Controlled Atmosphere Brazing of 3003 Aluminum Alloy Using Low-Melting-Point Filler Metal Fabricated by Melt-Spinning Technology
Abstract
:1. Introduction
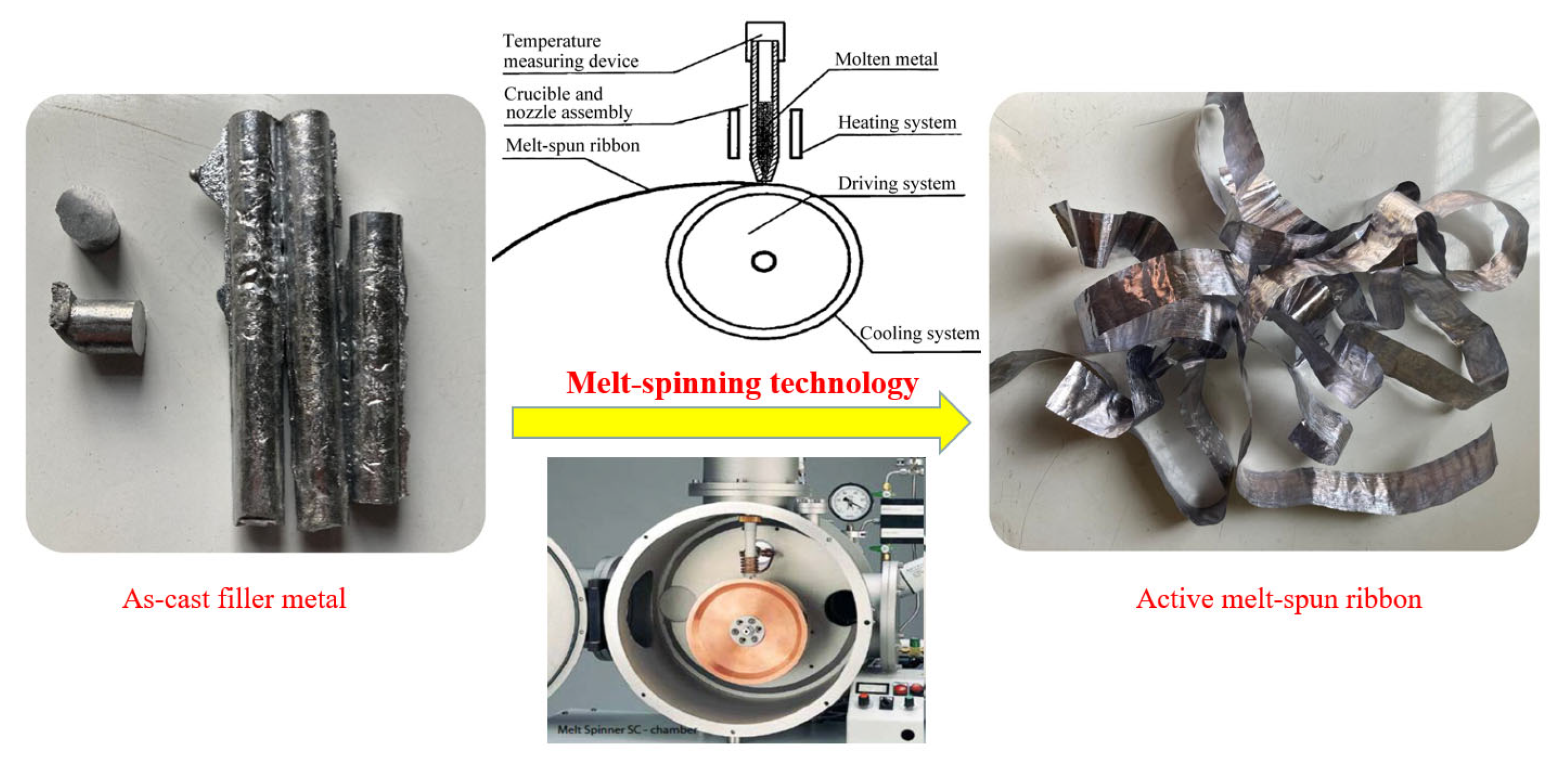
