Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions and Specimen Preparation
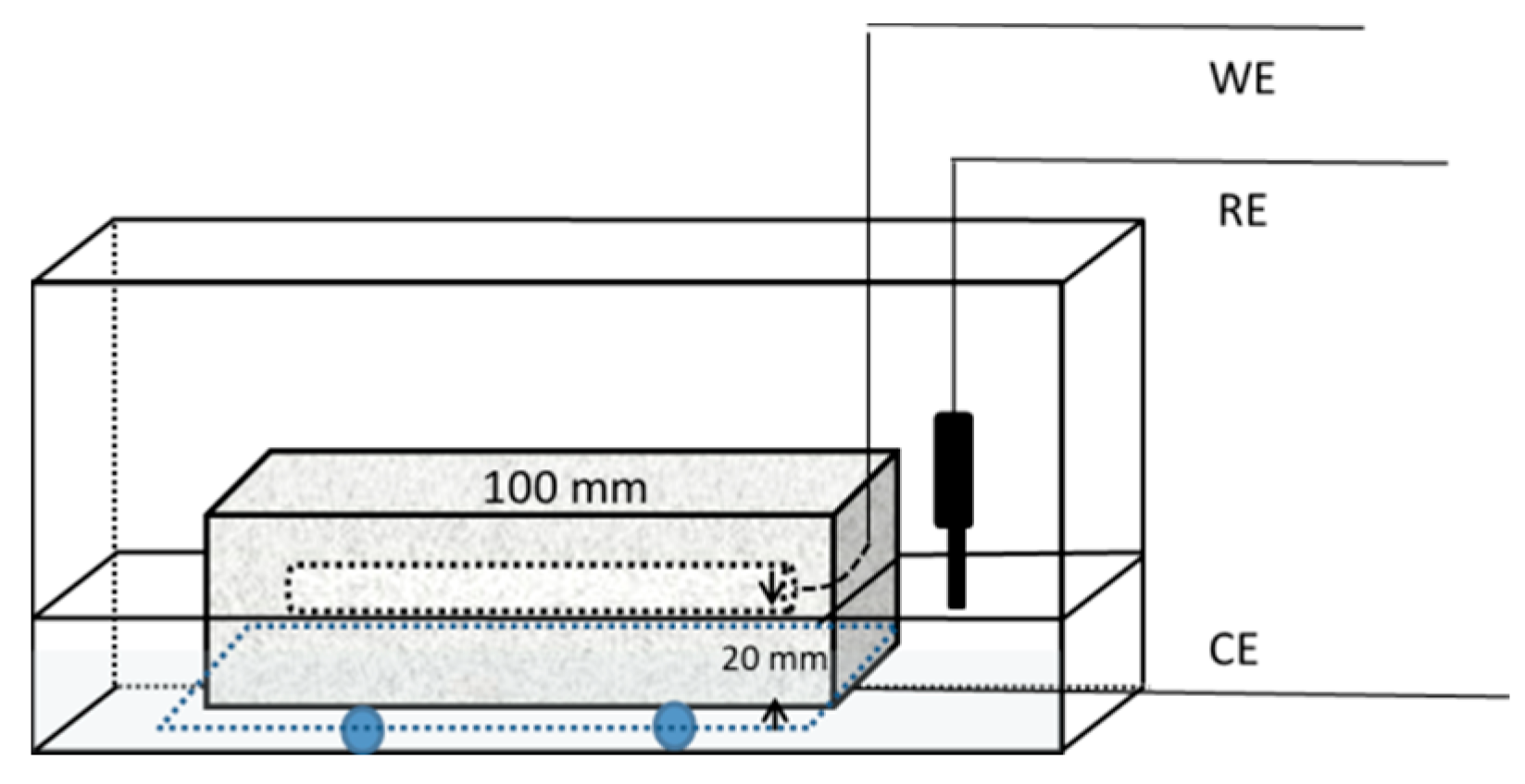
2.3. Specimens
2.4. Test Methods
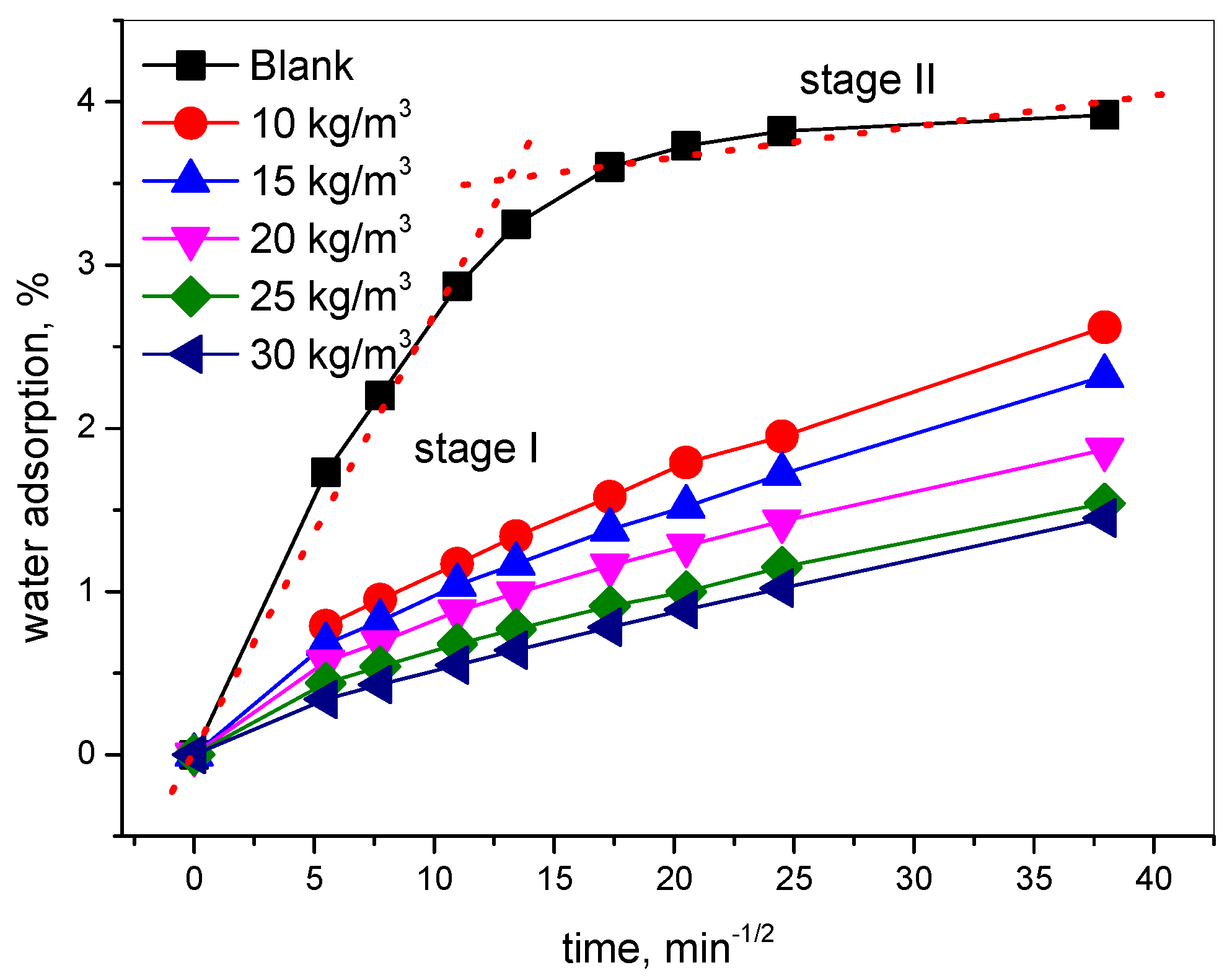
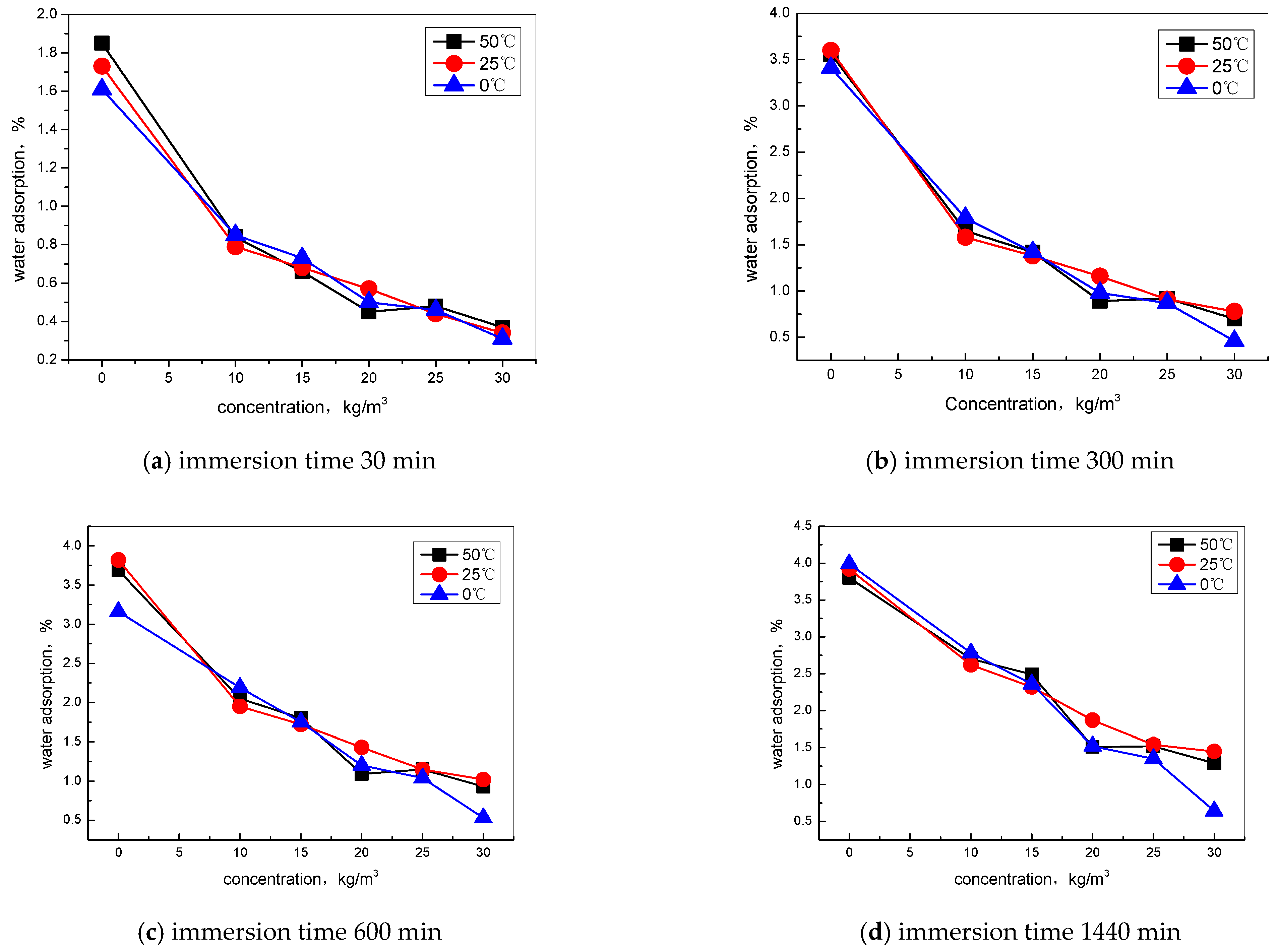
2.4.1. Water Absorption Test
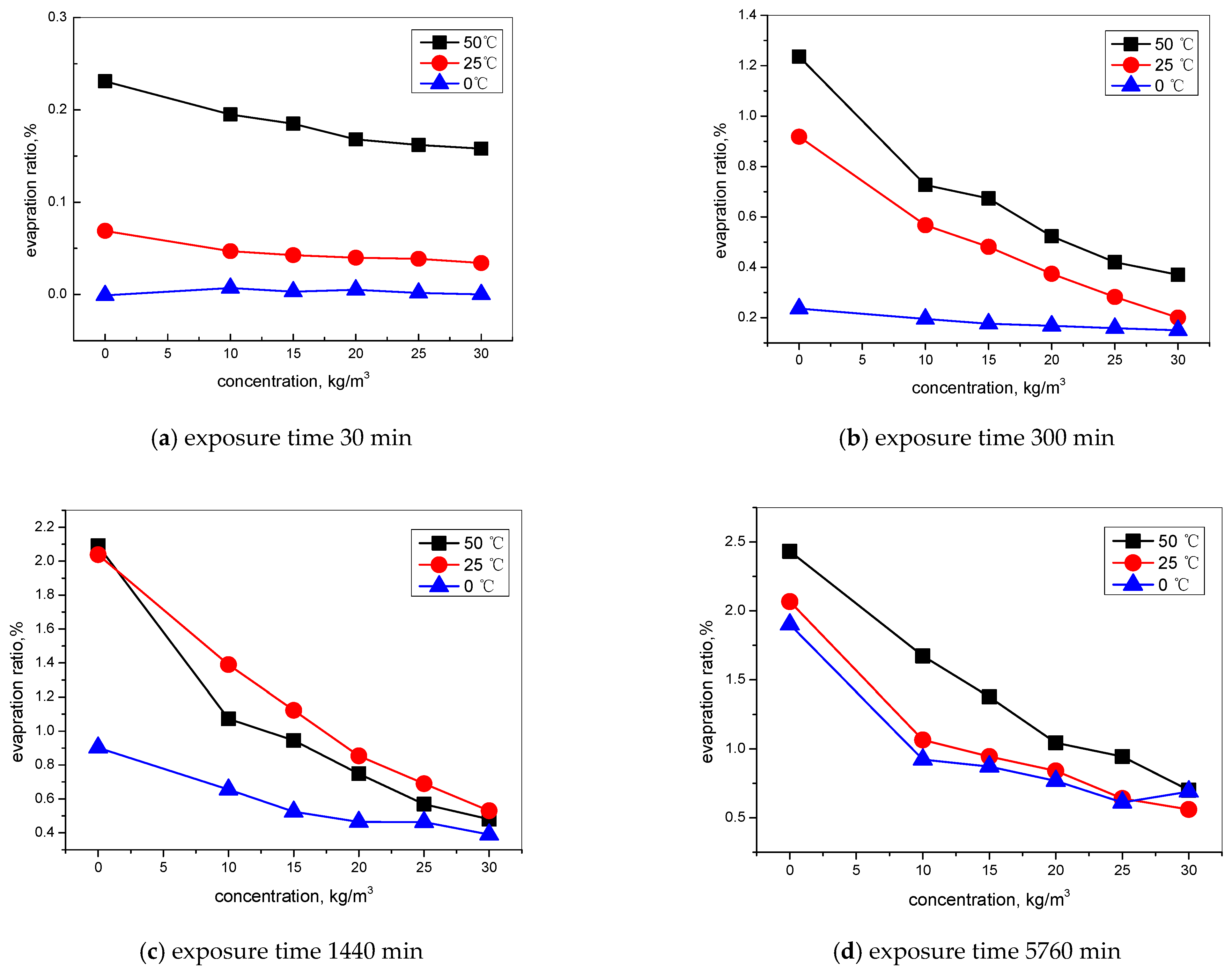
2.4.2. Evaporation Test
