Enhancement of Optical Telecommunication Bands: Pr3+-Doped Halide Phosphate Glasses Display Broadband NIR Photoluminescence Emission
Abstract
1. Introduction
2. Experimental Work
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Chen, B.J.; Pun, E.Y.B.; Lin, H. Ultra-broadband near-infrared emission in praseodymium ion doped germanium tellurite glasses for optical fiber amplifier at E-, S-, C-, and L-band. J. Appl. Phys. 2012, 111, 116101. [Google Scholar] [CrossRef]
- Zhou, B.; Pun, E.Y.-B. Superbroadband near-IR emission from praseodymium-doped bismuth gallate glasses. Opt. Lett. 2011, 36, 2958–2960. [Google Scholar] [CrossRef] [PubMed]
- Damak, K.; Yousef, E.; AlFaify, S.; Rüssel, C.; Maalej, R. Raman, green and infrared emission cross-sectionsof Er^3+ doped TZPPN tellurite glass. Opt. Mater. Express 2014, 4, 597. [Google Scholar] [CrossRef]
- Damak, K.; Yousef, E.S.; Al-Shihri, A.S.; Seo, H.J.; Rüssel, C.; Maâlej, R. Quantifying Raman and emission gain coefficients of Ho3+ doped TeO2·ZnO·PbO·PbF2·Na2O (TZPPN) tellurite glass. Solid State Sci. 2014, 28, 74–80. [Google Scholar] [CrossRef]
- Zhou, B.; Tao, L.; Tsang, Y.H.; Jin, W.; Pun, E. Superbroadband near-IR photoluminescence from Pr3+-doped fluorotellurite glasses. Opt. Express 2012, 20, 3803–3813. [Google Scholar] [CrossRef]
- Shen, L.; Chen, B.; Lin, H.; Pun, E. Praseodymium ion doped phosphate glasses for integrated broadband ion-exchanged waveguide amplifier. J. Alloys Compd. 2015, 622, 1093–1097. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, X.; Chen, D. Near-infrared emission from Pr-doped borophosphate glass for broadband telecommunication. J. Lumin. 2013, 135, 38–41. [Google Scholar] [CrossRef]
- Han, X.; Shen, L.; Pun, E.Y.B.; Ma, T.; Lin, H. Pr3+-doped phosphate glasses for fiber amplifiers operating at 1.38–1.53 μm of the fifth optical telecommunication window. Opt. Mater. 2014, 36, 1203–1208. [Google Scholar] [CrossRef]
- Belançon, M.P.; Marconi, J.D.; Ando, M.F.; Barbosa, L.C. Near-IR emission in Pr3+ single doped and tunable near-IR emission in Pr3+/Yb3+ codoped tellurite tungstate glasses for broadband optical amplifiers. Opt. Mater. 2014, 36, 1020–1026. [Google Scholar] [CrossRef]
- Nachimuthu, P.; Vithal, M.; Jagannathan, R. Absorption and Emission Spectral Properties of Pr3+, Nd3+, and Eu3+ Ions in Heavy-Metal Oxide Glasses. J. Am. Ceram. Soc. 2004, 83, 597–604. [Google Scholar] [CrossRef]
