Novel Efficient Reduction Route for Magnesium Production Using Silicothermic Process
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Materials
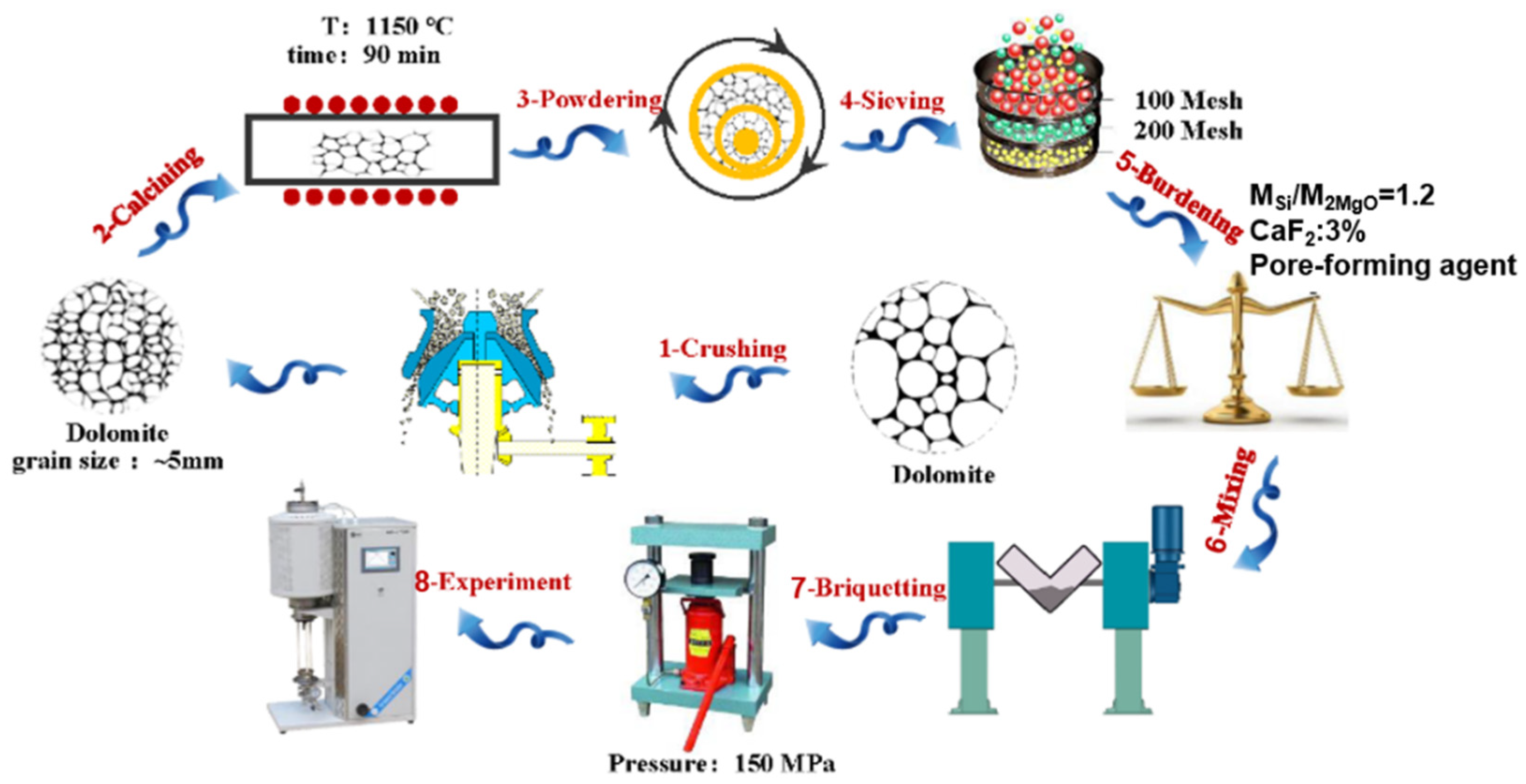
2.2. Preparation of Porous Pellet Precursors
- (1)
- A certain amount of dolomite with a uniform particle size was calcined in a muffle furnace at 1150 °C for 90 min; then, calcined dolomite with 30% activity and a 47.4% burning loss rate was obtained.
- (2)
- Ground calcined dolomite and 75% ferrosilicon were ground through a 100-mesh screen, and the ingredients were prepared according to the molar ratio of MSi:M2MgO = 1.2. Fluorite addition was carried out according to a mass ratio of 3%, then different ratios of ammonium bicarbonate were added and then fully mixed.
