Investigation of Long-Term Performance and Deicing Longevity Prediction of Self-Ice-Melting Asphalt Pavement
Abstract
:1. Introduction
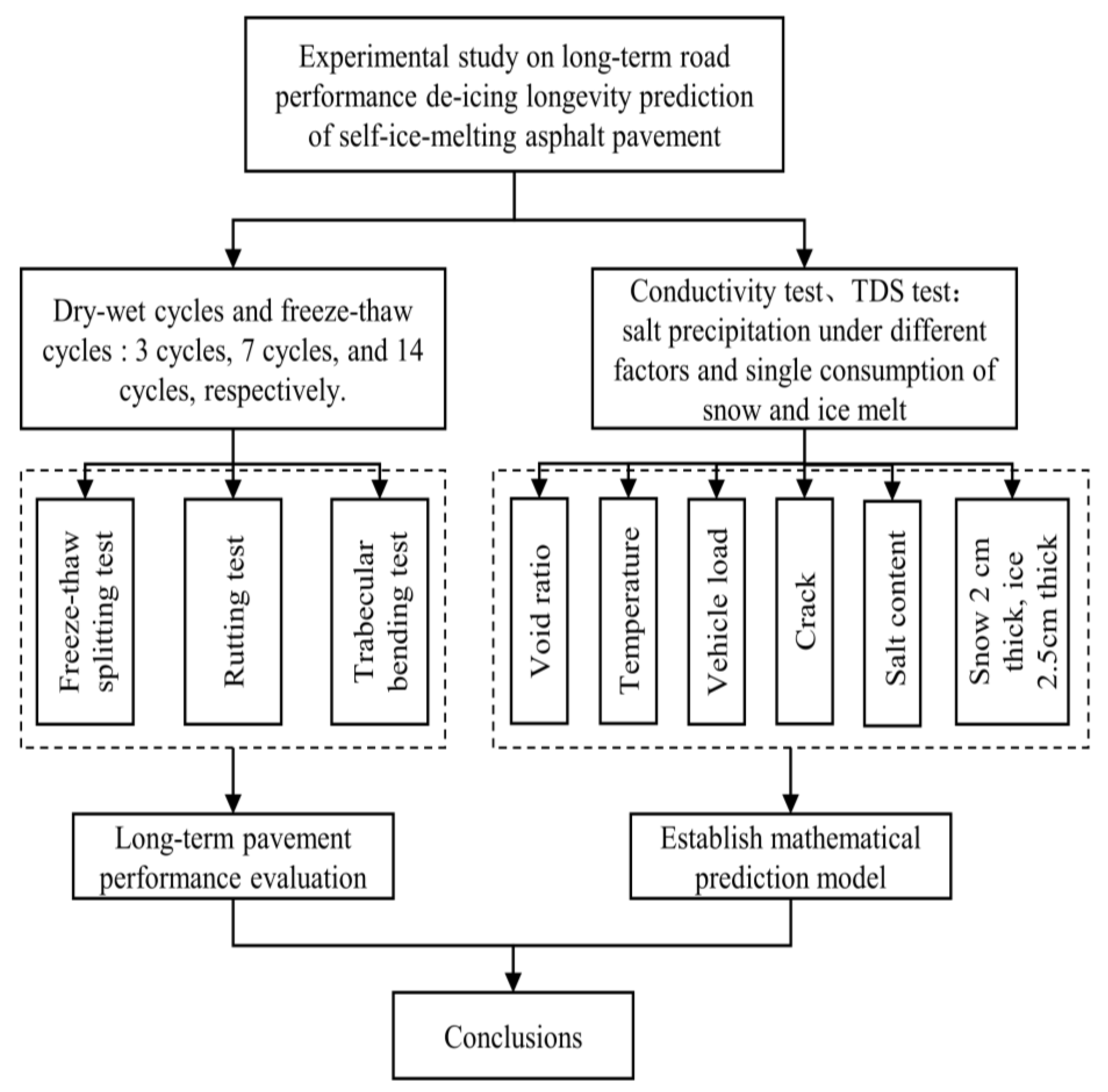
2. Materials and Methods
2.1. Materials
2.1.1. Asphalt and Aggregate
2.1.2. Salt-Storage Additive
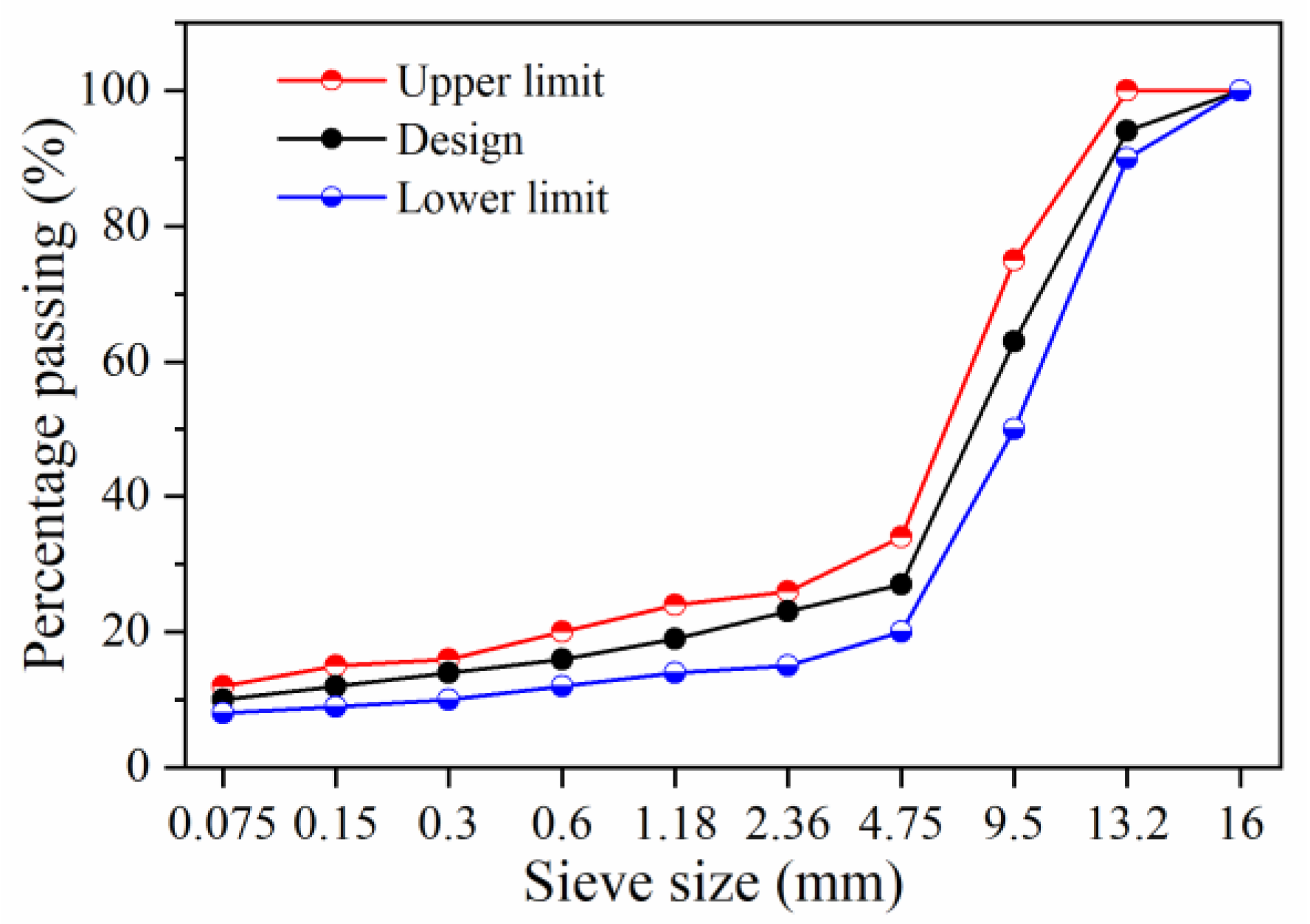
