Electrochemical Studies of Stainless Steel and Stainless Steel-TiO2 Composite in Reference to Molten Aluminum Alloy Using a Solid-State BaCO3 Electrolyte
Abstract
:1. Introduction
2. Experimental
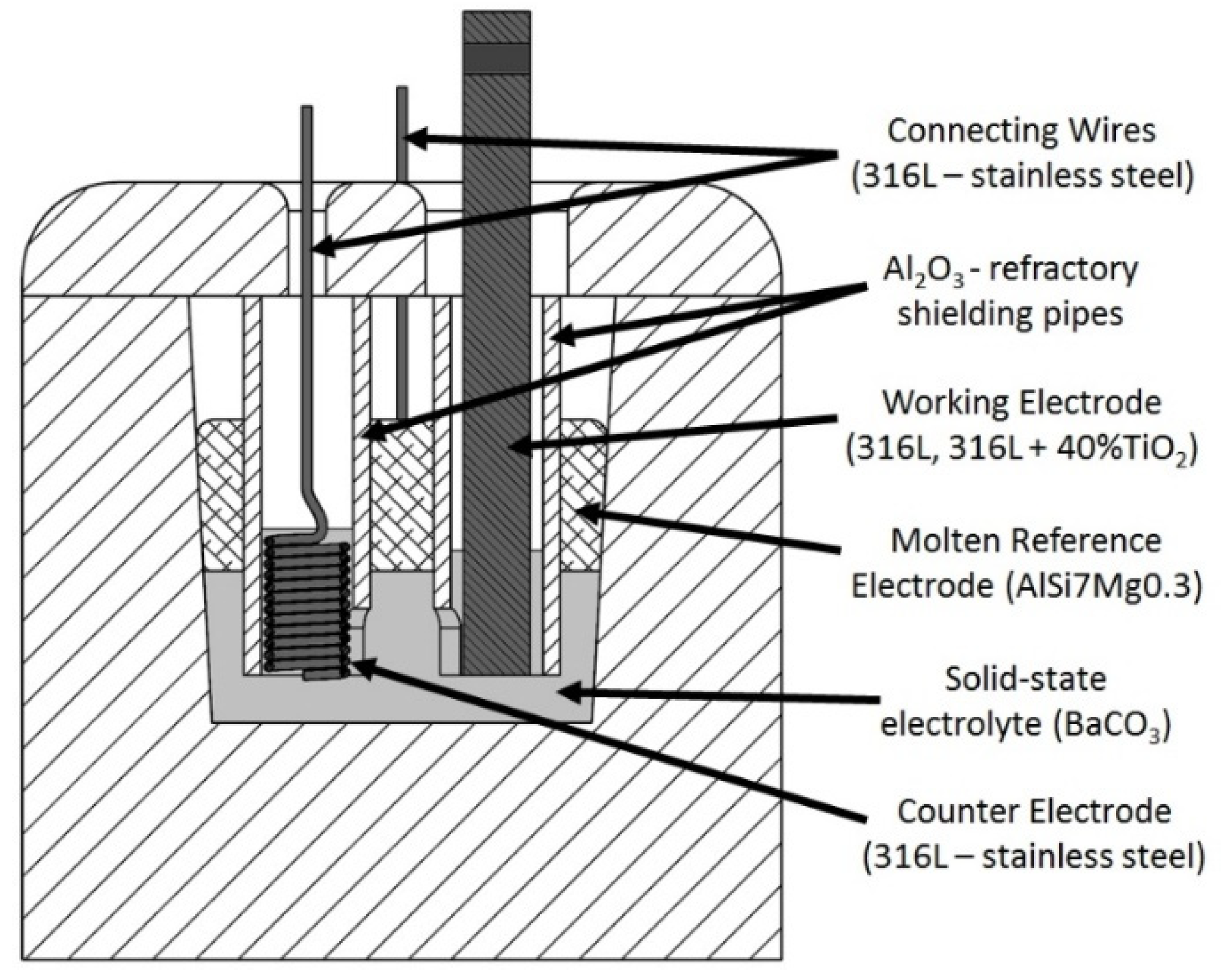
2.1. Materials and Manufacturing
2.2. Electrochemical Experiments