- (3)
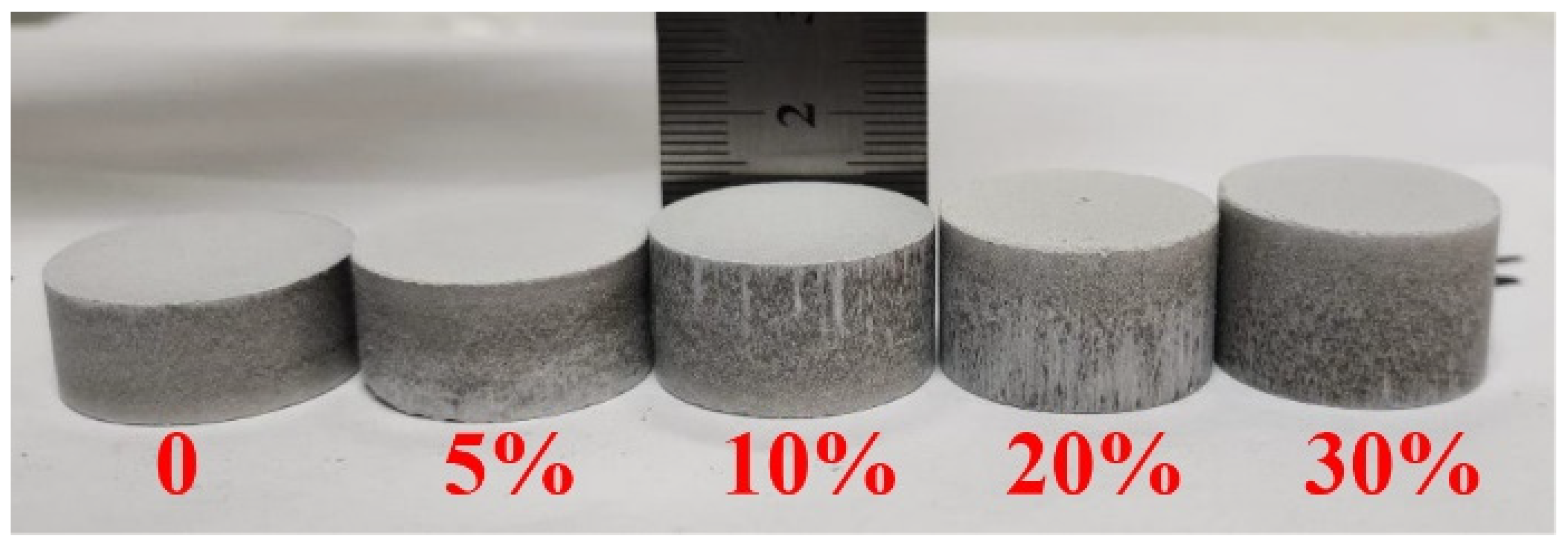
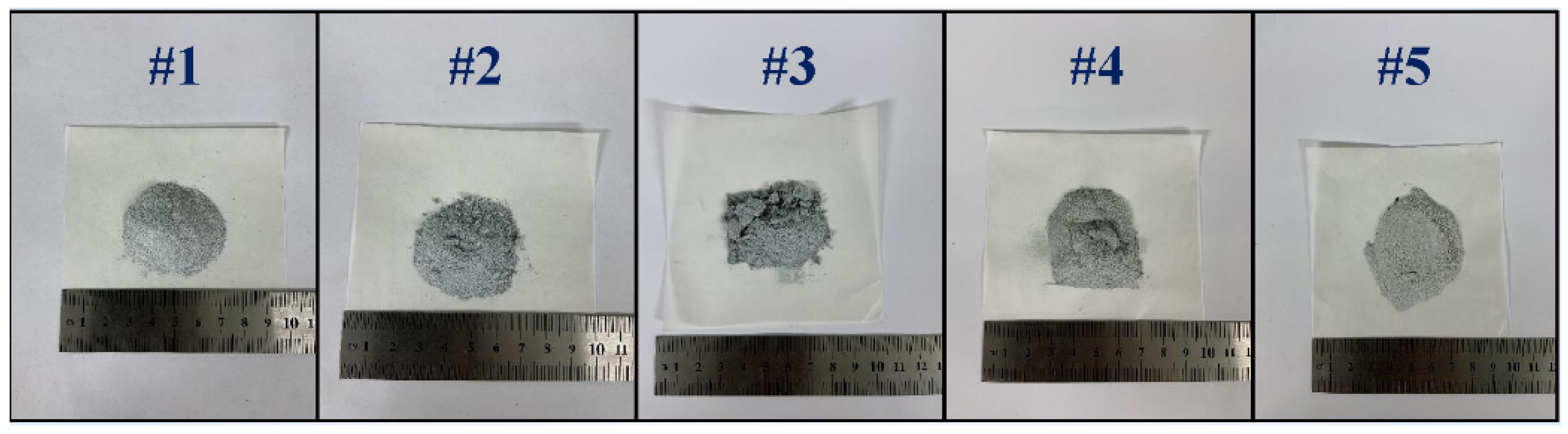
- Different ratios of the pore-forming agent NH4HCO3 were added to the mixed raw materials, and the mixture was fully mixed. Then, pellets with a net weight of approximately 10 g were prepared under a pressure of 150 MPa. The prepared pellets are shown in Figure 2.
- (4)
- The pellet sample was placed in a vacuum tube furnace, and the weight change in real-time was recorded. The pressure was lowered to 10 Pa by applying vacuum, and the pellet sample was heated from 100 °C to 1400 °C at a heating rate of 2 °C/min. Then, the weight loss curve of the pellet sample under the reaction conditions was obtained.
3. Results and Discussion
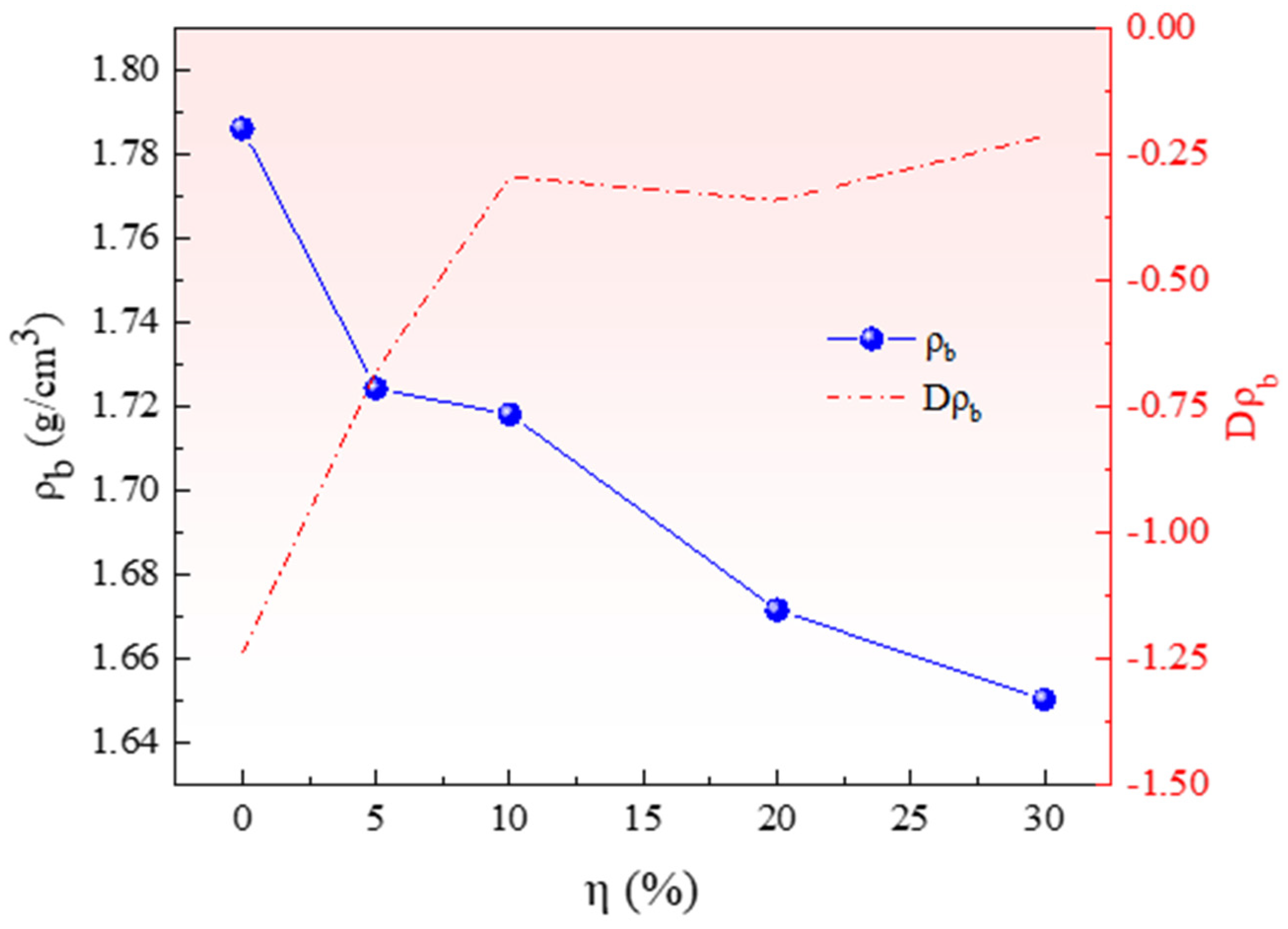
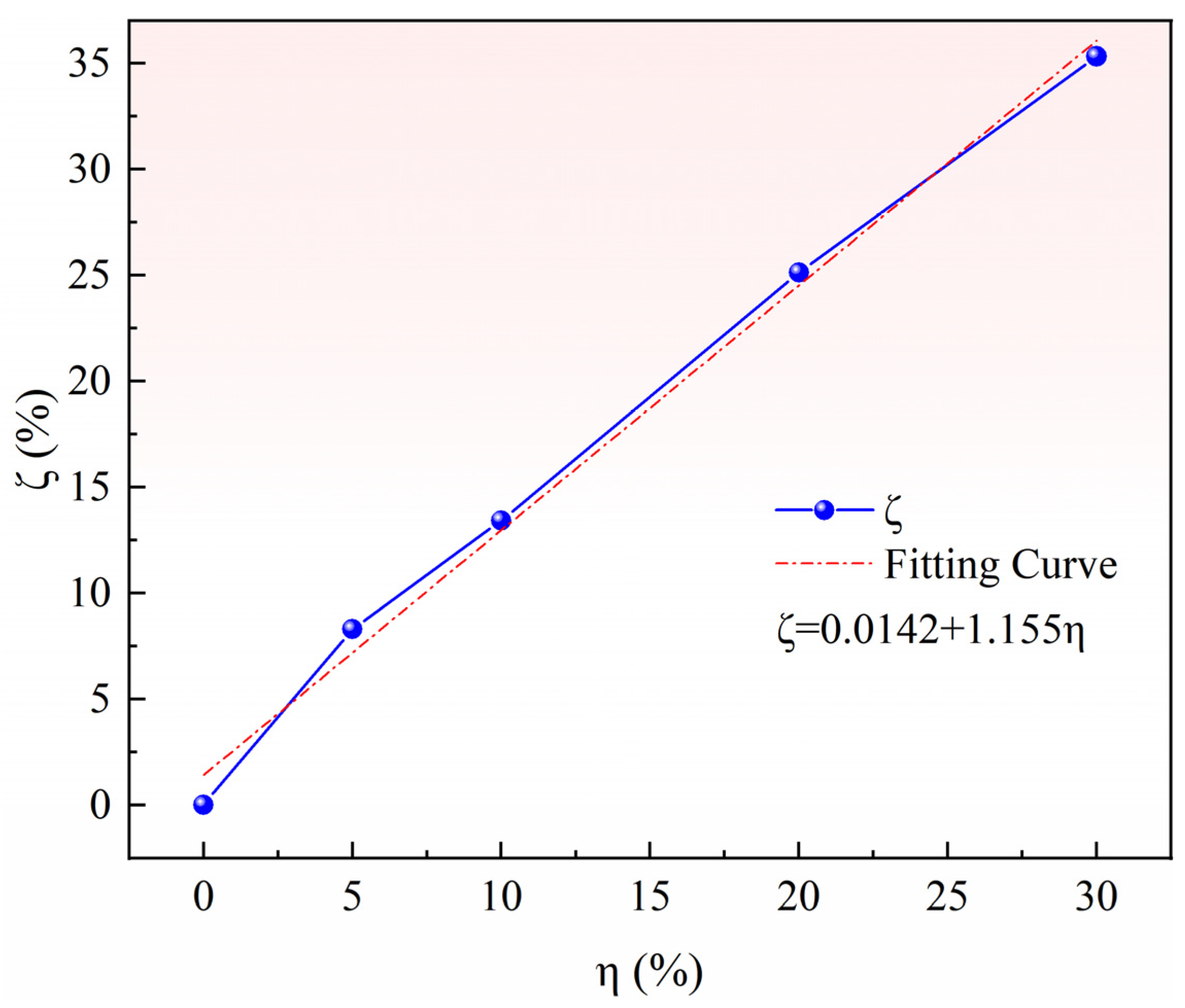
3.1. Effect of Pore-Forming Agent on Pellet Density and Porosity
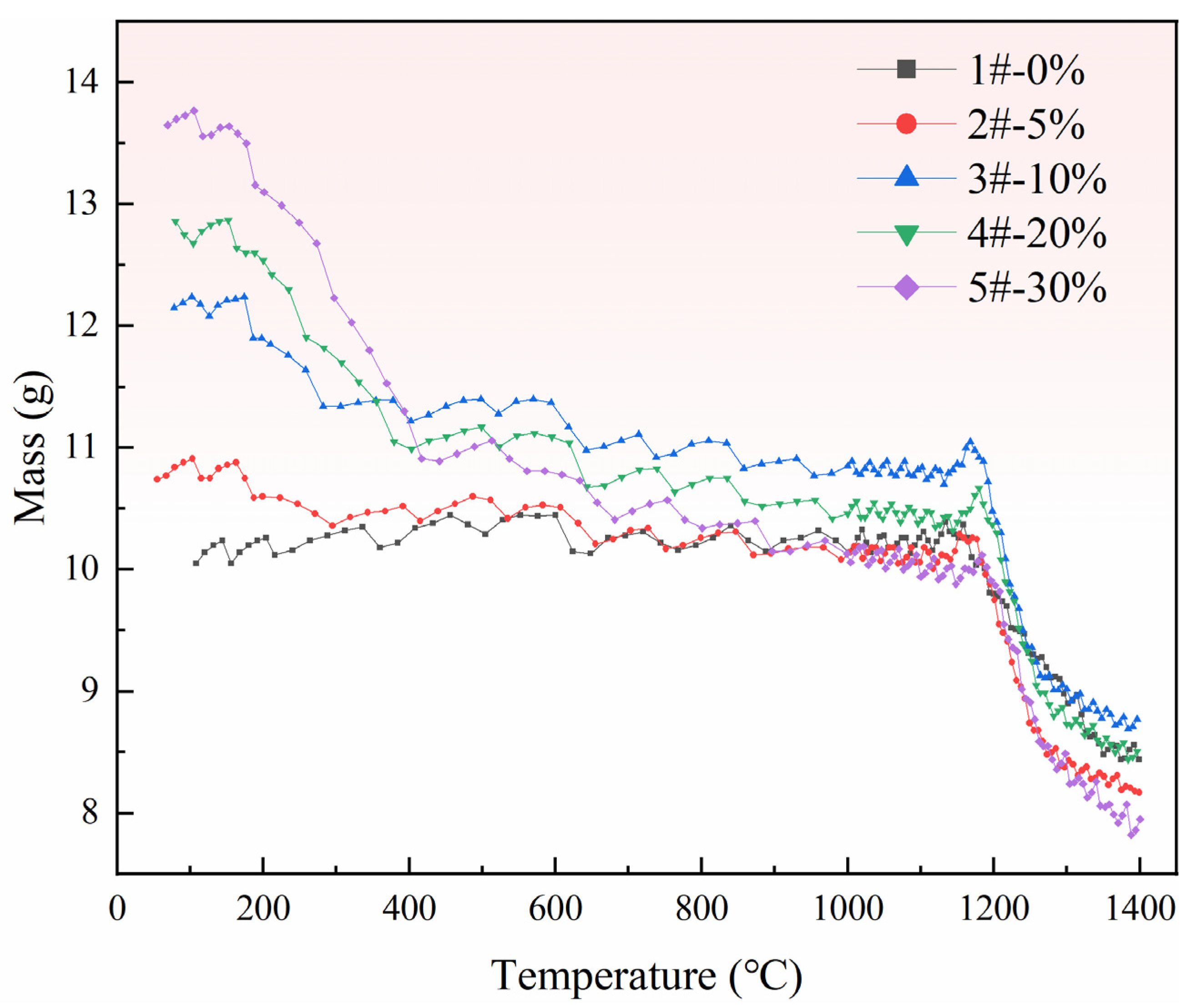
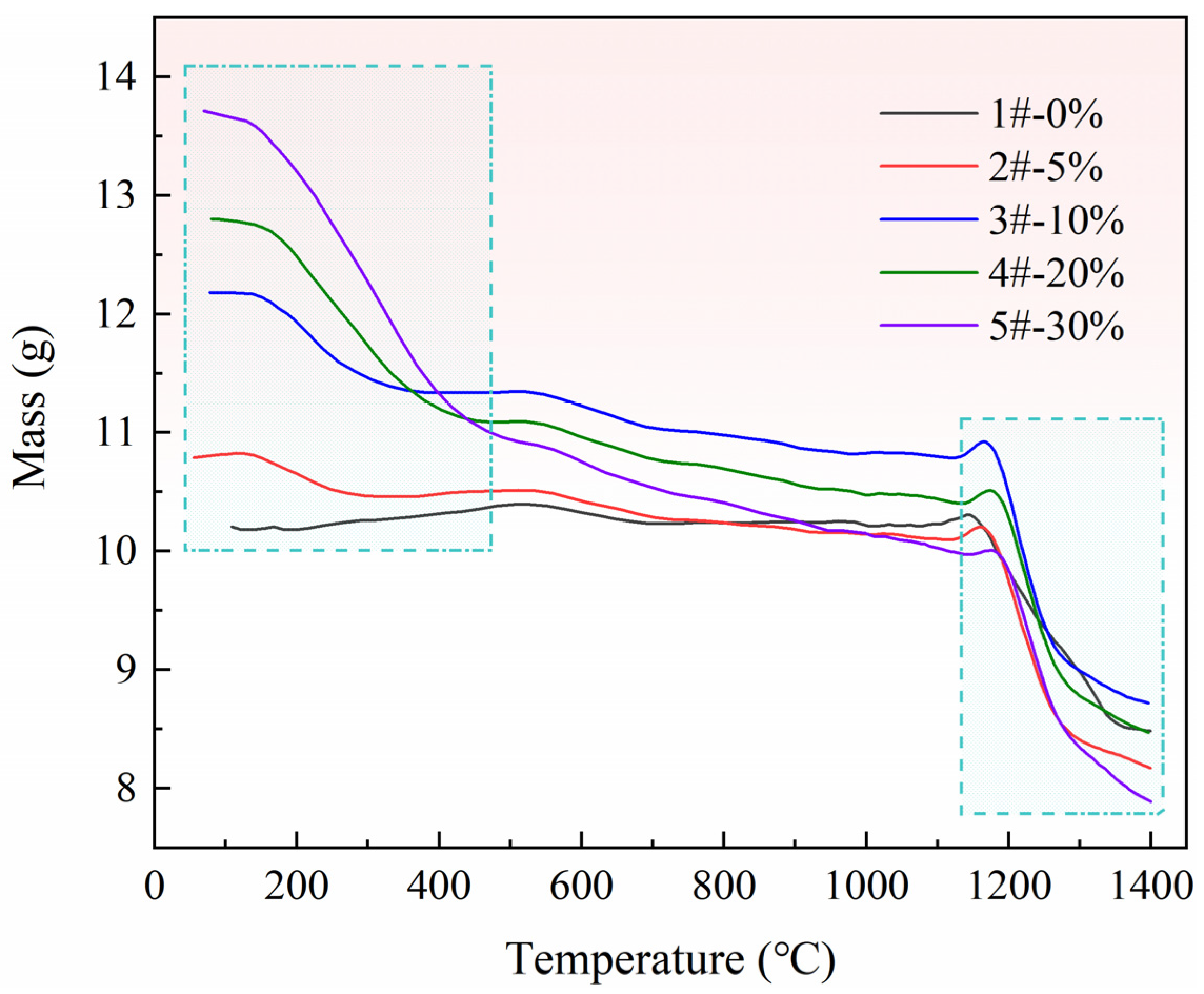
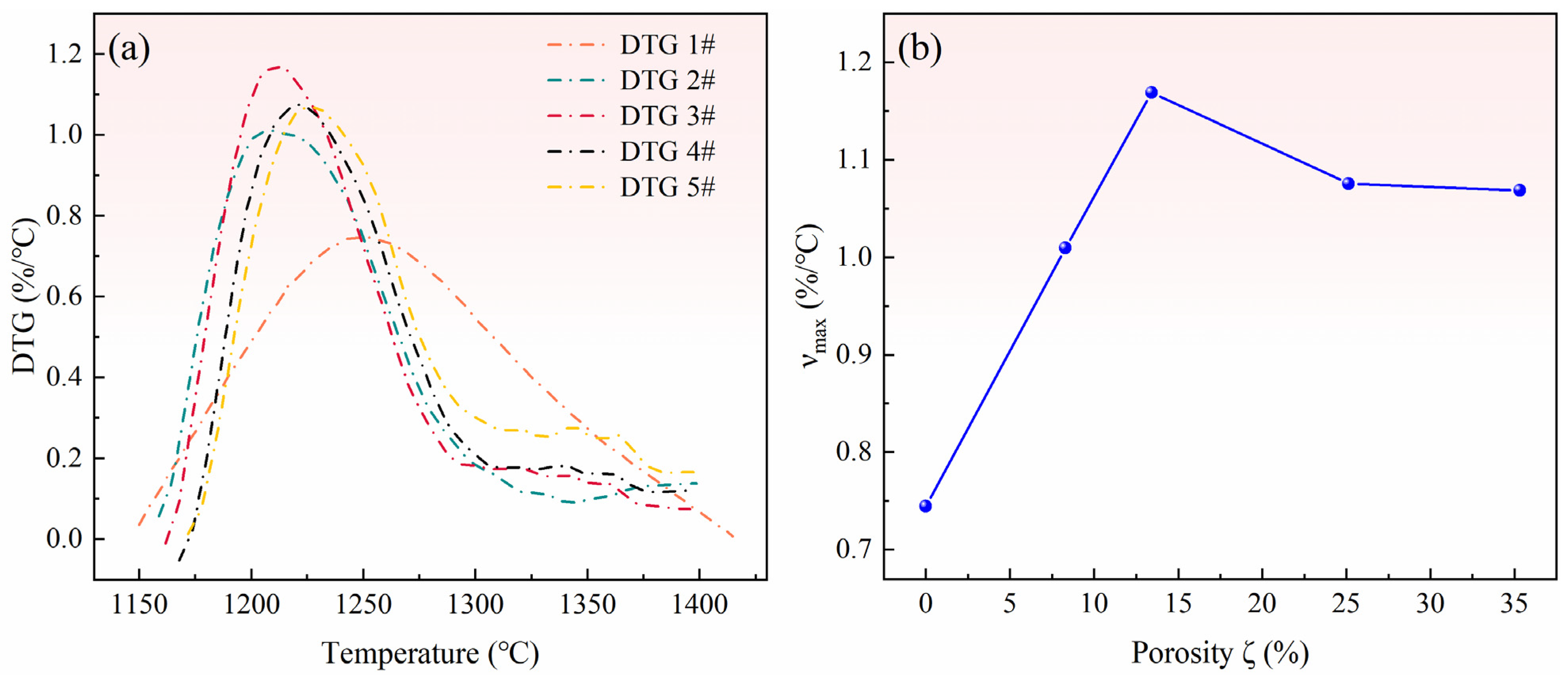
3.2. Reaction Characteristics of Pellets with Different Ratios of Pore-Forming Agents
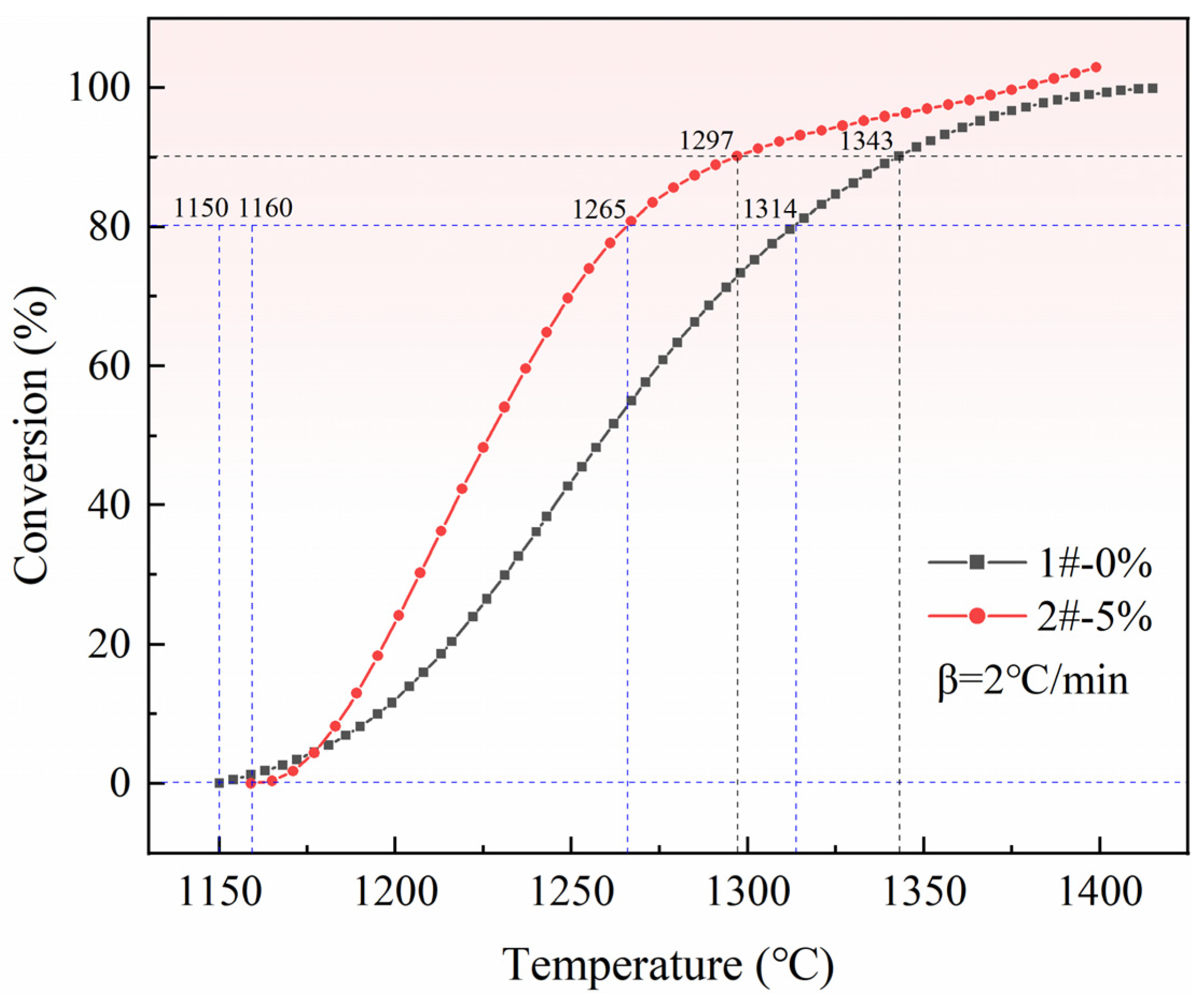
3.3. Effect of Pore Formation Rate on Reduction Conversion of Pellets
- (1)
- When the reduction conversion is 80%, the reduction efficiency of pellets with a 5% pore-forming agent is 36% higher than that of pellets without a pore-forming agent.