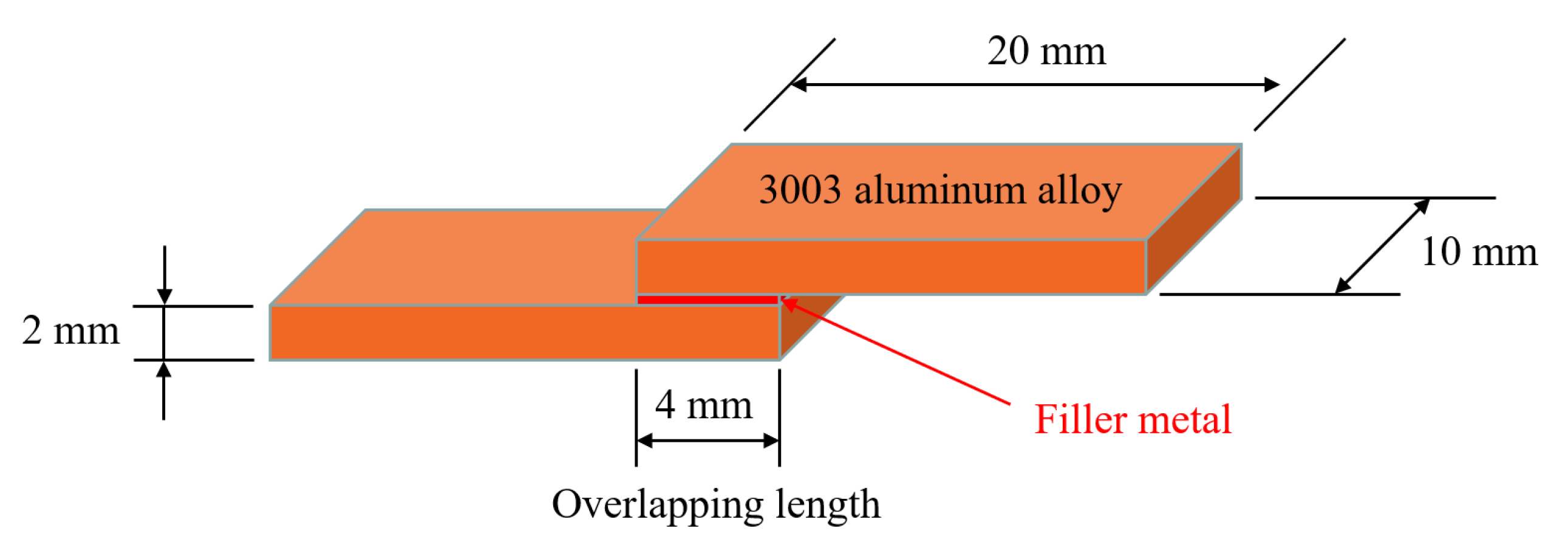
2. Materials and Methods
3. Results
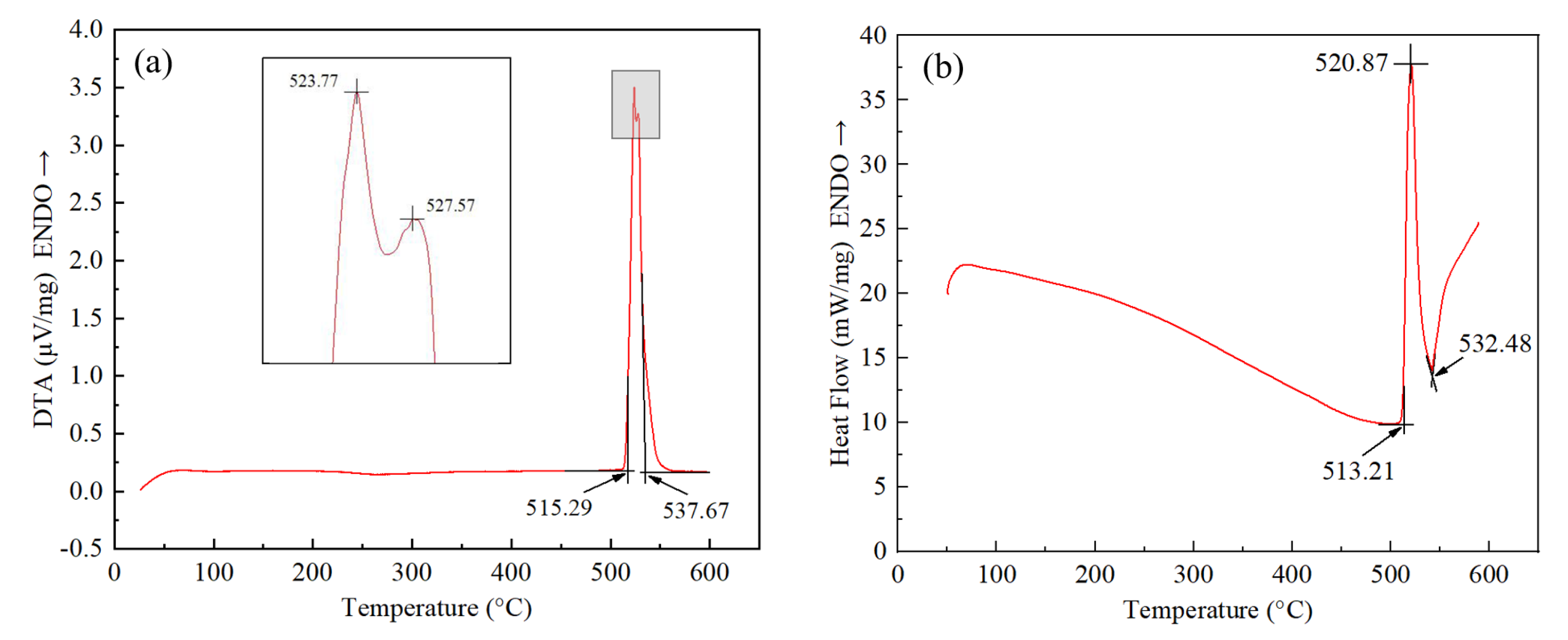
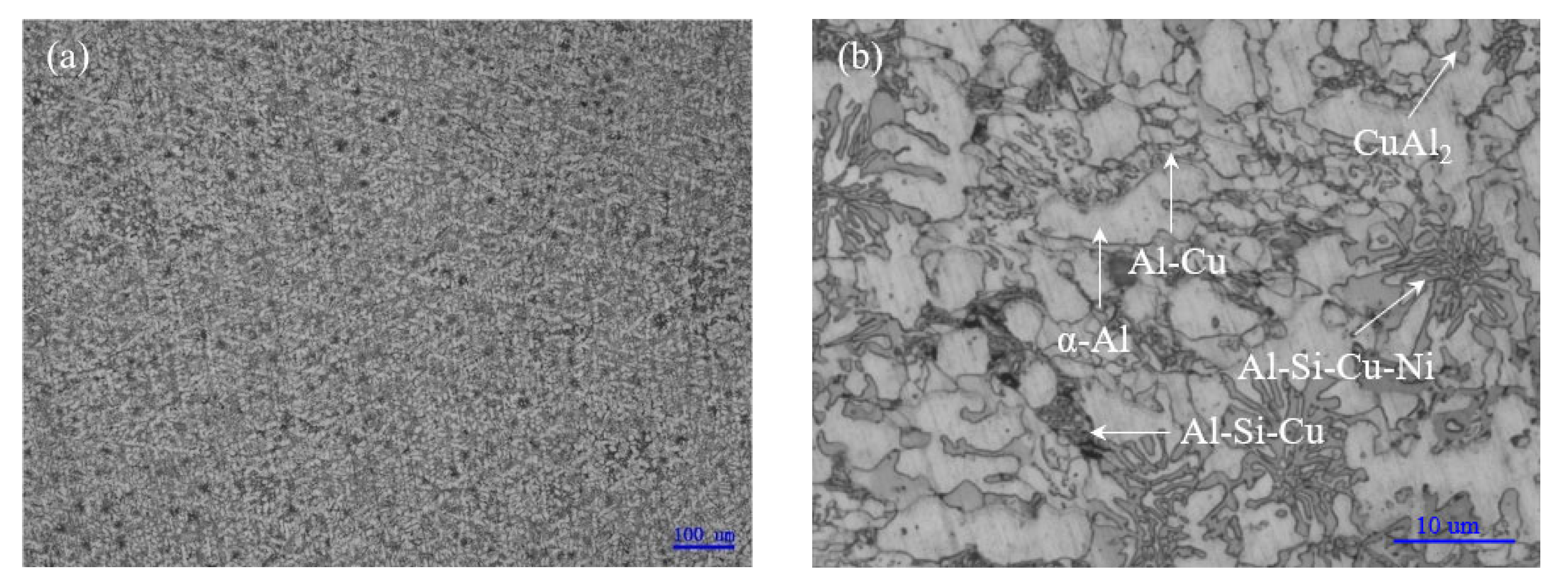
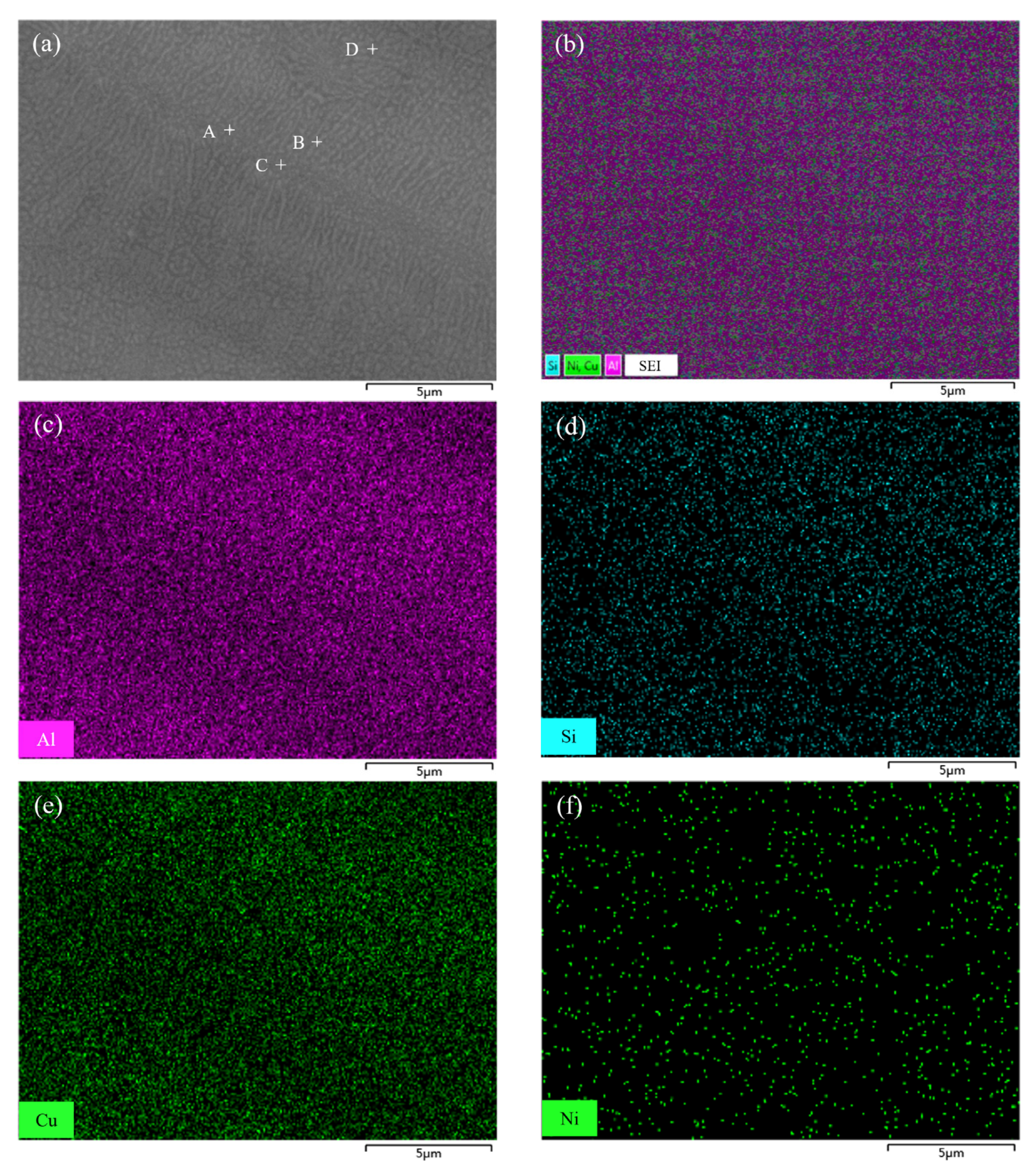
3.1. Melting Characteristics and