- Balda, R.; Fernández, J.; De Pablos, A.; Fdez-Navarro, J.M. Spectroscopic properties of Pr3+ions in lead germanate glass. J. Phys. Condens. Matter 1999, 11, 7411–7421. [Google Scholar] [CrossRef]
- Jacinto, C.; Oliveira, S.L.; Nunes, L.A.O.; Myers, J.D.; Myers, M.J.; Catunda, T. Normalized-lifetime thermal-lens method for the determination of luminescence quantum efficiency and thermo-optical coefficients: Application to Nd3+ -doped glasses. Phys. Rev. B 2006, 73, 125107. [Google Scholar] [CrossRef]
- Righini, G.; Ferrari, M. Photoluminescence of rare-earth-doped glasses. Riv. Nuovo Cim. 2006, 28, 1–53. [Google Scholar] [CrossRef]
- Burtan-Gwizdala, B.; Reben, M.; Cisowski, J.; Yousef, E.S.; Lisiecki, R.; Nosidlak, N. Strong emission at 1000 nm from Pr3+/Yb3+-codoped multicomponent tellurite glass. Pure Appl. Chem. 2021, 94, 147–156. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Q.; Huang, L.; Wang, J.; Su, Q. Near ultraviolet and visible-to-near-infrared spectral converting properties and energy transfer mechanism of Sr_2SiO_4:Ce^3+, Pr^3+ phosphor. Opt. Mater. Express 2014, 4, 227. [Google Scholar] [CrossRef]
- Mahlke, G.; Gössing, P. Corning Cable Systems; Fiber Optic Cables; Publicis MCD Corporate Publishin: Munich, Germany, 2001; pp. 13–30. [Google Scholar]
- Burtan-Gwizdala, B.; Reben, M.; Cisowski, J.; Lisiecki, R.; Ryba-Romanowski, W.; Jarząbek, B.; Mazurek, Z.; Nosidlak, N.; Grelowska, I. The influence of Pr3+ content on luminescence and optical behavior of TeO2-WO3-PbO-Lu2O3 glass. Opt. Mat. 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Seshadri, M.; Bell, M.J.V.; Anjos, V.; Messaddeq, Y. Spectroscopic investigations on Yb3+ doped and Pr3+/Yb3+ co-doped tellurite glasses for photonic applications. J. Rare Earths 2021, 39, 33–42. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Kesavulu, C.; Maheswari, T.; Pecharapa, W.; Depuru, S.R.; Jayasankar, C. Spectral characteristics of Pr3+-doped lead based phosphate glasses for optical display device applications. J. Lumin. 2020, 228, 117585. [Google Scholar] [CrossRef]
- Sangwaranatee, N.W.; Kiwsakunkran, N.; Kaewkhao, J. Investigation on Physical and Optical of Praseodymium Doped Sodium Aluminum Barium Phosphate Glasses. J. Phys. Conf. Ser. 2020, 1428, 12031. [Google Scholar] [CrossRef]
