Reaction Mechanism of CA6, Al2O3 and CA6-Al2O3 Refractories with Refining Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Crucibles and Refining Slag
2.2. Experimental
2.3. Characterization
2.4. Thermodynamic Simulation
3. Results and Discussion
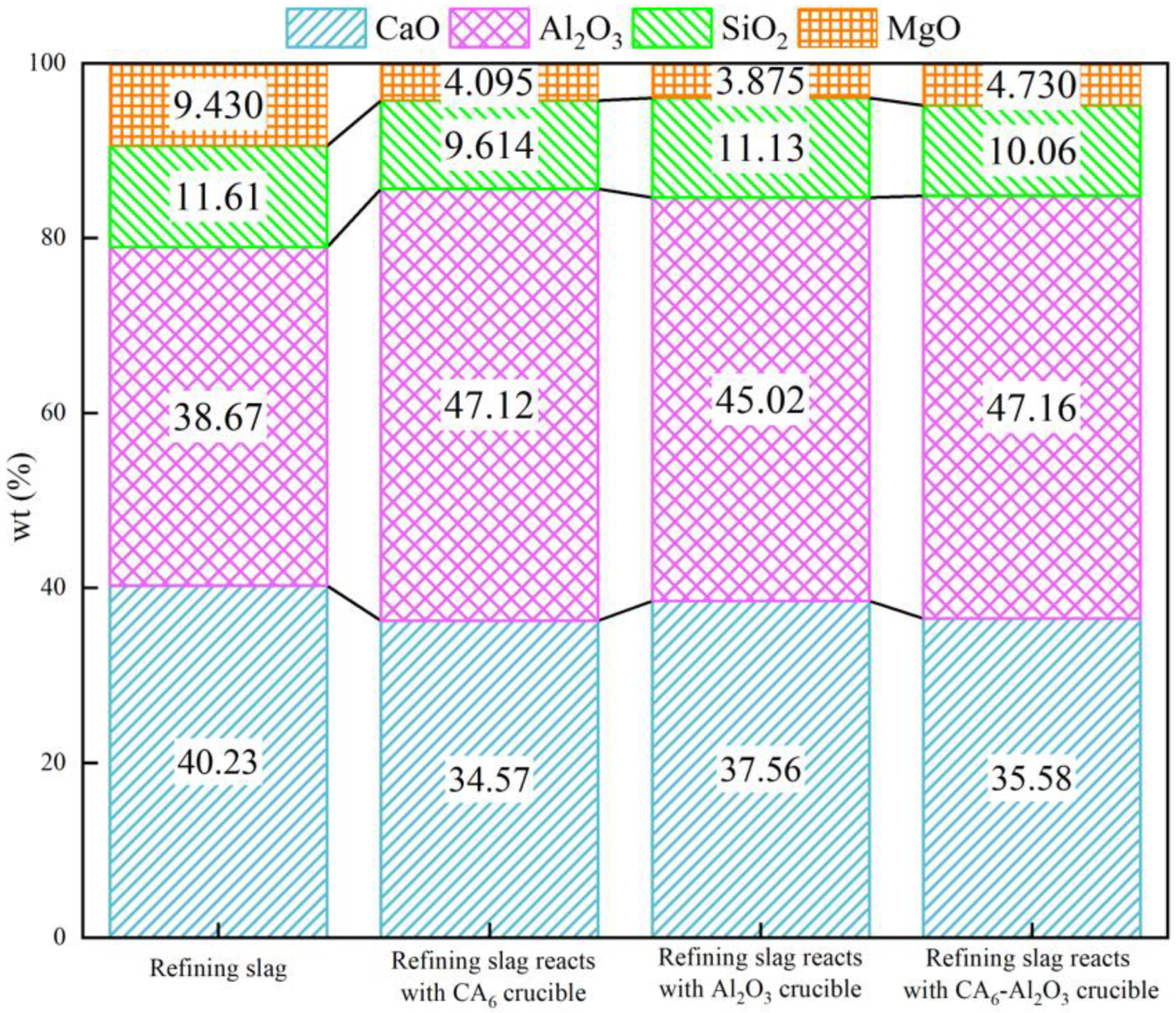
3.1. Composition Changes of Refining Slag
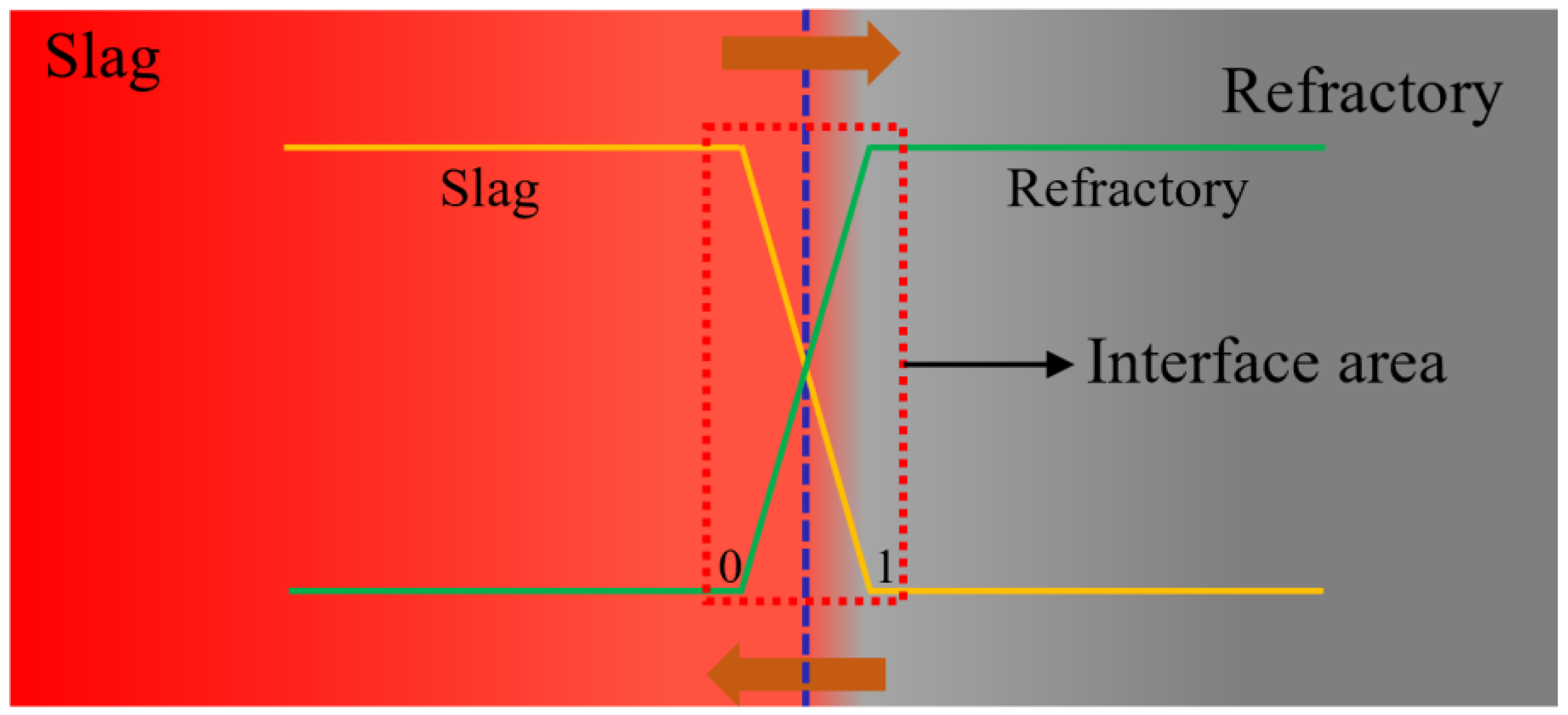
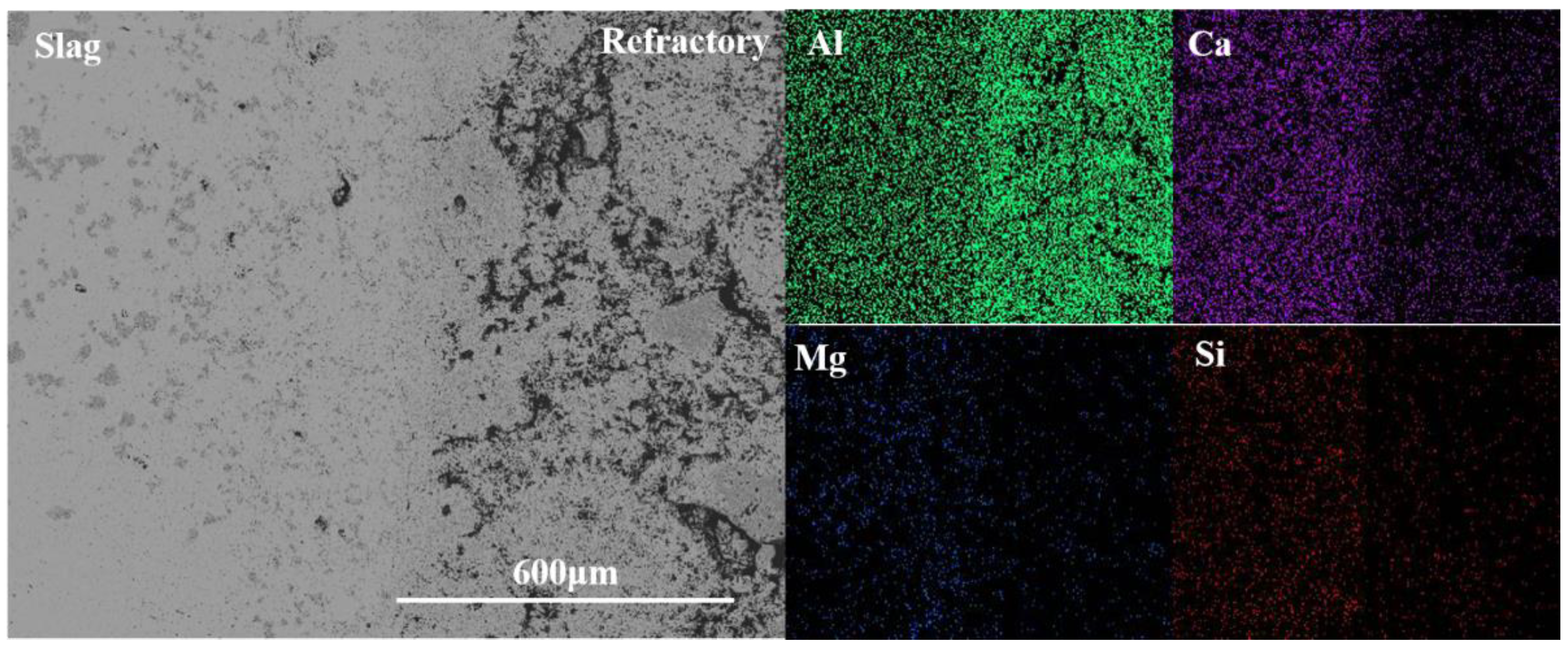
3.2. Microstructure and Element Distribution
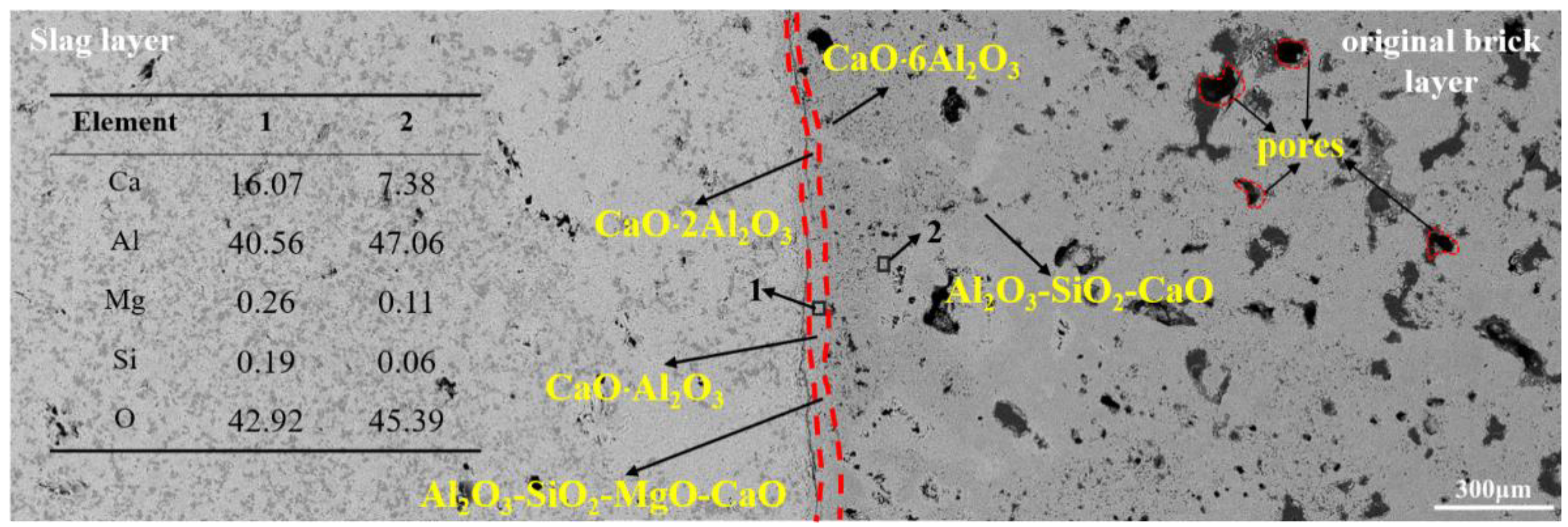
3.2.1. Corrosion of CA6 Crucible
3.2.2. Corrosion of Al2O3 Crucible
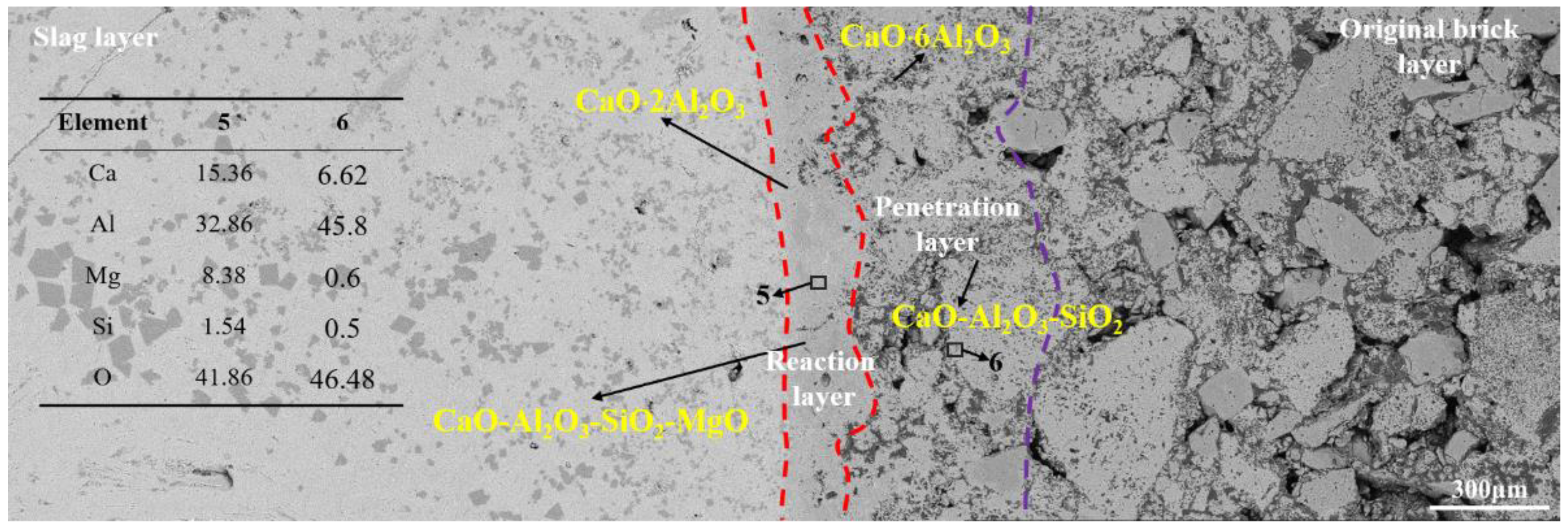
3.2.3. Corrosion of CA6-Al2O3 Crucible
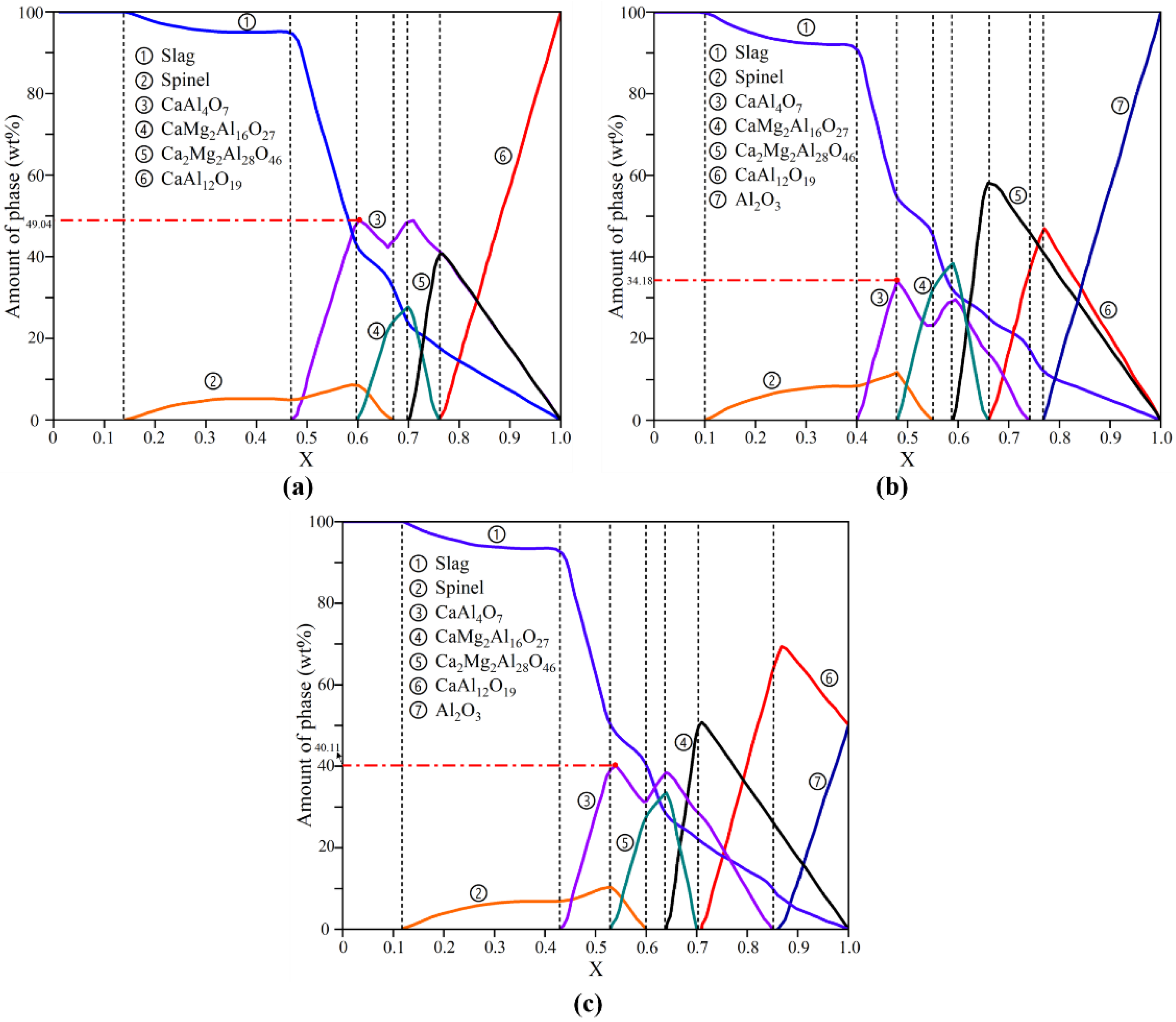
3.3. Thermodynamic Simulation of Corrosion of Crucibles by Refining Slag
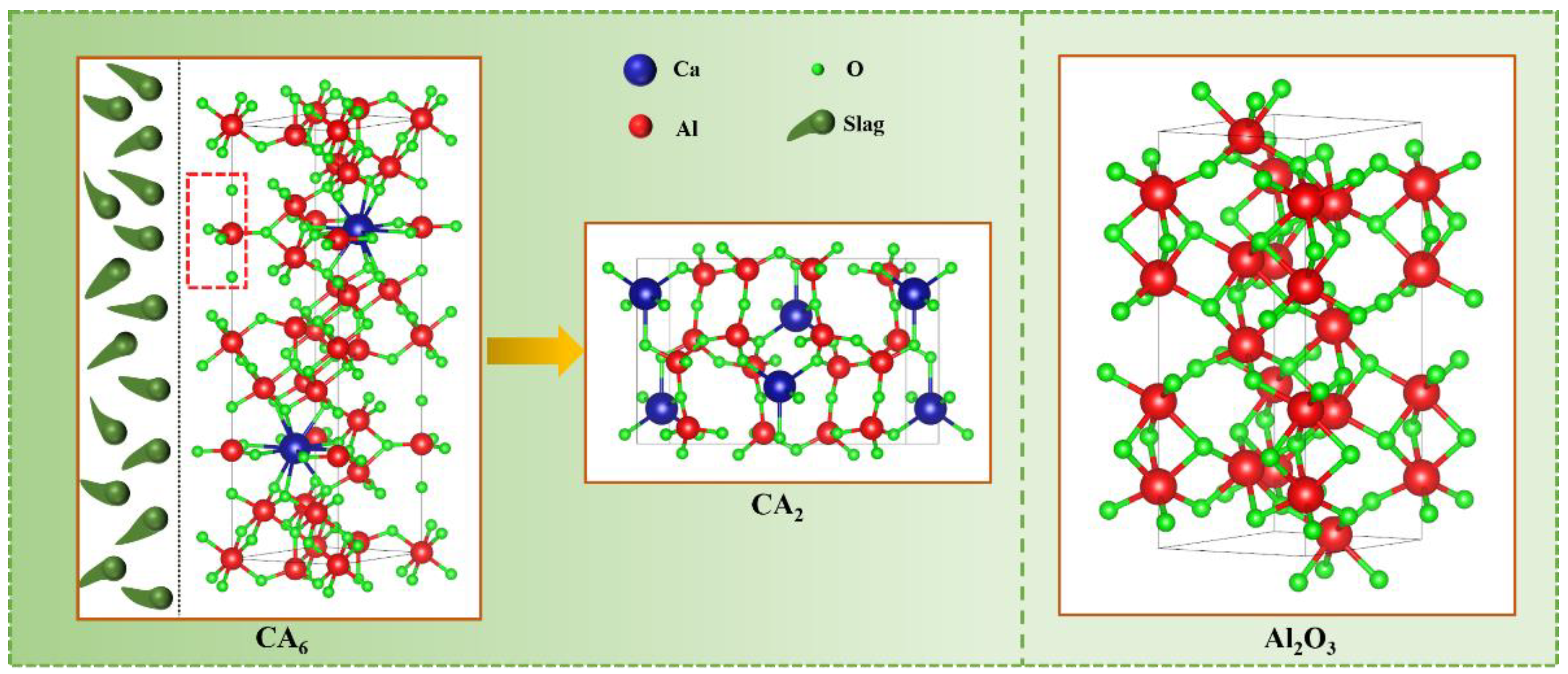
3.4. Crystal Structure Analysis of the Refractories
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Chen, L.; Wei, Y.; Li, N.; Zhang, S. Corrosion and penetration behaviors of slag/steel on the corroded interfaces of Al2O3-C refractories: Role of Ti3AlC2. Corros. Sci. 2018, 143, 166–176. [Google Scholar] [CrossRef]
- Xu, L.; Chen, M.; Wang, N.; Yin, X.L. Corrosion mechanism of MgAl2O4–CaAl4O7–CaAl12O19 composite by steel ladle slag: Effect of additives. J. Eur. Ceram. Soc. 2017, 37, 2737–2746. [Google Scholar] [CrossRef]