- (2)
- When the reduction conversion is 90%, the reduction efficiency of pellets with a 5% pore-forming agent is 29% higher than that of pellets without a pore-forming agent.
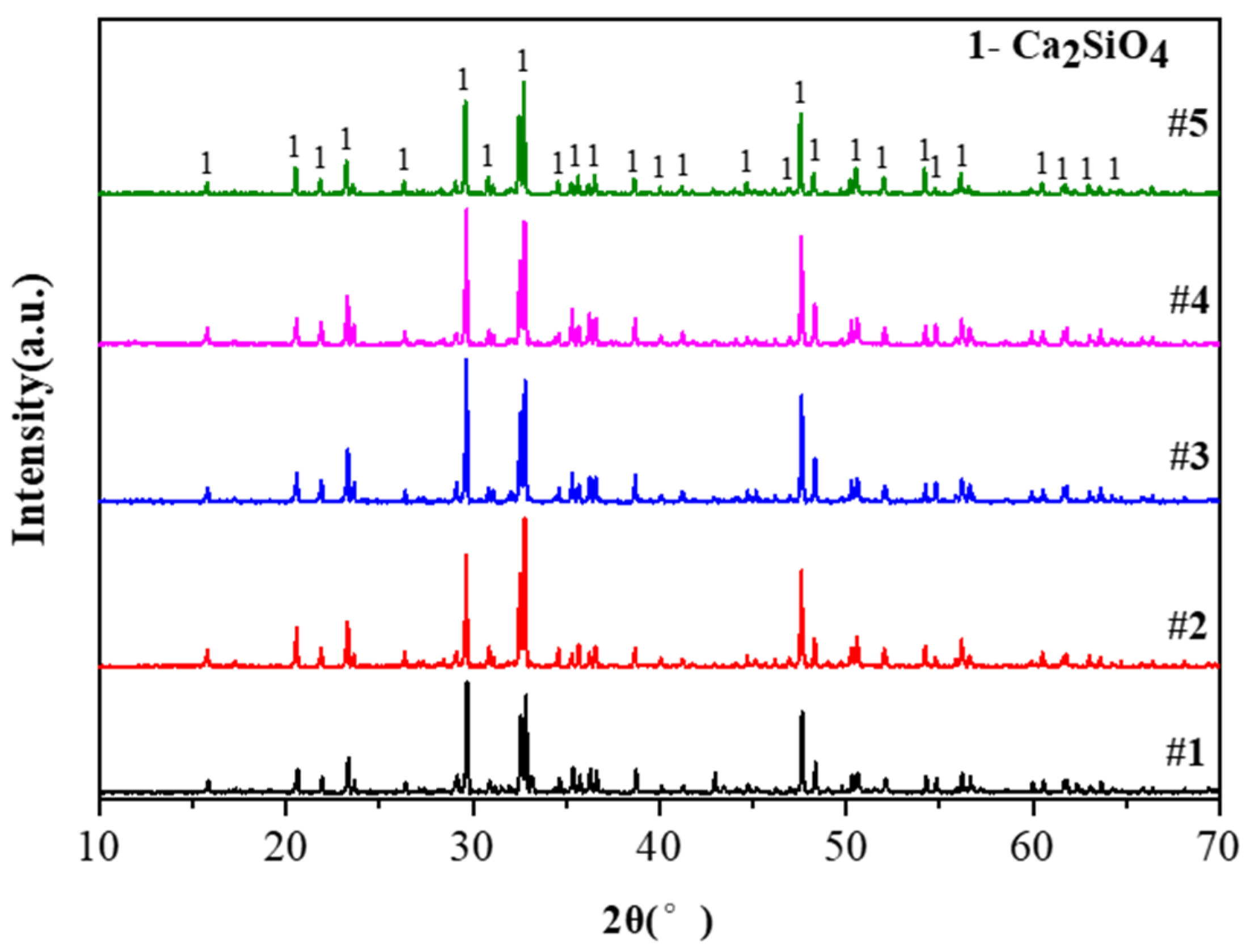
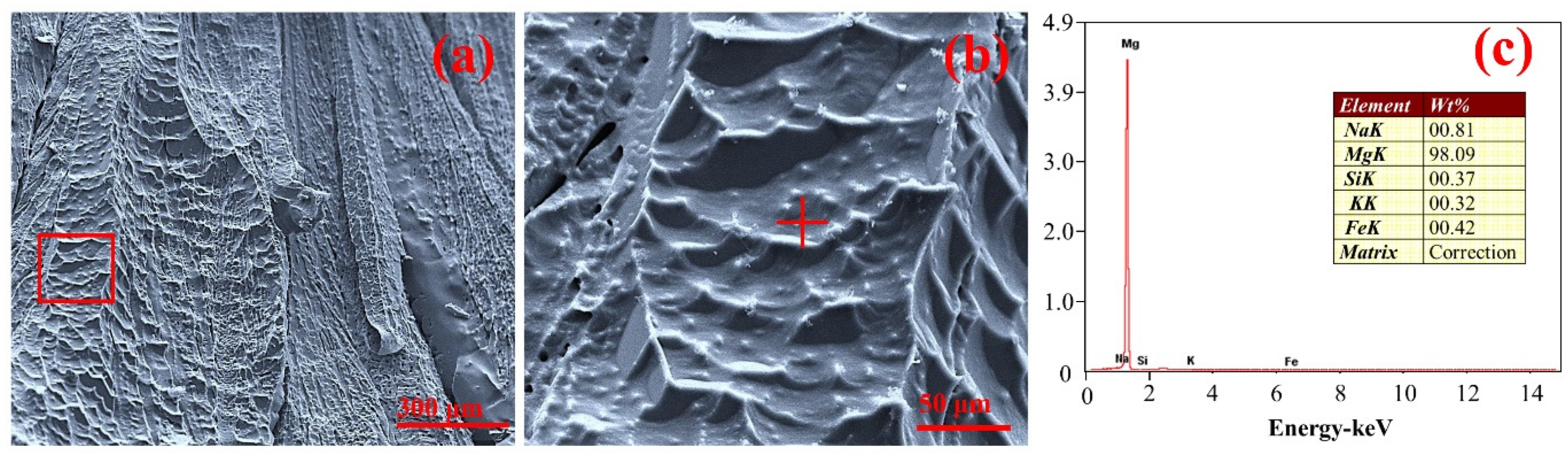
3.4. Characterization and Analysis of Crystallization Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aghion, E.; Bronfin, B. Magnesium Alloys Development towards the 21st Century. Mater. Sci. Forum 2000, 350–351, 19–30. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesium: Properties–applications–potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Pan, F.-S.; Zhang, J.; Wang, J.-F.; Yang, M.-B.; Han, E.-H.; Chen, R.-S. Key R&D activities for development of new types of wrought magnesium alloys in China. Trans. Nonferrous Met. Soc. China 2010, 20, 1249–1258. [Google Scholar] [CrossRef]
- Easton, M.; Beer, A.; Barnett, M.; Davies, C.; Dunlop, G.; Durandet, Y.; Blacket, S.; Hilditch, T.; Beggs, P. Magnesium alloy applications in automotive structures. JOM 2008, 60, 57–62. [Google Scholar] [CrossRef]
- Ogawa, Y.; Ando, D.; Sutou, Y.; Koike, J. A lightweight shape-memory magnesium alloy. Science 2016, 353, 368–370. [Google Scholar] [CrossRef]
- Erbel, R.; Mario, C.D.; Bartunek, J.; Bonnier, J.; Bruyne, B.D.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar] [CrossRef]