- Ritu, S.; Rao, A.S.; Deopa, N.; Venkateswarlu, M.; Jayasimhadri, M.; Haranath, D.; Vijaya Prakash, G. Spectroscopic study of Pr3+ ions doped Zinc Lead Tungsten, Tellurite glasses for visible photonic device applications. Opt. Mater. 2018, 78, 457e464. [Google Scholar]
- Flizikowski, G.A.S.; Zanuto, V.S.; Nunes, L.A.O.; Baesso, M.L.; Malacarne, L.C.; Astrath, N.G.C. Standard and modified Judd-Ofelt theories in Pr3+-doped calcium, aluminosilicateglasses: A comparative analysis. J. Alloys Compd. 2018, 780, 705–710. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar] [CrossRef]
- Afef, B.; Hegazy, H.H.; Algarni, H.; Yang, Y.; Damak, K.; Yousef, E.; Maalej, R. Spectroscopic analysis of trivalent Nd3+ /Yb3+ ions co-doped in PZS host glasses as a new laser material at 1.06 μm. J. Rare Earths 2017, 35, 361–367. [Google Scholar] [CrossRef]
- Afef, B.; Alqahtani, M.M.; Hegazy, H.H.; Yousef, E.; Damak, K.; Maâlej, R. Green and near-infrared emission of Er3+ doped PZS and PZC glasses. J. Lumin. 2018, 194, 706–712. [Google Scholar] [CrossRef]
- Hussein, K.I.; Alqahtani, M.S.; Alzahrani, K.J.; Alqahtani, F.F.; Zahran, H.Y.; Alshehri, A.M.; Yahia, I.S.; Reben, M.; Yousef, E.S. The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System. Materials 2022, 15, 1844. [Google Scholar] [CrossRef] [PubMed]
- Charfi, B.; Damak, K.; Alqahtani, M.S.; Hussein, K.I.; Alshehri, A.M.; Elkhoshkhany, N.; Assiri, A.L.; Alshehri, K.F.; Reben, M.; Yousef, E.S. Luminescence and Gamma Spectroscopy of Phosphate Glass Doped with Nd3+/Yb3+ and Their Multifunctional Applications. Photonics 2022, 9, 406. [Google Scholar] [CrossRef]
- Rayan, D.A.; Elbashar, Y.H.; Rashad, M.M.; El-Korashy, A. Optical spectroscopic analysis of cupric oxide doped barium phos-phate glass for bandpass absorption filter. J. Non-Cryst. Solids 2013, 382, 52–56. [Google Scholar] [CrossRef]
- Ghauri, M.; Siddiqi, S.; Shah, W.; Ashiq, M.; Iqbal, M. Optical properties of zinc molybdenum phosphate glasses. J. Non-Cryst. Solids 2009, 355, 2466–2471. [Google Scholar] [CrossRef]
- Elbashar, Y.H. Structural and spectroscopic analyses of copper doped P2O5-ZnO-K2O-Bi2O3 glasses. Process Appl. Ceram. 2015, 9, 169–173. [Google Scholar] [CrossRef]