- Berjonneau, J.; Prigent, P.; Poirier, J. The development of a thermodynamic model for Al2O3–MgO refractory castable corrosion by secondary metallurgy steel ladle slags. Ceram. Int. 2009, 35, 623–635. [Google Scholar] [CrossRef]
- Riaz, S.; Mills, K.; Bain, K. Experimental examination of slag/refractory interface. Ironmak. Steelmak. 2022, 29, 107–113. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, A.; Gu, H. Novel phenomenon of quasi-volcanic corrosion on the alumina refractory-slag-air interface. J. Am. Ceram. Soc. 2020, 103, 6639–6649. [Google Scholar] [CrossRef]
- Mills, K.C.; Su, Y.; Fox, A.B.; Li, Z.; Thackray, R.P.; Tsai, H. A review of slag splashing. ISIJ Int. 2005, 45, 619–633. [Google Scholar] [CrossRef]
- Liu, C.; Huang, F.; Wang, X. The effect of refining slag and refractory on inclusion transformation in extra low oxygen steels. Metall. Mater. Trans. B 2016, 47, 999–1009. [Google Scholar] [CrossRef]
- Huang, A.; Wang, Y.; Gu, H.; Zou, Y. Dynamic interaction of refractory and molten steel: Effect of alumina-magnesia castables on alloy steel cleanness. Ceram. Int. 2018, 44, 22146–22153. [Google Scholar] [CrossRef]
- Jones, P.T.; Vleugels, J.; Volders, I.; Blanpain, B.; van der Biest, O.; Wollants, P. A study of slag-infiltrated magnesia-chromite refractories using hybrid microwave heating. J. Eur. Ceram. Soc. 2002, 22, 903–916. [Google Scholar] [CrossRef]
