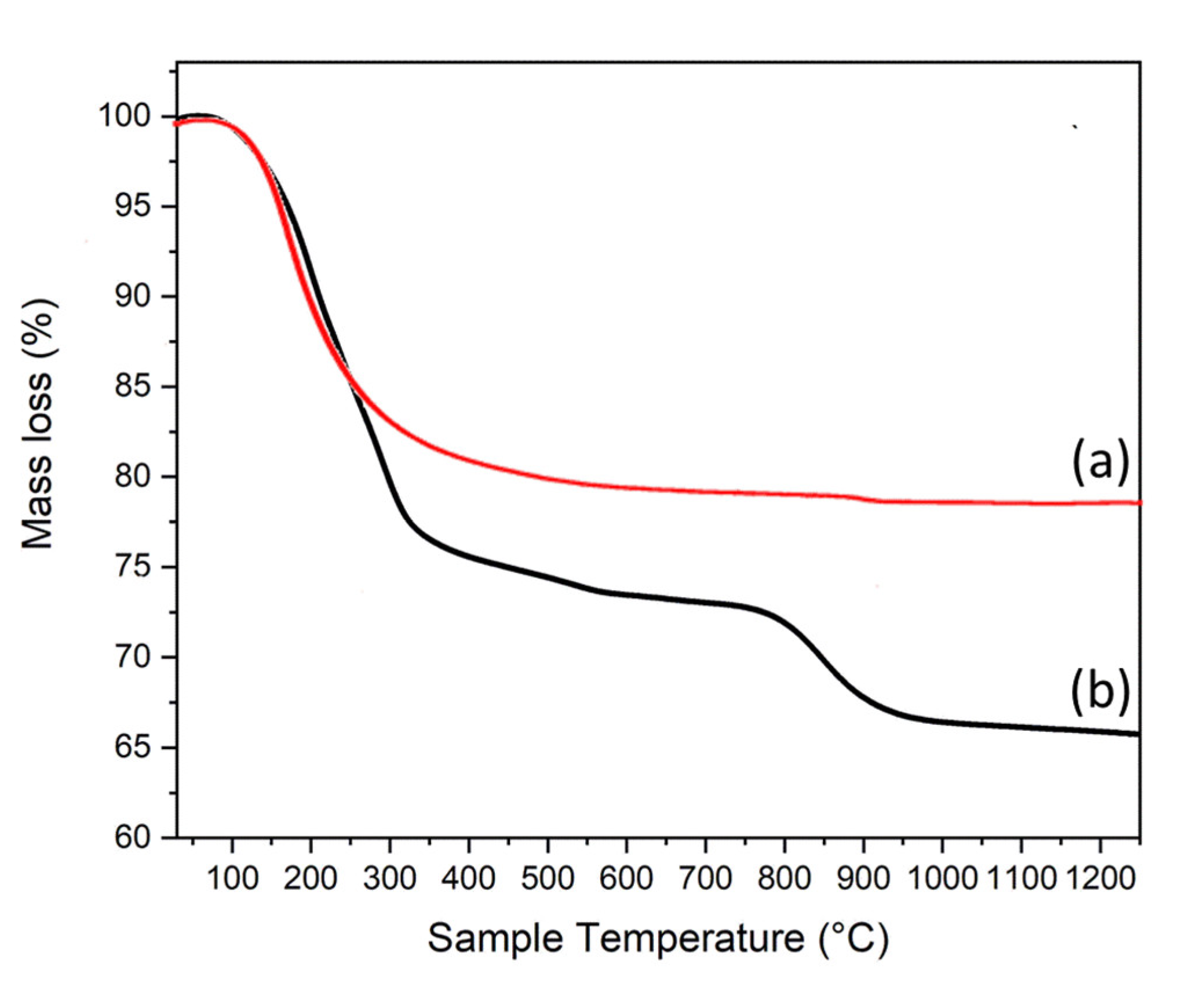
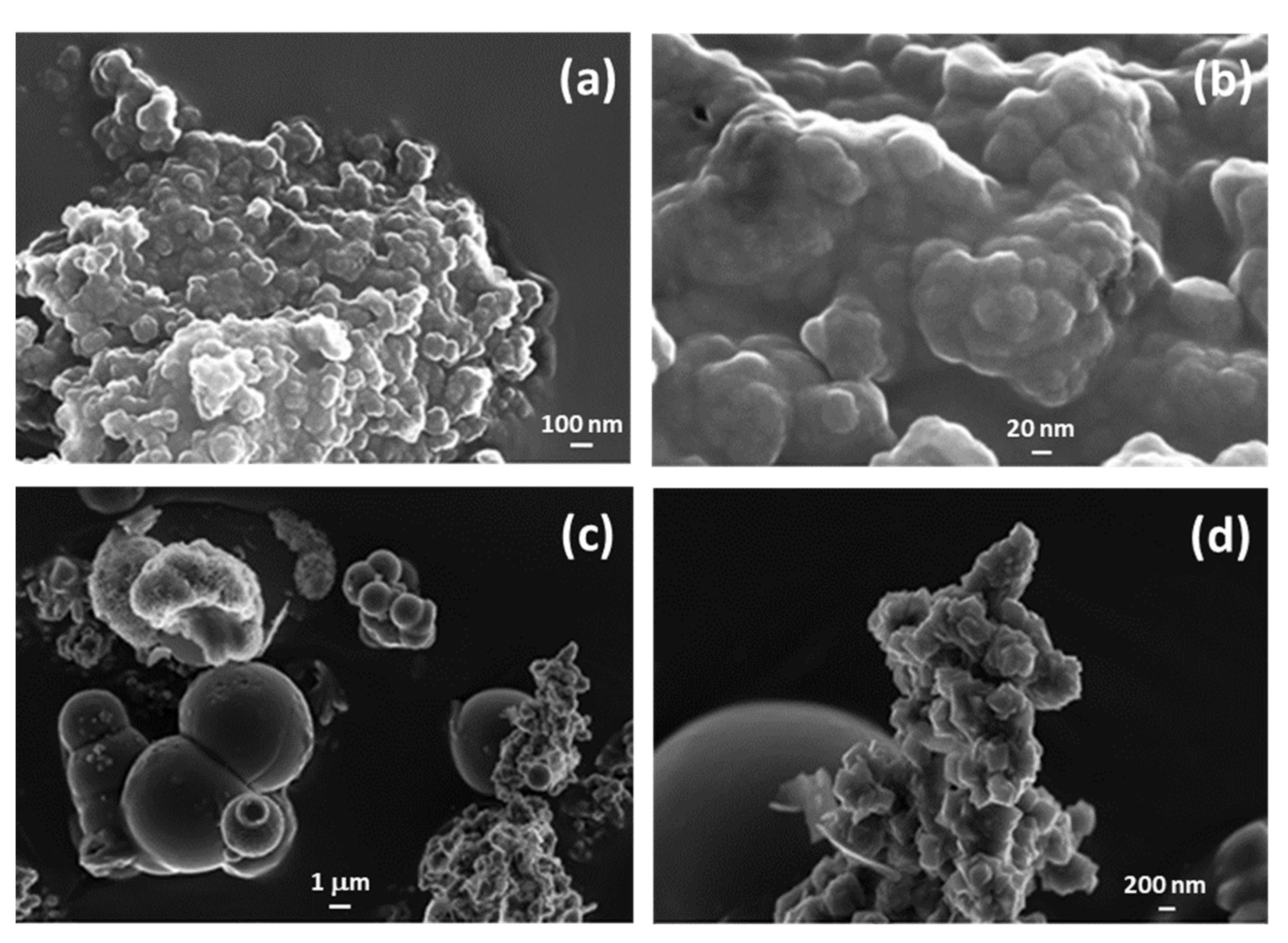
3.1. Structural (PXRD, IR and TG) and Morphological (FESEM) Analysis
3.2. Tuning of Sorbent Properties
3.3. Pollutant Adsorption
- -
- In terms of adsorption capacity qe, allophane, ZnAl-SO4 LDH and their combination offer a greater affinity towards Cr(VI) anions rather than cations such as Cu(II) and Fe(III). However, reflecting on removal rate rather than adsorption capacity, one can observe a symmetrical behavior between allophane and ZnAl-SO4 LDH. In fact, allophane showed a satisfactory efficiency for both cations, while it is barely performant for chromate anion. On the other hand, LDH is a better absorbent for chromate anion than for copper cation. Actually, with the data at our disposal, it is difficult to draw definitive conclusions about the performance for Fe(III) owing to its very low initial concentration in wastewater.