Microstructure of Low-Melting-Point Filler Metal
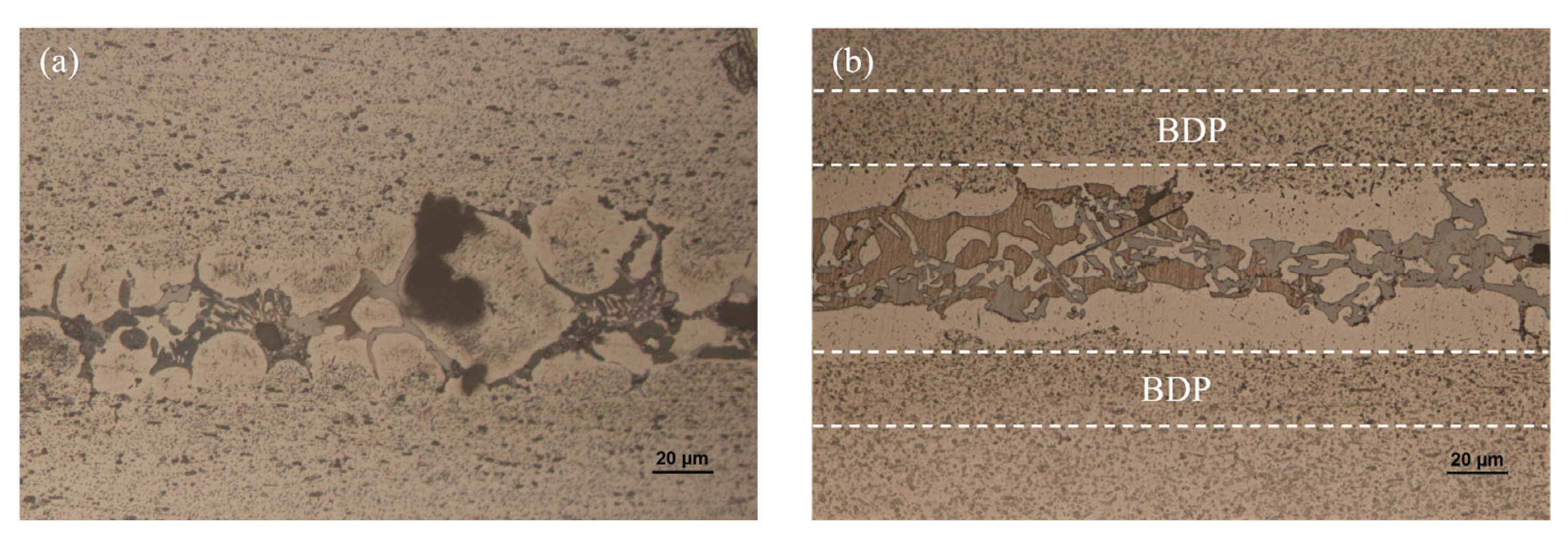
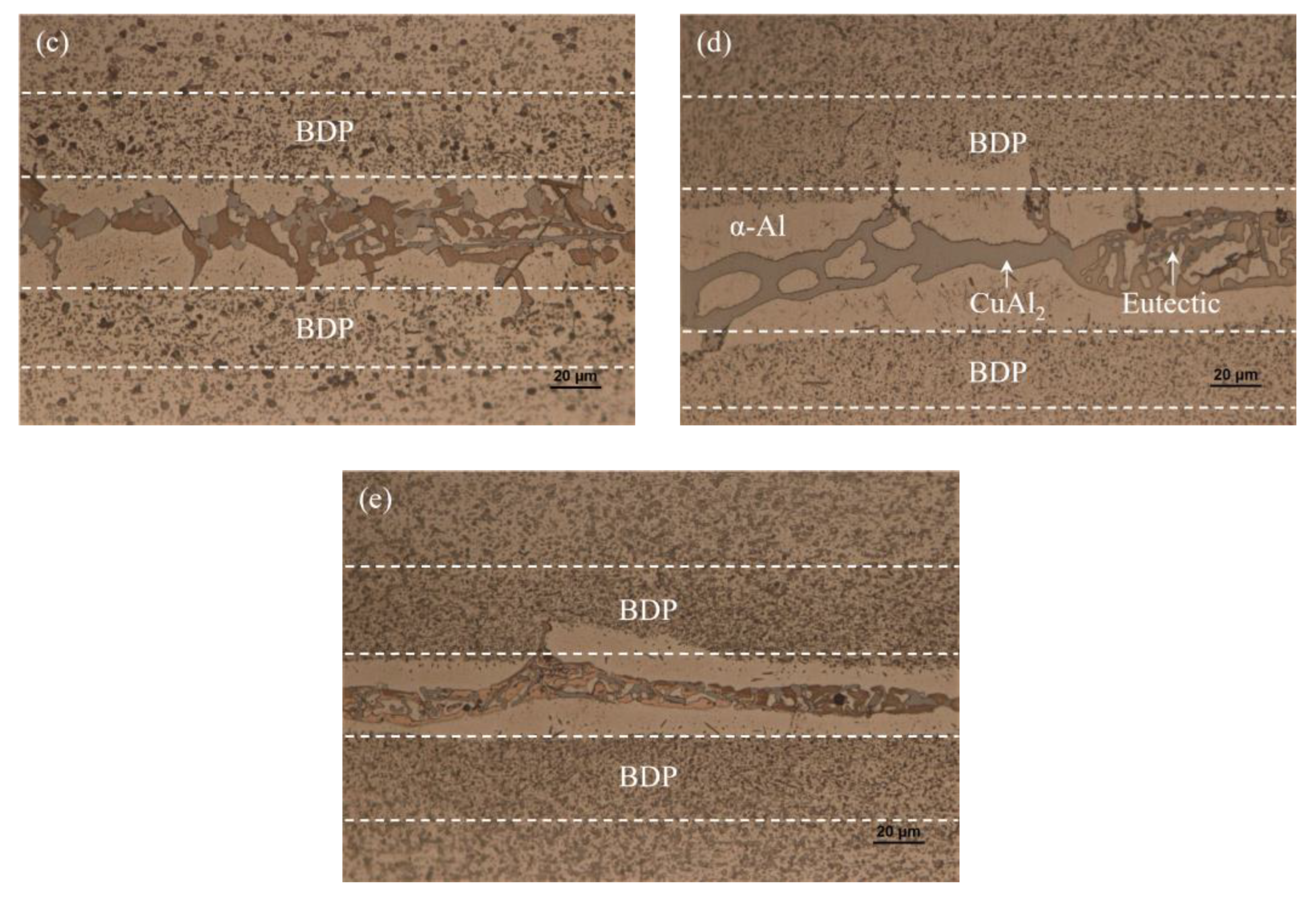
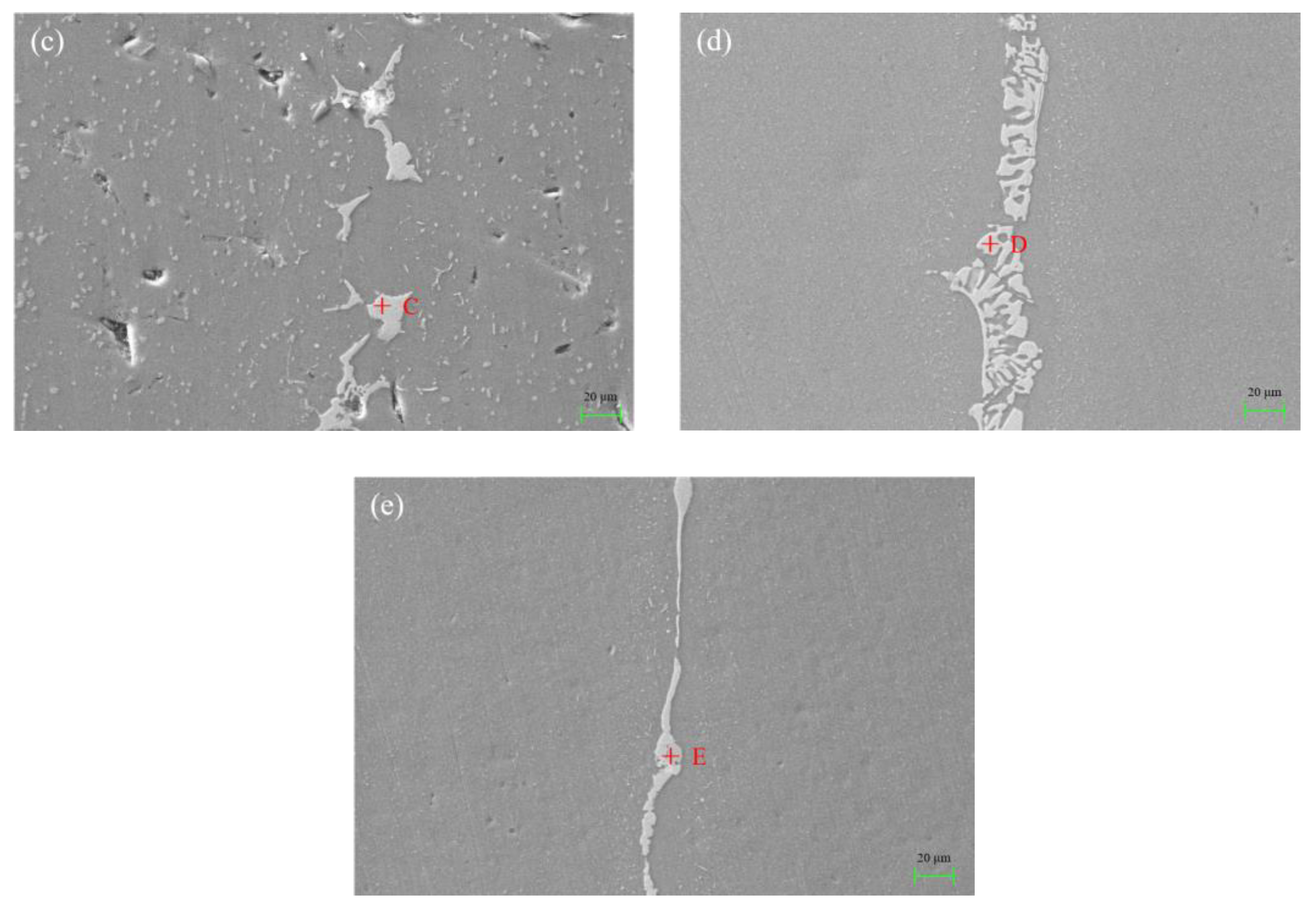
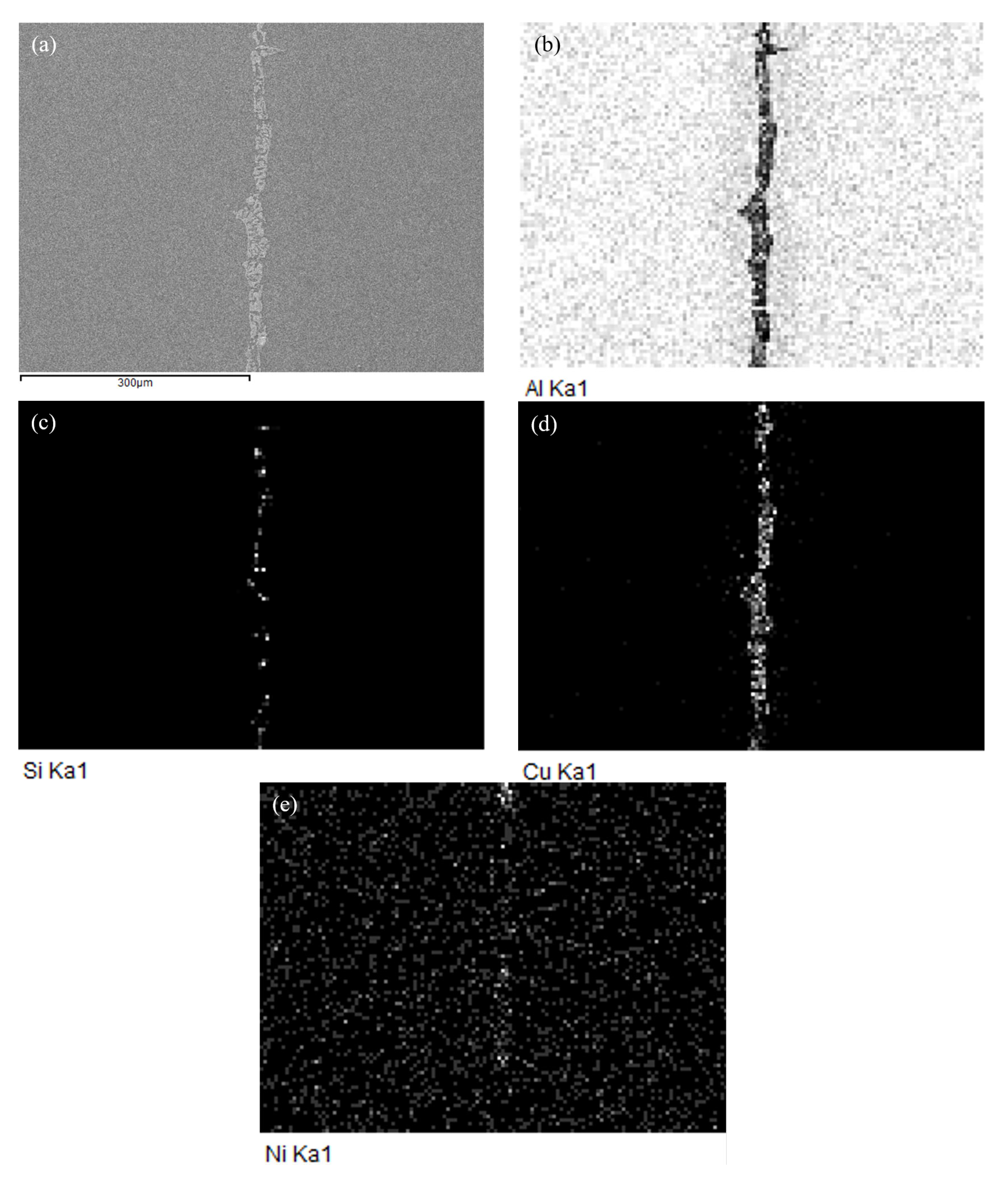