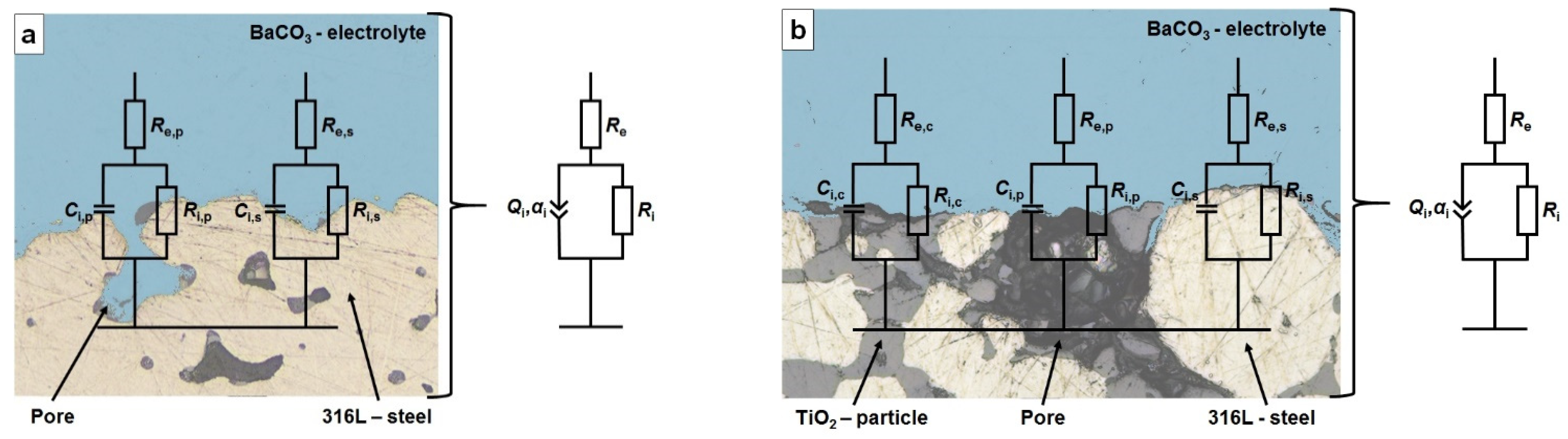
2.3. Analysis of the Electrode–Electrolyte Interfaces
3. Results and Discussion
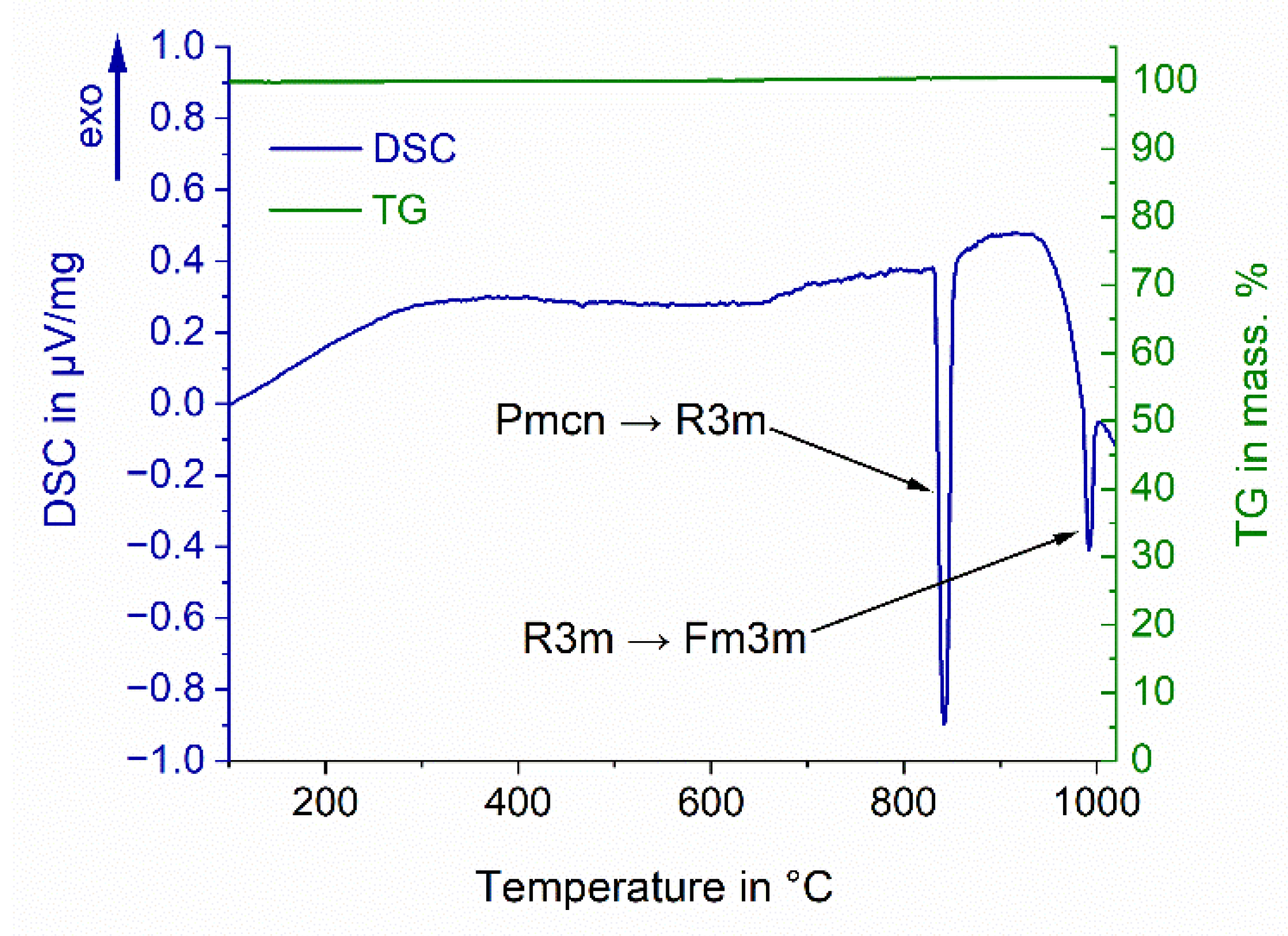
3.1. Thermal Analysis of BaCO3
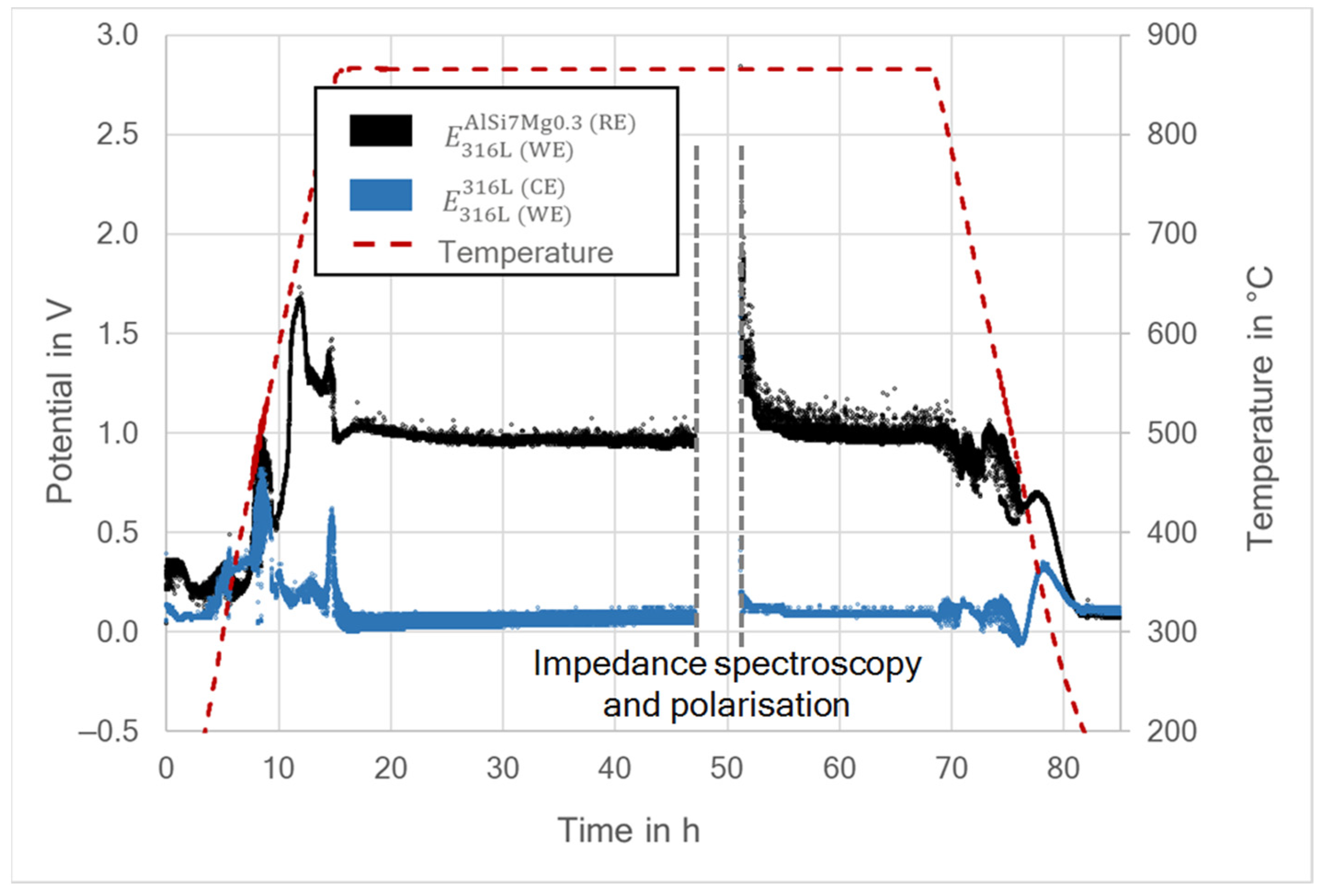
3.2. Differential Electrode Potential
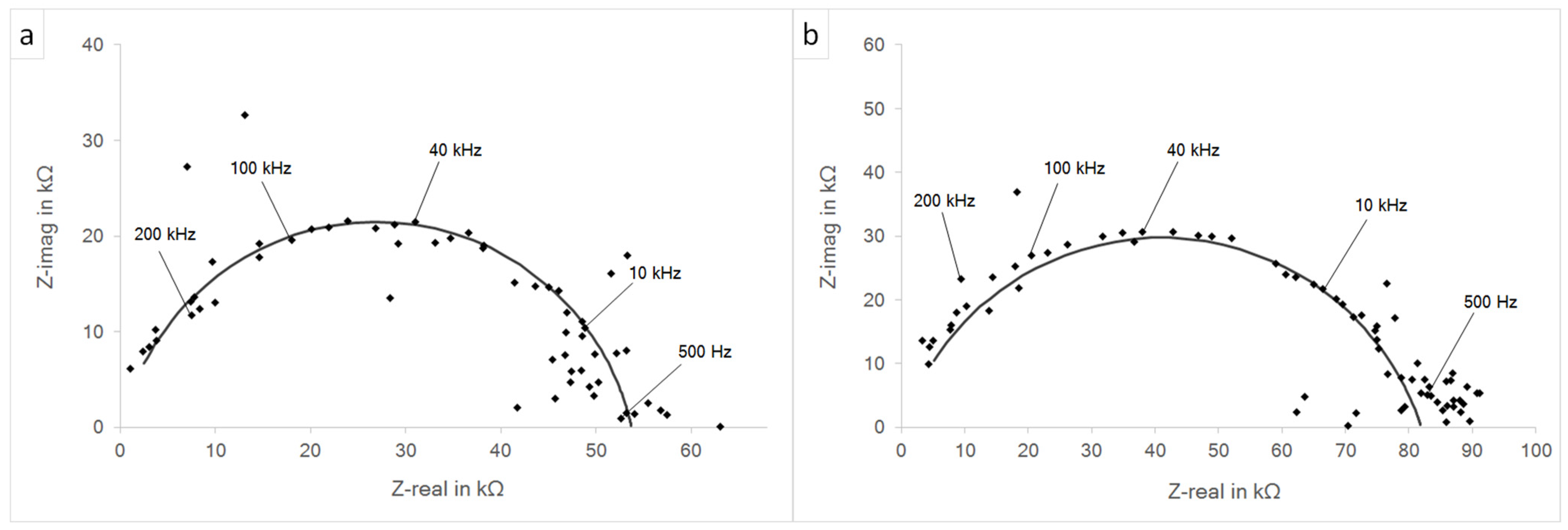