- Judd, B.R. Optical Absorption Intensities of Rare-Earth Ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare−earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Damak, K.; Maâlej, R.; Yousef, E.S.; Qusti, A.H.; Rüssel, C. Thermal and spectroscopic properties of Tm3+ doped TZPPN transparent glass laser material. J. Non-Cryst. Solids 2012, 358, 2974–2980. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Singh, R.K.; Pal, S. Praseodymium Ion Doped Sodium Borosilicate Glasses: Energy Interaction and Radiative Properties. Am. J. Condens. Matter Phys. 2015, 5, 10–18. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Lu, X.; You, Z.; Li, J.; Zhu, Z.; Tu, C. Optical properties of Pr3+-doped SrWO4 crystal. Appl. Phys. A 2007, 90, 497–502. [Google Scholar] [CrossRef]
- Carnall, W.T.; Crosswhite, H.; Crosswhite, H.M. Energy Level Structure and Transition Probabilities in the Spectra of the Trivalent Lanthanides in LaF3. United States: N. p.; Technical Report 1978; TRN: 79-005910. Available online: https://www.osti.gov/biblio/6417825/ (accessed on 4 August 2022). [CrossRef]
- Kaminskii, A.A. Laser Crystals: Their Physics and Properties; Springer: Berlin, Germany, 1981; pp. 157–158. [Google Scholar]
- Lalla, E.A.; Konstantinidis, M.; De Souza, I.; Daly, M.G.; Martín, I.R.; Lavín, V.; Rodríguez-Mendoza, U.R. Judd-Ofelt parameters of RE3+-doped fluorotellurite glass, (RE3: Pr3+, Nd3+, Sm3+, Tb3+, Dy3+, Ho3+, Er3+, and Tm3+). J. Alloys Compd. 2020, 845, 156028. [Google Scholar] [CrossRef]
- Ajithkumar, G.; Gupta, P.K.; Gin Jose, N.V. Unnikrishnan Judd±Ofelt intensity parameters and laser analysis of Pr3+.doped phosphate glasses sensitized by Mn2+ Ions. J. Non-Cryst. Solids 2000, 275, 93–106. [Google Scholar] [CrossRef]
- Malta, O.; Carlos, L. Intensities of 4f-4f transitions in glass materials. Quim. Nova 2003, 26, 889–895. [Google Scholar] [CrossRef]
- Goldner, P.; Auzel, F. Application of standard and modified Judd–Ofelt theories to a praseodymiumdoped fluorozirconate glass. J. Appl. Phys. 1996, 79, 7972. [Google Scholar] [CrossRef]
- Kornienko, A.A.; Kaminskii, A.A.; Dunina, E.B. Dependence of the Line Strength of f–f Transitions on the Manifold Energy. II. Analysis of Pr3+ in KPrP4O12. Phys. Status Solidi 1990, 157, 267–273. [Google Scholar] [CrossRef]