2.1.3. Asphalt Mixture
2.2. Methods
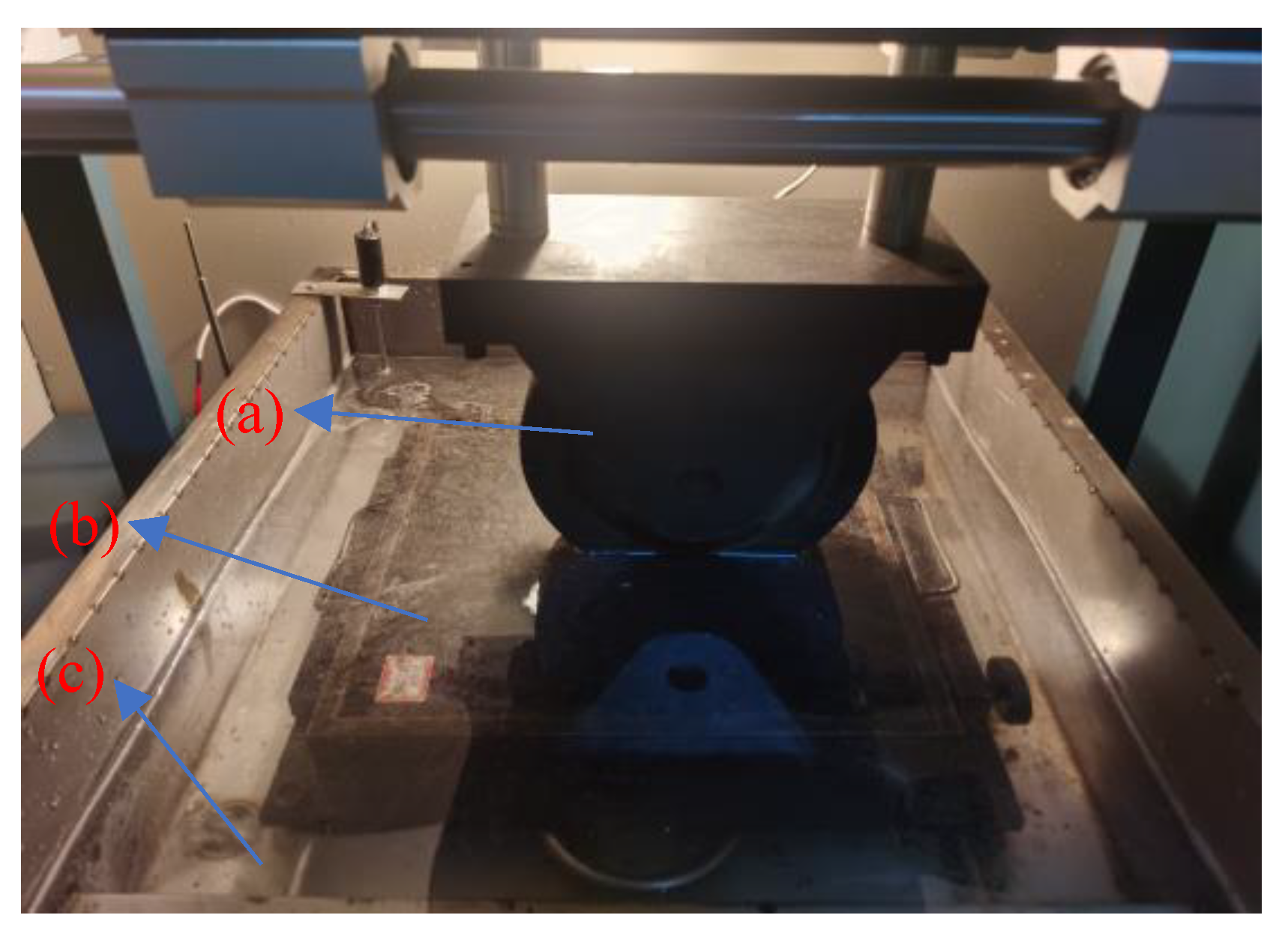
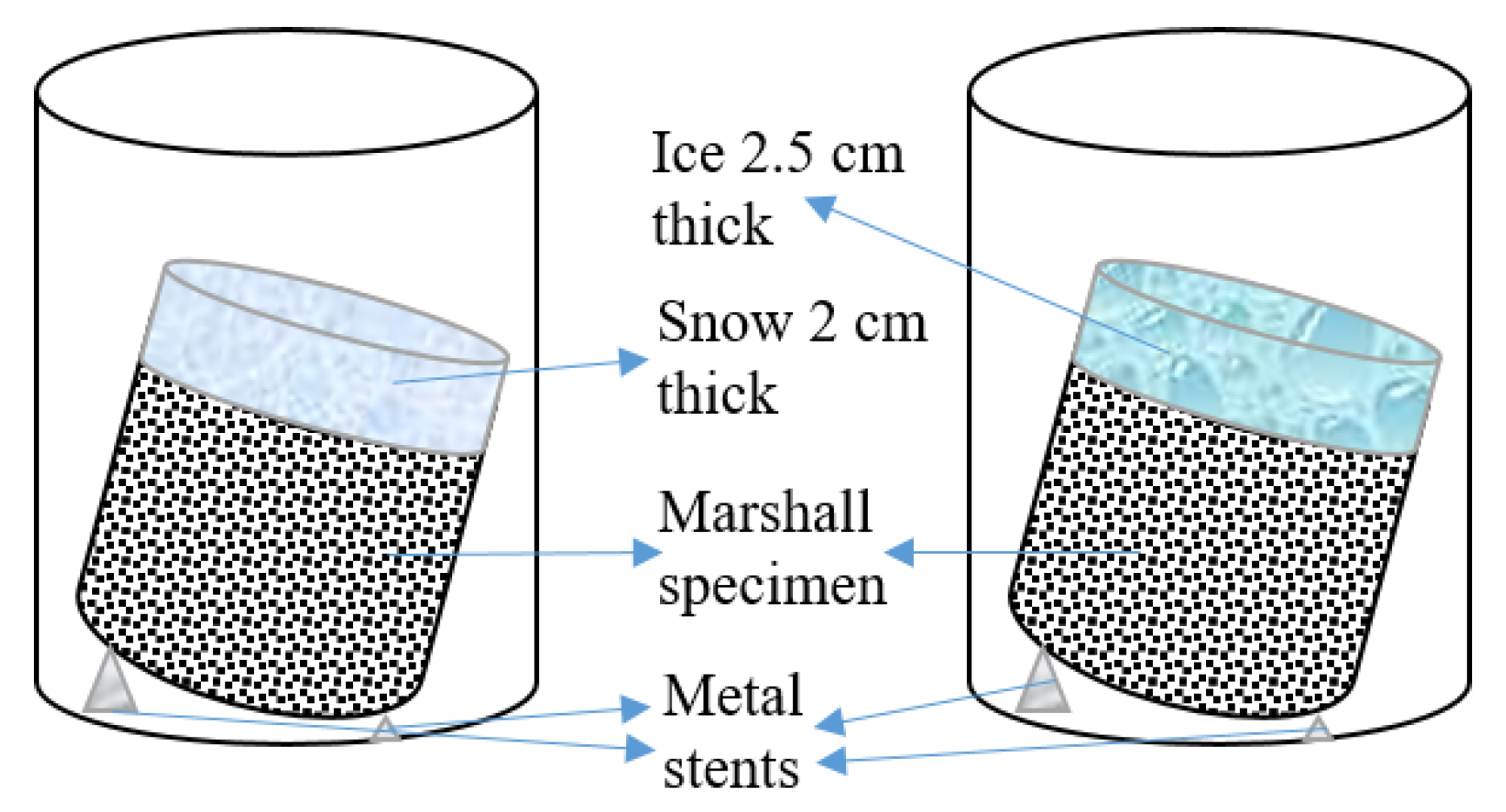
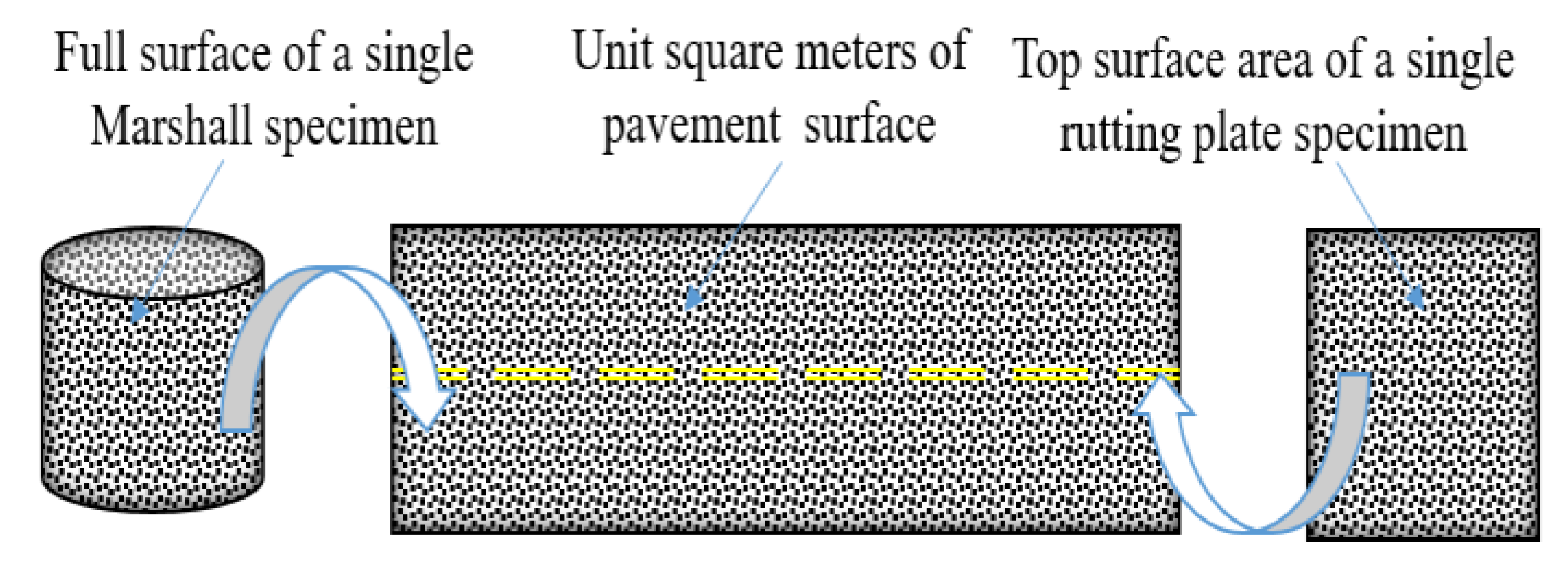
2.2.1. Long-Term Pavement Performance Test
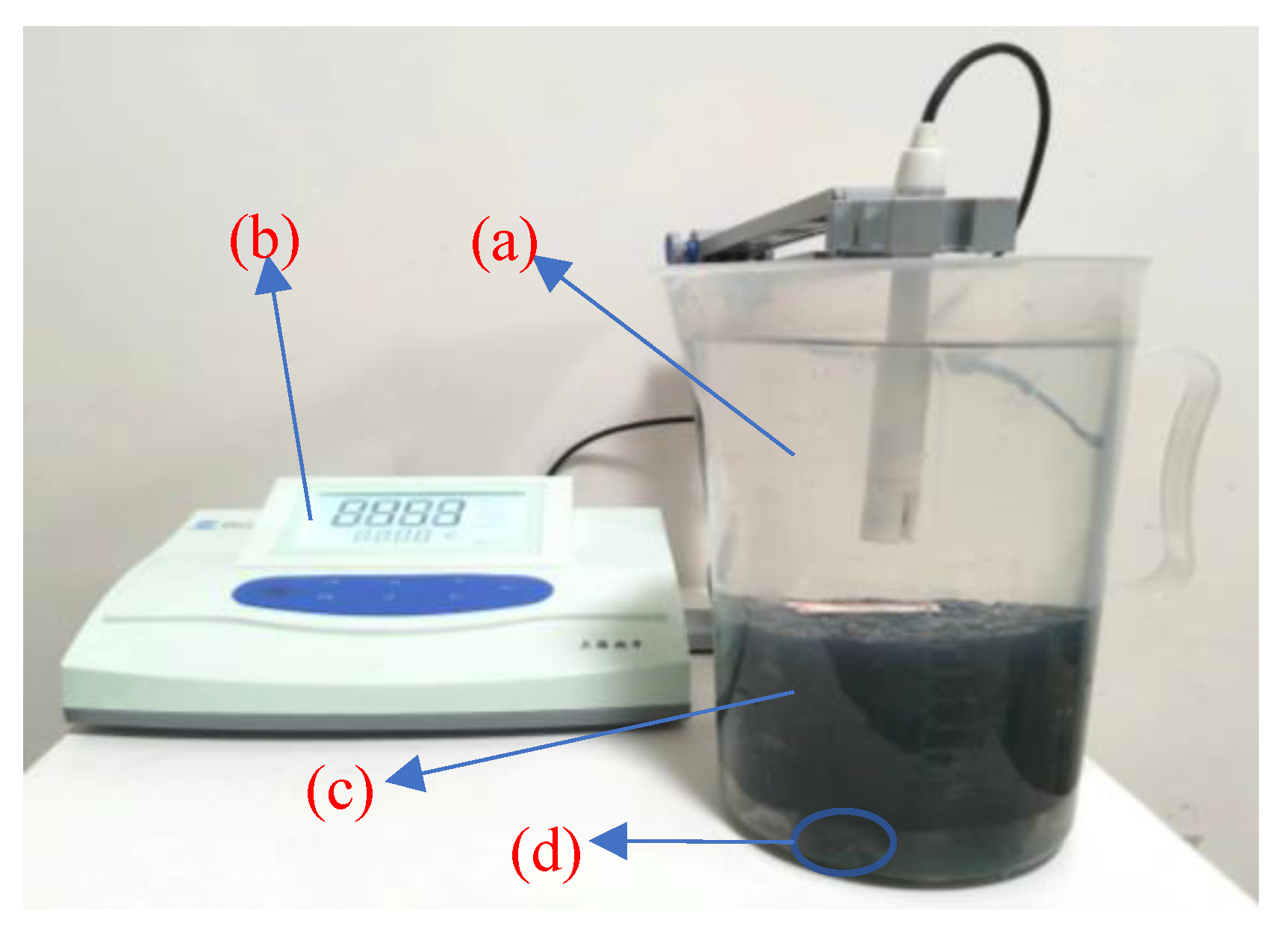
2.2.2. Salt Precipitation Test
2.2.3. Single-Consumption of Snow and Ice Melt Test
