Metal–Organic Frameworks (MOFs) Derived Materials Used in Zn–Air Battery
Abstract
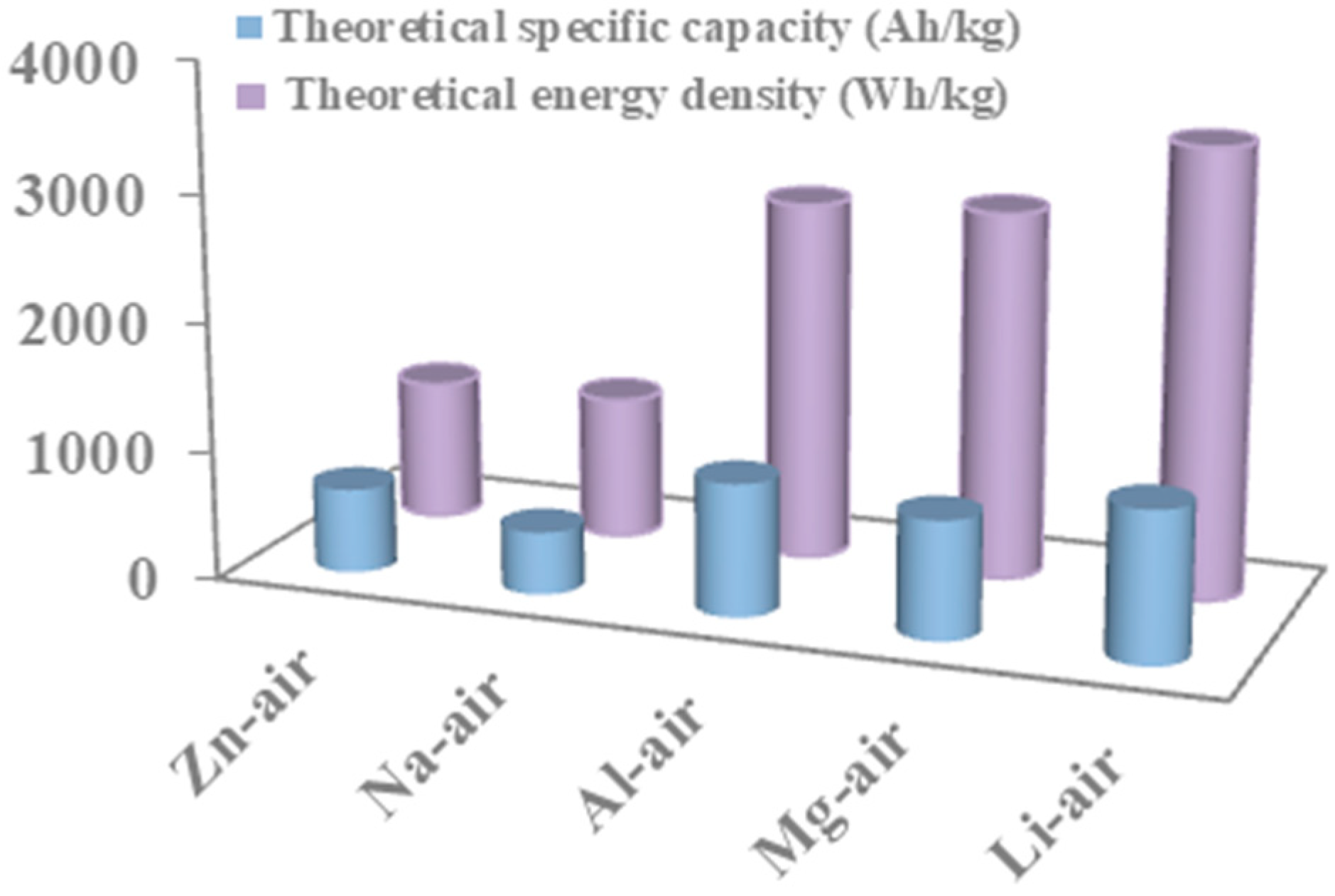
:1. Introduction
2. Catalytic Mechanism in Zinc-Air Battery