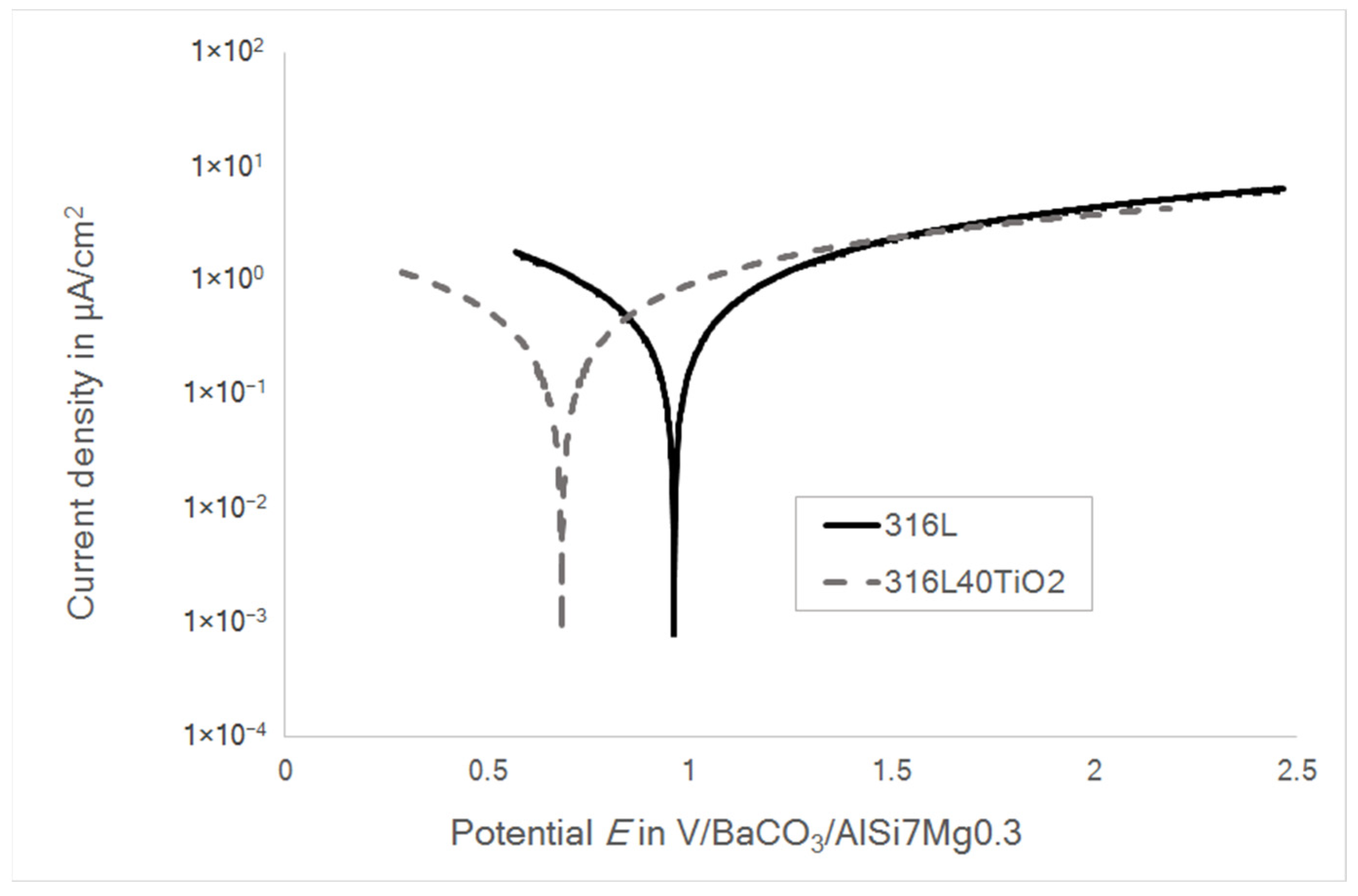
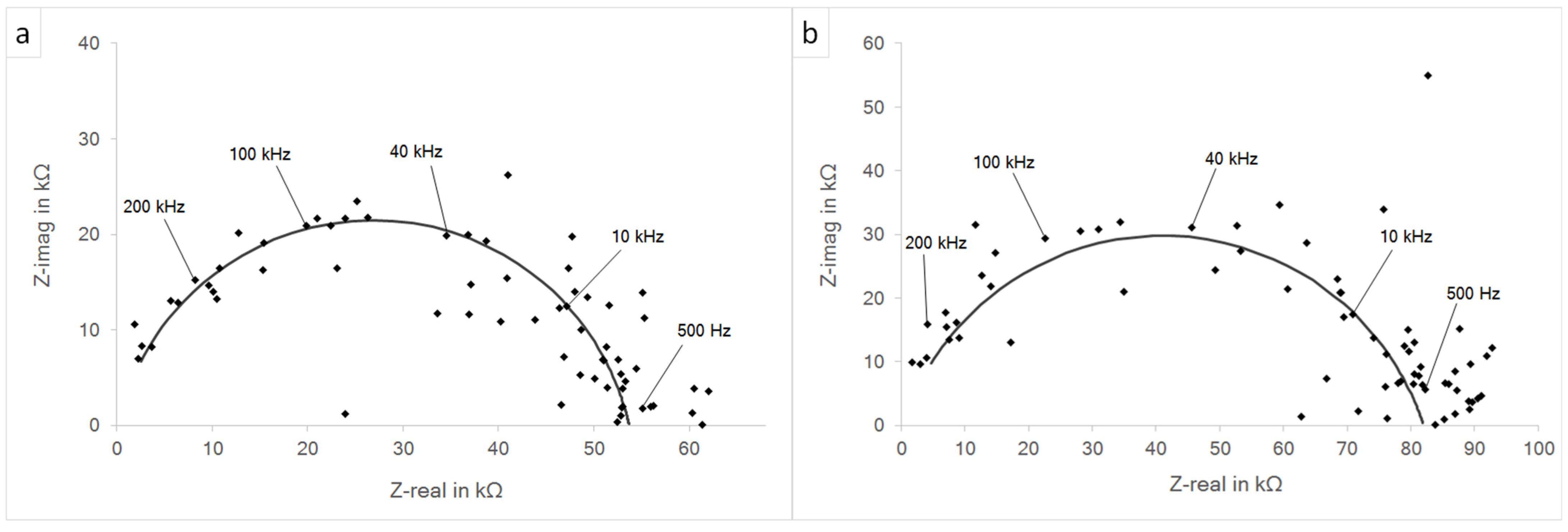
3.3. Impedance Spectroscopy and Potentiodynamic Polarization
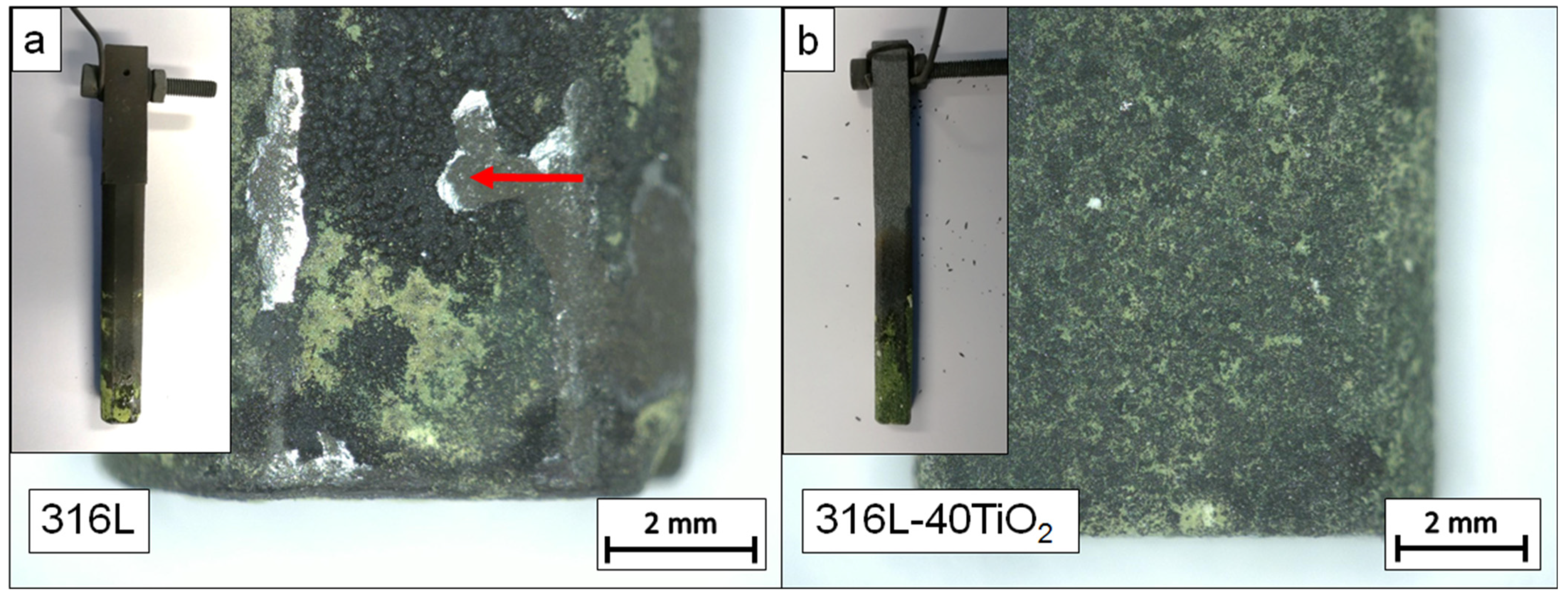
3.4. Analysis of the Electrode Surfaces
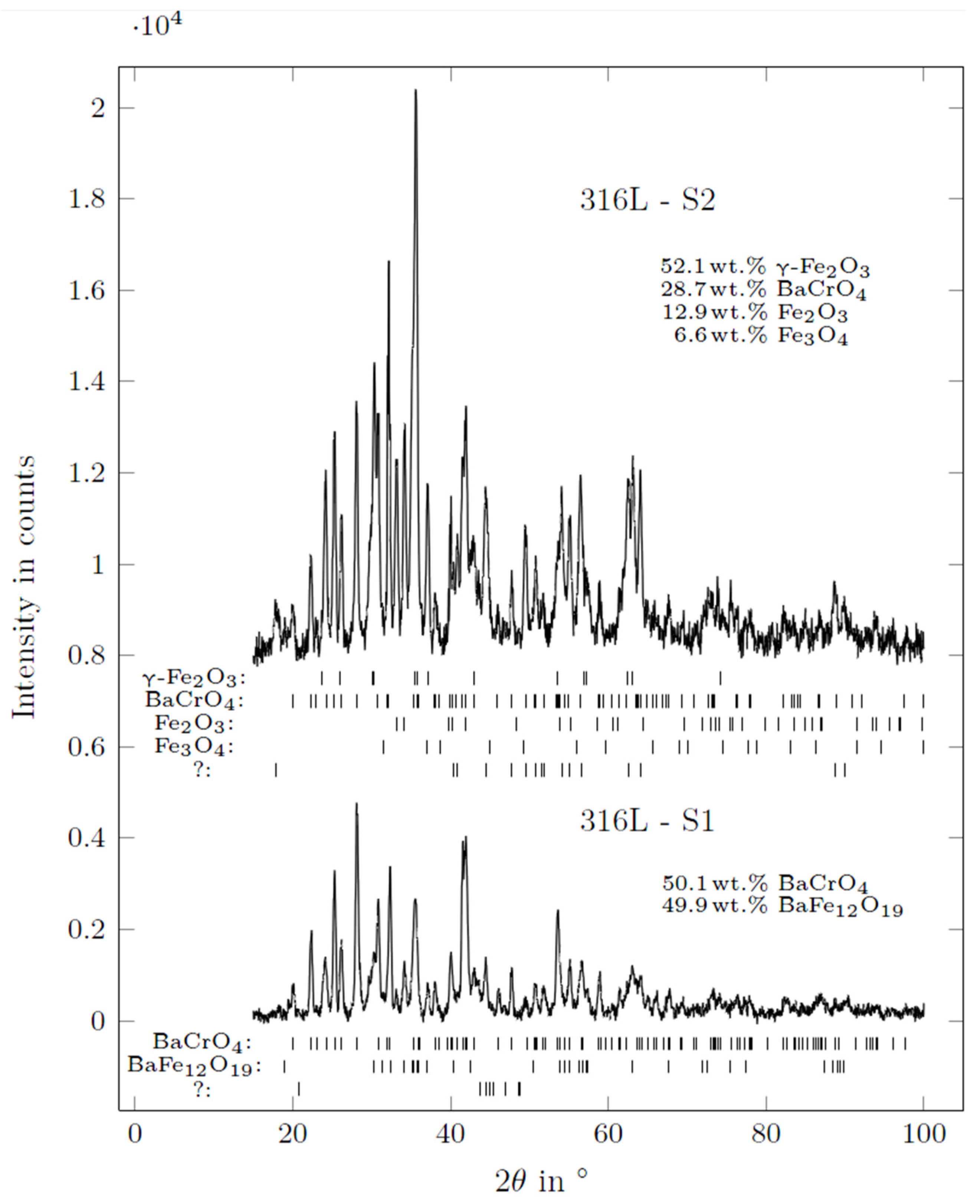
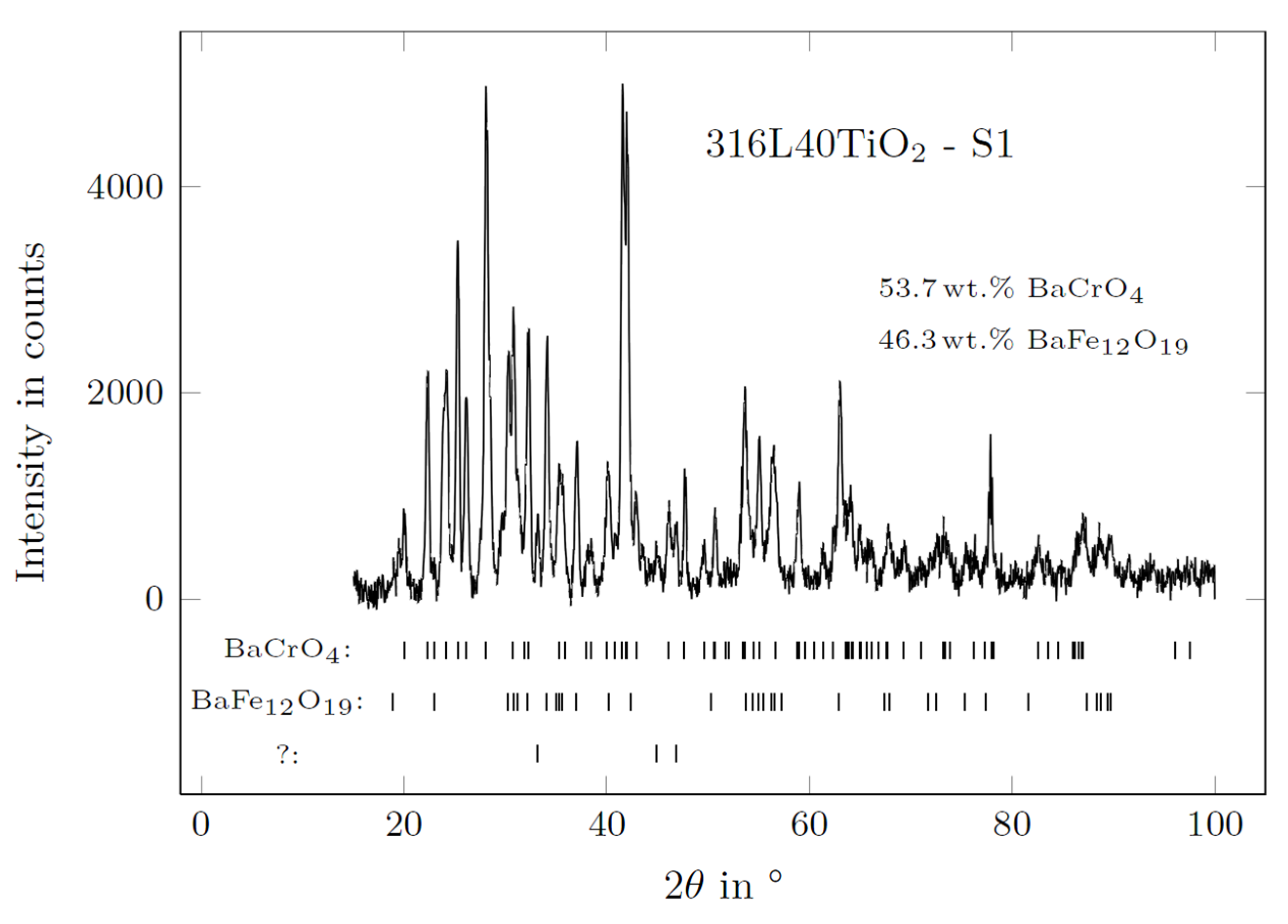
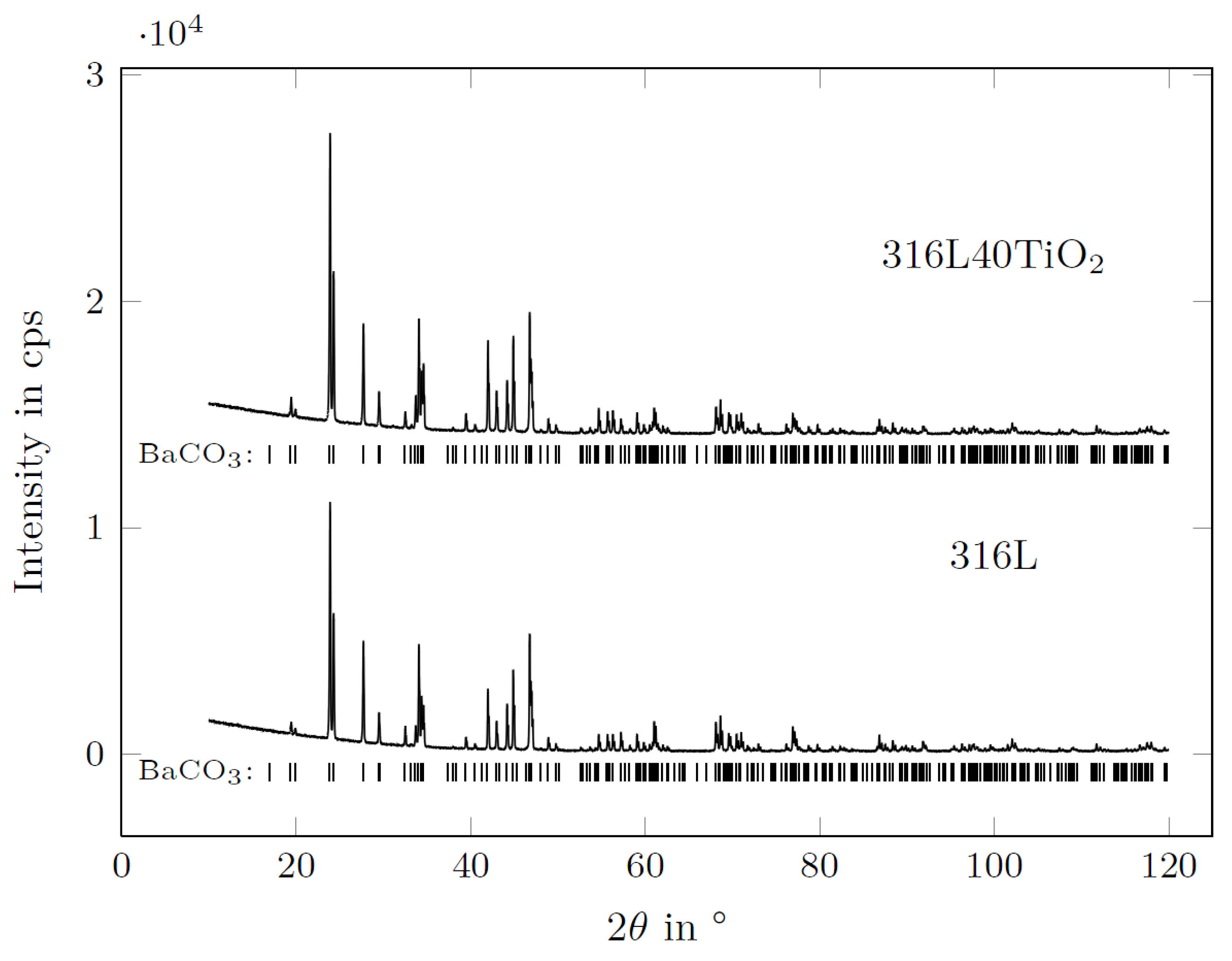
3.5. XRD of BaCO3 in Contact with the AlSi7Mg0.3 Reference Electrode
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malczyk, P.; Zienert, T.; Kerber, F.; Weigelt, C.; Sauke, S.-O.; Semrau, H.; Aneziris, C. Corrosion-Resistant Steel–MgO Composites as Refractory Materials for Molten Aluminum Alloys. Materials 2020, 13, 4737. [Google Scholar] [CrossRef] [PubMed]
- Tsirlina, G.A. Electrode and Cell Potential. In Encyclopedia of Interfacial Chemistry, Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–128. [Google Scholar] [CrossRef]
- ASTM G102; ASTM G102-89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2010.