2.2.4. Data Processing Methods
3. Results and Discussion
3.1. Evaluation of Long-Term Pavement Performance
3.2. Establishment of a Long-Term Predictive Model
3.2.1. Basic Assumptions
3.2.2. Original Model
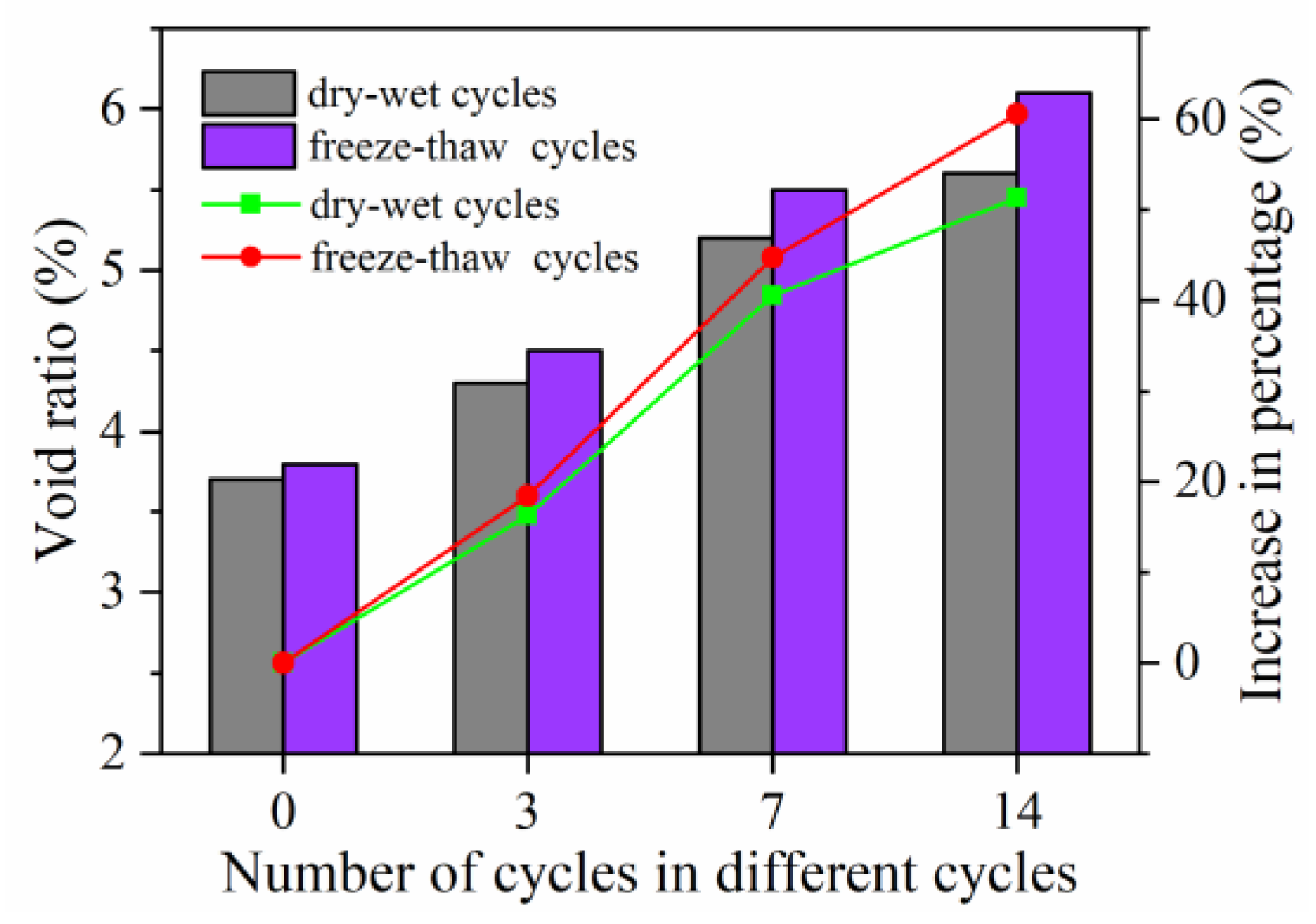
3.2.3. Void Ratio Influence Coefficient
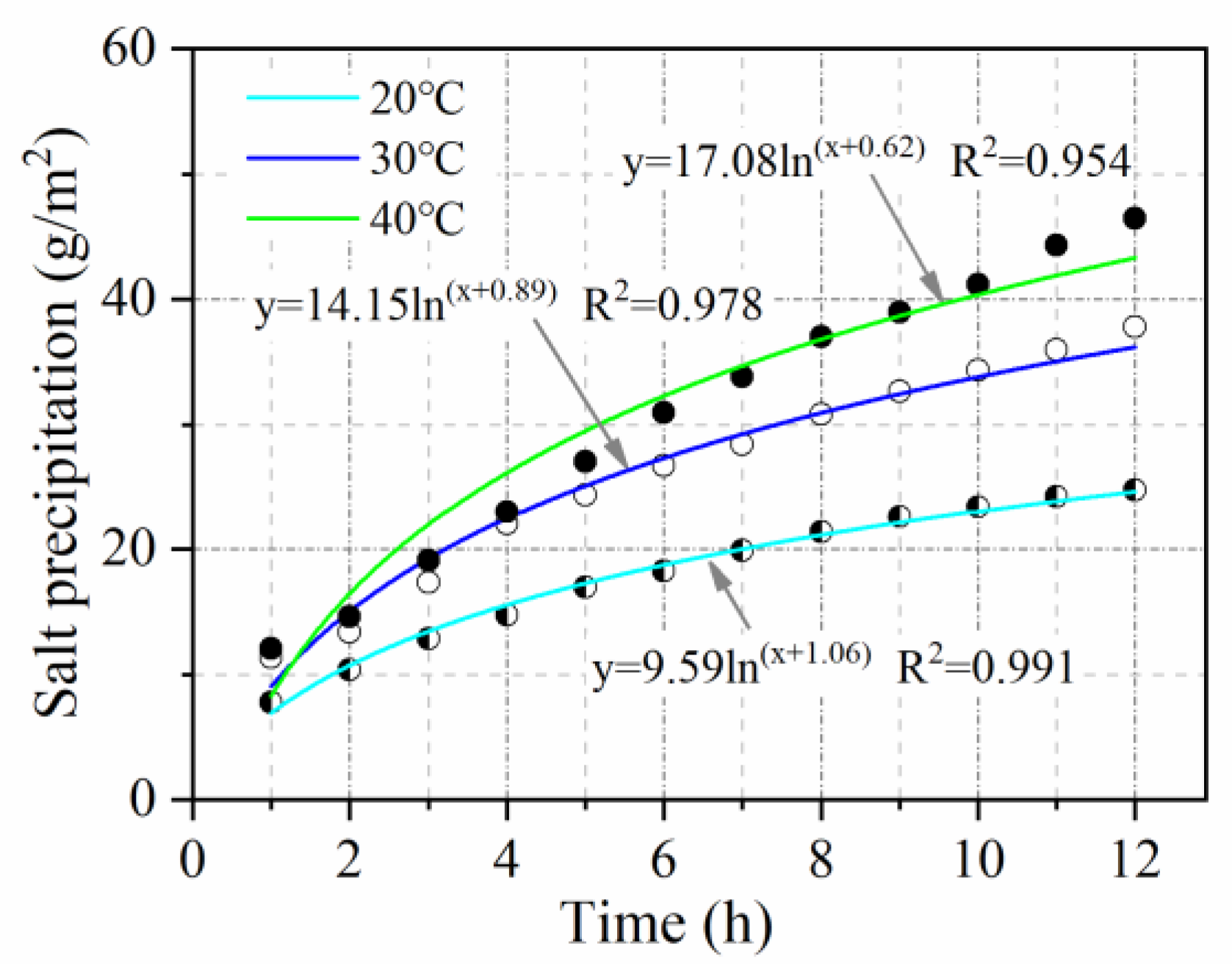