- Kumar, M.V.V.; Rama Gopal, K.; Reddy, R.R.; Lokeswara Reddy, G.V.; Sooraj Hussain, N.; Jamalaiah, B.C. Application of modified Judd–Ofelt theory and the evaluation of radiative properties of Pr3+-doped lead telluroborate glasses for laser applications. J. Non-Cryst. Solids 2013, 364, 20–27. [Google Scholar] [CrossRef]
- Sattayaporn, S.; Loiseau, P.; Aka, G.; Klimin, S.; Boldyrev, K.; Mavrin, B. Fine spectroscopy and Judd-Ofelt analysis of Pr3+ doped Sr0.7La0.3Mg0.3Al11.7O19 (Pr:ASL). J. Lumin. 2019, 219, 116895. [Google Scholar] [CrossRef]
- Guo, W.; Lin, Y.; Gong, X.; Chen, Y.; Luo, Z.; Huang, Y. Growth and spectroscopic properties of Pr3+:NaLa(MoO4)2 crystal. J. Appl. Phys. 2008, 104, 53105. [Google Scholar] [CrossRef]
- Génova, R.T.; Martin, I.R.; Rodriguez-Mendoza, U.R.; Lahoz, F.; Lozano-Gorrin, A.D.; Nunez, P.; Gonzalez-Platas, J.; Lavin, V. Optical intensities of Pr3+ ions in transparent oxyfluoride glass and glass–ceramic. Applications of the standard and modified Judd–Ofelt theories. J. Alloys Compd. 2004, 380, 167–172. [Google Scholar] [CrossRef]
- Sojka, L.; Tang, Z.; Furniss, D.; Sakr, H.; Oladeji, A.; Beres-Pawlik, E.; Dantanarayana, H.; Faber, E.; Seddon, A.B.; Benson, T.M.; et al. Broadband, mid-infrared emission from Pr3+ doped GeAsGaSe chalcogenide fiber, optically clad. Opt. Mater. 2014, 36, 1076–1082. [Google Scholar] [CrossRef]
- Sardar, D.K.; Russell, C.C., III. Optical transitions, absorption intensities, and intermanifold emission cross sections of Pr3+(4f2) in Ca5(PO4)3F crystal host. Appl. Phys. 2004, 95, 10. [Google Scholar] [CrossRef]
- Mazurak, Z.; Bodyl, S.; Lisiecki, R.; Gabrys-Pisarska, J.; Czaja, M. Optical properties of Pr3+, Sm3+ and Wr3+ doped P2O5–CaO–SrO–BaO phosphate glass. Opt. Mater. 2010, 32, 547–553. [Google Scholar] [CrossRef]
- Hegde, V.; Dwaraka Viswanath, C.S.; Chauhan, N.; Mahato, K.K.; Kamath, S.D. Photoluminescence and thermally stimulated luminescence properties of Pr3+-doped zinc sodium bismuth borate glasses. Opt. Mater. 2018, 84, 268–277. [Google Scholar] [CrossRef]
- Herrera, A.; Jacinto, C.; Becerra, A.R.; Franzen, P.L.; Balzaretti, N.M. Multichannel emission from Pr3+ doped heavy-metal oxide glass B2O3–PbO–GeO2–Bi2O3 for broadband signal amplification. J. Lumin. 2016, 180, 341–347. [Google Scholar] [CrossRef]
