Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel
Abstract
:1. Introduction
2. Test Stand
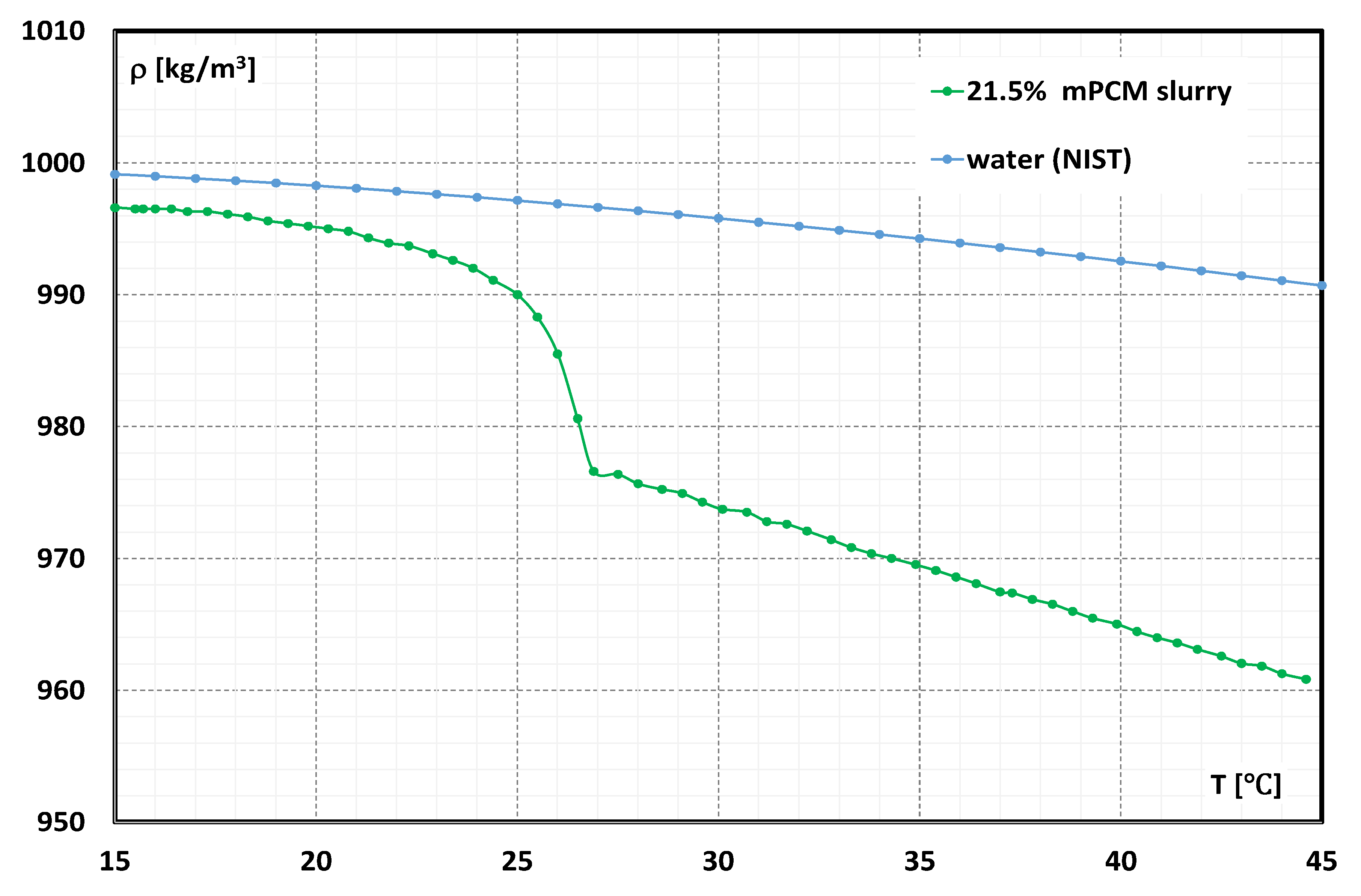
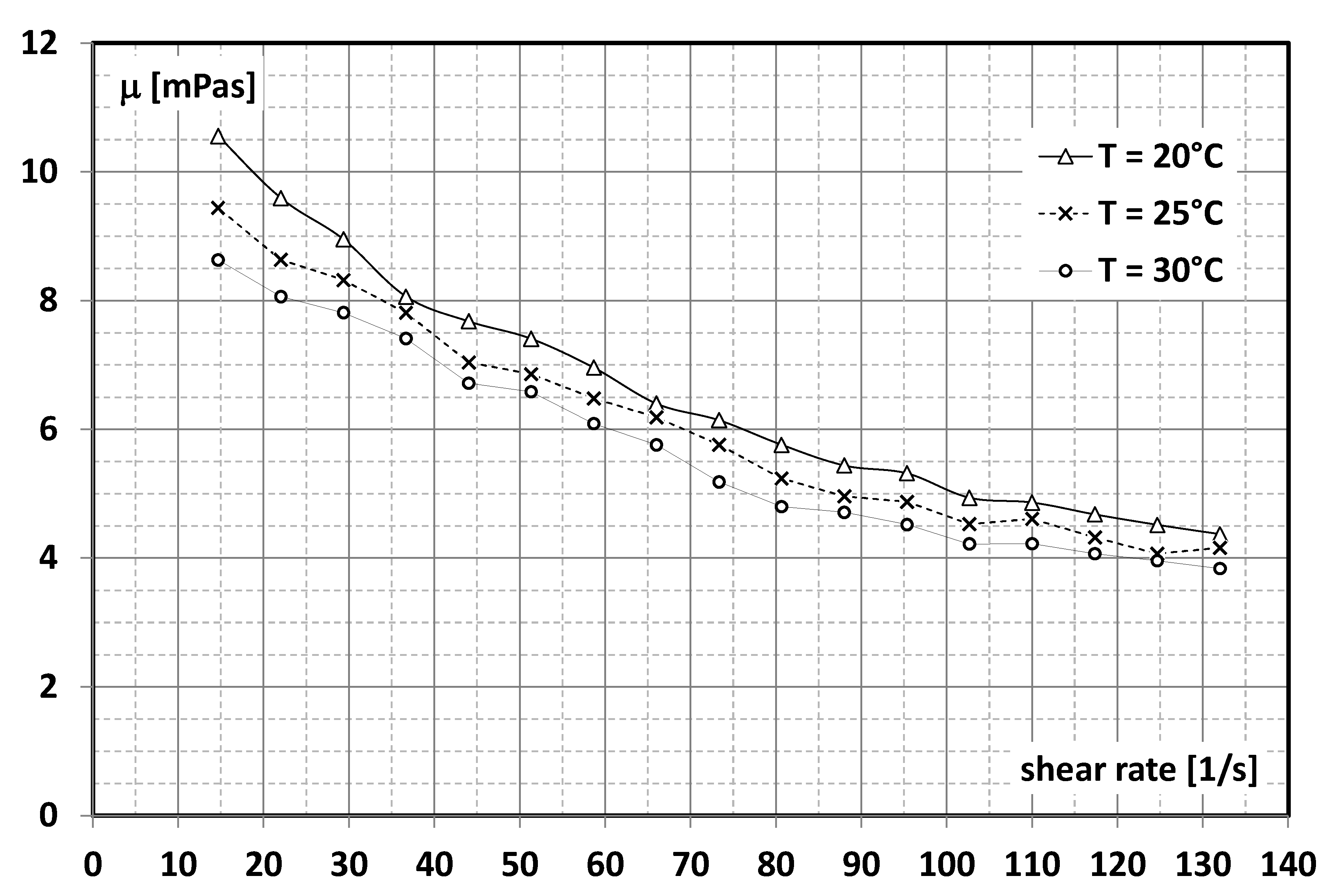
2.1. Working Fluid
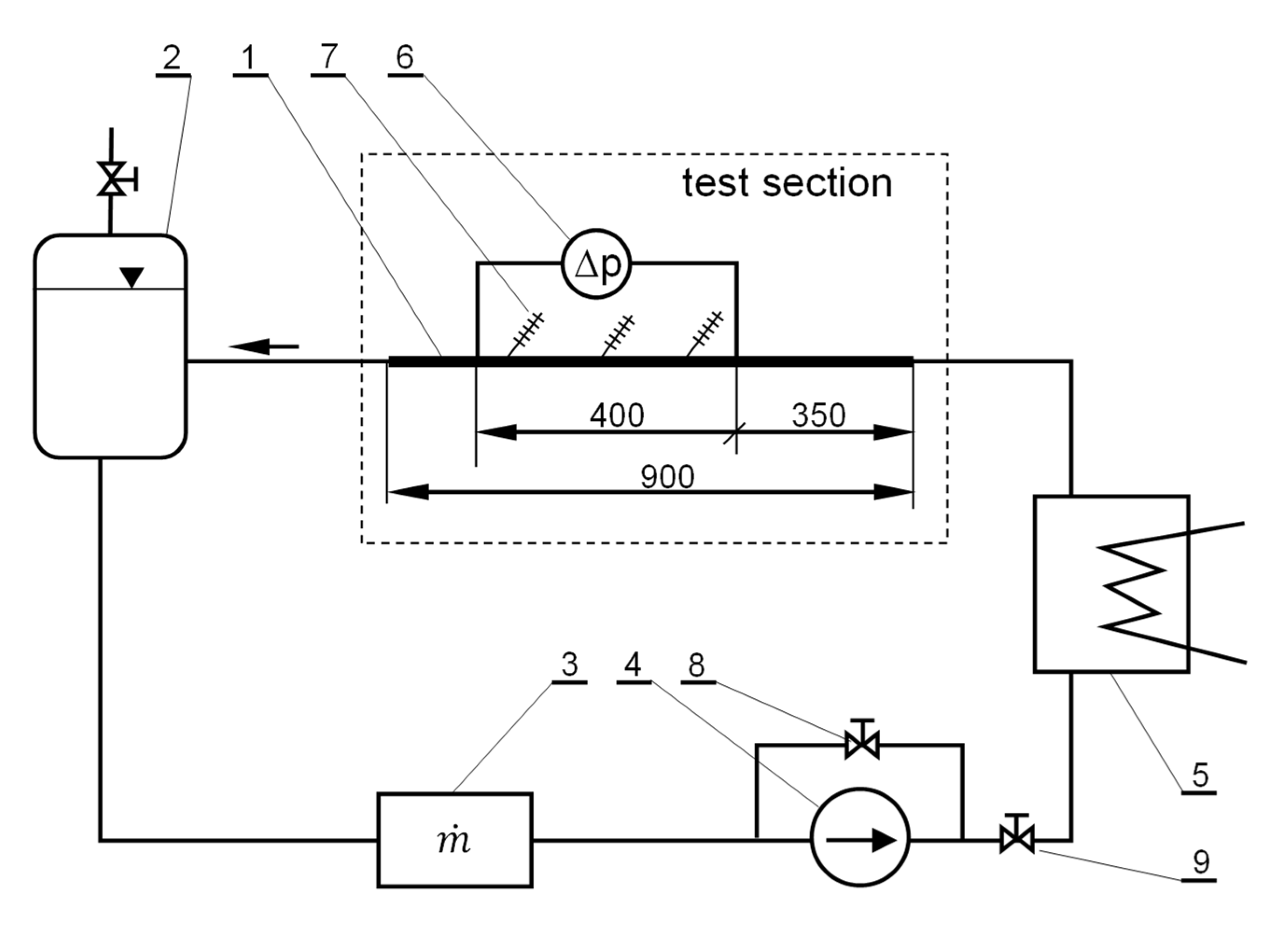
2.2. Experimental Facility
2.3. Research Procedure and Scope of the Experiment
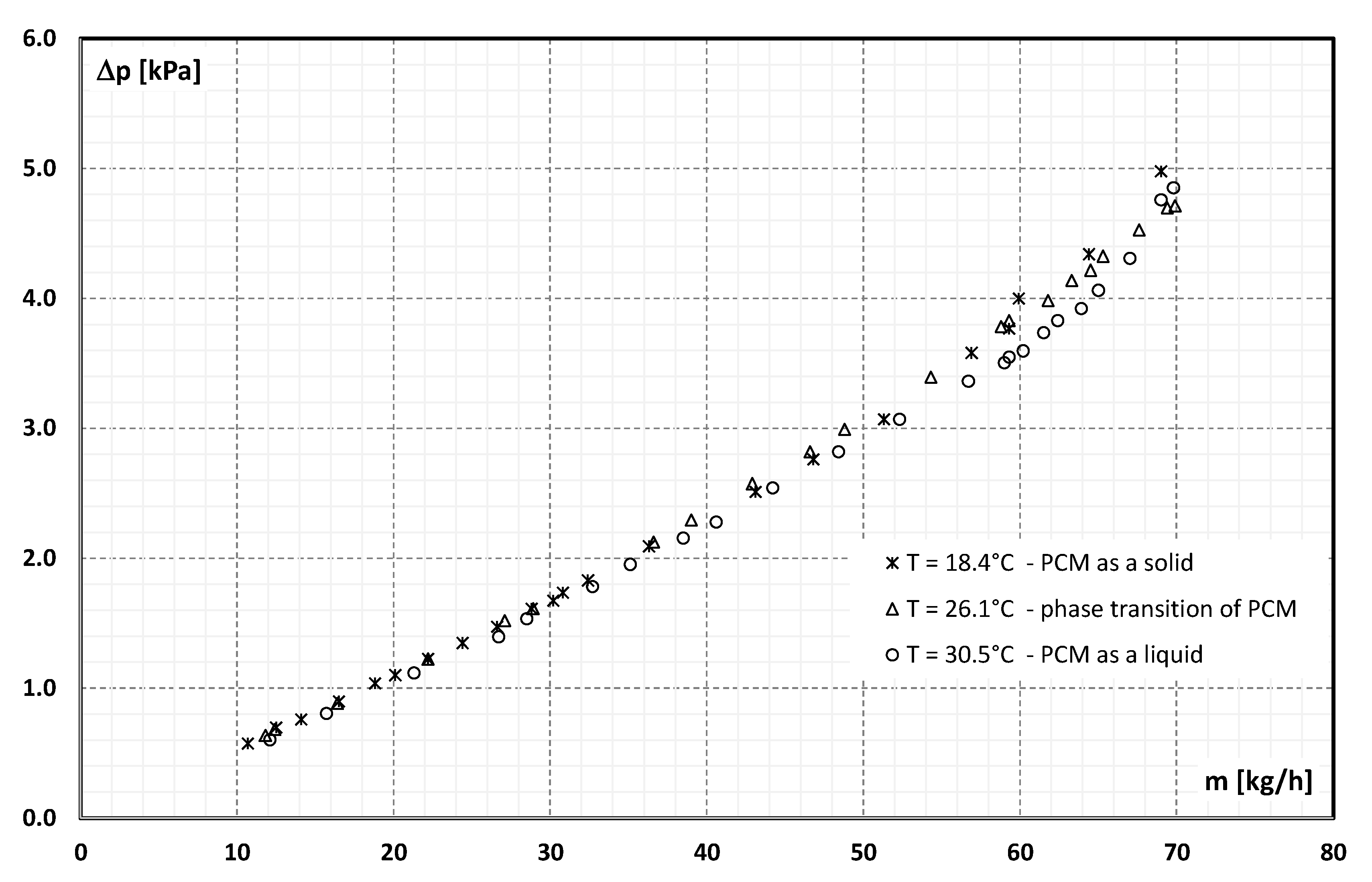
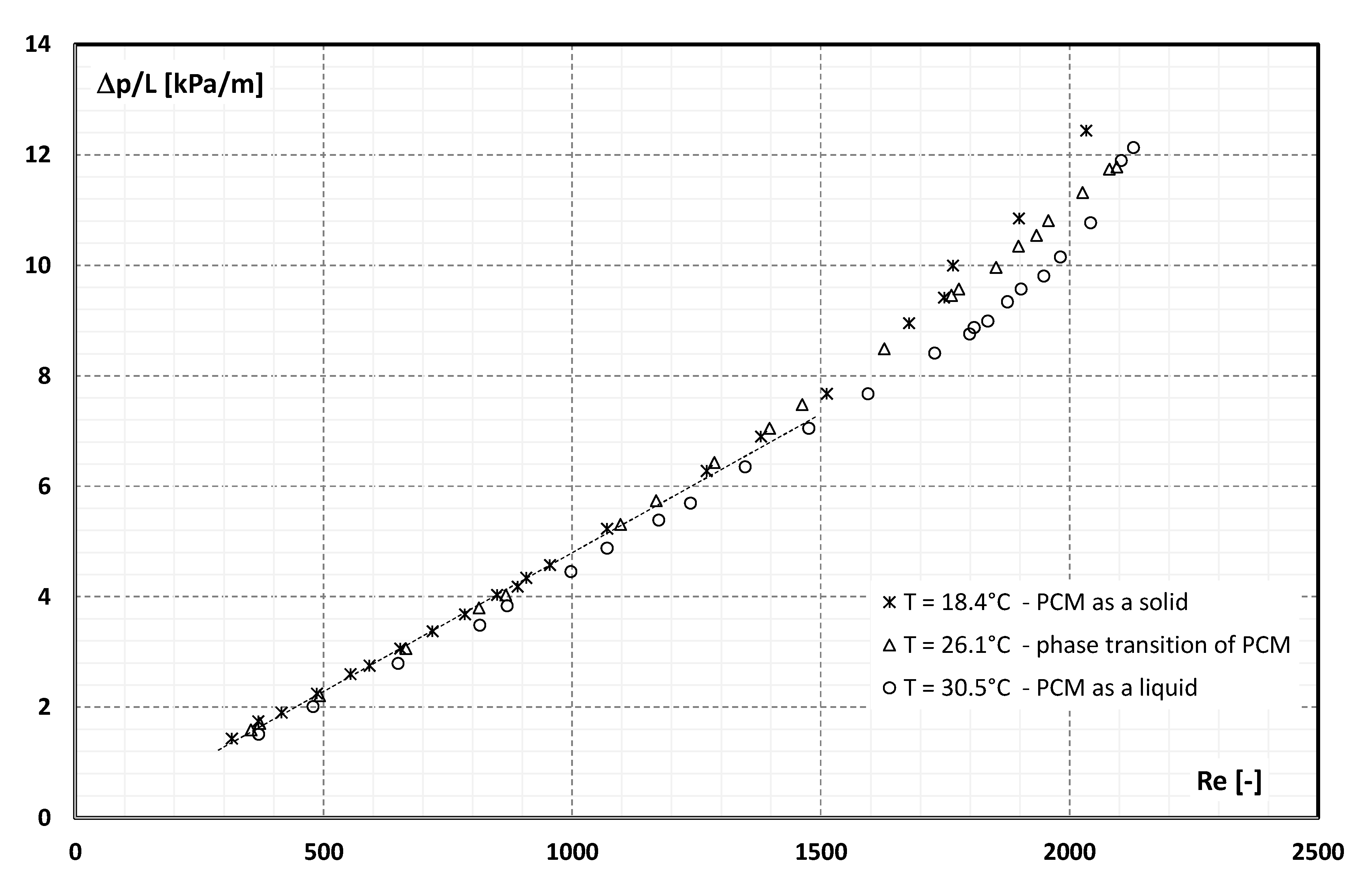
3. Experimental Data
4. Summary and Conclusions
- pressure drop during the flow of 21.5 wt.% of MPCM water slurry in a pipe channel with an internal diameter of 4 mm and a length of 400 mm varied from 0.5 kPa to 5 kPa,
- the flow resistance of the slurry depended on the Reynolds number and, for Re >1500, on the aggregate state of the PCM in the microcapsules,
- the more the Re number was higher than the value of 1500, the more the slurry flow resistance depended on the aggregate state of the PCM,
- the highest flow resistance was recorded when the PCM in microcapsules was in the form of a solid, and the lowest when the PCM was in the form of a liquid,
- the flow resistance in the range Re = 300 ÷ 1500 changed from 1.5 kPa/m to about 7 kPa/m and created a rectilinear characteristic, which is consistent with the theory of laminar fluid flow in pipe channels,