3.2.4. Temperature Influence Coefficient
3.2.5. Vehicle Load Influence Coefficient
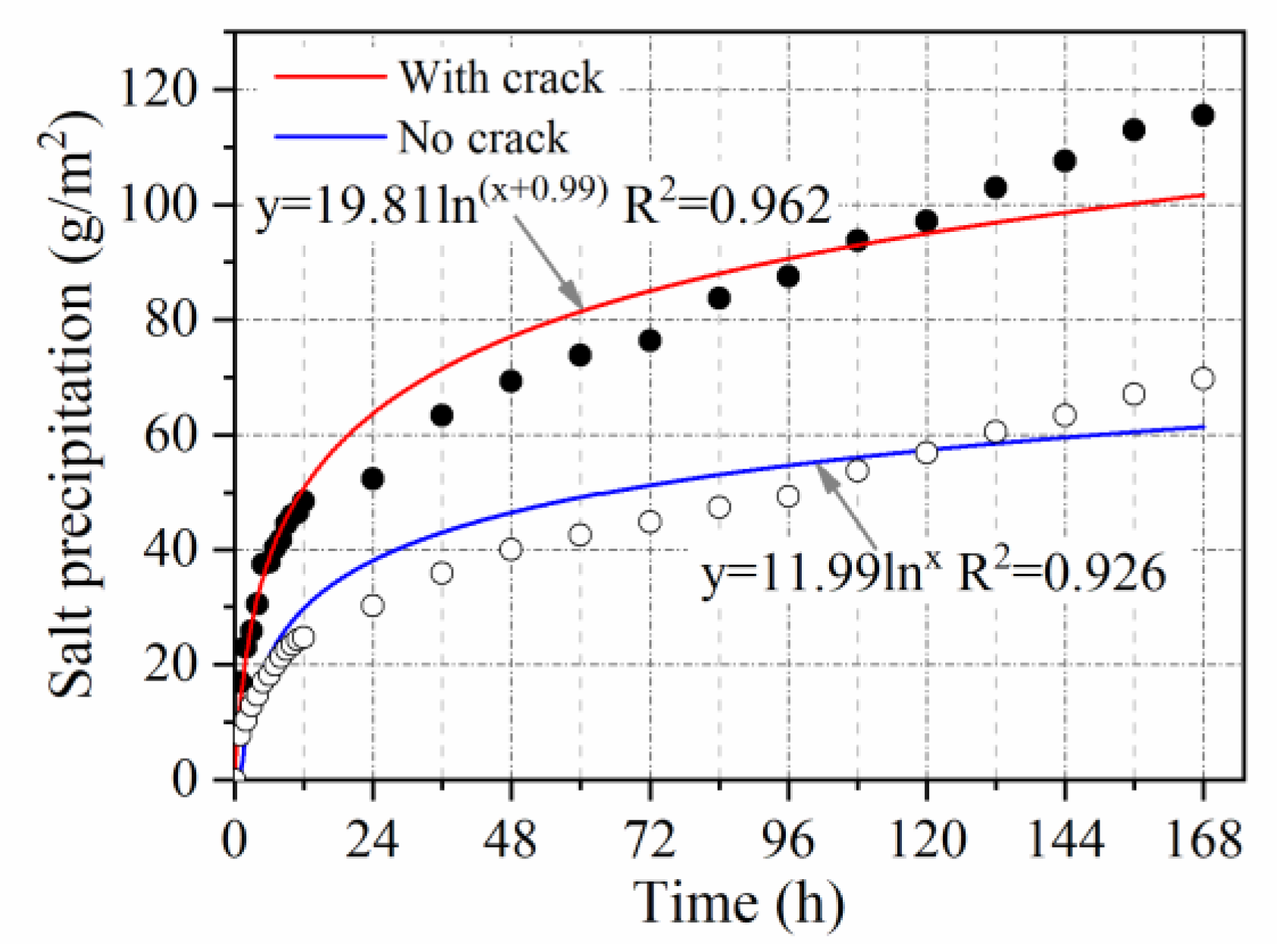
3.2.6. Crack Influence Coefficient
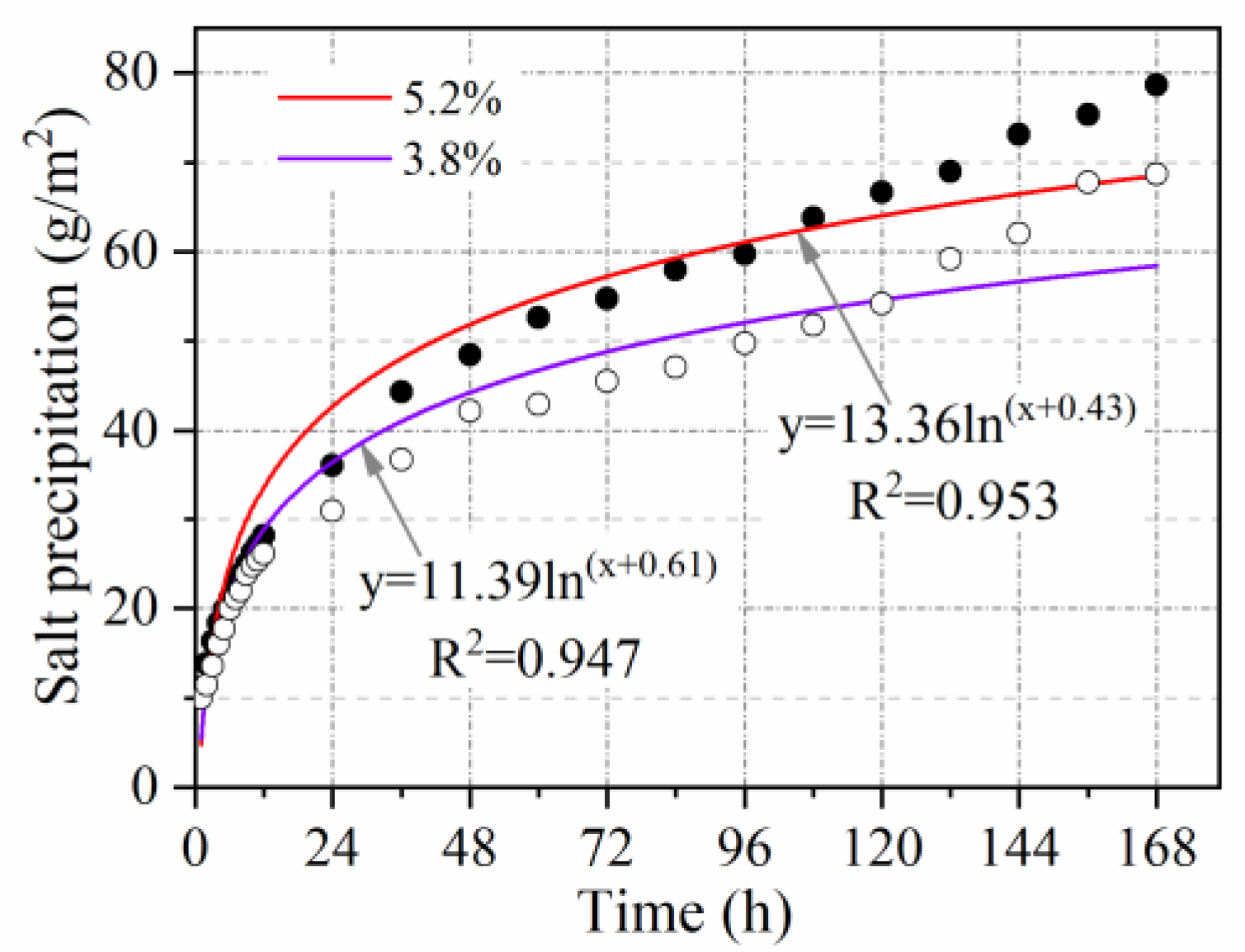
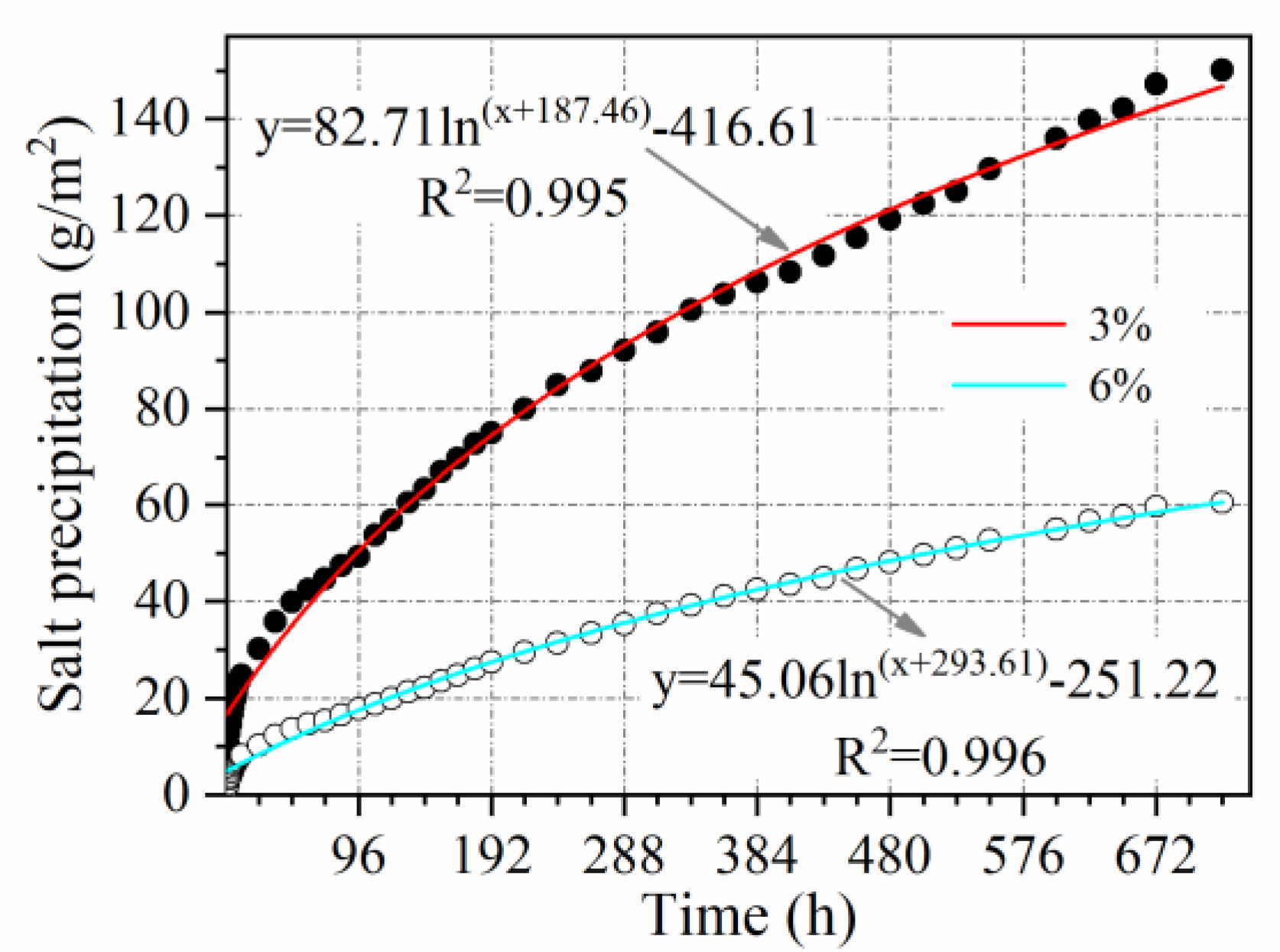
3.2.7. Salt Content Influence Coefficient
3.2.8. Original Model Modification