- Zhang, M.-Y.; Qin, Y.; Liu, Z.-Q.; Zhang, J.; Ritchie, R.O. 3D printed Mg-NiTi interpenetrating-phase composites with high strength, damping capacity, and energy absorption efficiency. Science 2020, 6, eaba5581. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Xu, Z.-G.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium matrix composites for biomedical applications: A review. J. Magnes. Alloy. 2019, 7, 8. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koc, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Xu, H.-X.; Zhang, X.-L. Green Enviroment-friendly Materials for 21 Century—Magnesium Alloys. J. Shanghai Univ. Eng. Sci. 2017, 04, 322–325. [Google Scholar]
- Xu, X.-Z.; Liu, Q.; Cheng, J. Application of Magnesium Alloy in Industry and National Defence. J. N. China Inst. Technol. 2002, 23, 190–192. [Google Scholar] [CrossRef]
- Yin, Z.-Z.; Qi, W.-C.; Zeng, R.-C.; Chen, X.-B.; Gu, C.-D.; Guan, S.-K.; Zheng, Y.-F. Advances in coatings on biodegradable magnesium alloys. J. Magnes. Alloy. 2020, 8, 42–65. [Google Scholar] [CrossRef]
- Gao, F.; Nie, Z.-R.; Wang, Z.-H.; Gong, X.-Z.; Zuo, T.-Y. Assessing environmental impact of magnesium production using Pidgeon process in China. Trans. Nonferrous Met. Soc. China 2008, 18, 749–754. [Google Scholar] [CrossRef]