2.4.3. Chloride Diffusion Test
2.4.4. Electrochemical Measurement
3. Results
3.1. Effect of Hydrophobic Admixture on the Water Adsorption of Concrete
3.2. Chloride Ion Diffusion
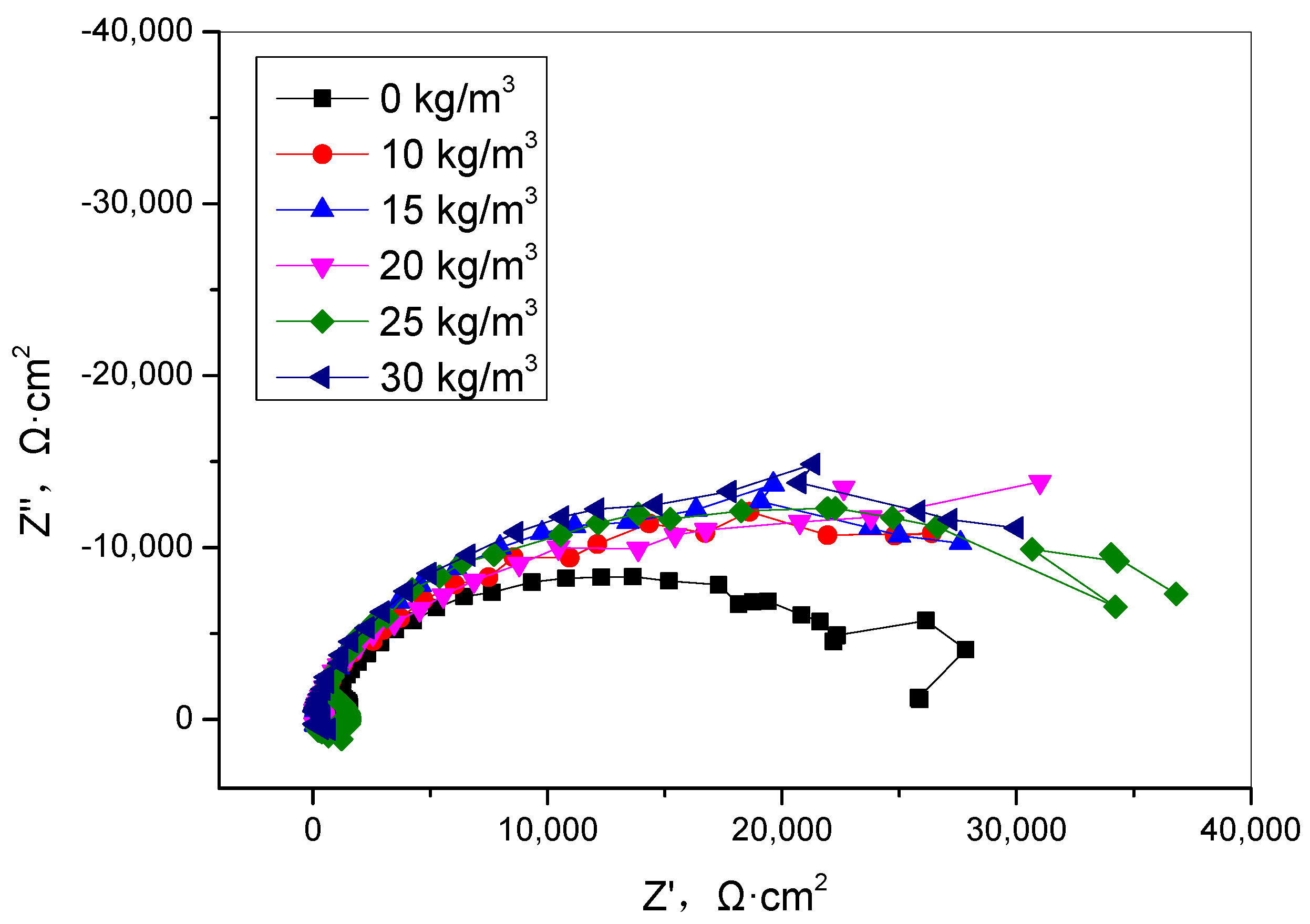
3.3. Electrochemical Measurements
4. Discussion
5. Conclusions
- (1)
- The nano-hydrophobic admixture exhibits a significant water adsorption inhibition behavior; the higher the content of TIA incorporated into concrete, the more water transportation-restraint performance was obtained. During the water adsorption process, the main influencing factor is the structure of the concrete pore modified by TIA, while the temperature has less effect on water transportation.
- (2)
- The nano-hydrophobic admixture also has an effective inhibition behavior on water evaporation, which is also influenced by environmental temperature. A higher content of TIA and a lower temperature favor reducing water evaporation.
- (3)
- Because the water adsorption and water evaporation process were both inhibited, the transportation of chloride ions was effectively restrained by the hydrophobic admixture. In the present investigation, the chloride ion concentration was reduced more than 50% compared with the blank concrete, which is normal marine engineering concrete.