3.3. Snow and Ice Melt Consumption
3.3.1. Single Consumption of Snow and Ice Melt
3.3.2. Annual Consumption of Snow and Ice Melt
3.4. Application of Prediction Model
4. Conclusions
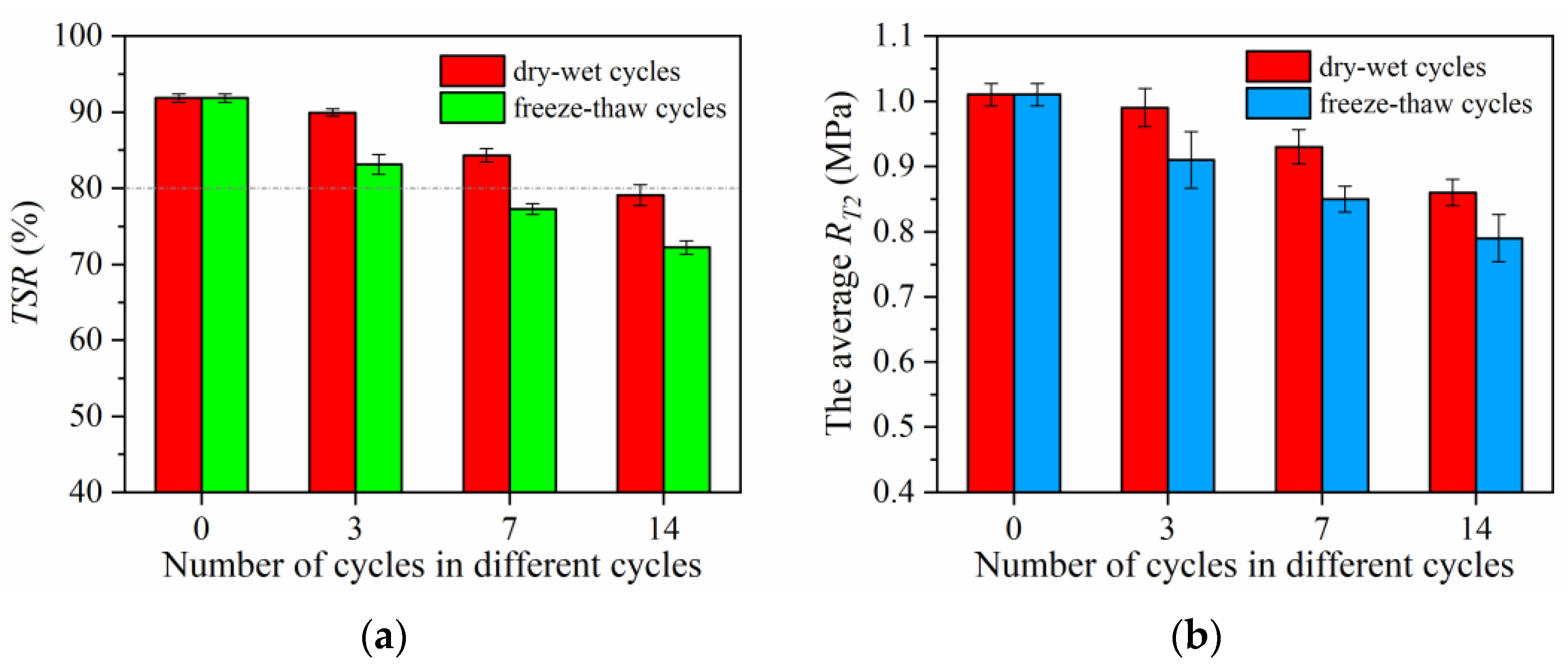
- The long-term water stability, long-term high-temperature stability, and long-term low-temperature crack resistance of self-ice-melting asphalt pavements are reduced by 14.19%, 18.79%, and 11.96%, respectively, under dry–wet cycles and by 21.35%, 29.18%, and 14.57%, respectively, under freeze–thaw cycles. With respect to long-term water stability, with 14 dry–wet cycles or 7 freeze-thaw cycles, the TSR is less than 80%.