- Caines, S.; Khan, F.; Zhang, Y.; Shirokoff, J. Simplified electrochemical potential noise method to predict corrosion and corrosion rate. J. Loss Prev. Process Ind. 2017, 47, 72–84. [Google Scholar] [CrossRef]
- Schottky, W.; Rothe, H. Physik der Glühelektroden. In Handbuch der Experimentalphysik; Wien, W., Harms, F., Lenz, H., Eds.; Akademische Verlagsgesellschaft, M.B.H.: Lepzig, Germany, 1928; Volume 13.2, pp. 145–155. [Google Scholar]
- Milazzo, G.; Caroli, S. Tables of Standard Electrode Potentials; Wiley: Chichester, UK, 1978; ISBN 9780471995340. [Google Scholar]
- Vanysek, P. Electrochemical series. In CRC Handbook of Chemistry and Physics, 91st ed.; Haynes, W.M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 8-20–8-29. ISBN 978-1439820773. [Google Scholar]
- Pletcher, D.; Walsh, F.C. Industrial Electrochemistry, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1990; ISBN 9789401121545. [Google Scholar]
- Grundler, P. In-Situ Thermoelectrochemistry—Working with Heated Electrodes; Springer: Berlin Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Rossiter, B.W.; Hamilton, J.F. Electrochemical Methods. In Physical Methods of Electrochemistry, 2nd ed.; Wiley: New York, NY, USA, 1986; ISBN 978-0471080275. [Google Scholar]
- Flitt, H.J.; Schweinsberg, P.D. A guide to polarisation curve interpretation: Deconstruction of experimental curves typical of the Fe/H2O/H+/O2 corrosion system. Corros. Sci. 2005, 47, 2125–2156. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Qu, Y.; Dong, L.; Guo, J.; Li, D.; Xue, W. In-situ high temperature electrochemical investigation of ZrO2/CrN ceramic composite film on zirconium alloy. Surf. Coat. Technol. 2019, 359, 366–373. [Google Scholar] [CrossRef]
- Gomes, A.; Navas, M.; Uranga, N.; Paiva, T.; Figueira, I.; Diamantino, T.C. High-temperature corrosion performance of austenitic stainless steels type AISI 316L and AISI 321H, in molten Solar Salt. Sol. Energy 2019, 177, 408–419. [Google Scholar] [CrossRef]
- Karthick, S.; Muralidharan, S.; Saraswathy, V. Corrosion performance of mild steel and galvanized iron in clay soil environment. Arab. J. Chem. 2018, 13, 3301–3318. [Google Scholar] [CrossRef]
- Alawi Al-Sodani, K.A.; Baghabra Al-Amoudi, O.S.; Maslehuddin, M.; Shameem, M. Efficiency of corrosion inhibitors in mitigating corrosion of steel under elevated temperature and chloride concentration. Constr. Build. Mater. 2018, 163, 97–112. [Google Scholar] [CrossRef]
- Holm, T.; Dahlstrøm, P.K.; Sunde, S.; Seland, F.; Harrington, D.A. Dynamic electrochemical impedance study of methanol oxidation at Pt at elevated temperatures. Electrochim. Acta 2019, 295, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Euch, S.E.; Bricault, D.; Cachet, H.; Sutter, E.M.M.; Tran, M.T.T.; Vivier, V.; Engler, N.; Marion, A.; Skocic, M.; Huerta-Ortega, B. Temperature dependence of the electrochemical behavior of the 690 Ni-base alloy between 25 and 325 °C. Electrochim. Acta 2019, 317, 509–520. [Google Scholar] [CrossRef]
- Elgaddafi, R.; Naidu, A.; Ahmed, R.; Shah, S.; Hassani, S.; Osisanya, S.O.; Saasen, A. Modeling and experimental study of CO2 corrosion on carbon steel at elevated pressure and temperature. J. Nat. Gas Sci. Eng. 2015, 27, 1620–1629. [Google Scholar] [CrossRef]
- Sah, S.P.; Tada, E.; Nishikata, A. Corrosion behaviour of austenitic stainless steels in carbonate melt at 923 K under controlled CO2-O2 environment. Corros. Sci. 2018, 133, 310–317. [Google Scholar] [CrossRef]
- Subari, F.; Maksom, H.F.; Zawawi, A. Corrosion Behavior of Eutectic Molten Salt solution on Stainless Steel 316L. Procedia Soc. Behav. Sci. 2015, 195, 2699–2708. [Google Scholar] [CrossRef]
- Kelley, K.K.; Anderson, C.T. Metal Carbonates—Correlations and Applications of Thermodynamic Properties. In Contributions to the Data on Theoretical Metallurgy, Part IV; U.S. Bulletin 384; Bureau of Mines: Washington, DC, USA, 1935; Available online: https://digital.library.unt.edu/ark:/67531/metadc12552/m2/1/high_res_d/Bulletin0384.pdf (accessed on 15 August 2022).
- Stern, K.H.; Weise, E.L. High Temperature Properties and Decomposition of Inorganic Salts; National Bureau of Standards: Washington, DC, USA, 1966. [Google Scholar]
- Antao, S.M.; Hassan, I. BaCO3: High-temperature crystal structures and the Pmcn→R3m phase transition at 811 °C. Phys. Chem. Miner. 2007, 34, 573–580. [Google Scholar] [CrossRef]
- Srivastava, N.; Joshi, K.V.; Thakur, A.K.; Menon, S.K.; Shahi, V.K. BaCO3 nanoparticles embedded retentive and cation selective membrane for separation/recovery of Mg2+ from natural water sources. Desalination 2014, 352, 142–149. [Google Scholar] [CrossRef]
- Dudczig, S. Werkstoffentwicklung von Feuerbetonen für Schlüsselbauteile zur Erfassung von Wechselwirkungen zwischen Stahlschmelzen und Feuerfestmaterialien in einem Stahlgusssimulator. Ph.D. Thesis, Technische Universität Bergakademie Freiberg, Freiberg, Germany, 2017. [Google Scholar]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Mansfeld, F.; Hsu, C.H. Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Mandel, M.; Krüger, L.; Decker, S. Electrochemical corrosion studies of spark plasma sintered zirconia particle-reinforced high-alloy steel at different temperatures. Corros. Sci. 2015, 90, 323–330. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Bordbar-Khiabani, A.; Yarmand, B. Immobilization of rGO/ZnO hybrid composites on the Zn substrate for enhanced photocatalytic activity and corrosion stability. J. Alloys Compd. 2020, 845, 156219. [Google Scholar] [CrossRef]
- Lentz, A.; Büchele, W.; Schöllhorn, H. Crystal Growth from Silica Gels and Single Crystal Structure of Bariumchromate. Cryst. Res. Technol. 1986, 21, 827–833. [Google Scholar] [CrossRef]
- Shin, H.-S. Crystal Structures of Ba-ferrites Synthesize by Coprecipitation-Oxidation Method. J. Korean Ceram. Soc. 1997, 34, 1045–1052. [Google Scholar]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-s.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Polshettiwar, V. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef]
- Shin, H.-S. A Study on the Structure of Maghemite (gamma-Fe2O3) I—Rietveld Analysis of Powder XRD Patterns. J. Korean Ceram. Soc. 1998, 35, 1113–1119. [Google Scholar]
- Blake, R.L.; Hassevick, R.E.; Zoltai, T.; Finger, L.W. Refineement of the Hematite Structure. Am. Mineral. 1966, 51, 123–129. [Google Scholar]
- Fleet, M.E. The Structure of Magnetite: Symmetry of Cubic Spinels. J. Solid State Chem. 1986, 62, 75–82. [Google Scholar] [CrossRef]
- “FactSage 7.2—List of Stored Phase Diagrams (6100)”. Available online: http://www.crct.polymtl.ca/fact/documentation/FS_All_PDs.htm (accessed on 10 April 2019).
