Facile Synthesis of CoSe/Co3O4-CNTs/NF Composite Electrode for High-Performance Asymmetric Supercapacitor
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
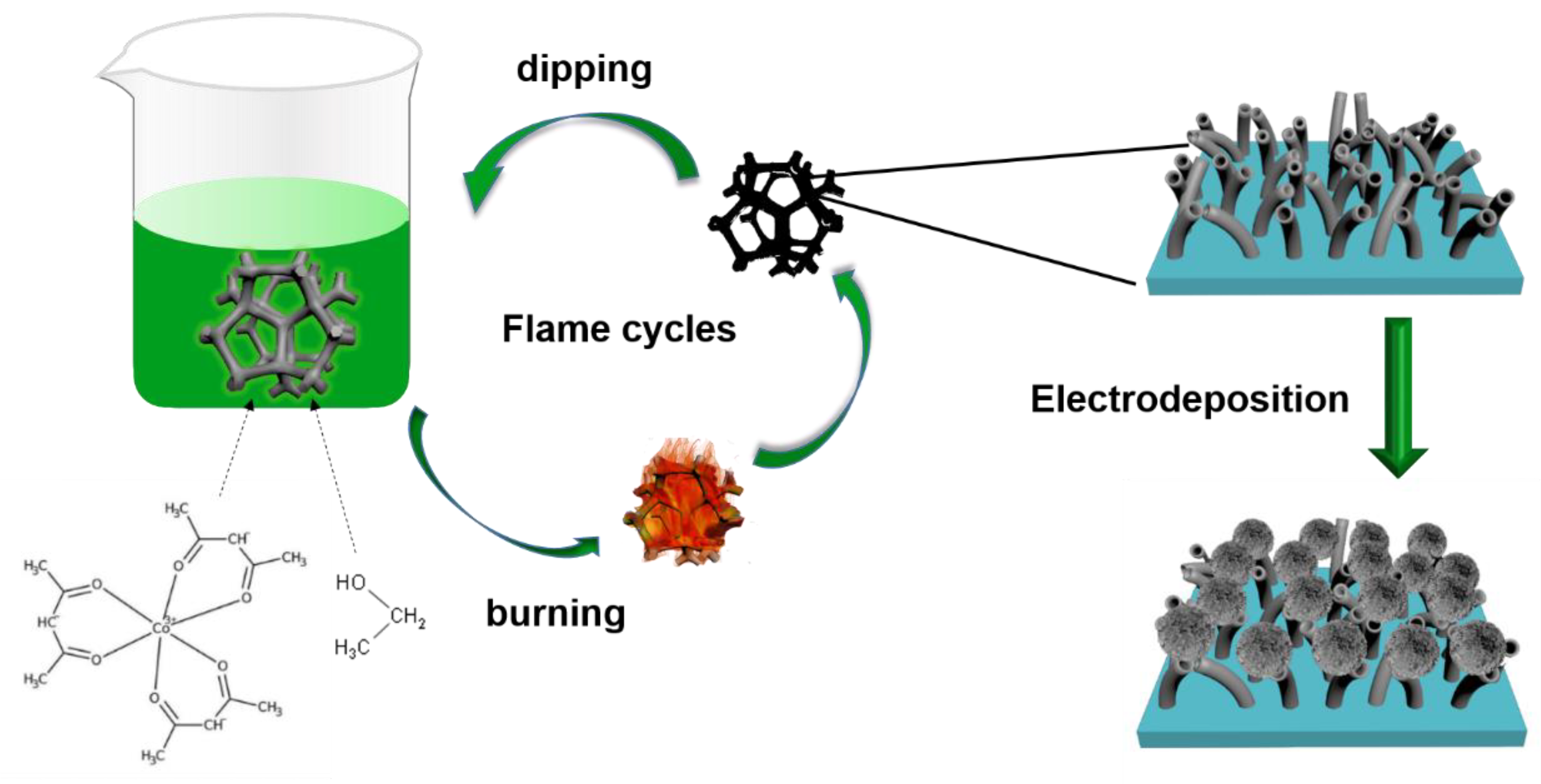
2.2. The Synthesis of Co3O4-CNTs/NF
2.3. The Synthesis of CoSe
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
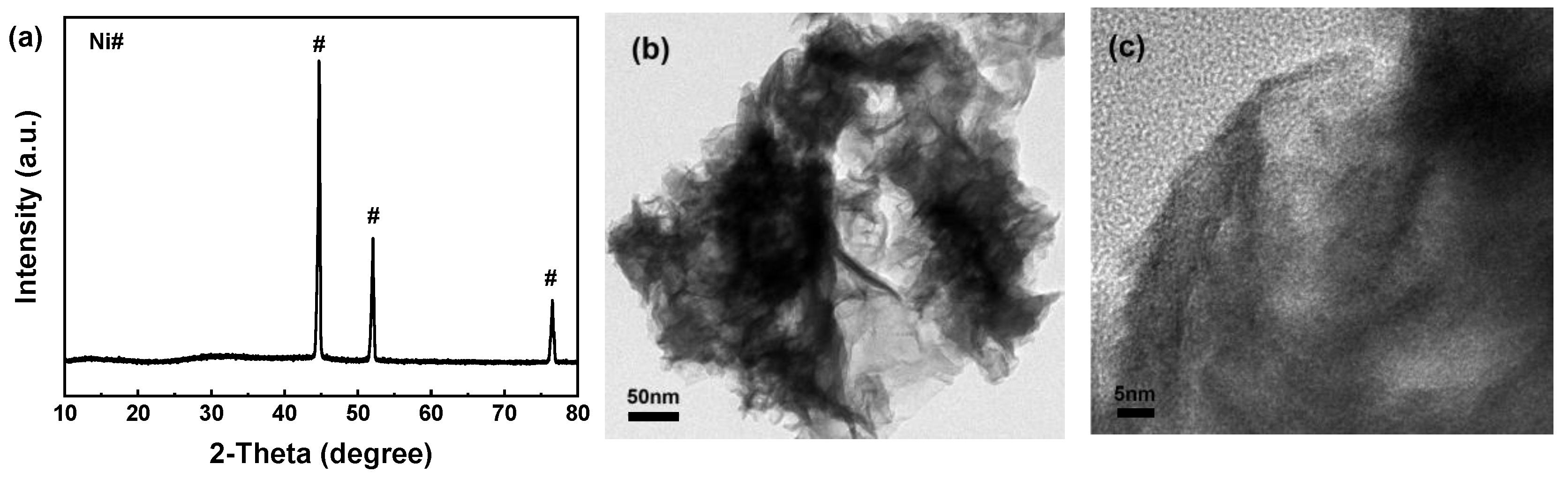
3.1. Characterization of Materials
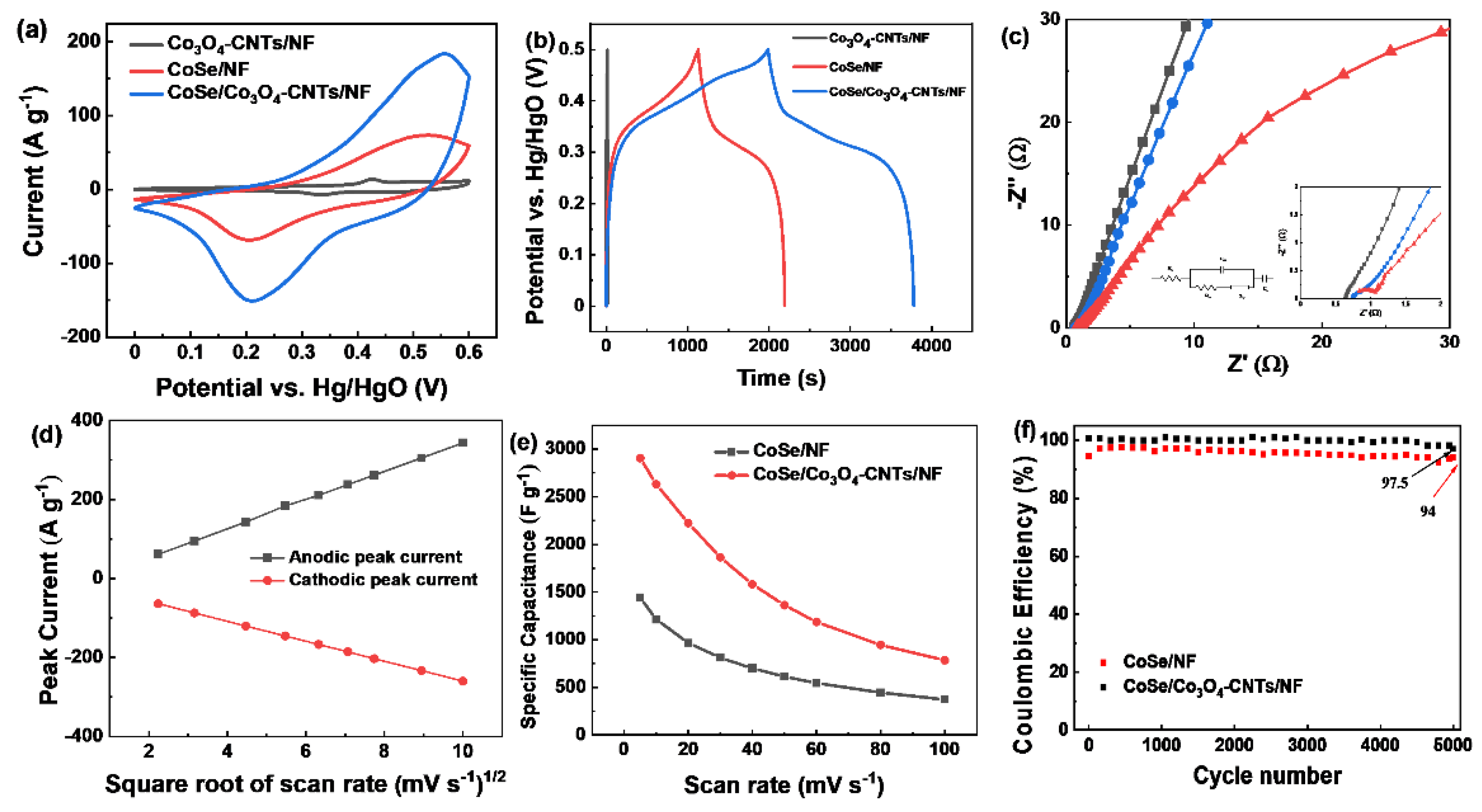
3.2. Electrochemical Performances of CoSe/Co3O4-CNTs/NF
3.3. Electrochemical Performances of CoSe/Co3O4-CNTs/NF//AC ASC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Berrueta, A.; Ursua, A.; San Martin, I.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Yan, T.; Li, R.Y.; Li, Z.J.; Fang, Y.J. A facile and scalable strategy for synthesis of size-tunable NiCo2O4 with nanocoral-like architecture for high-performance. Electrochim. Acta 2014, 134, 384–392. [Google Scholar]
- Chia, X.; Eng, A.Y.; Ambrosi, A.; Tan, S.M.; Pumera, M. Electrochemistry of Nanostructured Layered Transition-Metal Dichalcogenides. Chem. Rev. 2015, 115, 11941–11966. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhang, S.; Zhao, H.; Feng, Y.; Wang, M.; An, L.; Zhang, X.; Mi, J. One-pot synthesis CoFe2O4/CNTs composite for asymmetric supercapacitor electrode. Solid State Ionics 2019, 329, 15–24. [Google Scholar] [CrossRef]
- Peng, Y.J.; Wu, T.H.; Hsu, C.T.; Li, S.M.; Chen, M.G.; Hu, C.C. Electrochemical characteristics of the reduced graphene oxide/carbon nanotube/polypyrrole composites for aqueous asymmetric supercapacitors. J. Power Sources 2014, 272, 970–978. [Google Scholar] [CrossRef]
- Lei, X.Y.; Li, M.; Lu, M.; Guan, X.H. Electrochemical Performances Investigation of New Carbon-Coated Nickel Sulfides as Electrode Material for Supercapacitors. Materials 2019, 12, 12. [Google Scholar] [CrossRef]
- Liu, F.; He, J.T.; Liu, X.Y.; Chen, Y.K.; Liu, Z.; Chen, D.; Liu, H.; Zhou, W.J. MoC nanoclusters anchored Ni@N-doped carbon nanotubes coated on carbon fiber as three-dimensional and multifunctional electrodes for flexible supercapacitor and self-heating device. Carbon Energy 2021, 3, 129–141. [Google Scholar] [CrossRef]