- Cherubini, F.; Raugei, M.; Ulgiati, S. LCA of magnesium production Technological overview and worldwide estimation of environmental burdens. Resour. Conserv. Recycl. 2008, 52, 1093–1100. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhang, S.-J.; Guo, L.-J. Experimental and Numerical Studies on a One-Step Method for the Production of Mg in the Silicothermic Reduction Process. Ind. Eng. Chem. Res. 2015, 54, 8883–8892. [Google Scholar] [CrossRef]
- Xiong, N.; Tian, Y.; Yang, B.; Xu, B.-Q.; Dai, T.; Dai, Y.-N. Results of recent investigations of magnesia carbothermal reduction in vacuum. Vacuum 2019, 160, 213–225. [Google Scholar] [CrossRef]
- Qu, T.; Yang, B.; Tian, Y.; Dai, Y.-N. Carbothermal Production of Magnesium in Vacuum; Springer International Publishing: Cham, Switzerland, 2015; pp. 55–59. [Google Scholar] [CrossRef]
- Tian, Y.; Qu, T.; Yang, B.; Dai, Y.-N.; Xu, B.-Q.; Geng, S. Behavior Analysis of CaF2 in Magnesia Carbothermic Reduction Process in Vacuum. Metall. Mater. Trans. B 2012, 43, 657–661. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.-Q.; Yang, B.; Liu, D.-C.; Qu, T.; Liu, H.; Dai, Y.-N. Experimental Study on the Reversion Reaction Between Magnesium and CO Vapor in the Carbothermic Reduction of Magnesia Under Vacuum; Springer: Cham, Switzerland, 2018; pp. 165–170. [Google Scholar]