- -
- A presence of cations such as Cu(II) and Fe(III), almost ubiquitous in real metalworking wastewaters, does not interfere with the adsorption of Cr(VI), here dissolved in anionic form. Even despite the presence of these host cations, a 50% combination of allophane and LDH gives a value of qe for Cr(VI) adsorption very close to the maximum between the two components.
- -
- Notwithstanding the small values of qe for Cu(II) and Fe(III) adsorption obtainable from the data of Table 1, a synergistic effect in terms of Cu(II) removal rate is observable for a 50% allophane/LDH combination.
4. Conclusions
- The synthesis is simple, cost-effective and sustainable, both for allophane and for ZnAl-SO4 LDH: according to inherent safety guideword “substitution”, no reagents hazardous for health or environment were employed.
- In a context pertaining to the adsorption capacity, allophane, ZnAl-SO4 LDH and their combination proved to be efficient in the adsorption of Cr(VI).
- The removal rate of each sorbent species showed different trends according to their physicochemical properties, leading to different scenarios in wastewater decontamination. Being an anionic clay, allophane proved to be more efficient for cation extraction, while LDH showed a greater affinity for the anion which can be exchanged with the one originally present in the interlayer of the pristine substrate.
- The synergy effect between allophane and ZnAl-SO4 LDH for Cu(II) adsorption has been quantified according to the synergy index approach.
- The affinity typical of ZnAl-SO4 LDH for the chromate anion, though attaining the highest value with respect to the other sorbent and mixture tested here, was lower than other LDH-type sorbents considered in another work [48]. Nevertheless, its application in wastewater remediation can be interesting if the release of sulphate instead of other anions (eg. carbonate, nitrate) from the LDH during the reaction is preferred.
- While the LDH exchange mechanism for the anions occurs in the interlayer, a cationic substitution in the brucite layer can be hypothesized if the ionic rays of the new and leaving species are similar. Regarding iron adsorption, the characterization analysis did not provide precise information on where it may have taken place. It is possible that iron has been removed via interaction with the functional groups on the surface of the mineral.