- Bag, S.; Samanta, A.; Bhunia, P.; Raj, C.R. Rational functionalization of reduced graphene oxide with imidazolium-based ionic liquid for supercapacitor application. Int. J. Hydrogen Energy 2016, 41, 22134–22143. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Cao, F.; Zhang, Y.Q.; Xia, X.H. The Development Trend of Graphene Derivatives. J. Electron. Mater. 2022, 51, 4107–4114. [Google Scholar] [CrossRef]
- Li, Y.T.; Pi, Y.T.; Lu, L.M.; Xu, S.H.; Ren, T.Z. Hierarchical porous active carbon from fallen leaves by synergy of K2CO3 and their supercapacitor performance. J. Power Sources 2015, 299, 519–528. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.F.; He, H.W.; Peng, Y.Z.; Wu, Y.H. Engineering RuO2 on CuCo2O4/CuO nanoneedles as multifunctional electrodes for the hybrid supercapacitors and water oxidation catalysis. J. Alloys Compd. 2020, 832, 154962. [Google Scholar] [CrossRef]
- Yang, G.W.; Li, X.M.; Chen, T.; Gao, W.L.; Dai, Y.J.; Li, X.Y. Self-Supported PANI@MnO2 Coaxial Nanowire Network Sponge as a Binder Free Electrode for Supercapacitors. J. Nanosci. Nanotechnol. 2020, 20, 4203–4209. [Google Scholar] [CrossRef] [PubMed]
- Thulasi-Varma, C.V.; Balakrishnan, B.; Kim, H.J. Exploration of Ni-X(O, S, Se) for high performance supercapacitor with long-term stability via solution phase synthesis. J. Ind. Eng. Chem. 2020, 81, 294–302. [Google Scholar] [CrossRef]
- Zhou, J.J.; Han, X.; Tao, K.; Li, Q.; Li, Y.L.; Chen, C.; Han, L. Shish-kebab type MnCo2O4@Co3O4 nanoneedle arrays derived from MnCo-LDH@ZIF-67 for high-performance supercapacitors and efficient oxygen evolution reaction. Chem. Eng. J. 2018, 354, 875–884. [Google Scholar] [CrossRef]
- Dai, Z.Y.; Xue, L.C.; Zhang, Z.B.; Gao, Y.; Wang, J.; Gao, Q.S.; Chen, D.J. Construction of Single-Phase Nickel Disulfide Microflowers as High-Performance Electrodes for Hybrid Supercapacitors. Energy Fuels 2020, 34, 10178–10187. [Google Scholar] [CrossRef]