3.2. Microstructure and Element Distribution Analysis of Brazed Joint
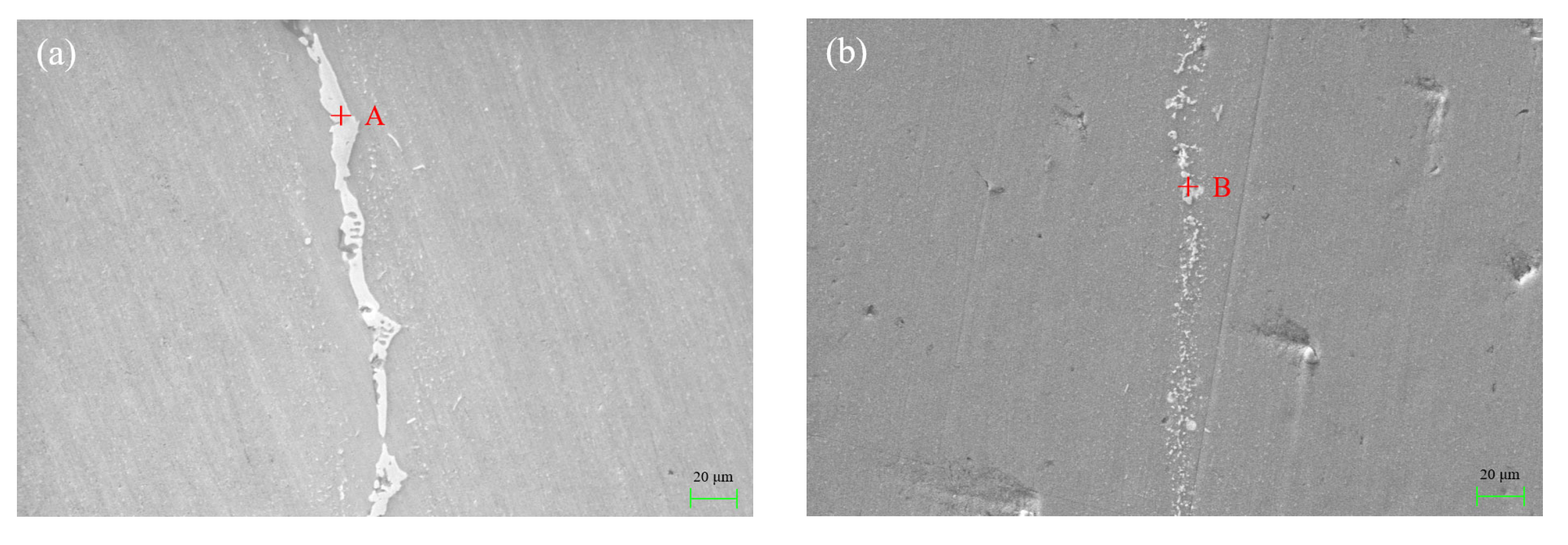
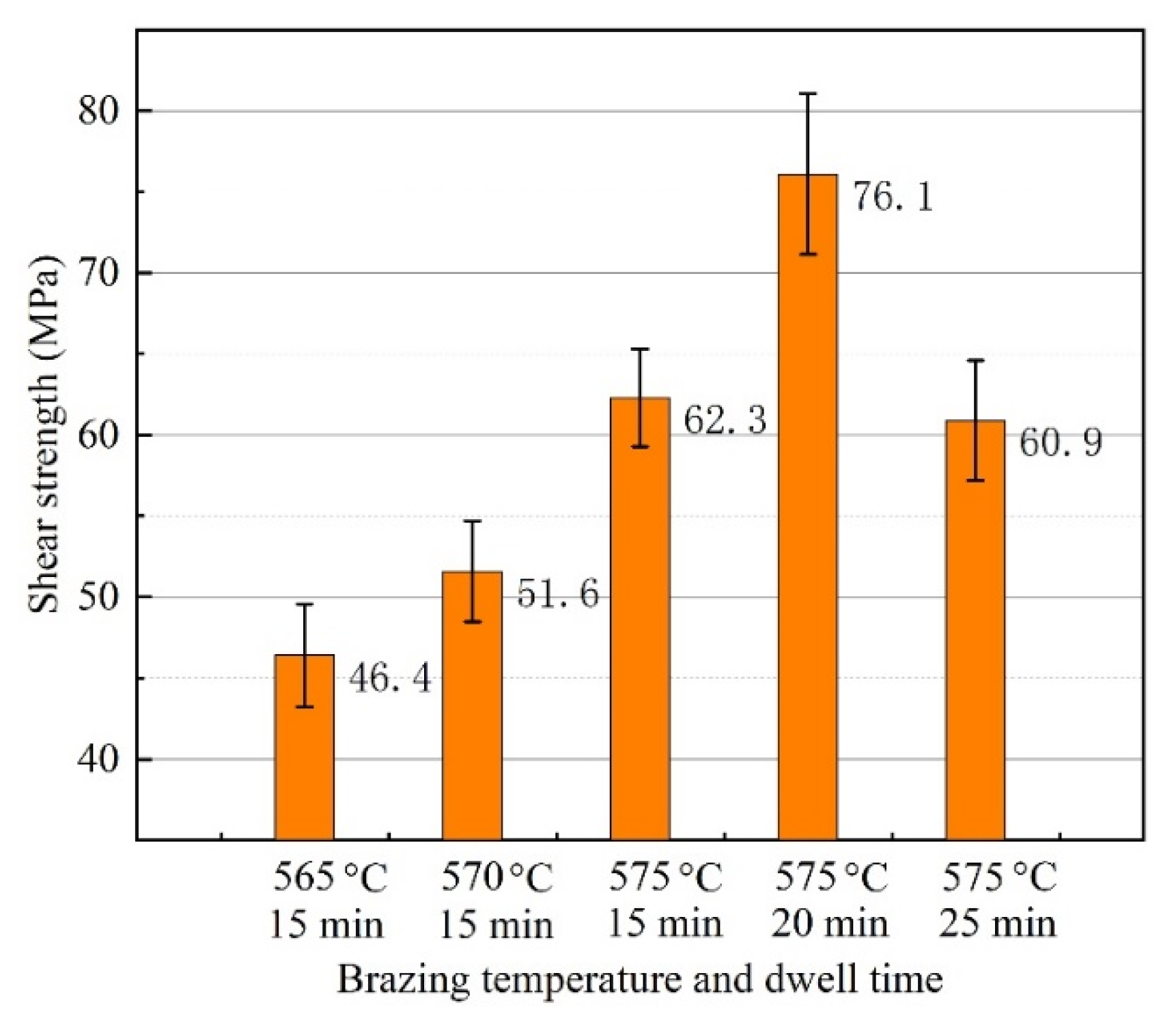
3.3. Gas Tightness and Mechanical Property of Brazed Joint
3.4. Open Circuit Potential and Corrosion Current Density of Filler Metal and Base Metal
4. Conclusions
- (1)
- The solidus and liquidus of brazing filler metal Al-5.0Si-20.5Cu-2.0Ni made by melt-spinning technology was 513.21 °C and 532.48 °C, respectively. Elements of Al, Si, Cu, and Ni in melt-spun ribbon were evenly distributed and elemental segregation was not found. The microstructure of brazing filler metal was uniform and small.