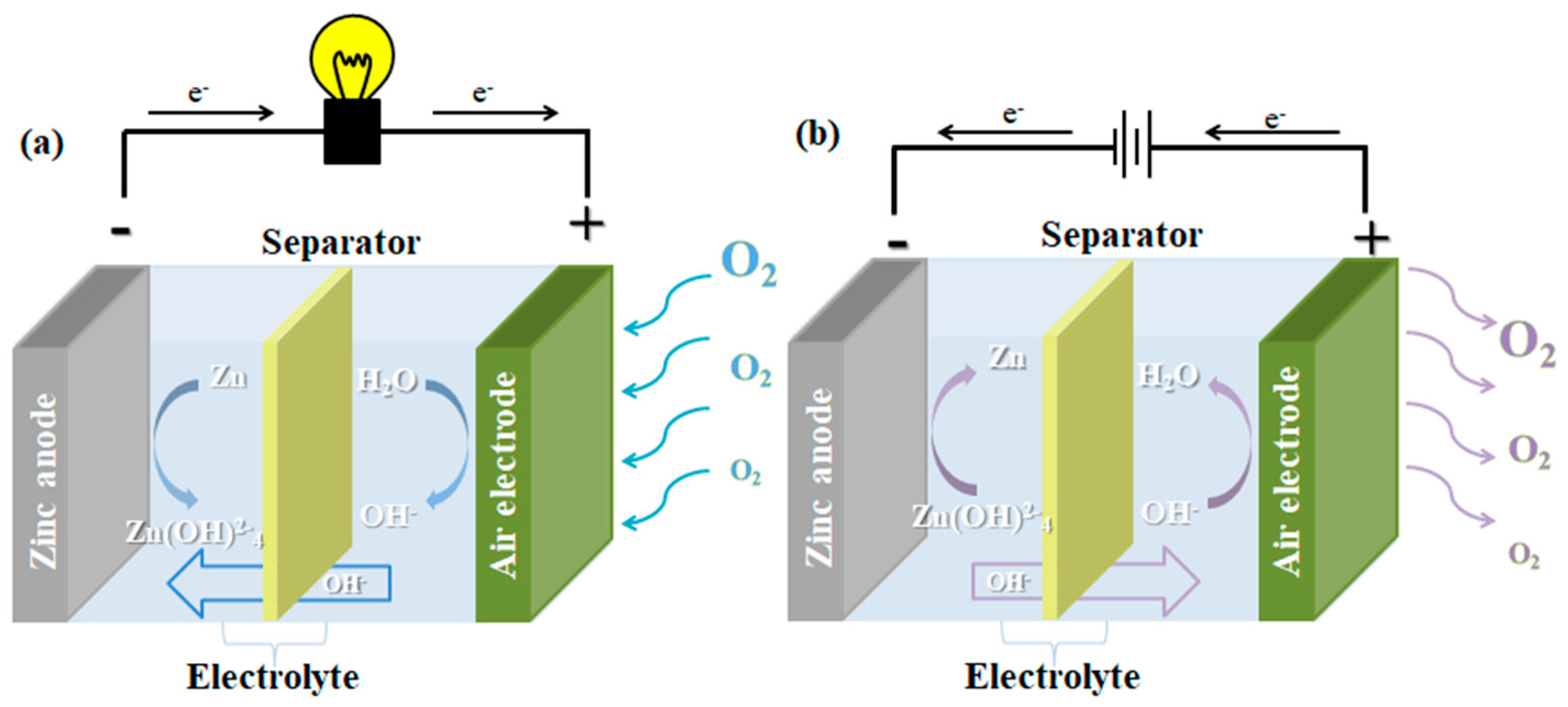
2.1. The Structure and Working Principle of the Zn–Air Battery
2.2. ORR Reaction Mechanism and Evaluation Parameters