- A future development of the present study will be devoted to realizing the highest-performing experimental setup in order to optimize the remediation purposes by combining the solid sorbents here proposed in different proportions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A review of adsorbents for heavy metal decontamination: Growing approach to wastewater treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Hu, Y.H. Design, synthesis, and performance of adsorbents for heavy metal removal from wastewater: A review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Cheremisina, O.; Litvinova, T.; Sergeev, V.; Ponomareva, M.; Mashukova, J. Application of the organic waste-based sorbent for the purification of aqueous solutions. Water 2021, 13, 3101. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Wiśniewska, M.; Wołowicz, A.; Gun’ko, V.M.; Zarko, V.I. Mixed silica-alumina oxide as sorbent for dyes and metal ions removal from aqueous solutions and wastewaters. Microporous Mesoporous Mater. 2017, 250, 128–147. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.-H.; Wu, J. Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Anbarasu, A.; Pasupuleti, R.R.; Manigandan, S.; Praveenkumar, T.R.; Kumar, J.A. Treatment of heavy metals containing wastewater using biodegradable adsorbents: A review of mechanism and future trends. Chemosphere 2022, 295, 133724. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mukherjee, S.; Bhattacharyya, N. Microorganism and agricultural based biosorbents towards removal of cadmium from waste-water: An overview. Recent Pat. Biotechnol. 2017, 11, 204–217. [Google Scholar] [CrossRef]
- Gómez-Aguilar, D.L.; Rodríguez-Miranda, J.P.; Salcedo-Parra, O.J. Fruit peels as a sustainable waste for the biosorption of heavy metals in wastewater: A review. Molecules 2022, 27, 2124. [Google Scholar] [CrossRef]
- Tofan, L.; Paduraru, C.; Robu, B.; Miron, A.; Amalinei, R.L.M. Removal of Cd(II) ions from aqueous solution by retention on pine bark. Environ. Eng. Manag. J. 2012, 11, 199–205. [Google Scholar] [CrossRef]
- Hossain, N.; Nizamuddin, S.; Shah, K. Thermal-chemical modified rice husk-based porous adsorbents for Cu (II), Pb (II), Zn (II), Mn (II) and Fe (III) adsorption. J. Water Process Eng. 2022, 46, 102620. [Google Scholar] [CrossRef]
- Castro, D.; Rosas-Laverde, N.M.; Aldás, M.B.; Almeida-Naranjo, C.E.; Guerrero, V.H.; Pruna, A.I. Chemical modification of agro-industrial waste-based bioadsorbents for enhanced removal of Zn(II) ions from aqueous solutions. Materials 2021, 14, 2134. [Google Scholar] [CrossRef]
- Popa, A.; Visa, A.; Maranescu, B.; Hulka, I.; Lupa, L. Chemical modification of chitosan for removal of Pb(II) ions from aqueous Solutions. Materials 2021, 14, 7894. [Google Scholar] [CrossRef] [PubMed]
- Simonič, M.; Zemljič, L.F. Functionalized wool as an efficient and sustainable adsorbent for removal of Zn(II) from an aqueous solution. Materials 2020, 13, 3208. [Google Scholar] [CrossRef] [PubMed]
- Martín, D.M.; Ahmed, M.M.; Rodríguez, M.; García, M.A.; Faccini, M. Aminated polyethylene terephthalate (PET) nanofibers for the selective removal of Pb(II) from polluted water. Materials 2017, 10, 1352. [Google Scholar] [CrossRef]
- Fabiano, B.; Reverberi, A.P.; Varbanov, P. Safety opportunities for the synthesis of metal nanoparticles and short-cut approach to workplace risk evaluation. J. Clean. Prod. 2019, 209, 297–308. [Google Scholar] [CrossRef]
- Lehmann, M.; Zouboulis, A.I.; Matis, K.A. Removal of metal ions from dilute aqueous solutions: A comparative study of inorganic sorbent materials. Chemosphere 881–892. [CrossRef]
- Agayeva, Z.R.; Bayramova, S.S.; Rafiyeva, H.I.; Gahramanova, Y.B.; Kazimova, E.M.; Ilyazova, K.N. The use of aluminosilicate clays as sorbents in the purification of media from heavy metal ions. Azerbaijan Chem. J. 2022, 2022, 100–106. [Google Scholar] [CrossRef]