- for Re = 1500, the characteristic of flow resistance begins to diverge from a linear trend, taking the shape of a different parabola for each of the PCM aggregate states,
- The hypothesis about the significant influence of the microcapsule state of aggregation on the resistance of the turbulent flow of MPCM slurries requires further detailed verification tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Li, P.; Zhao, X.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and building applications. Renew. Sustain. Energy Rev. 2017, 77, 246–262. [Google Scholar] [CrossRef]
- Ghasemi, K.; Tasnim, S.; Mahmud, S. PCM, nano/microencapsulation and slurries: A review of fundamentals, categories, fabrication, numerical models and applications. Sustain. Energy Technol. Assess. 2022, 52. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A review on effect of phase change material encapsulation on the thermal performance of a system. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Jurkowska, M.; Szczygieł, I. Review on properties of microencapsulated phase change materials slurries (MPCMS). Appl. Therm. Eng. 2016, 98, 365–373. [Google Scholar] [CrossRef]
- Shao, J.; Darkwa, J.; Kokogiannakis, G. Review of phase change emulsions (PCMEs) and their applications in HVAC systems. Energy Build. 2015, 94, 200–217. [Google Scholar] [CrossRef]
- Youssef, Z.; Delahaye, A.; Huang, L.; Trinquet, F.; Fournaison, L.; Pollerberg, C.; Doetsch, C. State of the art on phase change material slurries. Energy Convers. Manag. 2013, 65, 120–132. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, X.-Y.; Wang, S.-B.; Zhao, Y.-H.; Huang, Z. Fabrication and properties of microencapsulated-paraffin/gypsum-matrix building materials for thermal energy storage. Energy Convers. Manag. 2012, 55, 101–107. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Farid, M. Innovative method of metal coating of microcapsules containing phase change materials. Sol. Energy 2016, 129, 54–64. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Takeuchi, H.; Pyatenko, A.T.; Kayukawa, N. Characteristics of Microencapsulated PCM Slurry as a Heat-Transfer Fluid. AIChE J. 1999, 45, 696–707. [Google Scholar] [CrossRef]
- Wang, X.; Niu, J.; Li, Y.; Zhang, Y.; Wang, X.; Chen, B.; Zeng, R.; Song, Q. Heat transfer of microencapsulated PCM slurry flow in a circular tube. AIChE J. 2008, 54, 1110–1120. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zeng, R.; Zhang, Y.; Wang, X.; Niu, J.; Li, Y.; Di, H. An experimental study of convective heat transfer with microencapsulated phase change material suspension: Laminar flow in a circular tube under constant heat flux. Exp. Therm. Fluid Sci. 2008, 32, 1638–1646. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Marín, J.M.; Zalba, B. Experimental analysis of a microencapsulated PCM slurry as thermal storage system and as heat transfer fluid in laminar flow. Appl. Therm. Eng. 2012, 36, 370–377. [Google Scholar] [CrossRef]
- Taherian, H.; Alvarado, J.L.; Tumuluri, K.; Thies, C.; Park, C.-H. Fluid flow and heat transfer characteristics of microencapsulated phase change material slurry in turbulent flow. J. Heat Transf. 2014, 136, 061704. [Google Scholar] [CrossRef]
- Kostic, M. On turbulent drag and heat transfer reduction phenomena and laminar heat transfer enhancement in non-circular duct flow of certain non-Newtonian fluids. Int. J. Heat Mass Transf. 1994, 37, 133–147. [Google Scholar] [CrossRef]
- Alvarado, J.L.; Marsh, C.; Sohn, C.; Phetteplace, G.; Newell, T. Thermal performance of microencapsulated phase change material slurry in turbulent flow under constant heat flux. Int. J. Heat Mass Transf. 2007, 50, 1938–1952. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Microencapsulated PCM slurries’ dynamic viscosity experimental investigation and temperature-dependent prediction model. Int. J. Heat Mass Transf. 2019, 145, 118741. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental research of viscosity of microencapsulated PCM slurry at the phase change temperature. Int. J. Heat Mass Transf. 2019, 134, 1209–1217. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation on influence of microcapsules with PCM on propylene glycol rheological properties. E3S Web Conf. 2018, 70, 02005. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of MPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159, 120083. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Bohdal, T. Experimental studies of the influence of microencapsulated phase change material on thermal parameters of a flat liquid solar collector. Energies 2021, 14, 5135. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B. Determining the heat of fusion and specific heat of microencapsulated phase change material slurry by thermal delay method. Energies 2020, 14, 179. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M. Experimental investigation of the apparent thermal conductivity of microencapsulated phase-change-material slurry at the phase-transition temperature. Materials 2021, 14, 4124. [Google Scholar] [CrossRef]
- MICRONAL ® 5428 X. Available online: www.microteklabs.com (accessed on 29 August 2022).