- Klimesz, B.; Dominiak-Dzik, G.; Zelechower, M.; Ryba-Romanowski, W.J. Optical study of GeO2-PbO-PbF2 oxyfluoride glass singly doped with Pr3+, Nd3+, Sm3+ and Eu3+. J. Alloys Compd. 2005, 403, 76–85. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Crystalline Lasers: Physical Processes and Operating Schemes; CRC Press: New York, NY, USA, 1996; 296p. [Google Scholar]
- Oliveira, A.S.; Gouveia, E.A.; De Araujo, M.T.; Gouveia-Neto, A.S.; De Araujo, C.B.; Messaddeq, Y. Twentyfold blue upcon-version emission enhancement through thermal effects in Pr3+/Yb3+-co-doped fluoroindate glasses excited at 1.064 µm. J. Appl. Phys. 2000, 87, 4274–4278. [Google Scholar] [CrossRef]
- Shojiya, M.; Kawamoto, Y.; Kadono, K. Judd–Ofelt parameters and multiphonon relaxation of Ho3+ ions in ZnCl2-based glass. J. Appl. Phys. 2001, 89, 4944–4950. [Google Scholar] [CrossRef]
- Naresh, V.; Ham, B.S. Influence of multiphonon and cross relaxations on 3P0 and 1D2 emission levels of Pr3+ doped borosilicate glasses for broad band signal amplification. J. Alloys Compd. 2016, 664, 321–330. [Google Scholar] [CrossRef]
- Lei, W.H.; Chen, B.J.; Zhang, X.L.; Pun, E.Y.B.; Lin, H. Optical evaluation on Nd(3+)-doped phosphate glasses for O-band amplification. Appl. Opt. 2011, 50, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.C.; Wang, R.F.; Yang, Z.W.; Song, Z.G.; Yin, Z.Y.; Qiu, J.B. Spectroscopic properties of Tm3+ doped TeO2-R2O-La2O3 glasses for 1.47 μm optical amplifiers. J. Non-Cryst. Solids 2011, 357, 2409–2412. [Google Scholar] [CrossRef]
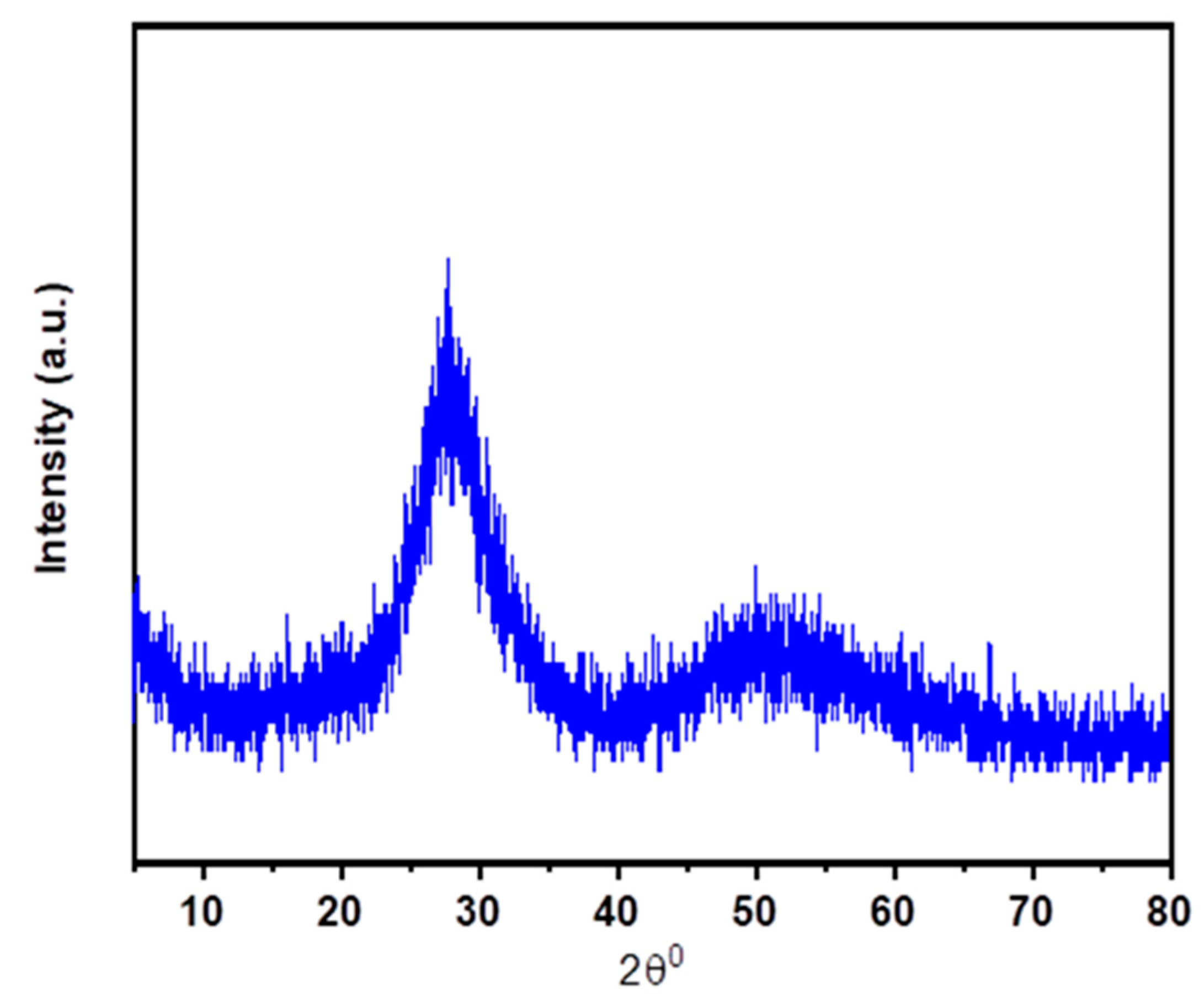
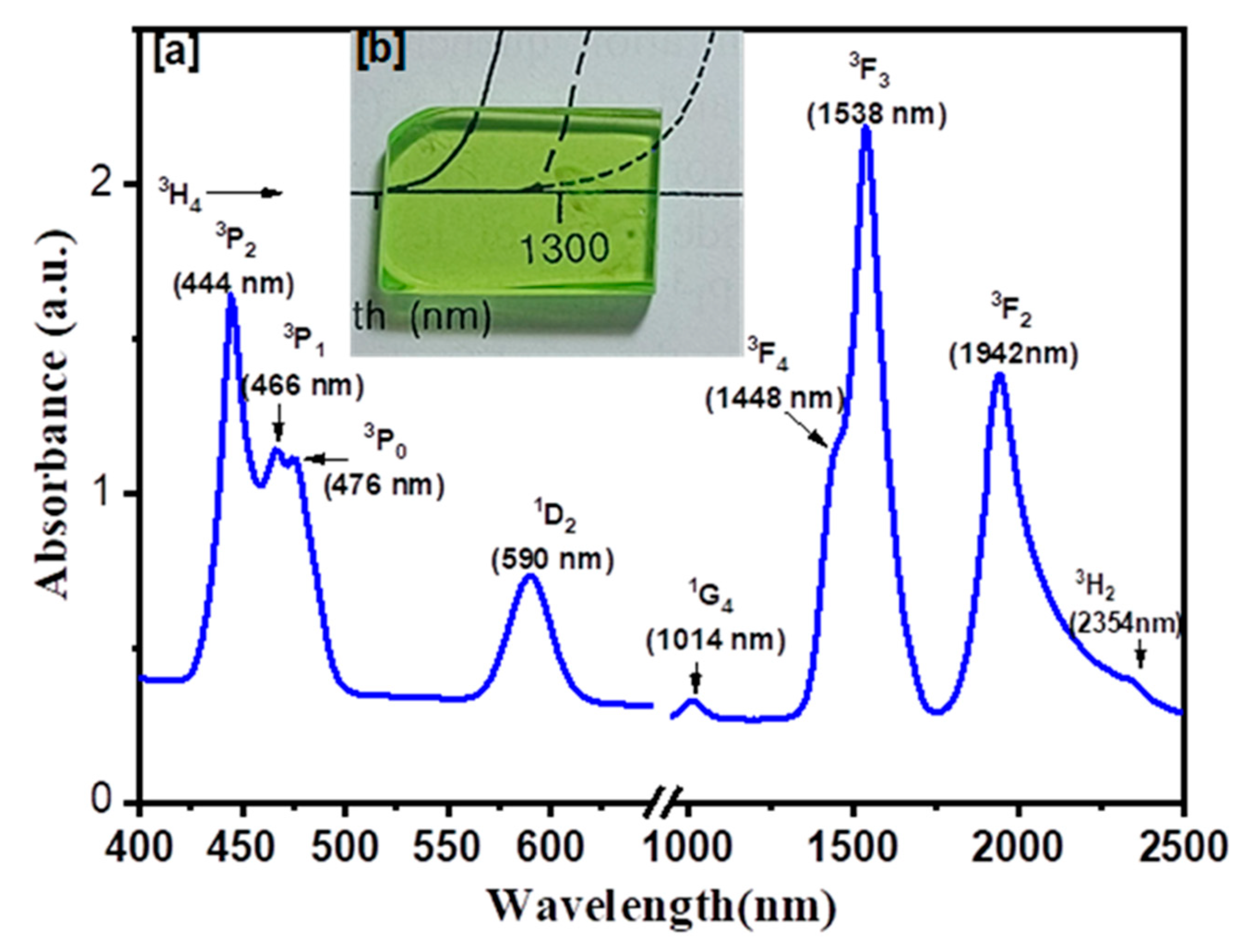
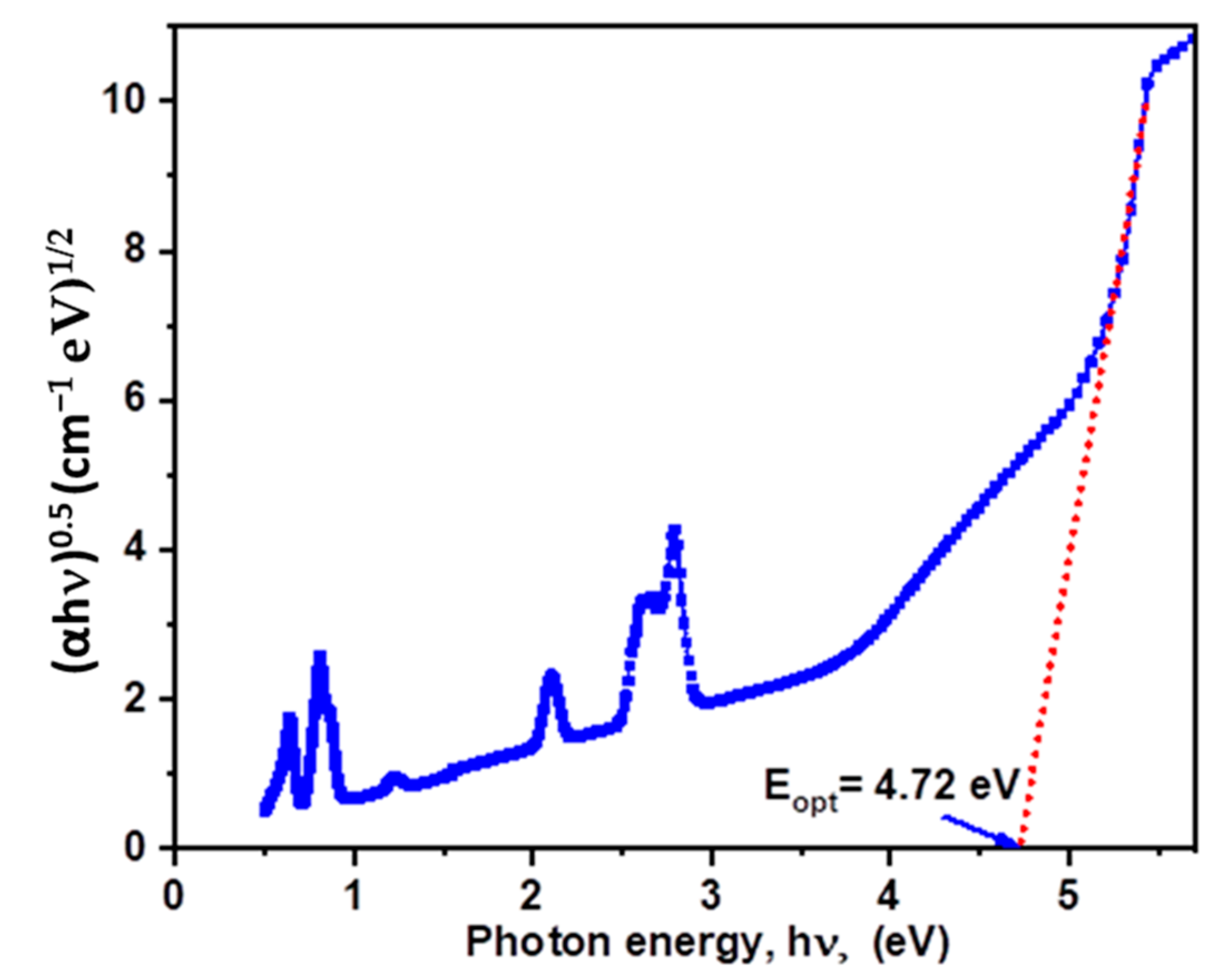
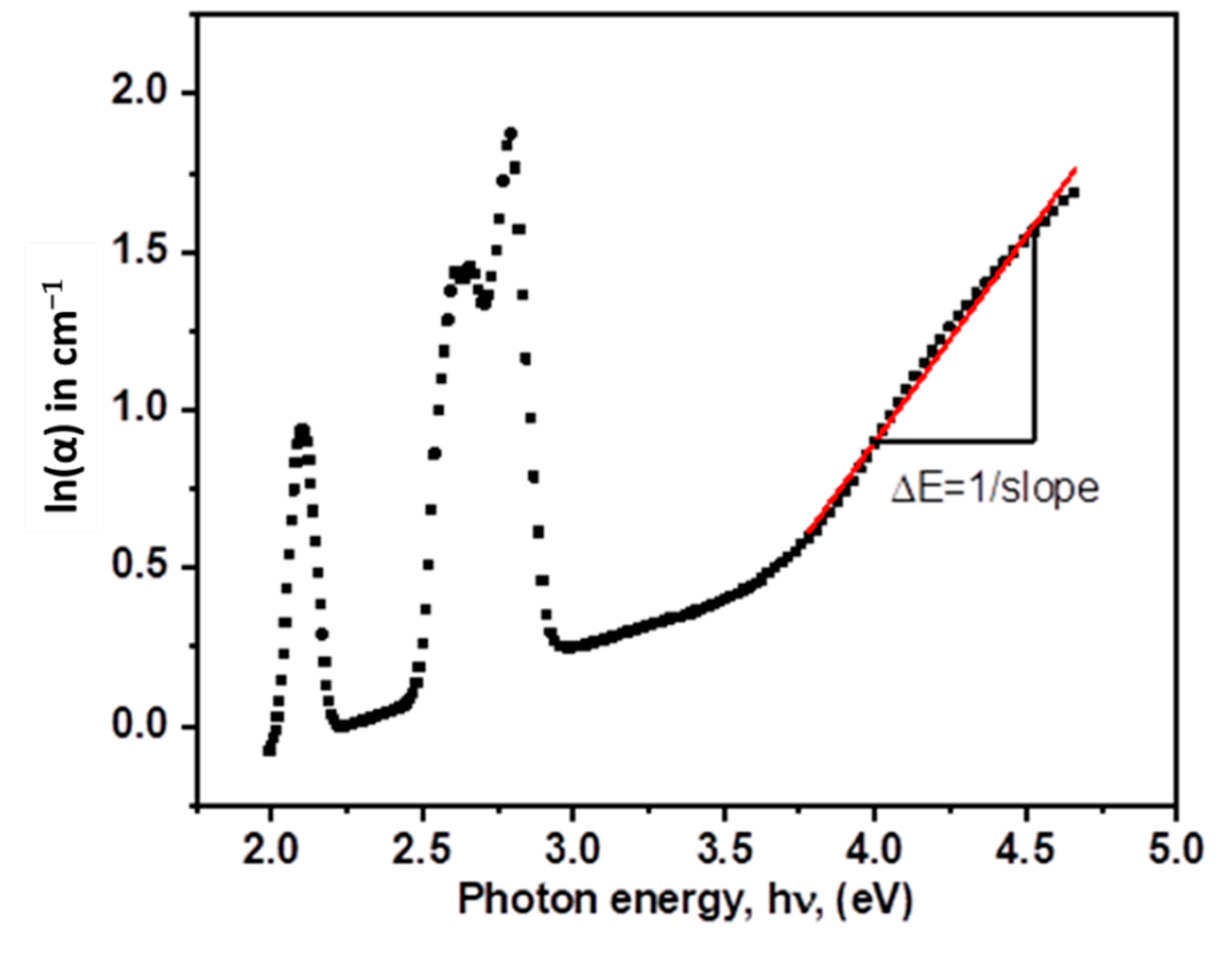
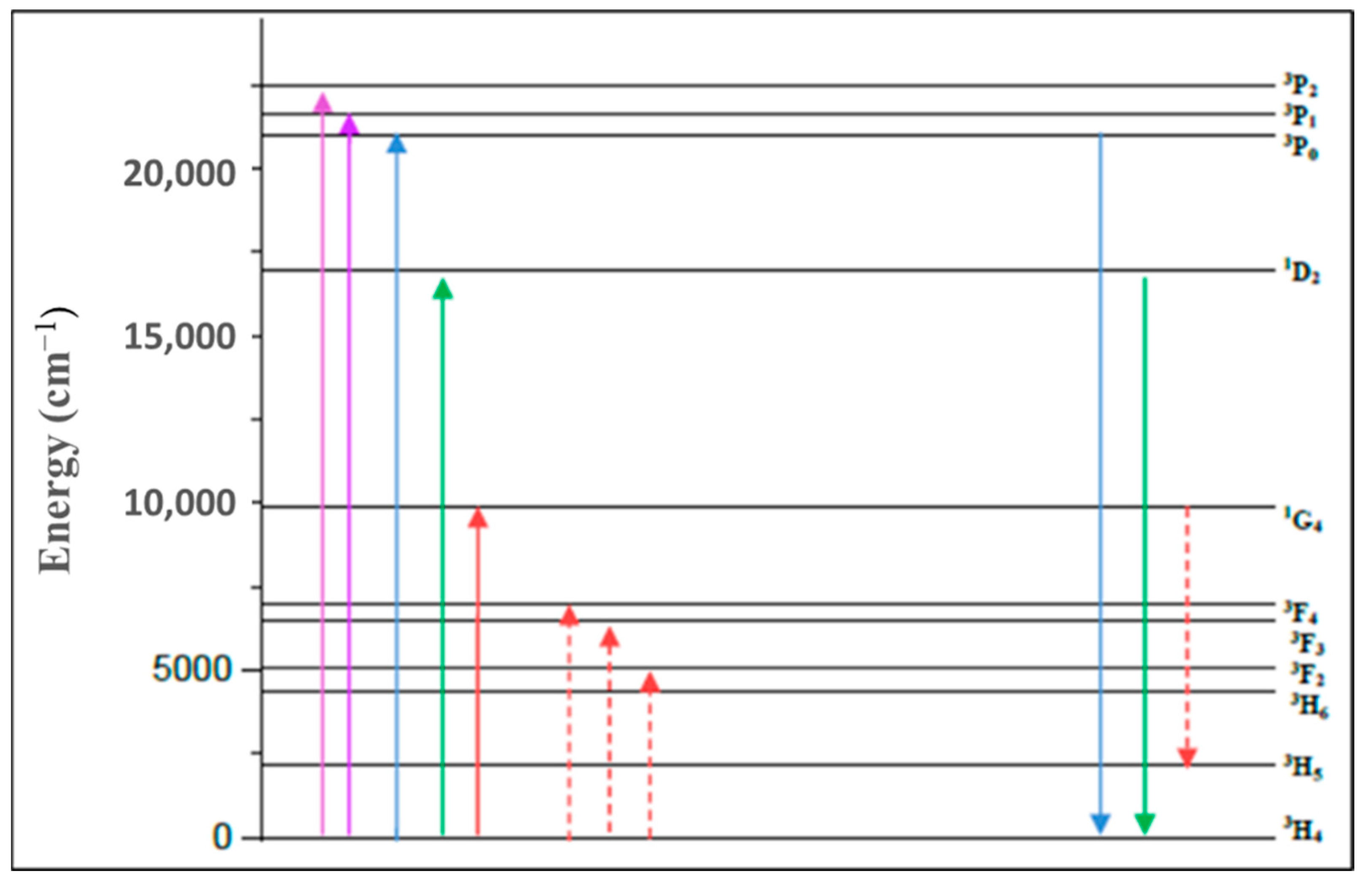
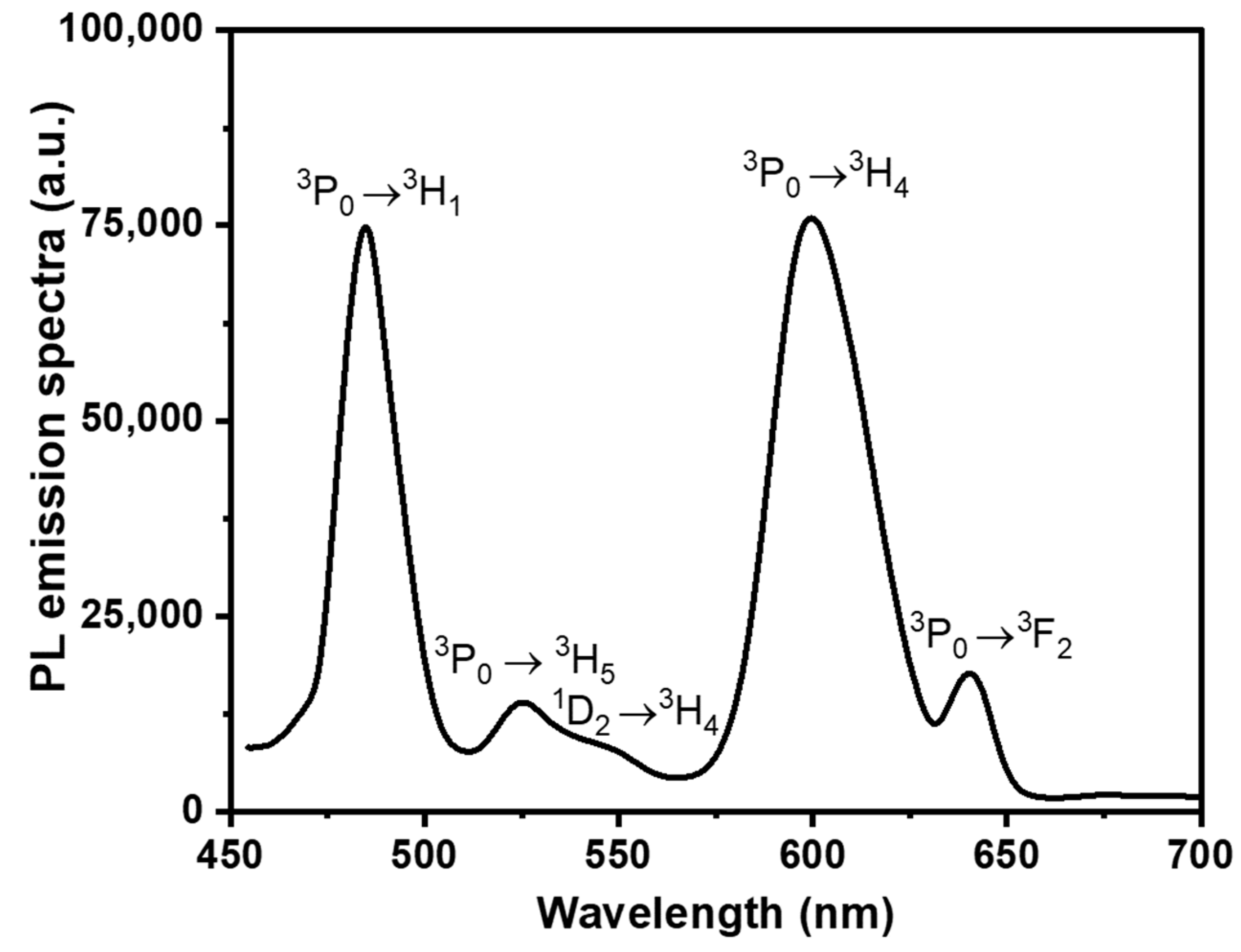
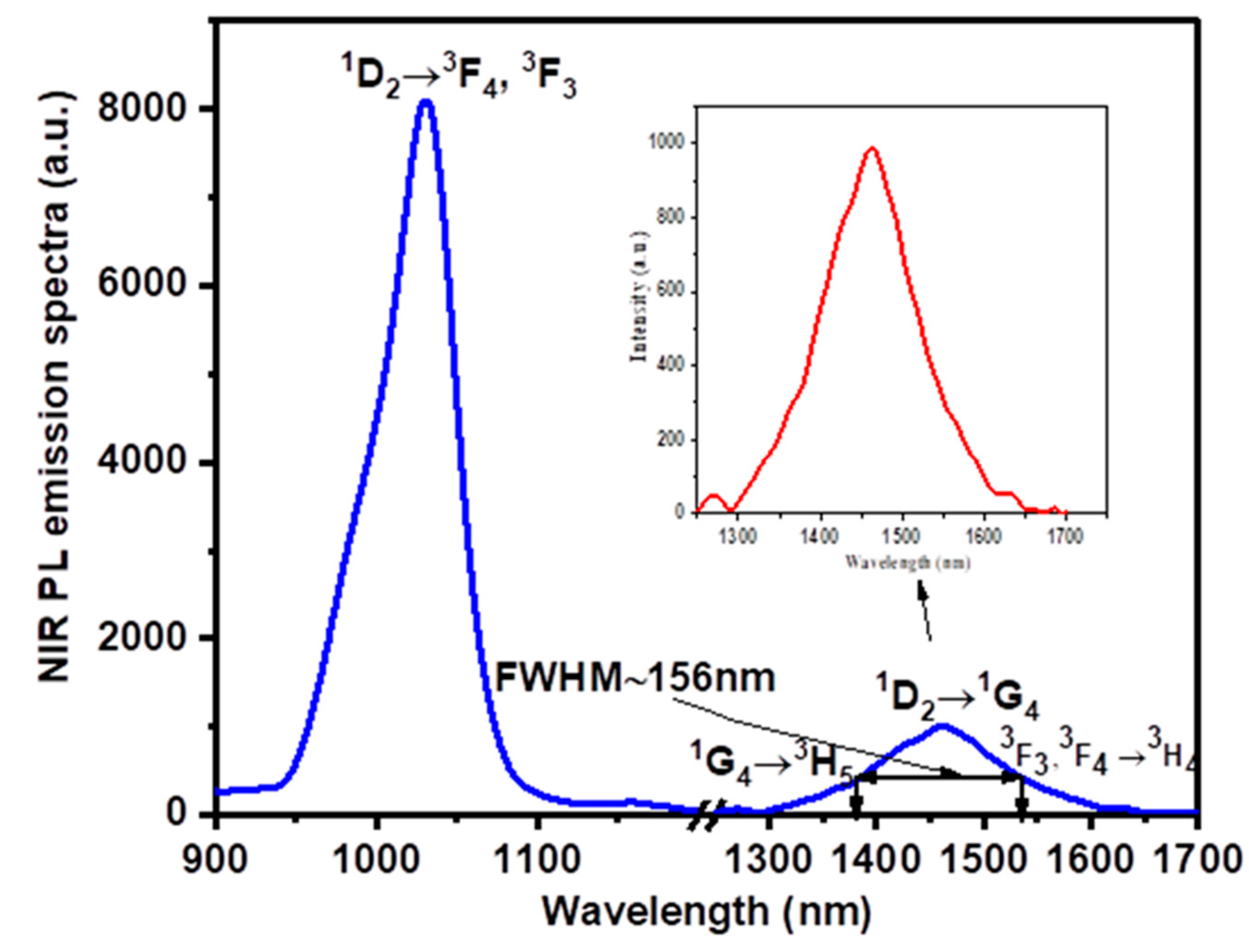
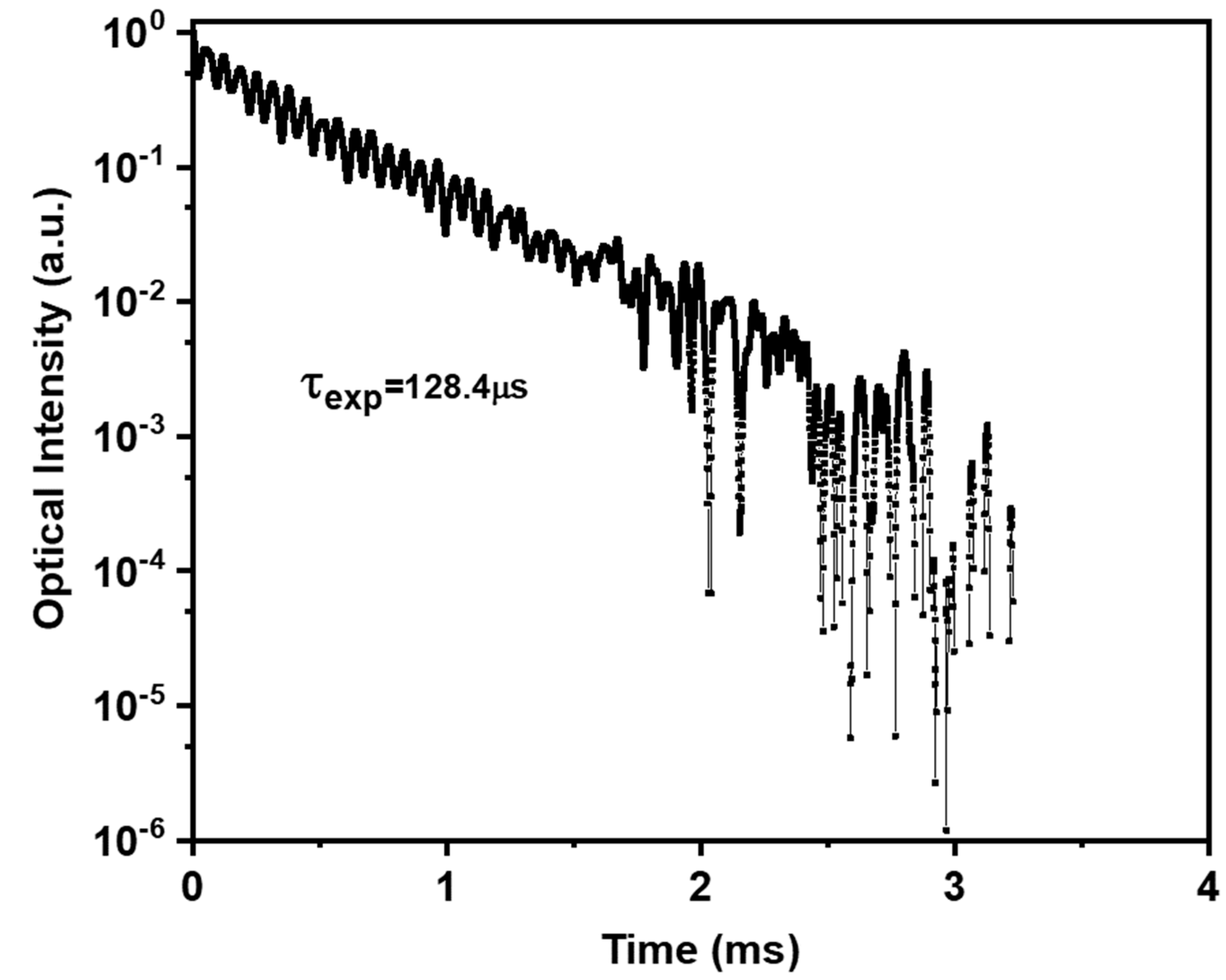
| λ (nm) | ν (cm−1) | ||U2||2 | ||U4||2 | ||U6||2 | Γ (nm·cm−1) | Sexp (10−24 m2) | Scal (10−24 m2) | |
|---|---|---|---|---|---|---|---|---|
| 3H4 → 3P2 | 445 | 22471 | 0 | 0.0362 | 0.1355 | 19.11 | 0.54705 | 0.30554 |
| 3P1 | 462 | 21652 | 0 | 0.1707 | 0 | 7.51 | 0.20720 | 0.28026 |
| 3P0 | 476 | 21012 | 0 | 0.1728 | 0 | 14.61 | 0.39129 | 0.28371 |
| 1D2 | 590 | 16956 | 0.0026 | 0.017 | 0.052 | 10.31 | 0.22271 | 0.12241 |
| 3F3 | 1541 | 6490 | 0.0654 | 0.3469 | 0.6983 | 215.68 | 1.78360 | 1.83910 |
| 3F2 | 1958 | 5106 | 0.5089 | 0.4032 | 0.1177 | 137.06 | 0.89182 | 0.88521 |