- Goto, Y.; Takada, T. Phase Diagram of the System BaO-Fe2O3. J. Am. Ceram. Soc. 2006, 43, 150–153. [Google Scholar] [CrossRef]
- Frost, R.; Gal, P.L. Oxidation reactions in natural Fe-Ti oxide spinels. Acta Crystallogr. A 1980, 36, 678–682. [Google Scholar] [CrossRef]
- Charilaou, M.; Löffler, J.F.; Gehring, A.U. Fe–Ti-O exchange at high temperature and thermal hysteresis. Geophys. J. Int. 2011, 185, 647–652. [Google Scholar] [CrossRef]
- Abdel-Karim, A.-A.M.; Elwan, W.I.; Helmy, H.; El-Shafey, S.A. Spinels, Fe-Ti oxide minerals, apatites, and carbonates hosted in the ophiolites of Eastern Desert of Egypt: Mineralogy and chemical aspects. Arab. J. Geosci. 2014, 7, 693–709. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between alpha-Fe2O3, gamma-Fe2O3 and Fe3O4 during the large scale and low temperature sol-gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- De Villiers, J. Crystal structures of aragonite, strontianite, and witherite. Am. Mineral. 1971, 56, 758–766. Available online: https://rruff.info/uploads/AM56_758.pdf (accessed on 15 August 2022).


| Steel | Fe | Cr | Ni | Si | Mo | Mn | Ti | Nb | S | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| 316L | Bal. | 17.6 | 10.9 | 0.5 | 2.66 | 0.2 | 0.01 | 0.01 | 0.01 | 0.04 |
| Raw Material | Particle Size in µm | True Density in g·cm−3 | ||
|---|---|---|---|---|
| D10 | D50 | D90 | ||
| 316L | 4 | 30 | 53 | 7.94 |
| TiO2 | 0.1 | 1.9 | 4 | 4.30 |
| Alloy | Al | Si | Mg | Fe | Cu | Mn | Zn | Ti | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| AlSi7Mg0.3 | 92.30 | 7.17 | 0.27 | 0.081 | 0.002 | 0.002 | 0.007 | 0.12 | 0.001 | 0.003 |
| Working Electrode | Re in Ω | Ri in kΩ | Qi in S*sα | αi | Ceff in pF | τeff in ns |
|---|---|---|---|---|---|---|
| 316L | 100 | 53.6 | 3.50 × 10−10 | 0.86 | 21.56 | 2.15 |
| 316L40TiO2 | 100 | 81.9 | 5.60 × 10−10 | 0.8 | 8.73 | 0.87 |
| Working Electrode | Re in Ω | Ri in kΩ | Qi in S*sα | αi | Ceff in pF | τeff in ns |
|---|---|---|---|---|---|---|
| 316L | 100 | 53.6 | 3.50 × 10−10 | 0.86 | 21.56 | 2.15 |
| 316L40TiO2 | 100 | 81.9 | 5.60 × 10−10 | 0.8 | 8.73 | 0.87 |
| No. | Composition in at. % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | Ba | Fe | Cr | Ni | Mo | Mn | Al | Si | Na | |
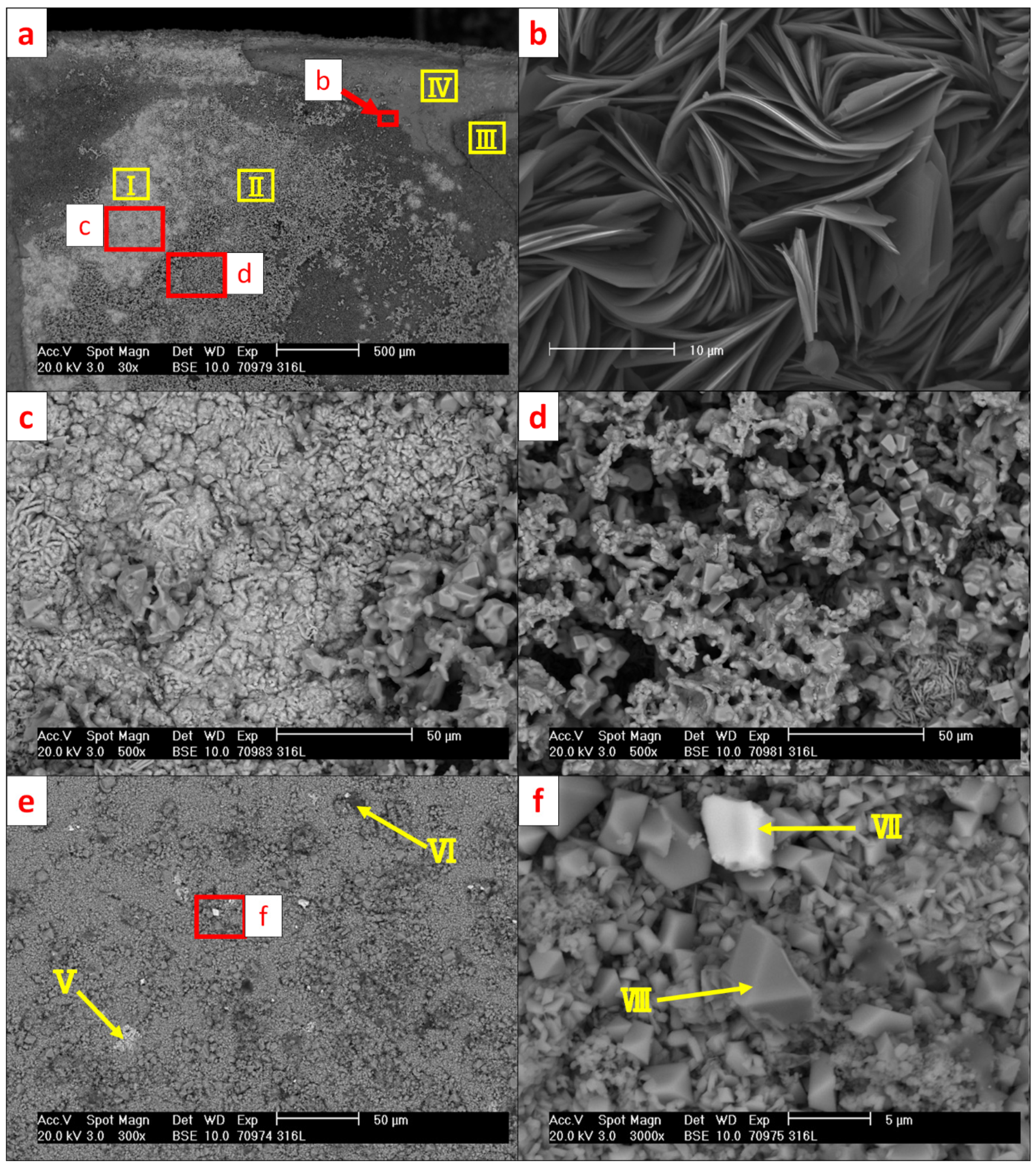
| Ⅰ | 57.9 | 15.1 | 23.6 | - | - | - | 0.7 | - | 0.9 | 1.8 |
| Ⅱ | 62.4 | 18.3 | 2.7 | 13.1 | - | 0.4 | - | 3.1 | - | - |