- Chen, H.C.; Fan, M.Q.; Li, C.; Tian, G.L.; Lv, C.J.; Chen, D.; Shu, K.Y.; Jiang, J.J. One-pot synthesis of hollow NiSe-CoSe nanoparticles with improved performance for hybrid supercapacitors. J. Power Sources 2016, 329, 314–322. [Google Scholar] [CrossRef]
- Ye, A.N.; Zhu, Q.; Zhang, X.H.; Yang, Z.H. Spatial Distribution Control on the Energy Storage Performance of PANI@PVA@ACNT-Based Flexible Solid-State Supercapacitors. ACS Appl. Energ. Mater. 2020, 3, 3082–3091. [Google Scholar] [CrossRef]
- BoopathiRaja, R.; Parthibavarman, M. Desert rose like heterostructure of NiCo2O4/NF@PPy composite has high stability and excellent electrochemical performance for asymmetric super capacitor application. Electrochim. Acta 2020, 346, 136270. [Google Scholar] [CrossRef]
- Duy, L.T.; Seo, H. Construction of stretchable supercapacitors using graphene hybrid hydrogels and corrosion-resistant silver nanowire current collectors. Appl. Surf. Sci. 2020, 521, 146467. [Google Scholar] [CrossRef]
- Jayaraman, T.; Karuppasamy, K.; Govindarajan, D.; Abu Ul Hassan Sarwar, R.; Prabhakarn, A.; Kirubanandam, S.; Parasuraman, K.; Hyun-Seok, K. Recent Advances in Metal Chalcogenides (MX.; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. [Google Scholar]
- Kim, H.C.; Huh, S. Porous Carbon-Based Supercapacitors Directly Derived from Metal-Organic Frameworks. Materials 2020, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.P.; Nsabimana, A.; Luque, R.; Xu, G.B. 3D Porous Carbonaceous Electrodes for Electrocatalytic Applications. Joule 2018, 2, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Q.; Wen, L.; Tong, Y.Y.; Wang, S.H.; Hou, X.G.; An, X.D.; Dou, S.X.; Liang, J. Photo-rechargeable batteries and supercapacitors: Critical roles of carbon-based functional materials. Carbon Energy 2021, 3, 225–252. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Tian, Y.R.; Sarwar, S.; Luo, J.J.; Zhang, X.Y. Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors. J. Power Sources 2020, 452, 227793. [Google Scholar] [CrossRef]
- Li, Y.J.; Song, C.M.; Chen, J.C.; Shang, X.N.; Chen, J.P.; Li, Y.; Huang, M.; Meng, F.B. Sulfur and nitrogen Co-doped activated CoFe2O4@C nanotubes as an efficient material for supercapacitor applications. Carbon 2020, 162, 124–135. [Google Scholar] [CrossRef]