- Ali, M.; Sayet, T.; Gasser, A.; Blond, E. Transient Thermo-Mechanical Analysis of Steel Ladle Refractory Linings Using Mechanical Homogenization Approach. Ceramics 2020, 3, 171–189. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M.; Sichen, D. Effect of refractory on nonmetallic inclusions in Al-killed steel. Metall. Mater. Trans. B 2016, 47, 3158–3167. [Google Scholar] [CrossRef]
- Deng, Z.; Cheng, L.; Chen, L.; Zhu, M. Effect of Refractory on Nonmetallic Inclusions in Si–Mn-Killed Steel. Steel Res. Int. 2019, 90, 1900268. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Zhang, L. Formation mechanism of MgO containing inclusions in the molten steel refined in MgO refractory crucibles. Metals 2020, 10, 444. [Google Scholar] [CrossRef]
- Beskow, K.; Tripathi, N.N.; Nzotta, M.; Sandberg, A.; Sichen, D. Impact of slag–refractory lining reactions on the formation of inclusions in steel. Ironmak. Steelmak. 2004, 31, 514–518. [Google Scholar] [CrossRef]
- Brabie, V. Mechanism of reaction between refractory materials and aluminum deoxidised molten steel. ISIJ Int. 1996, 36, S109–S112. [Google Scholar] [CrossRef]