- Dungan, T.A.; Aime, M. Production of Magnesium by the Carbothermic Process at Permanente. Am. Inst. Min. Metall. Eng. 1944, 308–314. [Google Scholar]
- Brooks, G.; Trang, S.; Witt, P.; Khan, M.N.H.; Nagle, M. The Carbothermic Route to Magnesium. JOM J. Miner. Met. Mater. Soc. 2006, 58, 51–55. [Google Scholar] [CrossRef]
- Prentic, L.H.; Nagle, M.W.; Barton, T.R.D.; Tassios, S.; Kuan, B.T.; Witt, P.J.; Constanti-Carey, K.K. Carbothermal Production of Magnesium: Csiro’s Magsonic Process; Springer International Publishing: Cham, Switzerland, 2012; pp. 31–35. [Google Scholar] [CrossRef]
- Mathaudhu, S.N.; Luo, A.A.; Neelameggham, N.R.; Nyberg, E.A.; Sillekens, W.H. Essential Readings in Magnesium Technology; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Fu, D.-X.; Zhang, T.-A.; Dou, Z.-H.; Guan, L.-K. A new energy-efficient and environmentally friendly process to produce magnesium. Can. Metall. Q. 2017, 56, 418–425. [Google Scholar] [CrossRef]
- Fu, D.-X.; Zhang, T.-A.; Guan, L.-K.; Dou, Z.-H.; Wen, M. Magnesium Production by Silicothermic Reduction of Dolime in Pre-prepared Dolomite Pellets. JOM 2016, 68, 3208–3213. [Google Scholar] [CrossRef]
- Che, Y.-S.; Hao, Z.-H.; Zhu, J.-P.; Fu, Z.-H.; He, J.-L.; Song, J.-X. Kinetic mechanism of Magnesium Production by Silicothermy in Argon Flowing. Thermochim. Acta 2019, 681, 178397. [Google Scholar] [CrossRef]