- Kirubasankar, B.; Murugadoss, V.; Lin, J.; Ding, T.; Dong, M.; Liu, H.; Zhang, J.; Li, T.; Wang, N.; Guo, Z.; et al. In situ grown nickel selenide on graphene nanohybrid electrodes for high energy density asymmetric supercapacitors. Nanoscale 2018, 10, 20414–20425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Liu, W.J.; Liu, Y.; Shi, W.D. Construction of porous interface on CNTs@NiCo-LDH core-shell nanotube arrays for supercapacitor applications. Chem. Eng. J. 2020, 383, 123150. [Google Scholar] [CrossRef]
- Esteves, L.M.; Oliveira, H.A.; Passos, F.B. Carbon nanotubes as catalyst support in chemical vapor deposition reaction: A review. J. Ind. Eng. Chem. 2018, 65, 1–12. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Cheminform 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Q.; Lili, Z.; Dai-Ming, T.; Ruixue, M.; Yongzhao, W.; Bingsen, Z.; Feng, D.; Chang, L.; Hui-Ming, C. Precise Identification of the Active Phase of Cobalt Catalyst for Carbon Nanotube Growth by In Situ Transmission Electron Microscopy. ACS Nano 2020, 14, 16823–16831. [Google Scholar]
- Zhang, H.; Yang, B.; Wu, X.; Li, Z.; Lei, L.; Zhang, X. Polymorphic CoSe2 with Mixed Orthorhombic and Cubic Phases for Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2015, 7, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.B.; Wang, L.; Li, D.G.; Gao, S.B.; Sun, Q.; Lu, P.; Xing, P.F.; An, M.Z. Nucleation and growth mechanism of a nanosheet-structured NiCoSe2 layer prepared by electrodeposition. Nanotechnology 2019, 30, 245602. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, J.; Li, X.; Li, W.; Zhang, X.; Koratkar, N.; Yu, Z.Z. Flame Synthesis of Superhydrophilic Carbon Nanotubes/Ni Foam Decorated with Fe2O3 Nanoparticles for Water Purification via Solar Steam Generation. ACS Appl. Mater. Interfaces 2020, 12, 13229–13238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, R.; Sun, S.; Wu, X. Facile synthesis of nickel-cobalt selenide nanoparticles as battery-type electrode for all-solid-state asymmetric supercapacitors. J. Colloid Interface Sci. 2019, 549, 16–21. [Google Scholar] [CrossRef]