- Shin, J.; Chung, Y.; Park, J. Refractory–Slag–Metal–Inclusion Multiphase Reactions Modeling Using Computational Thermodynamics: Kinetic Model for Prediction of Inclusion Evolution in Molten Steel. Metall. Mater. Trans. B 2016, 48, 46–59. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z. Effect of initial large-sized inclusion content on inclusion removal during electroslag remelting of H13 die steel. Ironmak. Steelmak. 2018, 45, 919–923. [Google Scholar] [CrossRef]
- Lei, Z.; Hong, Y.; Xie, J.; Sun, C.; Zhao, A. Effects of inclusion size and location on very-high-cycle fatigue behavior for high strength steels. Mater. Sci. Eng. A. 2012, 558, 234–241. [Google Scholar] [CrossRef]
- Holappa, L.; Helle, A. Inclusion control in high-performance steels. J. Mater. Process. Technol. 1995, 53, 177–186. [Google Scholar] [CrossRef]
- Karr, U.; Sandaiji, Y.; Tanegashima, R.; Murakami, S.; Schoenbauer, B.; Fitzka, M.; Mayer, H. Inclusion initiated fracture in spring steel under axial and torsion very high cycle fatigue loading at different load ratios. Int. J. Fatigue 2020, 134, 105525. [Google Scholar] [CrossRef]
- Zhang, S.; Rezaie, H.R.; Sarpoolaky, H.; Lee, W.E. Alumina dissolution into silicate slag. J. Am. Ceram. Soc. 2000, 83, 897–903. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.G.; Kim, J.S. Dissolution rate of Al2O3 into molten CaO-SiO2-Al2O3 slags. ISIJ Int. 2002, 42, 852–860. [Google Scholar] [CrossRef]