- (4)
- The nano-hydrophobic admixture effectively prolongs steel corrosion by suppressing of chloride ion transport, which has less effect on electrochemical corrosion behavior when the chloride ions directly contact the steel surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef]
- Coppola, L.; Beretta, S.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Caputo, D.; Carsana, M.; Cioffi, R.; et al. The Improvement of Durability of Reinforced Concretes for Sustainable Structures: A Review on Different Approaches. Materials 2022, 15, 2728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or superhydrophobic modification of cement-based materials: A systematic review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- Page, C.L.; Treadaway, K.W.J. Aspects of the electrochemistry of steel in concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yu, J.; Kong, X. Water absorption behavior of hydrophobized concrete using silane emulsion as admixture. Cem. Concr. Res. 2022, 154, 106738. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. The effect of silane-based hydrophobic admixture on corrosion of reinforcing steel in concrete. Cem. Concr. Res. 2008, 38, 1354–1357. [Google Scholar] [CrossRef]
- Tittarelli, F.; Moriconi, G. Comparison between surface and bulk hydrophobic treatment against corrosion of galvanized reinforcing steel in concrete. Cem. Concr. Res. 2011, 41, 609–614. [Google Scholar] [CrossRef]
- Tittarelli, F. Oxygen diffusion through hydrophobic cement-based materials. Cem. Concr. Res. 2009, 39, 924–928. [Google Scholar] [CrossRef]
- Tittarelli, F.; Mobili, A.; Giosuè, C.; Belli, A.; Bellezze, T. Corrosion behaviour of bare and galvanized steel in geopolymer and Ordinary Portland Cement based mortars with the same strength class exposed to chlorides. Corros. Sci. 2018, 134, 64–77. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An alternative admixture to reduce sorptivity of alkali-activated slag cement by optimising pore structure and introducing hydrophobic film. Cem. Concr. Compos. 2018, 95, 183–192. [Google Scholar] [CrossRef]
- Corinaldesi, V. Combined effect of expansive, shrinkage reducing and hydrophobic admixtures for durable self compacting concrete. Constr. Build. Mater. 2012, 36, 758–764. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Javed, S.; Khan, T.M.Y.; Baig, R.U. Assessment of Destructive and Nondestructive Analysis for GGBS Based Geopolymer Concrete and Its Statistical Analysis. Polymers 2022, 14, 3132. [Google Scholar] [CrossRef] [PubMed]
- Polder, R.B.; Borsje, H.; Vries, H. Prevention of reinforcement corrosion by hydrophobic treatment of concrete. Hero 2001, 46, 227–238. [Google Scholar]
- Medeiros, M.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Medeiros, M.; Helene, P. Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete. Mater. Struct. 2008, 41, 59–71. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kozhukhova, M.; Sobolev, K.; Nosonovsky, M. Anti-Icing Superhydrophobic Surfaces: Controlling Entropic Molecular Interactions to Design Novel Icephobic Concrete. Entropy 2016, 18, 132. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Azab, W.; Cuellar-Camacho, J.L.; Guday, G.; Lippitz, A.; Unger, W.E.S.; Osterrieder, K.; Adeli, M.; Haag, R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale 2019, 11, 15804–15809. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, P. Effect of hydrophobic agent in cement and concrete: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1116, 012175. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Chen, R.; Hou, D.; Xu, J.; Lv, K.; Zheng, Q. The design and evaluation of a smart polymer-based fluids transport inhibitor. J. Clean. Prod. 2020, 257, 120528. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Hou, D.; Chang, H.; Yu, J. The inhibiting effect and mechanisms of smart polymers on the transport of fluids throughout nano-channels. Appl. Surf. Sci. 2020, 500, 144019. [Google Scholar] [CrossRef]