- Qian, W.Z.; Liu, T.; Wei, F.; Yuan, H.Y. Quantitative Raman characterization of the mixed samples of the single and multi-wall carbon nanotubes. Carbon 2003, 41, 1851–1854. [Google Scholar] [CrossRef]
- Ruan, J.; Mo, F.; Chen, Z.; Liu, M.; Zheng, S.; Wu, R.; Fang, F.; Song, Y.; Sun, D. Rational Construction of Nitrogen-Doped Hierarchical Dual-Carbon for Advanced Potassium-Ion Hybrid Capacitors. Adv. Energy Mater. 2020, 10, 1904045. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Gungunes, H.; Korkmaz, A.D.; Zubar, T.; Trukhanov, S.; Trukhanov, A.; Manikandan, A.; Alahmari, F.; Baykal, A. Influence of Dy3+ Ions on the Microstructures and Magnetic, Electrical, and Microwave Properties of [Ni0.4Cu0.2Zn0.4](Fe2-x Dyx)O4 (0.00 ≤ x ≤ 0.04) Spinel Ferrites. ACS Omega 2021, 6, 10266–10280. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Darwish, K.A.; Salem, M.M.; Hemeda, O.M.; Abdel Ati, M.I.; Darwish, M.A.; Kaniukov, E.Y.; Podgornaya, S.V.; Turchenko, V.A.; Tishkevich, D.I.; et al. Impact of the heat treatment conditions on crystal structure, morphology and magnetic properties evolution in BaM nanohexaferrites. J. Alloys Compd. 2021, 866, 158961. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, Y.; Zheng, Y.; Hu, X.; Liu, Z. Facile fabrication of flower-like CuCo2S4 on Ni foam for supercapacitor application. J. Mater. Sci. 2017, 52, 9531–9538. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Hu, Z.; Xi, L.; Ji, X.; Liu, Y. 3D interconnected ultrathin cobalt selenide nanosheets as cathode materials for hybrid supercapacitors. Electrochim. Acta 2018, 269, 30–37. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.Y.; Zhang, L.; Huang, J.; Liu, H. ZIF-67-Derived CoSe/NC Composites as Anode Materials for Lithium-Ion Batteries. Nanoscale Res. Lett. 2019, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ma, G.; Sun, K.; Zhang, Z.; Li, J.; Zhou, X.; Lei, Z. A novel aqueous asymmetric supercapacitor based on petal-like cobalt selenide nanosheets and nitrogen-doped porous carbon networks electrodes. J. Power Sources 2015, 297, 351–358. [Google Scholar] [CrossRef]