- Yan, P.; Webler, B.A.; Pistorius, P.C.; Fruehan, R.J. Nature of MgO and Al2O3 Dissolution in Metallurgical Slags. Metall. Mater. Trans. B 2015, 46, 2414–2418. [Google Scholar] [CrossRef]
- de Bilbao, E.; Poirier, J.; Dombrowski, M. Corrosion of high alumina refractories by Al2O3-CaO slag: Thermodynamic and kinetic approaches. Metall. Res. Technol. 2015, 112, 607–621. [Google Scholar] [CrossRef]
- Fernández, B.; Almanza, J.; Rodríguez, J.; Cortes, D.; Escobedo, J.; Gutiérrez, E. Corrosion mechanisms of Al2O3/MgAl2O4 by V2O5, NiO, Fe2O3 and vanadium slag. Ceram. Int. 2011, 37, 2973–2979. [Google Scholar] [CrossRef]
- Song, J.; Liu, Y.; Lv, X.; You, Z. Corrosion Behavior of Al2O3 Substrate by SiO2–MgO–FeO–CaO–Al2O3 Slag. J. Mater. Res. Technol. 2020, 9, 314–321. [Google Scholar] [CrossRef]
- Tang, H.; Wu, G.; Wang, Y.; Li, J.; Lan, P.; Zhang, J. Comparative evaluation investigation of slag corrosion on Al2O3 and MgO-Al2O3 refractories via experiments and thermodynamic simulations. Ceram. Int. 2017, 43, 16502–16511. [Google Scholar] [CrossRef]
- Wang, W.; Xue, L.; Zhang, T.; Zhou, L.; Chen, J.; Pan, Z. Thermodynamic corrosion behavior of Al2O3, ZrO2 and MgO refractories in contact with high basicity refining slag. Ceram. Int. 2019, 45, 20664–20673. [Google Scholar] [CrossRef]