- Brazdis, R.I.; Fierascu, I.; Avramescu, S.M.; Fierascu, R.C. Recent progress in the application of hydroxyapatite for the adsorption of heavy metals from water matrices. Materials 2021, 14, 6898. [Google Scholar] [CrossRef]
- Stoian, O.; Covaliu, C.I.; Paraschiv, G.; Catrina (Traistaru), G.-A.; Niţă-Lazăr, M.; Matei, E.; Biriş, S.Ş.; Tudor, P. Magnetite oxide nanomaterial used for lead ions removal from industrial wastewater. Materials 2021, 14, 2831. [Google Scholar] [CrossRef]
- Zheng, Y.; Rao, F.; Zhang, M.; Li, J.; Huang, W. Efficient, selective, and reusable metal–organic framework-based adsorbent for the removal of Pb(II) and Cr(VI) heavy-metal pollutants from wastewater. Clean. Eng. Technol. 2021, 5, 100344. [Google Scholar] [CrossRef]
- Nikolaev, A.I.; Gerasimova, L.G.; Maslova, M.V.; Shchukina, E.S. Synthetic analogues of natural titanosilicate mesoporous. minerals as potential functional materials. Synthesis and application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 704, 012003. [Google Scholar] [CrossRef]
- Oleksiienko, O.; Wolkersdorfer, C.; Sillanpää, M. Titanosilicates in cation adsorption and cation exchange—A review. Chem. Eng. J. 2017, 317, 570–585. [Google Scholar] [CrossRef]
- Baldermann, A.; Grießbacher, A.C.; Baldermann, C.; Purgstaller, B.; Letofsky-Papst, I.; Kaufhold, S.; Dietzel, M. Removal of barium, cobalt, strontium, and zinc from solution by natural and synthetic allophane adsorbents. Geosciences 2018, 8, 309. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup:Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Consani, S.; Balić-Žunić, T.; Cardinale, A.M.; Sgroi, W.; Giuli, G.; Carbone, C. A novel synthesis routine for woodwardite and its affinity towards light (La, Ce, Nd) and heavy (Gd and Y) rare earth elements. Materials 2018, 11, 130. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Vecchio Ciprioti, S.; Fortunato, M.; Catauro, M. Thermal behavior and antibacterial studies of a carbonate Mg–Al-based layered double hydroxide (LDH) for in vivo uses. J. Therm. Anal. Calorim. 2022, in press. [CrossRef]
- Li, X.; Fortunato, M.; Cardinale, A.M.; Sarapulova, A.; Njel, C.; Dsoke, S. Electrochemical study on nickel aluminum layered double hydroxides as high-performance electrode material for lithium-ion batteries based on sodium alginate binder. J. Solid State Electrochem. 2022, 26, 49–61. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Shi, R.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Efficient photocatalytic nitrogen fixation over Cuδ+-modified defective ZnAl-layered double hydroxide nanosheets. Adv. Energy Mater. 2020, 10, 1901973. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Jiang, J.-Q. Strategic phosphate removal/recovery by a re-usable Mg-Fe-Cl layered double hydroxide. Process Saf. Environ. Prot. 2017, 107, 454–462. [Google Scholar] [CrossRef]
- Mir, Z.M.; Bastos, A.; Höche, D.; Zheludkevich, M.L. Recent advances on the application of layered double hydroxides in concrete-A review. Materials 2020, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Doelsch, E.; Basile-Doelsch, I.; Abidin, Z.; Miche, H.; Masion, A.; Rose, J.; Borschneck, D.; Bottero, J.-Y. Structure and distribution of allophanes, imogolite and proto-imogolite involcanic soils. Geoderma 2012, 183–184, 100–108. [Google Scholar] [CrossRef]
- Filimonova, S.; Kaufhold, S.; Wagner F., E.; Ha¨usler, W.; Ko¨gel-Knabner, I. The role of allophane nano-structure and Fe oxide speciation for hosting soil organic matter in an allophanic Andosol. Geochim. Cosmochim. Acta 2016, 180, 284–302. [Google Scholar] [CrossRef]