- Xue, Z.; Li, X.; Liu, Q.; Cai, M.; Liu, K.; Liu, M.; Ke, Z.; Liu, X.; Li, G. Interfacial Electronic Structure Modulation of NiTe Nanoarrays with NiS Nanodots Facilitates Electrocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, e1900430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, A.; Ding, L.; Zhou, Z.; Wang, Y.; Niu, S.; Liang, S.; Cao, G. Nitrogen-Doped Yolk-Shell-Structured CoSe/C Dodecahedra for High-Performance Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3624–3633. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H.X. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Wu, C.; Zhang, Y.; Ma, Y.; Sun, X.; Sun, J.; Zhang, X.; Yuan, C. Monodisperse Metallic NiCoSe2 Hollow Sub-Microspheres: Formation Process, Intrinsic Charge-Storage Mechanism, and Appealing Pseudocapacitance as Highly Conductive Electrode for Electrochemical Supercapacitors. Adv. Funct. Mater. 2018, 28, 1705921. [Google Scholar]
- Wang, X.; Fang, Y.; Shi, B.; Huang, F.; Rong, F.; Que, R.; Shao, M. Fabrication of Isomorphous Co3O4@Co3O4 Hierarchical Core-Shell Nanoneedles for High-Performance Supercapacitors. Chemistryselect 2017, 2, 9267–9276. [Google Scholar]
- Wang, Y.; Yin, Z.; Yan, G.; Wang, Z.; Li, X.; Guo, H.; Wang, J. New insight into the electrodeposition of NiCo layered double hydroxide and its capacitive evaluation. Electrochim. Acta 2020, 336, 135734. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Gui, P.; Wen, J.; Fu, X.; Fang, G. Bifunctional bamboo-like CoSe2 arrays for high-performance asymmetric supercapacitor and electrocatalytic oxygen evolution. Nanotechnology 2018, 29, 205401. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Wen, J.; Gui, P.; Fang, G. Metal-Organic Framework Template Derived Porous CoSe2 Nanosheet Arrays for Energy Conversion and Storage. ACS Appl. Mater. Interfaces 2017, 9, 35927–35935. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Pan, A.Q.; Wang, Y.P.; Cao, X.X.; Zhou, Z.L.; Zhu, T.; Liang, S.Q.; Cao, G.Z. Self-templated synthesis of N-doped CoSe2/C double-shelled dodecahedra for high-performance supercapacitors. Energy Storage Mater. 2017, 8, 28–34. [Google Scholar] [CrossRef]