- Fu, L.; Huang, A.; Gu, H.; Lu, D.; Lian, P. Effect of nano-alumina sol on the sintering properties and microstructure of microporous corundum. Mater. Des. 2016, 89, 21–26. [Google Scholar] [CrossRef]
- Chen, J.; Yan, M.; Su, J.; Li, B.; Chou, K.C.; Hou, X.; Chen, M.; Zhao, B. Controllable Preparation of Al2O3-MgO·Al2O3-CaO·6Al2O3(AMC) Composite with Improved Slag Penetration Resistance. Int. J. Appl. Ceram. Technol. 2016, 13, 33–40. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Chen, H.; Chen, J.; Hou, X.; Li, Y. Physical and mechanical properties of hot-press sintering ternary CM2A8 (CaMg2Al16O27) and C2M2A14 (Ca2Mg2Al28O46) ceramics. J. Adv. Ceram. 2018, 7, 229–236. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Chen, J.; Wang, E.; Hou, X.; Li, Y. Preparation, growth mechanism and slag resistance behavior of ternary Ca2Mg2Al28O46(C2M2A14). Int. J. Appl. Ceram. Technol. 2019, 16, 1126–1137. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Mi, W.; Cao, Z.; Li, B.; Liang, C.; Vance, L. Substitution of Ba for Ca in the Structure of CaAl12O19. J. Am. Ceram. Soc. 2017, 100, 413–418. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Mi, W.; Cao, Z.; Li, B.; Li, G. Synthesis of CaO·2MgO·8Al2O3 (CM2A8) and its slag resistance mechanism. J. Eur. Ceram. Soc. 2017, 37, 1799–1804. [Google Scholar] [CrossRef]