- Baldermann, A.; Fleischhacker, Y.; Schmidthaler, S.; Wester, K.; Nachtnebel, M.; Eichinger, S. Removal of barium from solution by natural and iron(III) oxide-modified allophane, beidellite and zeolite adsorbents. Materials 2020, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Dharmawan, C.A.; Cari; Pranoto; Setyono, P. Combination of activated allophane-effective microorganisms for bioremediation of iron and manganese on pharmaceutical industry wastewater. IOP Conf. Ser. Earth Environ. Sci. 2018, 200, 012020. [Google Scholar] [CrossRef]
- Opiso, E.; Sato, T.; Yoneda, T. Adsorption and co-precipitation behavior of arsenate, chromate, selenate and boric acid with synthetic allophane-like materials. J. Hazard. Mater. 2009, 170, 79–86. [Google Scholar] [CrossRef]
- Reverberi, A.P.; D’Addona, D.M.; Bruzzone, A.A.G.; Teti, R.; Fabiano, B. Nanotechnology in machining processes: Recent advances. Procedia CIRP 2019, 79, 3–8. [Google Scholar] [CrossRef]
- Bîrjega, R.; Pavel, O.D.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A Gen. 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Iyoda, F.; Hayashi, S.; Arakawa, S.; John, B.; Okamoto, M.; Hayashi, H.; Yuan, G. Synthesis and adsorption characteristics of hollow spherical allophane nano-particles. Appl. Clay Sci. 2012, 56, 77–83. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. Powder Cell for Windows. Powder Diffr. 1998, 13, 256–259. [Google Scholar]
- King, G.; Schwarzenbach Latcon, D. Xtal3.7 System. In The Gnu Xtal System User’s Manual; Hall, S.R., du Boilay, D.J., Olthof-Hazekamp, R., Eds.; University of Western: Perth, Australia, 2002. [Google Scholar]
- Rodriguez-Carvajal, J. FULLPROF: A Program for Rietveld Refinement and Pattern Matching Analysis. In Abstracts of the Satellite Meeting on Powder Diffraction of the XV Congress of theIUCr, 127; Toulouse, France, 1990; Available online: https://www.bibsonomy.org/bibtex/1f3e4945ad804f38471c6ad3a513f1e76/jamasi (accessed on 26 August 2022).
- Harikrishnan, R.; Mani, G.; Mani, M.; Kaviyarasu, K.; Baskaran, I. One step microwave assisted synthesis of praseodymium orthoferrite nanoparticles: Rietveld refinement phase matching, optical, and magnetic property analysis. Physica B 2022, 639, 414019. [Google Scholar] [CrossRef]
- Ohashi, F.; Wada, S.-I.; Suzuki, M.; Maeda, M.; Tomura, S. Synthetic allophane from high-concentration solutions: Nanoengineering of the porous solid. Clay Miner. 2002, 37, 451–456. [Google Scholar] [CrossRef]
- Liao, L.; Zhao, N.; Xia, Z. Hydrothermal synthesis of Mg-Al layered double hydroxides (LDHs) from natural brucite and Al(OH)3. Mater. Res. Bull. 2012, 47, 3897–3901. [Google Scholar] [CrossRef]
- Xia, Y.; Li, Y.; Xu, Y. Adsorption of Pb(II) and Cr(VI) from aqueous solution by synthetic allophane suspension: Isotherm, kinetics, and mechanisms. Toxics 2022, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Boccalon, E.; Gorrasi, G.; Nocchetti, M. Layered double hydroxides are still out in the bloom: Syntheses, applications and advantages of three-dimensional flower-like structures. Adv. Colloid Interface Sci. 2020, 285, 102284. [Google Scholar] [CrossRef]
- Cardinale, A.M.; Carbone, C.; Consani, S.; Fortunato, M.; Parodi, N. Layered double hydroxides for remediation of industrial wastewater from a galvanic plant. Crystals 2020, 10, 443. [Google Scholar] [CrossRef]
- Pang, F.M.; Teng, S.P.; Teng, T.T.; Mohd Omar, A.K. Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Qual. Res. J. Can. 2009, 44, 174–182. [Google Scholar] [CrossRef]
- Guo, J.; Wu, Y.; Wang, Z.; Yu, J.; Li, J.-R. Review: Adsorbents for the recovery of precious metals from wastewater. J. Mater. Sci. 2022, 57, 10886–10911. [Google Scholar] [CrossRef]
- D.Lgs n.152 del 03-04-06 All.5, P.Terza . Available online: https://www.bosettiegatti.eu/info/norme/statali/2006_0152.htm (accessed on 26 August 2022).