| System | Ω2 | Ω4 | Ω6 | Trend | χ |
|---|---|---|---|---|---|
| PZLBPr [Present Work]: | 0.018 | 1.641 | 1.816 | Ω2 < Ω4 < Ω6 | 0.90 |
| PPbKANPr0.5 [19] | 1.51 | 18.03 | 19.81 | Ω2 < Ω4 < Ω6 | 0.91 |
| Phosphate [49] | 4.19 | 4.29 | 6.40 | Ω2 < Ω4 < Ω6 | 0.67 |
| ZNBBP [50] | 1.7 | 3.06 | 4.72 | Ω2 < Ω4 < Ω6 | 0.64 |
| BPGBPr [51] | 0.70 | 2.96 | 7.03 | Ω2 < Ω4 < Ω6 | 0.42 |
| Oxyfluoride [52] | 0.66 | 12.49 | 3.17 | Ω2 < Ω6 < Ω4 | 3.94 |
| Ca5(PO4)3F [48] | 0.32 | 1.59 | 3.82 | Ω2 < Ω4 < Ω6 | 0.41 |
| LaF3 [53] | 0.12 | 1.77 | 4.78 | Ω2 < Ω4 < Ω6 | 0.37 |
| LiPrP4O12 [53] | 1.82 | 2.83 | 6.54 | Ω2 < Ω4 < Ω6 | 0.43 |
| YAlO3 [53] | 2.00 | 6.00 | 7.00 | Ω2 < Ω4 < Ω6 | 0.85 |
| LiYF4 [53] | 0.00 | 8.07 | 7.32 | Ω2 < Ω6 < Ω4 | 1.10 |
| Oxy–Fluoride [21] | 0.13 | 4.09 | 6.33 | Ω2 < Ω4 < Ω6 | 0.64 |
| TPA(n = cst) [38] | 0.48 | 1.39 | 13.5 | Ω2 < Ω4 < Ω6 | 0.10 |
| TPA (n ≠ cst) [38] | 0.92 | 1.85 | 6.61 | Ω2 < Ω4 < Ω6 | 0.27 |
| TeO2-Li2CO3-Pr2O3 [38] | 3.81 | 5.81 | 4.1 | Ω2 < Ω6 < Ω4 | 1.41 |
| PTBPr [43] | 3.07 | 3.36 | 8.68 | Ω2 < Ω6 < Ω4 | 0.38 |
| Transition | Wavelength (nm) | A (s−1) | τ (ms) | β (%) | |
|---|---|---|---|---|---|
| 3P2 → | 3H4 | 431.77 | 4393.6 | 0.040 | 17.6 |
| 3H5 | 476.0 | 5891.9 | 23.7 | ||
| 3H6 | 532.72 | 7053.9 | 28.3 | ||
| 3F2 | 550.54 | 3420.7 | 13.7 | ||
| 3F3 | 597.18 | 2776.2 | 11.1 | ||
| 3F4 | 613.28 | 1078.3 | 4.3 | ||
| 1G4 | 755.32 | 268.29 | 1.1 | ||
| 1D2 | 1716.4 | 26.99 | 0.1 | ||
| 3P0 | 5647.2 | 0.023 | 0.0 | ||
| 3P1 | 8671.9 | 0.013 | 0.0 | ||
| 3P1 → | 3H4 | 454.39 | 5762.6 | 0.076 | 43.9 |
| 3H5 | 503.64 | 0 | 0.0 | ||
| 3H6 | 567.59 | 1391.1 | 10.6 | ||
| 3F2 | 587.86 | 47.37 | 0.4 | ||
| 3F3 | 641.35 | 2435.6 | 18.6 | ||
| 3F4 | 659.95 | 3141.5 | 24.0 | ||
| 1G4 | 827.39 | 338.42 | 2.6 | ||
| 1D2 | 2139.9 | 0.2739 | 0.0 | ||
| 3P0 | 16190 | 0 | 0.0 | ||
| 3P0 → | 3H4 | 467.51 | 16068 | 0.041 | 67.4 |
| 3H5 | 519.81 | 150.98 | 0.6 | ||
| 3H6 | 588.21 | 3329.6 | 14.0 | ||
| 3F2 | 610.01 | 139.41 | 0.6 | ||
| 3F3 | 667.80 | 0.00 | 0.0 | ||
| 3F4 | 687.99 | 3539.2 | 14.8 | ||
| 1G4 | 871.95 | 609.13 | 2.6 | ||
| 1D2 | 2465.8 | 0.10 | 0.0 | ||
| 1D2 → | 3H4 | 576.89 | 737.95 | 0.147 | 47.8 |
| 3H5 | 658.66 | 14.84 | 1.0 | ||
| 3H6 | 772.48 | 294.00 | 19.1 | ||
| 3F2 | 810.52 | 291.04 | 18.9 | ||
| 3F3 | 915.82 | 42.40 | 2.7 | ||