- Xu, L.; Wang, E.; Hou, X.; Chen, J.; He, Z.; Liang, T. Effect of incorporation of nitrogen on calcium hexaaluminate. J. Eur. Ceram. Soc. 2020, 40, 6155–6161. [Google Scholar] [CrossRef]
- Guo, C.; Wang, E.; Hou, X.; Kang, J.; Yang, T.; Liang, T.; Bei, G. Preparation of Zr4+ doped calcium hexaaluminate with improved slag penetration resistance. J. Am. Ceram. Soc. 2021, 104, 4854–4866. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, J.; Li, Y.; Cheng, Y.; Nath, M.; Zhang, Y.; Zheng, L.; Wei, Y.; Zhang, S.; Li, N. Corrosion mechanism of cement-bonded Al2O3–MgAl2O4 pre-cast castables in contact with molten steel and slag. Ceram. Int. 2022, 48, 5168–5173. [Google Scholar] [CrossRef]
- Darban, S.; Reynaert, C.; Ludwig, M.; Prorok, R.; Jastrzębska, I.; Szczerba, J. Corrosion of Alumina-Spinel Refractory by Secondary Metallurgical Slag Using Coating Corrosion Test. Materials 2022, 15, 3425. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, P.; Chen, J.; Zhao, H.; He, K. Corrosion resistance of Al–Cr-slag containing chromium–corundum refractories to slags with different basicity. Ceram. Int. 2018, 44, 12162–12168. [Google Scholar] [CrossRef]
- Yin, H.; Gao, K.; Wan, Q.; Xin, Y.; Tang, Y.; Yuan, H. A comparative study on the slag resistance of dense corundum-spinel refractory and lightweight corundum-spinel refractory with density gradient. Ceram. Int. 2021, 47, 21310–21318. [Google Scholar] [CrossRef]