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.-R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, P.; Wang, R.; Furui, S.; Xi, G. Forced flow and convective melting heat transfer of clathrate hydrate slurry in tubes. Int. J. Heat Mass Transf. 2010, 53, 3745–3757. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M.; Kaczmarek, D.; Nalepa, B.; Zajączkowski, B.; Valíček, J.; Harničárová, M. Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel. Materials 2022, 15, 6719. https://doi.org/10.3390/ma15196719
Dutkowski K, Kruzel M, Kaczmarek D, Nalepa B, Zajączkowski B, Valíček J, Harničárová M. Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel. Materials. 2022; 15(19):6719. https://doi.org/10.3390/ma15196719
Chicago/Turabian StyleDutkowski, Krzysztof, Marcin Kruzel, Dominika Kaczmarek, Bartłomiej Nalepa, Bartosz Zajączkowski, Jan Valíček, and Marta Harničárová. 2022. "Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel" Materials 15, no. 19: 6719. https://doi.org/10.3390/ma15196719
APA StyleDutkowski, K., Kruzel, M., Kaczmarek, D., Nalepa, B., Zajączkowski, B., Valíček, J., & Harničárová, M. (2022). Influence of the Physical State of Microencapsulated PCM on the Pressure Drop of Slurry in a Circular Channel. Materials, 15(19), 6719. https://doi.org/10.3390/ma15196719