- The relationship between salt precipitation under natural immersion and time follows a logarithmic function (R2 > 0.9). Salt precipitation is accelerated by void ratio, temperature, vehicle load, and cracking, with accelerating factors of V = 1.1, T = 1.65, L = 1.5, and C = 1.65, respectively. Salt precipitation slows down with decreasing salt content and is only 0.54 times the original salt precipitation when the salt content is reduced to half.
- Based on the proportion of each factor in the life cycle of pavement, the influence coefficients of void ratio, temperature, vehicle load, and cracking are incorporated into the mathematical regression model of salt precipitation over time. Furthermore, the prediction model of deicing longevity of self-ice-melting asphalt pavement is established considering the single consumption of snow and ice melt.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.J.; Wu, Y.K.; Zhu, P.H.; Ning, T. Snow Melting Performance of Graphene Composite Conductive Concrete in Severe Cold Environment. Materials 2021, 14, 6715. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ban, H.; Park, W.J. Deicing Concrete Pavements and Roads with Carbon Nanotubes (CNTs) as Heating Elements. Materials 2020, 13, 2504. [Google Scholar] [CrossRef] [PubMed]
- Shishegaran, A.; Daneshpajoh, F.; Taghavizade, H.; Mirvalad, S. Developing conductive concrete containing wire rope and steel powder wastes for route deicing. Constr. Build. Mater. 2020, 232, 117184. [Google Scholar] [CrossRef]
- Asfour, S.; Bernardin, F.; Toussaint, E.; Piau, J.M. Hydrothermal modeling of porous pavement for its surface de-freezing. Appl. Therm. Eng. 2016, 107, 493–500. [Google Scholar] [CrossRef]
- Liu, H.; Maghoul, P.; Holländer, H.M. Sensitivity analysis and optimum design of a hydronic snow melting system during snowfall. Phys. Chem. Earth. 2019, 113, 31–42. [Google Scholar] [CrossRef]
- Daniels, J.W.; Heymsfield, E.; Kuss, M. Hydronic heated pavement system performance using a solar water heating system with heat pipe evacuated tube solar collectors. Sol. Energy 2019, 179, 343–351. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.H.; Seo, U.J. Eco-Friendly Snow Melting Systems Developed for Modern Expressways. J. Test. Eval. 2019, 47, 3432–3447. [Google Scholar] [CrossRef]
- Ghalandari, T.; Hasheminejad, N.; Van den Bergh, W.; Vuye, C. A critical review on large-scale research prototypes and actual projects of hydronic asphalt pavement systems. Renew. Energy 2021, 177, 1421–1437. [Google Scholar] [CrossRef]
- Habibzadeh-Bigdarvish, O.; Yu, X.; Li, T.; Lei, G.; Banerjee, A.; Puppala, A.J. A novel full-scale external geothermal heating system for bridge deck de-icing. Appl. Therm. Eng. 2021, 185, 116365. [Google Scholar] [CrossRef]
- Ho, I.H.; Dickson, M. Numerical modeling of heat production using geothermal energy for a snow-melting system. Geomech. Energy Environ. 2017, 10, 42–51. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wu, S.P.; Liu, Q.T.; Hu, J.F.; Yuan, Y.; Ye, Q.S. Snow and ice melting properties of self-healing asphalt mixtures with induction heating and microwave heating. Appl. Therm. Eng. 2018, 129, 871–883. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yang, J.; Sun, X.Y.; Wang, C.; Yang, R.; Zhu, J. Preparation and characterization of anti-freezing asphalt pavement. Constr. Build. Mater. 2020, 236, 117579. [Google Scholar] [CrossRef]
- Liu, Z.; Xing, M.; Chen, S.; He, R.; Cong, P. Influence of the chloride-based anti-freeze filler on the properties of asphalt mixtures. Constr. Build. Mater. 2014, 51, 133–140. [Google Scholar] [CrossRef]
- Ma, T.; Geng, L.; Ding, X.; Zhang, D.; Huang, X. Experimental study of deicing asphalt mixture with anti-icing additives. Constr. Build. Mater. 2016, 127, 653–662. [Google Scholar] [CrossRef]
- Han, S.; Yin, Y.; Peng, B.; Dong, S.; Wu, S. Experimental Study of Asphalt Mixture with Acetate Anti-Icing Filler. Arab. J. Sci. Eng. 2022, 47, 4225–4237. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Liu, G.Q.; Yang, T.; Zhao, Y. Long-Term Performance and Deicing Effect of Sustained-Release Snow Melting Asphalt Mixture. Adv. Civ. Eng. 2019, 2019, 1940692. [Google Scholar] [CrossRef]
- Zhong, K.; Sun, M.Z.; Chang, R.H. Performance evaluation of high-elastic/salt-storage asphalt mixture modified with Mafilon and rubber particles. Constr. Build. Mater. 2018, 193, 153–161. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, M.; Chen, W.; Bi, S.; Wang, C.; Li, Y. Salt-dissolved regularity of the self-ice-melting pavement under rainfall. Constr. Build. Mater. 2019, 204, 371–383. [Google Scholar] [CrossRef]
- Luo, S.; Yang, X. Performance evaluation of high-elastic asphalt mixture containing deicing agent Mafilon. Constr. Build. Mater. 2015, 94, 494–501. [Google Scholar] [CrossRef]
- Ma, T.; Ding, X.H.; Wang, H.; Zhang, W. Experimental Study of High-Performance Deicing Asphalt Mixture for Mechanical Performance and Anti-Icing Effectiveness. J. Mater. Civ. Eng. 2018, 30, 04018180. [Google Scholar] [CrossRef]
- Zou, M. The Research on the Asphalt Mixture That Utilizing the Combination Physical and Chemical Interactions to Melt Ice and Deice; Chongqing Jiaotong University: Chongqing, China, 2016. [Google Scholar]
- Xu, O.M.; Han, S.; Zhang, C.L.; Liu, Y.; Xiao, F.; Xu, J. Laboratory investigation of andesite and limestone asphalt mixtures containing sodium chloride-based anti-icing filler. Constr. Build. Mater. 2015, 98, 671–677. [Google Scholar] [CrossRef]
- Zheng, M.L.; Zhou, J.L.; Wu, S.J.; Yuan, H.; Meng, J. Evaluation of long-term performance of anti-icing asphalt pavement. Constr. Build. Mater. 2015, 84, 277–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Shi, X.M. Model development and prediction of anti-icing longevity of asphalt pavement with salt-storage additive. J. Infrastruct. Preserv. Resil. 2022, 3, 00047. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, H.; Xiao, J.; Tan, Y. Effects of freeze-thaw cycles on fatigue performance of asphalt mixture and development of fatigue-freeze-thaw (FFT) uniform equation. Constr. Build. Mater. 2020, 242, 118043. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. Ministry of Transport of the People’s Republic of China: Beijing, China, 2011.
- Tan, Y.Q.; Zhang, C.; Xu, H.N.; Tian, D. Snow Melting and Deicing Characteristics and Pavement Performance of Active Deicing and Snow Melting Pavement. China J. Highw. Transp. 2019, 32, 1–17. [Google Scholar]
- Zhang, J.; Apeagyei, A.K.; Airey, G.D.; Grenfell, J.R.A. Influence of aggregate mineralogical composition on water resistance of aggregate–bitumen adhesion. Int. J. Adhes. Adhes. 2015, 62, 45–54. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.F.; Li, Z.H.; Zhao, Y.L.; Zhao, Y. Evaluation of Performance Deterioration Characteristics of Asphalt Mixture in Corrosion Environment Formed by Snow-Melting Agents. J. Mater. Civ. Eng. 2022, 34, 0004125. [Google Scholar] [CrossRef]
- JTG F40-2004; Technical Specifications for Construction of Highway Asphalt Pavements. Ministry of Transport of the People’s Republic of China: Beijing, China, 2004.