| 3F4 | 954.23 | 49.17 | 3.2 | ||
| 1G4 | 1349.0 | 113.84 | 7.4 | ||
| 1G4 → | 3H4 | 1007.9 | 18.33 | 3.096 | 5.7 |
| 3H5 | 1287.1 | 218.72 | 67.7 | ||
| 3H6 | 1807.6 | 71.32 | 22.1 | ||
| 3F2 | 2030.6 | 2.19 | 0.7 | ||
| 3F3 | 2852.3 | 2.42 | 0.7 | ||
| 3F4 | 3261.1 | 9.96 | 3.1 | ||
| 3F4 → | 3H4 | 1458.8 | 199.48 | 4.995 | 99.7 |
| 3H5 | 2126.5 | 0.00 | 0.0 | ||
| 3H6 | 4055.7 | 0.00 | 0.0 | ||
| 3F2 | 5381.7 | 0.69 | 0.3 | ||
| 3F3 | 22,753.0 | 0.01 | 0.0 | ||
| 3F3 → | 3H4 | 1558.8 | 401.43 | 2.489 | 99.9 |
| 3H5 | 2345.7 | 0.00 | 0.0 | ||
| 3H6 | 4935.5 | 0.00 | 0.0 | ||
| 3F2 | 7049.1 | 0.20 | 0.01 | ||
| 3F2 → | 3H4 | 2001.4 | 127.81 | 7.823 | 100 |
| 3H5 | 3515.5 | 0.00 | 0.0 | ||
| 3H6 | 16,460.0 | 0.00 | 0.0 | ||
| 3H6 → | 3H4 | 2278.4 | 11.59 | 86.286 | 100 |
| 3H5 | 4470.3 | 0.00 | 0.0 | ||
| 3H5 → | 3H4 | 4646.6 | 0.00 | 0.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charfi, B.; Damak, K.; Maâlej, R.; Alqahtani, M.S.; Hussein, K.I.; Alshehri, A.M.; Hussain, A.M.; Burtan-Gwizdala, B.; Reben, M.; Yousef, E.S. Enhancement of Optical Telecommunication Bands: Pr3+-Doped Halide Phosphate Glasses Display Broadband NIR Photoluminescence Emission. Materials 2022, 15, 6518. https://doi.org/10.3390/ma15196518
Charfi B, Damak K, Maâlej R, Alqahtani MS, Hussein KI, Alshehri AM, Hussain AM, Burtan-Gwizdala B, Reben M, Yousef ES. Enhancement of Optical Telecommunication Bands: Pr3+-Doped Halide Phosphate Glasses Display Broadband NIR Photoluminescence Emission. Materials. 2022; 15(19):6518. https://doi.org/10.3390/ma15196518
Chicago/Turabian StyleCharfi, Bilel, Kamel Damak, Ramzi Maâlej, Mohammed S. Alqahtani, Khalid I. Hussein, Ali M. Alshehri, Abdulrahman M. Hussain, Bozena Burtan-Gwizdala, Manuela Reben, and El Sayed Yousef. 2022. "Enhancement of Optical Telecommunication Bands: Pr3+-Doped Halide Phosphate Glasses Display Broadband NIR Photoluminescence Emission" Materials 15, no. 19: 6518. https://doi.org/10.3390/ma15196518
APA StyleCharfi, B., Damak, K., Maâlej, R., Alqahtani, M. S., Hussein, K. I., Alshehri, A. M., Hussain, A. M., Burtan-Gwizdala, B., Reben, M., & Yousef, E. S. (2022). Enhancement of Optical Telecommunication Bands: Pr3+-Doped Halide Phosphate Glasses Display Broadband NIR Photoluminescence Emission. Materials, 15(19), 6518. https://doi.org/10.3390/ma15196518