| Crucible | Bulk Density/(g/cm3) | Apparent Porosity/(%) |
|---|---|---|
| CA6 | 2.93 | 19.23 |
| Al2O3 | 2.92 | 24.41 |
| CA6-Al2O3 | 2.68 | 28.93 |
| CaO | Al2O3 | MgO | SiO2 | |
|---|---|---|---|---|
| Wt (%) | 40 | 39 | 10 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, Z.; Feng, J.; Li, B.; Chen, J.; Ren, B.; Jia, Y.; Yin, S. Reaction Mechanism of CA6, Al2O3 and CA6-Al2O3 Refractories with Refining Slag. Materials 2022, 15, 6779. https://doi.org/10.3390/ma15196779
Liu J, Liu Z, Feng J, Li B, Chen J, Ren B, Jia Y, Yin S. Reaction Mechanism of CA6, Al2O3 and CA6-Al2O3 Refractories with Refining Slag. Materials. 2022; 15(19):6779. https://doi.org/10.3390/ma15196779
Chicago/Turabian StyleLiu, Jie, Zheng Liu, Jisheng Feng, Bin Li, Junhong Chen, Bo Ren, Yuanping Jia, and Shu Yin. 2022. "Reaction Mechanism of CA6, Al2O3 and CA6-Al2O3 Refractories with Refining Slag" Materials 15, no. 19: 6779. https://doi.org/10.3390/ma15196779
APA StyleLiu, J., Liu, Z., Feng, J., Li, B., Chen, J., Ren, B., Jia, Y., & Yin, S. (2022). Reaction Mechanism of CA6, Al2O3 and CA6-Al2O3 Refractories with Refining Slag. Materials, 15(19), 6779. https://doi.org/10.3390/ma15196779