- Pranoto; Purnawan, C.; Hushina, A.N. Synthesis and characterization of allophane-like as chromium (Cr) ion adsorbent. IOP Conf. Ser.Mater. Sci. Eng. 2018, 333, 012062. [Google Scholar] [CrossRef]
- Babel, S.; Opiso, E.M. Removal of Cr from synthetic wastewater by sorption into volcanic ash soil. Int. J. Environ. Sci. Technol. 2007, 4, 99–107. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, L.; Liang, J.; Guo, X.; Li, W.; Huang, Z. Green synthesis of expanded graphite/layered double hydroxides nanocomposites and their application in adsorption removal of Cr(VI) from aqueous solution. J. Clean. Prod. 2019, 209, 1216–1227. [Google Scholar] [CrossRef]
- Abdi, J.; Sisi, A.J.; Hadipoor, M.; Khataee, A. State of the art on the ultrasonic-assisted removal of environmental pollutants using metal-organic frameworks. J. Hazard. Mater. 2022, 424, 127558. [Google Scholar] [CrossRef] [PubMed]
- Hassandoost, R.; Kotb, A.; Movafagh, Z.; Esmat, M.; Guegan, R.; Endo, S.; Jevasuwan, W.; Fukata, N.; Sugahara, Y.; Khataee, A.; et al. Nanoarchitecturing bimetallic manganese cobaltite spinels for sonocatalytic degradation of oxytetracycline. Chem. Eng. J. 2022, 431, 133851. [Google Scholar] [CrossRef]

| Wastewater Treatment | Metals | |||||
|---|---|---|---|---|---|---|
| Cr(VI) | Cu(II) | Fe(III) | ||||
| Concentration (ppm) | Removal Rate r (%) | Concentration (ppm) | Removal Rate r (%) | Concentration (ppm) | Removal Rate r (%) | |