- Sakthivel, M.; Ramaraj, S.; Chen, S.; Ho, K.-C. Bimetallic vanadium cobalt diselenide nanosheets with additional active sites for excellent asymmetric pseudocapacitive performance: Comparing the electrochemical performances with M–CoSe2 (M = Zn, Mn, and Cu). J. Mater. Chem. A 2019, 7, 12565–12581. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Fan, M.; Li, C.; Chen, D.; Tian, G.; Shu, K. Bimetallic nickel cobalt selenides: A new kind of electroactive material for high-power energy storage. J. Mater. Chem. A 2015, 3, 23653–23659. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Wen, J.; Gui, P.; Guo, Y.; Guan, C.; Liu, J.; Fang, G. Rational Construction of Hollow Core-Branch CoSe2 Nanoarrays for High-Performance Asymmetric Supercapacitor and Efficient Oxygen Evolution. Small 2018, 14, 1700979. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zheng, X.; Cao, X.; Yang, C.; Zhao, Q.; Zhang, Y.; Xia, X. Facile Synthesis of CoSe/Co3O4-CNTs/NF Composite Electrode for High-Performance Asymmetric Supercapacitor. Materials 2022, 15, 5841. https://doi.org/10.3390/ma15175841
Wang Y, Zheng X, Cao X, Yang C, Zhao Q, Zhang Y, Xia X. Facile Synthesis of CoSe/Co3O4-CNTs/NF Composite Electrode for High-Performance Asymmetric Supercapacitor. Materials. 2022; 15(17):5841. https://doi.org/10.3390/ma15175841
Chicago/Turabian StyleWang, Ying, Xiang Zheng, Xianjun Cao, Chengtao Yang, Qiang Zhao, Yongqi Zhang, and Xinhui Xia. 2022. "Facile Synthesis of CoSe/Co3O4-CNTs/NF Composite Electrode for High-Performance Asymmetric Supercapacitor" Materials 15, no. 17: 5841. https://doi.org/10.3390/ma15175841
APA StyleWang, Y., Zheng, X., Cao, X., Yang, C., Zhao, Q., Zhang, Y., & Xia, X. (2022). Facile Synthesis of CoSe/Co3O4-CNTs/NF Composite Electrode for High-Performance Asymmetric Supercapacitor. Materials, 15(17), 5841. https://doi.org/10.3390/ma15175841