- (2)
- When the brazing temperature was 565 °C, some voids appeared in the center of the brazing seam. As the brazing temperature reached 575 °C, the void in the joint disappeared completely. The morphology of CuAl2 was sensitive to the brazing temperature and dwell time. The appearance of net-like CuAl2 brazed at 575 °C for 20 min was more beneficial to improve joint mechanical properties.
- (3)
- The leakage rate of the joint was qualified to be 10−10 Pa·m3/s when the brazing temperature was 570 °C or higher, whether the gas tightness test was carried out immediately after brazing or after one week. The maximum shear strength of 76.1 MPa can be obtained when the joint was brazed at 575 °C for 20 min. More dwell time induced the growth of the interfacial layer and reduced the joint shear strength.
- (4)
- The brazing filler metal Al-5.0Si-20.5Cu-2.0Ni had better corrosion resistance than that of 3003 aluminum alloy. The average OCP of filler metal and 3003 aluminum alloy was −627 mV and −746 mV. The Icorr of filler metal was 43.8% lower than that of 3003 aluminum alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lacaze, J.; Tierce, S.; Lafont, M.C.; Thebault, Y.; Pebere, N.; Mankowski, G.; Blanc, C.; Robidou, H.; Vaumousse, D.; Daloz, D. Study of the microstructure resulting from brazed aluminium materials used in heat exchangers. Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing. Mater. Sci. Eng. A 2005, 413, 317–321. [Google Scholar] [CrossRef]
- Benoit, M.J.; Ogunsanya, I.G.; Winkler, S.; Worswick, M.J.; Wells, M.A.; Hansson, C.M. Internal corrosion of warm formed aluminum alloy automotive heat exchangers. Journal of Materials Engineering and Performance. J. Mater. Eng. Perform. 2021, 30, 2876–2889. [Google Scholar] [CrossRef]
- Yuan, Z.P.; Tu, Y.Y.; Yuan, T.; Huang, Y.H.; Zhang, Y.H. Gradient multilayer aluminium sheets used in automotive heat exchangers. J. Mater. Sci. 2021, 56, 5215–5232. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Q.Y. Investigation of pseudo-ternary system AlF3-KF-KCl. Thermochim. Acta 2003, 404, 3–7. [Google Scholar] [CrossRef]
- Cocian, G.; Popa, C. Measurement method for determining the residual flux deposits in a heat exchanger from a controlled atmosphere brazing line. Stud. Univ. Babes Bolyai Chem. 2015, 60, 187–193. [Google Scholar]
- Fei, W.P.; Wang, B.; Lou, Y.B.; Long, W.M.; Deng, J.F.; Zhang, L.; Yin, P.Z.; Wang, S.Q. Study on novel powder metallurgy Al-Si brazing filler metal with flux. Crystals 2022, 12, 544. [Google Scholar] [CrossRef]
- Feng, T.; Lou, S.N.; Wu, L.H.; Li, Y.J. XRD and TEM analysis of the microstructure in the brazing joint of 3003 cladding aluminum alloy. J. Univ. Sci. Technol. Beijing 2005, 12, 531–534. [Google Scholar]
- Lu, W.J.; Yu, W.Y.; Li, P.; Wang, H.L. Brazing mechanism of a new low melting point aluminum base composite filler metal. Rare Metal Mater. Eng. 2011, 40, 413–416. [Google Scholar]
- Peng, C.Y.; Zhu, D.D.; Li, K.F.; Du, X.; Zhao, F.; Wan, M.P.; Tan, Y.B. Research on a low melting point Al-Si-Cu (Ni) filler metal for 6063 aluminum alloy brazing. Appl. Sci. 2021, 11, 4296. [Google Scholar] [CrossRef]
- Cui, C.; Schulz, A.; Achelis, L.; Uhlenwinkel, V.; Leopold, H.; Piwek, V.; Tang, Z.; Seefeld, T. Development of low-melting-point filler materials for laser beam brazing of aluminum alloys. Mater. Werkst. 2014, 45, 717–726. [Google Scholar] [CrossRef]
- Lee, S.J.; Hyun, J.D.; Jung, J.-P. Aluminum brazing and its principle. J. Microelectron. Packag. Soc. 2017, 24, 1–7. [Google Scholar]
- Niu, Z.W.; Huang, J.H.; Chen, S.H.; Zhao, X.K. Effects of germanium additions on microstructures and properties of Al-Si filler metals for brazing aluminum. Trans. Nonferrous Metals Soc. China 2016, 26, 775–782. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.S.; Jung, D.S.; Han, J.W.; You, B.D. Fabrication and sagging behavior of three-layer Al-Si/Al-Mn-Zn/Al-Si clad sheets for automotive heat exchanger. In Eco-Materials Processing & Design; Kim, H.S., Park, S.Y., Lee, S.W., Eds.; Trans Tech Publications Ltd.: Bäch, Switzerland, 2003; Volume 439, pp. 221–226. [Google Scholar]
- Luo, W.; Wang, L.T.; Wang, Q.M.; Gong, H.L.; Yan, M. A new filler metal with low contents of Cu for high strength aluminum alloy brazed joints. Mater. Des. 2014, 63, 263–269. [Google Scholar] [CrossRef]
- Pei, C.; Wu, X.; Zhang, G.Q.; Cheng, Y.Y.; Ren, X.Y.; Wang, W.; Xiong, H.P. Microstructures and mechanical properties of brazed 6063 aluminum alloy joint with Al-Cu-Si-Ni filler metal. Weld. World 2020, 64, 1933–1938. [Google Scholar] [CrossRef]
- Voncina, M.; Medved, J.; Boncina, T.; Zupanic, F. Effect of Ce on morphology of alpha(Al)-Al2Cu eutectic in Al-Si-Cu alloy. Trans. Nonferrous Metals Soc. China 2014, 24, 36–41. [Google Scholar] [CrossRef]
- Lanzutti, A.; Andreatta, F.; Magnan, M.; Fedrizzi, L. Microstructural and in-depth electrochemical characterization of Zn diffusion layers on aluminum 3xxx alloy. Surf. Interface Anal. 2019, 51, 1165–1172. [Google Scholar] [CrossRef]