- Liu, J.; Mu, S.; Cai, J.; Jiang, Q. Preparation and performance evaluation of cement hydration responsive nanomaterial. J. Build. Struct. 2019, 40, 181–187. [Google Scholar]
- Zhu, Z.; Cai, J.S.; Hong, J.X.; Mu, S.; Zhou, X.C.; Ma, Q.; Chen, C.C. Effect of Hydration Response Nanomaterials on Corrosion Resistance of Reinforced Concrete. J. Chin. Soc. Corros. Prot. 2021, 41, 732–736. [Google Scholar]
- Feng, Z.; Wang, F.; Xie, T.; Ou, J.; Xue, M.; Li, W. Integral hydrophobic concrete without using silane. Constr. Build. Mater. 2019, 227, 116678. [Google Scholar] [CrossRef]
- Wang, F.; Lei, S.; Ou, J.; Li, W. Effect of PDMS on the waterproofing performance and corrosion resistance of cement mortar. Appl. Surf. Sci. 2019, 507, 145016. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Mu, S.; Liu, J.; Hong, J.; Zhou, X.; Ma, Q.; Shi, L. Corrosion inhibition effect of three imidazolium ionic liquids on carbon steel in chloride contaminated environment. Int. J. Electrochem. Sci. 2020, 15, 1287–1301. [Google Scholar] [CrossRef]
- Liu, J.; Cai, J.; Shi, L.; Liu, J.; Zhou, X.; Mu, S.; Hong, J. The inhibition behavior of a water-soluble silane for reinforcing steel in 3.5% NaCl saturated Ca(OH)2 solution. Constr. Build. Mater. 2018, 189, 95–101. [Google Scholar] [CrossRef]
- Liu, J.Z.; Cai, J.S.; Shi, L.; Zhao, D.; Chen, C.C.; Jiang, Q.; Sha, J.F. Corrosion behavior of carbon steel in chloride contaminated simulated concrete pore solution with carboxylate of benzoic acid and dimethylethanolamine. Anti-Corros. Methods Mater. 2017, 64, 555–562. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Rahman, M.M.; Chamberlain, D.A. Fundamental interaction of hydrophobic materials in concrete with different moisture contents in saline environment. Constr. Build. Mater. 2019, 207, 122–135. [Google Scholar] [CrossRef]
- Luzar, A.; Leung, K. Dynamics of capillary evaporation. I. Effect of morphology of hydrophobic surfaces. J. Chem. Phys. 2000, 113, 5836–5844. [Google Scholar] [CrossRef]
- Dash, S.; Garimella, S.V. Droplet evaporation on heated hydrophobic and superhydrophobic surfaces. Phys. Rev. E 2014, 89, 042402. [Google Scholar] [CrossRef] [PubMed]


| Composition | C | Si | Mn | P | S | Ceq |
|---|---|---|---|---|---|---|
| % | 0.25 | 0.8 | 1.6 | 0.045 | 0.045 | 0.54 |
| Composition | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| % | 22.16 | 6.17 | 58.27 | 3.61 | 0.93 | 1.84 | 0.14 | 0.32 |
| NO. | Cement | Flyash | Slag Powder | Sand | Gravel | Water | Water Reducer | TIA |
|---|---|---|---|---|---|---|---|---|
| 1 | 220 | 110 | 100 | 730 | 1100 | 150 | 5 | - |
| 2 | 220 | 110 | 100 | 730 | 1100 | 110 | 5 | 10 |
| 3 | 220 | 110 | 100 | 730 | 1100 | 115 | 5 | 15 |
| 4 | 220 | 110 | 100 | 730 | 1100 | 130 | 5 | 20 |
| 5 | 220 | 110 | 100 | 730 | 1100 | 125 | 5 | 25 |
| 6s | 220 | 110 | 100 | 730 | 1100 | 120 | 5 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Ran, Q.; Ma, Q.; Zhang, H.; Liu, K.; Zhou, Y.; Mu, S. Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion. Materials 2022, 15, 6842. https://doi.org/10.3390/ma15196842
Cai J, Ran Q, Ma Q, Zhang H, Liu K, Zhou Y, Mu S. Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion. Materials. 2022; 15(19):6842. https://doi.org/10.3390/ma15196842
Chicago/Turabian StyleCai, Jingshun, Qianping Ran, Qi Ma, Hao Zhang, Kai Liu, Yang Zhou, and Song Mu. 2022. "Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion" Materials 15, no. 19: 6842. https://doi.org/10.3390/ma15196842
APA StyleCai, J., Ran, Q., Ma, Q., Zhang, H., Liu, K., Zhou, Y., & Mu, S. (2022). Influence of a Nano-Hydrophobic Admixture on Concrete Durability and Steel Corrosion. Materials, 15(19), 6842. https://doi.org/10.3390/ma15196842