- Che, Y.-S.; Zhang, C.; Song, J.-X.; Shang, X.-J.; Chen, X.-P.; He, J.-L. The silicothermic reduction of magnesium in flowing argon and numerical simulation of novel technology. J. Magnes. Alloy. 2020, 8, 752–760. [Google Scholar] [CrossRef]
- Pidgeon, L.M.; King, J.A. The vapour pressure of magnesium in the thermal reduction of MgO by ferrosilicon. Discuss. Faraday Soc. 1948, 4, 197–206. [Google Scholar] [CrossRef]
- Toguri, J.M.; Pidgeon, L.M. High-temperature studies of metallurgical processes: Part II. The thermal reduction of calcined dolomite with silicon. Can. J. Chem. 1962, 40, 1769–1776. [Google Scholar] [CrossRef]
- Che, Y.-S.; Mai, G.-P.; Li, S.-L.; He, J.-L.; Song, J.-X.; Yi, J.-H. Kinetic mechanism of magnesium production by silicothermic reduction of CaO·MgO in vacuum. Trans. Nonferrous Met. Soc. China 2020, 30, 2812–2822. [Google Scholar] [CrossRef]
- Xu, R. Magnesium Production by Siliconthermic Reduction Method; Central South University Press: Changsha, China, 2003. [Google Scholar]
- Li, R.-B.; Zhang, S.-J.; Guo, L.-J.; Wei, J.-J. Numerical study of magnesium (Mg) production by the Pidgeon process: Impact of heat transfer on Mg reduction process. Int. J. Heat Mass Transfer. 2013, 59, 328–337. [Google Scholar] [CrossRef]