- Yang, J.L.; Xue, S.B.; Dai, W.; Xue, P. Saturation phenomenon of Ce and Ti in the modification of Al-Zn-Si filler metal. Int. J. Miner. Metall. Mater. 2015, 22, 184–189. [Google Scholar] [CrossRef]
- Lin, H.; Hwang, J.R.; Fung, C.P. Fatigue properties of 6061-T6 aluminum alloy butt joints processed by vacuum brazing and tungsten inert gas welding. Adv. Mech. Eng. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Yang, T.Y.; Zhang, D.K.; Wang, K.H.; Huang, J. Aluminium-silicon-magnesium filler metal for aluminium vacuum brazing wettability and characteristics of brazing microstructure. Mater. Trans. 2016, 57, 983–987. [Google Scholar] [CrossRef]
- Rullik, L.; Bertram, F.; Niu, Y.R.; Evertsson, J.; Stenqvist, T.; Zakharov, A.A.; Mikkelsen, A.; Lundgren, E. Surface development of an aluminum brazing sheet during heating studied by XPEEM and XPS. Mater. Res. Express 2016, 3, 106506. [Google Scholar] [CrossRef]
- Niu, Z.W.; Huang, J.H.; Liu, K.K.; Xu, F.Z.; Chen, S.H.; Zhao, X.K. Brazing of 6061 aluminum alloy with the novel Al-Si-Ge-Zn filler metal. Mater. Lett. 2016, 179, 47–51. [Google Scholar] [CrossRef]
- Wang, Z.J.; Huang, Y.H.; Liu, C.T.; Li, J.J.; Wang, J.C. Atomic packing and size effect on the Hume-Rothery rule. Intermetallics 2019, 109, 139–144. [Google Scholar] [CrossRef]
- He, Q.F.; Ye, Y.F.; Yang, Y. Formation of Random Solid Solution in Multicomponent Alloys: From Hume-Rothery Rules to Entropic Stabilization. J. Ph. Equilib. Diffus. 2017, 38, 416–425. [Google Scholar] [CrossRef]
- Mizutani, U.; Inukai, M.; Sato, H.; Zijlstra, E.S. Hume-Rothery stabilization mechanism and e/a determination in MI-type Al-Mn, Al-Re, Al-Re-Si, Al-Cu-Fe-Si and Al-Cu-Ru-Si 1/1-1/1-1/1 approximants-a proposal for a new Hume-Rothery electron concentration rule. Philos. Mag. 2012, 92, 1691–1715. [Google Scholar] [CrossRef]
- Calcatelli, A.; Bergoglio, M.; Mari, D. Leak detection, calibrations and reference flows: Practical example. Vacuum 2007, 81, 1538–1544. [Google Scholar] [CrossRef]
- Magielko, H. Measurement of gas flow rate from helium standard leaks. Measurement 2012, 45, 2445–2448. [Google Scholar] [CrossRef]
- Cheng, D.F. Study on Laser Welding of SiC Particle Reinforced Aluminum Matrix Composite Based on Nano Effect. Ph.D. Thesis, Henan Polytechnic University, Jiaozuo, China, 2017. [Google Scholar]
- Zhang, J.; Yu, H.S.; Zhao, Q.; Wang, H.T.; Min, G.H. Study on preparing Al2O3 particles reinforced ZL109 composite by in situ reaction of Fe2O3/Al system. Int. J. Modern Phys. B 2009, 23, 1413–1418. [Google Scholar] [CrossRef]



| Element | Si | Fe | Cu | Mn | Al |
|---|---|---|---|---|---|
| wt.% | 0.12 | 0.70 | 0.12 | 1.20 | Balance |
| Test Points | Al | Si | Cu | Ni |
|---|---|---|---|---|
| A | 73.7 | 4.5 | 19.9 | 1.9 |
| B | 71.6 | 4.9 | 21.4 | 2.1 |
| C | 72.1 | 5.1 | 20.9 | 1.9 |
| D | 74.3 | 4.8 | 18.8 | 2.1 |
| Test Points | Al | Si | Cu | Ni | C |
|---|---|---|---|---|---|
| A | 57.19 | - | 20.81 | 12.70 | 9.30 |
| B | 63.92 | - | 16.07 | 9.10 | 10.91 |
| C | 55.79 | - | 24.02 | 8.80 | 11.39 |
| D | 59.01 | 0.96 | 25.37 | 3.23 | 11.43 |
| E | 62.37 | 5.74 | 13.10 | 8.77 | 10.02 |
| CAB Temperature (°C) | Dwell Time (min) | Leak Rate after Brazing (Pa·m3/s) | Leak Rate after One Week (Pa·m3/s) |
|---|---|---|---|
| 565 | 15 | 10−8 | 10−8 |
| 570 | 15 | 10−10 | 10−10 |
| 575 | 15 | 10−10 | 10−10 |
| 575 | 20 | 10−10 | 10−10 |
| 575 | 25 | 10−10 | 10−10 |
| Specimen No. | OCP (mV) | Average OCP (mV) | ||
|---|---|---|---|---|
| 3000 s | 3300 s | 3600 s | ||
| AlSiCuNi-1 | −618 | −619 | −619 | −627 |
| AlSiCuNi-2 | −625 | −624 | −620 | |
| AlSiCuNi-3 | −638 | −642 | −640 | |
| 3003-1 | −745 | −747 | −747 | −746 |
| 3003-2 | −747 | −748 | −747 | |
| 3003-3 | −745 | −747 | −747 | |
| Specimen | Icorr (A/cm2) |
|---|---|
| AlSiCuNi | 1.3276 × 10−4 |
| 3003 | 2.3605 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Qin, Z.; Lu, Q. Controlled Atmosphere Brazing of 3003 Aluminum Alloy Using Low-Melting-Point Filler Metal Fabricated by Melt-Spinning Technology. Materials 2022, 15, 6080. https://doi.org/10.3390/ma15176080
Gao Z, Qin Z, Lu Q. Controlled Atmosphere Brazing of 3003 Aluminum Alloy Using Low-Melting-Point Filler Metal Fabricated by Melt-Spinning Technology. Materials. 2022; 15(17):6080. https://doi.org/10.3390/ma15176080
Chicago/Turabian StyleGao, Zeng, Zhen Qin, and Qingsong Lu. 2022. "Controlled Atmosphere Brazing of 3003 Aluminum Alloy Using Low-Melting-Point Filler Metal Fabricated by Melt-Spinning Technology" Materials 15, no. 17: 6080. https://doi.org/10.3390/ma15176080
APA StyleGao, Z., Qin, Z., & Lu, Q. (2022). Controlled Atmosphere Brazing of 3003 Aluminum Alloy Using Low-Melting-Point Filler Metal Fabricated by Melt-Spinning Technology. Materials, 15(17), 6080. https://doi.org/10.3390/ma15176080