- Nian, T.; Li, P.; Mao, Y.; Zhang, G.; Liu, Y. Connections between chemical composition and rheology of aged base asphalt binders during repeated freeze-thaw cycles. Constr. Build. Mater. 2018, 159, 338–350. [Google Scholar] [CrossRef]
- Menapace, I.; Yiming, W.; Masad, E. On the Effects of Environmental Factors on the Chemical Composition of Asphalt Binders. Energ. Fuel. 2018, 33, 2614–2624. [Google Scholar] [CrossRef]
- Nian, T.; Li, P.; Wei, X.; Wang, P.; Li, H.; Guo, R. The effect of freeze-thaw cycles on durability properties of SBS-modified bitumen. Constr. Build. Mater. 2018, 187, 77–88. [Google Scholar] [CrossRef]
- Chen, M.; Geng, J.; Xia, C.; He, L.; Liu, Z. A review of phase structure of SBS modified asphalt: Affecting factors, analytical methods, phase models and improvements. Constr. Build. Mater. 2021, 294, 123610. [Google Scholar] [CrossRef]
- Chen, H.; Chen, M.; Geng, J.; He, L.; Xia, C.; Niu, Y.; Luo, M. Effect of multiple freeze-thaw on rheological properties and chemical composition of asphalt binders. Constr. Build. Mater. 2021, 308, 125086. [Google Scholar] [CrossRef]
- Xu, G.; Chen, X.; Cai, X.; Yu, Y.; Yang, J. Characterization of Three-Dimensional Internal Structure Evolution in Asphalt Mixtures during Freeze–Thaw Cycles. App. Sci. 2021, 11, 4316. [Google Scholar] [CrossRef]
- Xu, H.; Guo, W.; Tan, Y. Internal structure evolution of asphalt mixtures during freeze–thaw cycles. Mater. Des. 2015, 86, 436–446. [Google Scholar] [CrossRef]
- Wu, S.Y.; Yang, J.; Yang, R.C.; Zhu, J.; Liu, S.; Wang, C. Investigation of microscopic air void structure of anti-freezing asphalt pavement with X-ray CT and MIP. Constr. Build. Mater. 2018, 178, 473–483. [Google Scholar] [CrossRef]
- Luo, R.; Liu, Z.; Huang, T.; Tu, C.; Feng, G. Effect of Freezing-thawing Cycles on Water Vapor Diffusion in Asphalt Mixtures. China J. Highw. Transp. 2018, 31, 20–26. [Google Scholar]
- Xu, H.; Shi, H.; Zhang, H.; Li, H.; Leng, Z.; Tan, Y. Evolution of dynamic flow behavior in asphalt mixtures exposed to freeze-thaw cycles. Constr. Build. Mater. 2020, 255, 119320. [Google Scholar] [CrossRef]
- Xu, H.; Guo, W.; Tan, Y. Permeability of asphalt mixtures exposed to freeze–thaw cycles. Cold Reg. Sci. Technol. 2016, 123, 99–106. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y. Influence Factors of Crack Resistance of Asphalt Mixture under the Damage of Deicing Salt and Freezing-Thawing Cycles. J. Build. Mater. 2016, 19, 773–778. [Google Scholar]
- Li, Z.; Tan, Y.; Wu, S.; Yang, F. The effects of the freeze-thaw cycle on the mechanical properties of the asphalt mixture. J. Harbin Eng. Univ. 2014, 35, 378–382. [Google Scholar]
- Hu, Y.; Kang, Y.; Yang, X.; Jia, X.; Min, J.; Wang, S.; Shang, K. An analysis on the temporal-spatial characteristics of highway surface freezing in Beijing from 2008 to 2015. J. Glaciol. Geocryol. 2017, 39, 811–823. [Google Scholar]
- Wei, H.; He, Q.; Jiao, Y.; Chen, J.; Hu, M. Evaluation of anti-icing performance for crumb rubber and diatomite compound modified asphalt mixture. Constr. Build. Mater. 2016, 107, 109–116. [Google Scholar] [CrossRef]






| Technical Indicator | Measured Value | Standard Value | Test Method [26] | |
|---|---|---|---|---|
| Penetration at 25 °C (0.1 mm) | 55.0 | 40–60 | T 0604 | |
| Penetration index (PI) | 0.16 | ≥0 | T 0604 | |
| Softening point TR&B (°C) | 80.6 | ≥60 | T 0606 | |
| Ductility 5 °C (cm) | 33.0 | ≥20 | T 0605 | |
| After TFOT | Quality change (%) | 0.59 | ≤1.0 | T 0609 |
| Penetration ratio 25 (%) | 70 | ≥65 | T 0604 | |
| Ductility at 5 °C (cm) | 26.4 | ≥15 | T 0605 | |
| Technical Indicator | Value | Specification |
|---|---|---|
| Apparent relative density (g/cm3) | 1.05 | ≤1.25 |
| Water content (%) | 0.36 | ≤0.75 |
| Fiber content (%) | 0.34 | ≤0.75 |
| Slender and flat particle content (%) | 6 | ≤10 |
| Technical Indicator | Value | Specification |
|---|---|---|
| Density (g/cm3) | 2.27 | 2.25–2.35 |
| Salt precipitation (%) | ≤0.4 | ≤0.4 |
| PH value | 8.23 | 8.0–8.5 |
| Moisture absorption rate | 0.5 | ≤0.7 |
| Main components | NaCl, CaCO3, Fe2O3, etc. | — |
| Performance Indicator | Decline Rate of Each Performance Index with the Number of Cycles (%) | |||||
|---|---|---|---|---|---|---|
| 3 Cycles | 7 Cycles | 14 Cycles | ||||
| D-WC | F-TC | D-WC | F-TC | D-WC | F-TC | |
| TSR | 2.08 | 9.48 | 8.18 | 15.88 | 14.19 | 21.35 |
| T2 | 1.98 | 9.90 | 7.92 | 15.84 | 14.85 | 21.78 |
| DS | 4.78 | 8.56 | 10.92 | 20.19 | 18.79 | 29.18 |
| εB | 1.66 | 2.67 | 5.84 | 9.83 | 11.96 | 14.57 |
| Type of Test | TDS (mg/L) | Average TDS (mg/L) | Average Volume (mL) | ||
|---|---|---|---|---|---|
| Snow melting | 758 | 684 | 660 | 700.67 | 31.4 |
| Ice melting | 968 | 951 | 973 | 964 | 198.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Guo, R. Investigation of Long-Term Performance and Deicing Longevity Prediction of Self-Ice-Melting Asphalt Pavement. Materials 2022, 15, 6026. https://doi.org/10.3390/ma15176026
Zhang H, Guo R. Investigation of Long-Term Performance and Deicing Longevity Prediction of Self-Ice-Melting Asphalt Pavement. Materials. 2022; 15(17):6026. https://doi.org/10.3390/ma15176026
Chicago/Turabian StyleZhang, Haihu, and Runhua Guo. 2022. "Investigation of Long-Term Performance and Deicing Longevity Prediction of Self-Ice-Melting Asphalt Pavement" Materials 15, no. 17: 6026. https://doi.org/10.3390/ma15176026
APA StyleZhang, H., & Guo, R. (2022). Investigation of Long-Term Performance and Deicing Longevity Prediction of Self-Ice-Melting Asphalt Pavement. Materials, 15(17), 6026. https://doi.org/10.3390/ma15176026