- Zhang, C.; Chu, H.-Q.; Gu, M.-Y.; Zheng, S. Experimental and numerical investigation of silicothermic reduction process with detailed chemical kinetics and thermal radiation. Therm. Eng. 2018, 135, 454–462. [Google Scholar] [CrossRef]


| Composition | MgO | CaO | Al2O3 | Fe2O3 | K2O | Na2O | CaF2 | Si | Fe | Other | CO2 | Hydration Activity (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcined dolomite | 22.13 | 30.12 | 0.05 | 0.05 | 0.02 | 0.01 | 0.58 | 47.04 | 30.75 | |||
| Ferrosilicon | 76.10 | 19.69 | 4.21 | |||||||||
| Fluorite | 2.18 | 0.02 | 0.01 | 93.06 | 4.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Mai, G.; Che, Y.; He, J. Novel Efficient Reduction Route for Magnesium Production Using Silicothermic Process. Materials 2022, 15, 6009. https://doi.org/10.3390/ma15176009
Chen Y, Mai G, Che Y, He J. Novel Efficient Reduction Route for Magnesium Production Using Silicothermic Process. Materials. 2022; 15(17):6009. https://doi.org/10.3390/ma15176009
Chicago/Turabian StyleChen, Yongqiang, Gengpeng Mai, Yusi Che, and Jilin He. 2022. "Novel Efficient Reduction Route for Magnesium Production Using Silicothermic Process" Materials 15, no. 17: 6009. https://doi.org/10.3390/ma15176009
APA StyleChen, Y., Mai, G., Che, Y., & He, J. (2022). Novel Efficient Reduction Route for Magnesium Production Using Silicothermic Process. Materials, 15(17), 6009. https://doi.org/10.3390/ma15176009