| Ⅲ | 41.6 | 2.3 | 50.8 | - | 0.5 | - | 2.0 | - | 1.3 | 1.5 |
| Ⅳ | 45.1 | - | 52.2 | 1.1 | 0.6 | - | 0.3 | - | 0.7 | - |
| Ⅴ | 14.5 | - | 69.2 | 5.7 | 5.3 | 0.7 | 0.2 | - | 4.4 | - |
| Ⅵ | 53.3 | - | 0.7 | 0.7 | - | - | 0.2 | 43.4 | 1.1 | 0.7 |
| Ⅶ | 66.4 | - | 6.1 | 16.8 | 0.4 | - | 10.3 | - | - | - |
| Ⅷ | 74.0 | 15.5 | 4.8 | 4.4 | - | - | 1.3 | - | - | - |
| Scan Area | Composition in at. % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Ba | Fe | Cr | Ni | Mo | Mn | Ti | Al | Si | Na | |
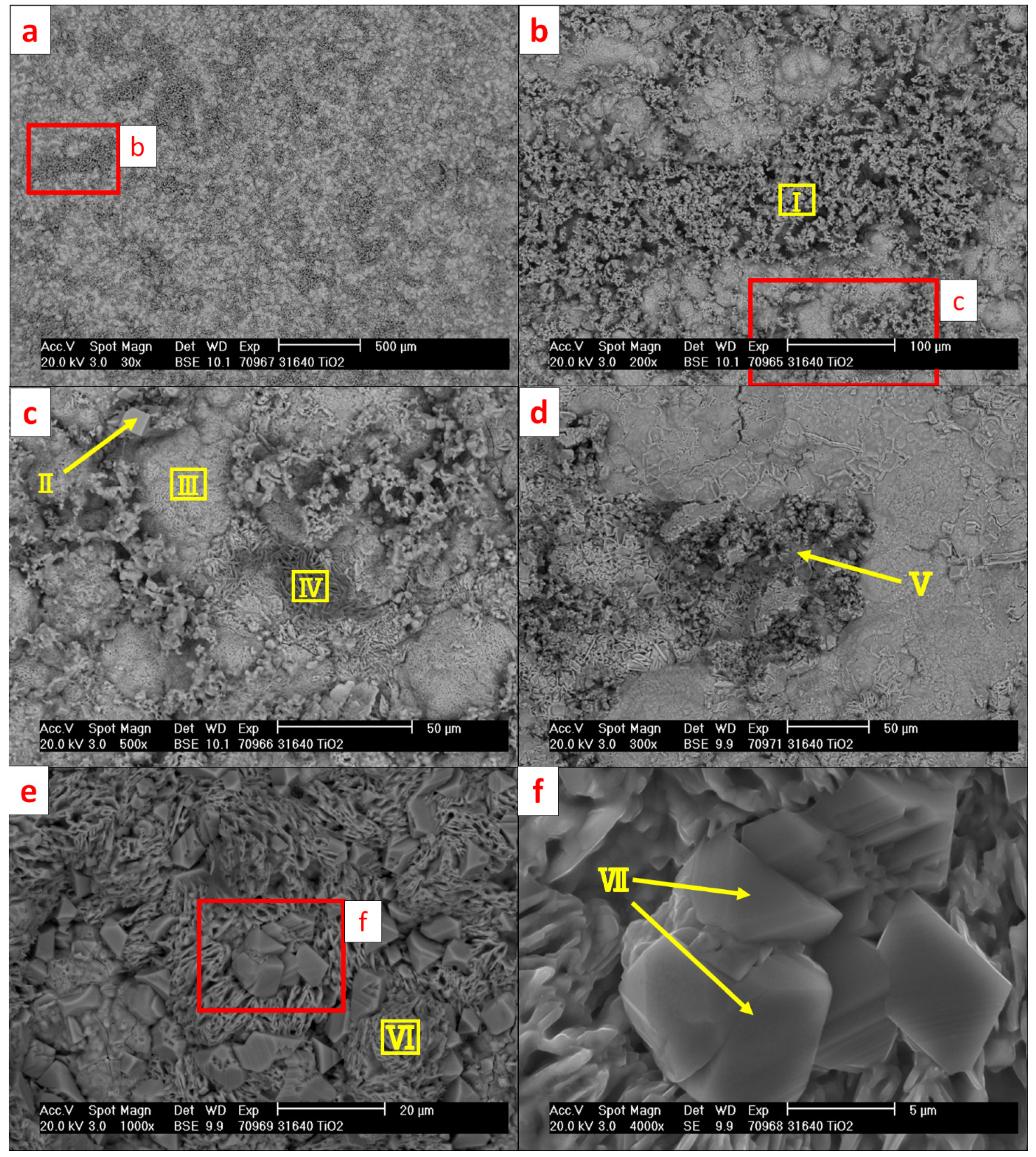
| Ⅰ | 63.2 | 19.7 | 1.5 | 10.1 | - | 1.7 | 0.5 | - | 0.5 | 0.1 | 2.7 |
| Ⅱ | 70.6 | 12.9 | 0.5 | 11.4 | - | 1.8 | 0.1 | 1.8 | - | - | 0.9 |
| Ⅲ | 51.1 | 16.3 | 26.9 | - | - | 0.4 | 0.2 | 2.1 | - | 1.2 | 1.8 |
| Ⅳ | 48.1 | 1.4 | 28.5 | - | 1.5 | - | 7.6 | 0.3 | - | 0.3 | 12.3 |
| Ⅴ | 45.9 | - | 30.8 | 0.2 | - | - | 3.4 | 15.7 | - | - | 4.0 |
| Ⅵ | 50.1 | - | 47.4 | - | - | - | 2.5 | - | - | - | - |
| Ⅶ | 58.3 | - | 24.7 | - | - | - | 8.2 | 4.6 | - | - | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malczyk, P.; Mandel, M.; Zienert, T.; Weigelt, C.; Krüger, L.; Hubalkova, J.; Schmidt, G.; Aneziris, C.G. Electrochemical Studies of Stainless Steel and Stainless Steel-TiO2 Composite in Reference to Molten Aluminum Alloy Using a Solid-State BaCO3 Electrolyte. Materials 2022, 15, 6723. https://doi.org/10.3390/ma15196723
Malczyk P, Mandel M, Zienert T, Weigelt C, Krüger L, Hubalkova J, Schmidt G, Aneziris CG. Electrochemical Studies of Stainless Steel and Stainless Steel-TiO2 Composite in Reference to Molten Aluminum Alloy Using a Solid-State BaCO3 Electrolyte. Materials. 2022; 15(19):6723. https://doi.org/10.3390/ma15196723
Chicago/Turabian StyleMalczyk, Piotr, Marcel Mandel, Tilo Zienert, Christian Weigelt, Lutz Krüger, Jana Hubalkova, Gert Schmidt, and Christos G. Aneziris. 2022. "Electrochemical Studies of Stainless Steel and Stainless Steel-TiO2 Composite in Reference to Molten Aluminum Alloy Using a Solid-State BaCO3 Electrolyte" Materials 15, no. 19: 6723. https://doi.org/10.3390/ma15196723
APA StyleMalczyk, P., Mandel, M., Zienert, T., Weigelt, C., Krüger, L., Hubalkova, J., Schmidt, G., & Aneziris, C. G. (2022). Electrochemical Studies of Stainless Steel and Stainless Steel-TiO2 Composite in Reference to Molten Aluminum Alloy Using a Solid-State BaCO3 Electrolyte. Materials, 15(19), 6723. https://doi.org/10.3390/ma15196723