| Untreated | 102 | / | 0.57 | / | 0.03 | / |
| Allophane | 86 | 15.7 | 0.07 | 87.7 | 0.01 | 66.6 |
| ZnAl-SO4 LDH | 50.6 | 50.4 | 0.41 | 28.0 | <d.l. | ~100 |
| Allophane + LDH | 53.0 | 48 | 0.06 | 89.5 | <d.l. | ~100 |
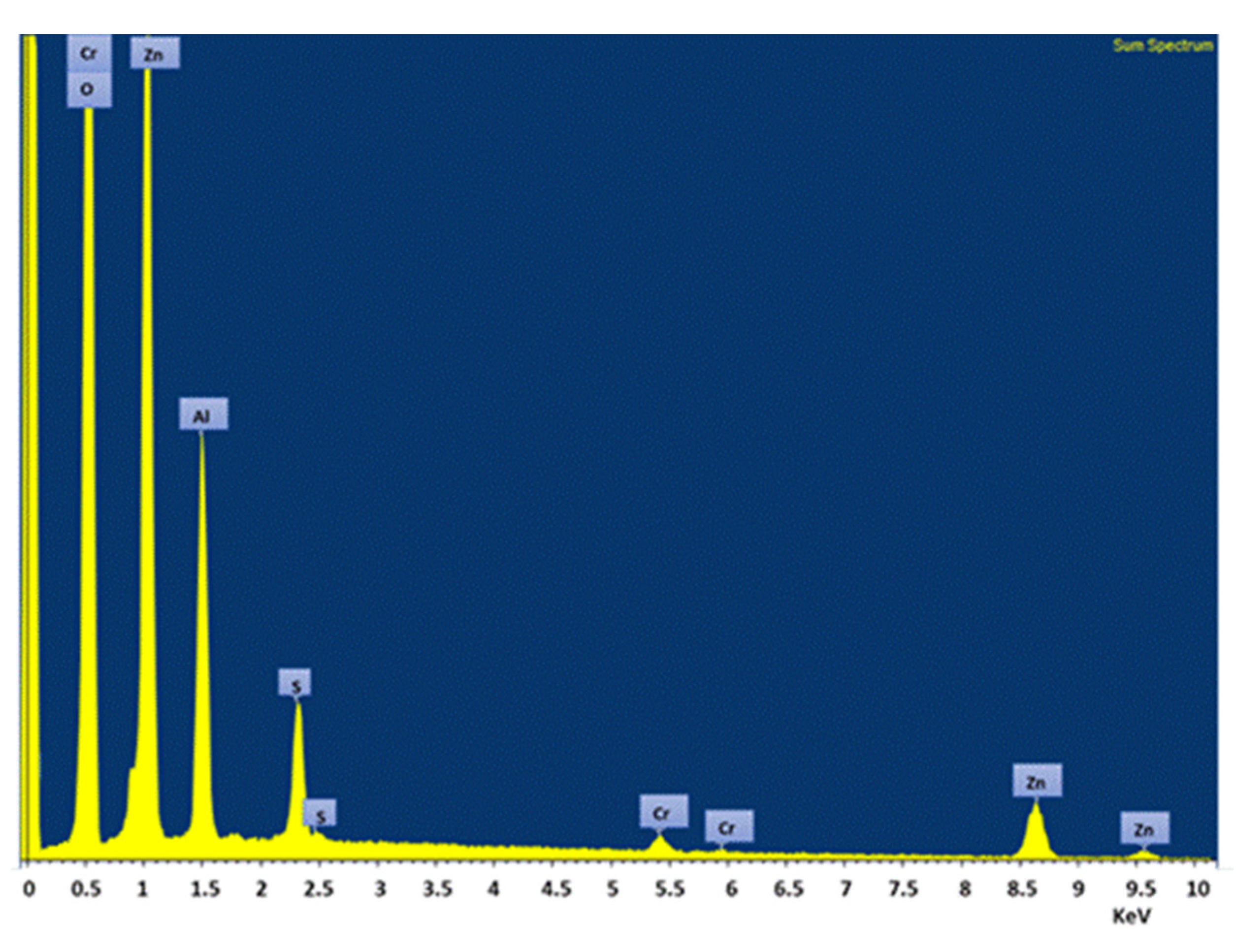
| Element | Mass % (Average Value) |
|---|---|
| Al | 13.39 |
| S | 5.29 |
| Cr | 0.44 |
| Zn | 45.14 |
| O | 35.74 |
| Total | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardinale, A.M.; Carbone, C.; Fortunato, M.; Fabiano, B.; Reverberi, A.P. ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects. Materials 2022, 15, 6887. https://doi.org/10.3390/ma15196887
Cardinale AM, Carbone C, Fortunato M, Fabiano B, Reverberi AP. ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects. Materials. 2022; 15(19):6887. https://doi.org/10.3390/ma15196887
Chicago/Turabian StyleCardinale, Anna Maria, Cristina Carbone, Marco Fortunato, Bruno Fabiano, and Andrea Pietro Reverberi. 2022. "ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects" Materials 15, no. 19: 6887. https://doi.org/10.3390/ma15196887
APA StyleCardinale, A. M., Carbone, C., Fortunato, M., Fabiano, B., & Reverberi, A. P. (2022). ZnAl-SO4 Layered Double Hydroxide and Allophane for Cr(VI), Cu(II) and Fe(III) Adsorption in Wastewater: Structure Comparison and Synergistic Effects. Materials, 15(19), 6887. https://doi.org/10.3390/ma15196887