2.3. OER Reaction Mechanism and Evaluation Parameters
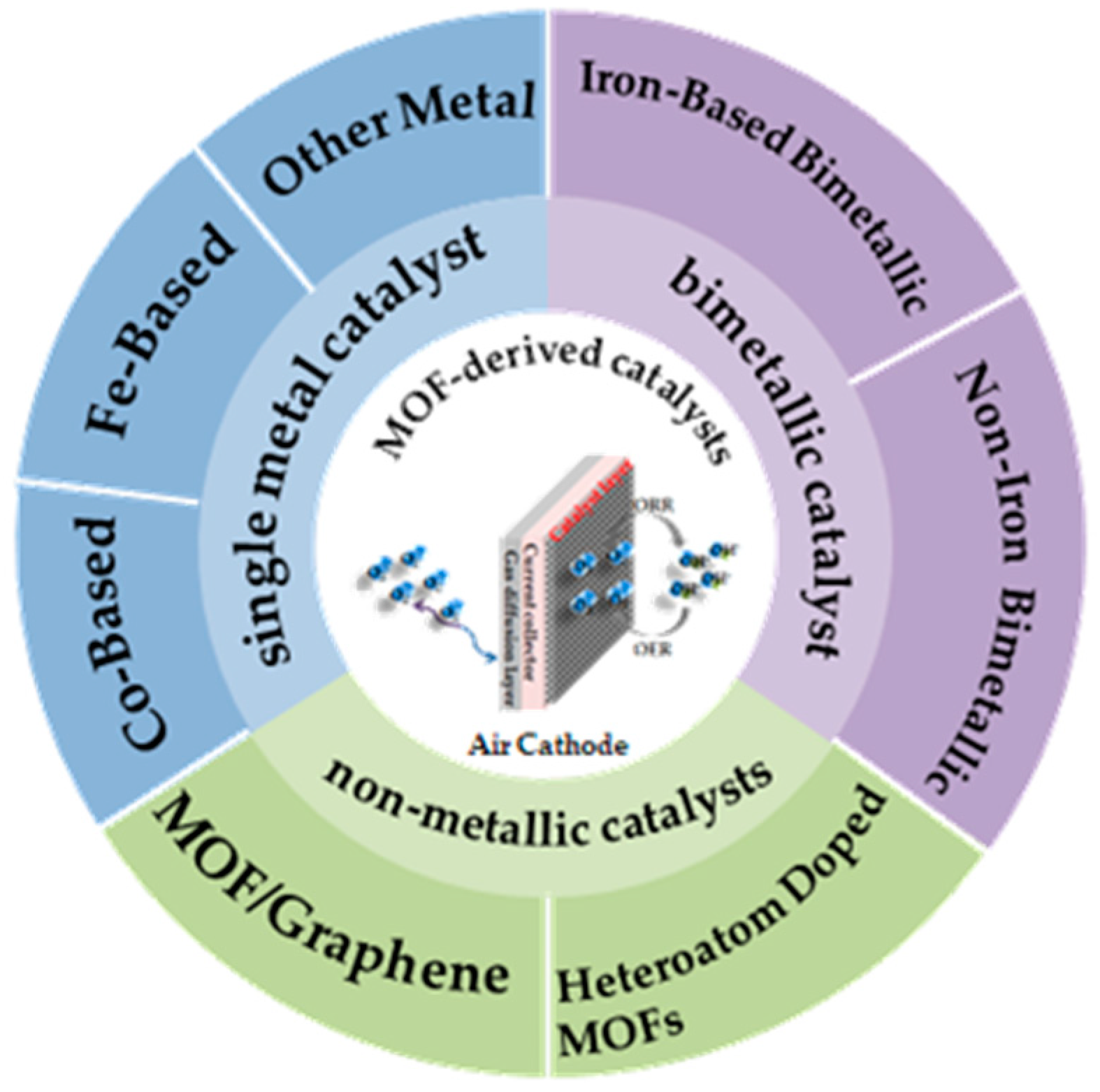
3. MOF-Derived Non-Precious Metal Catalysts
3.1. MOF-Derived Single Metal Catalyst
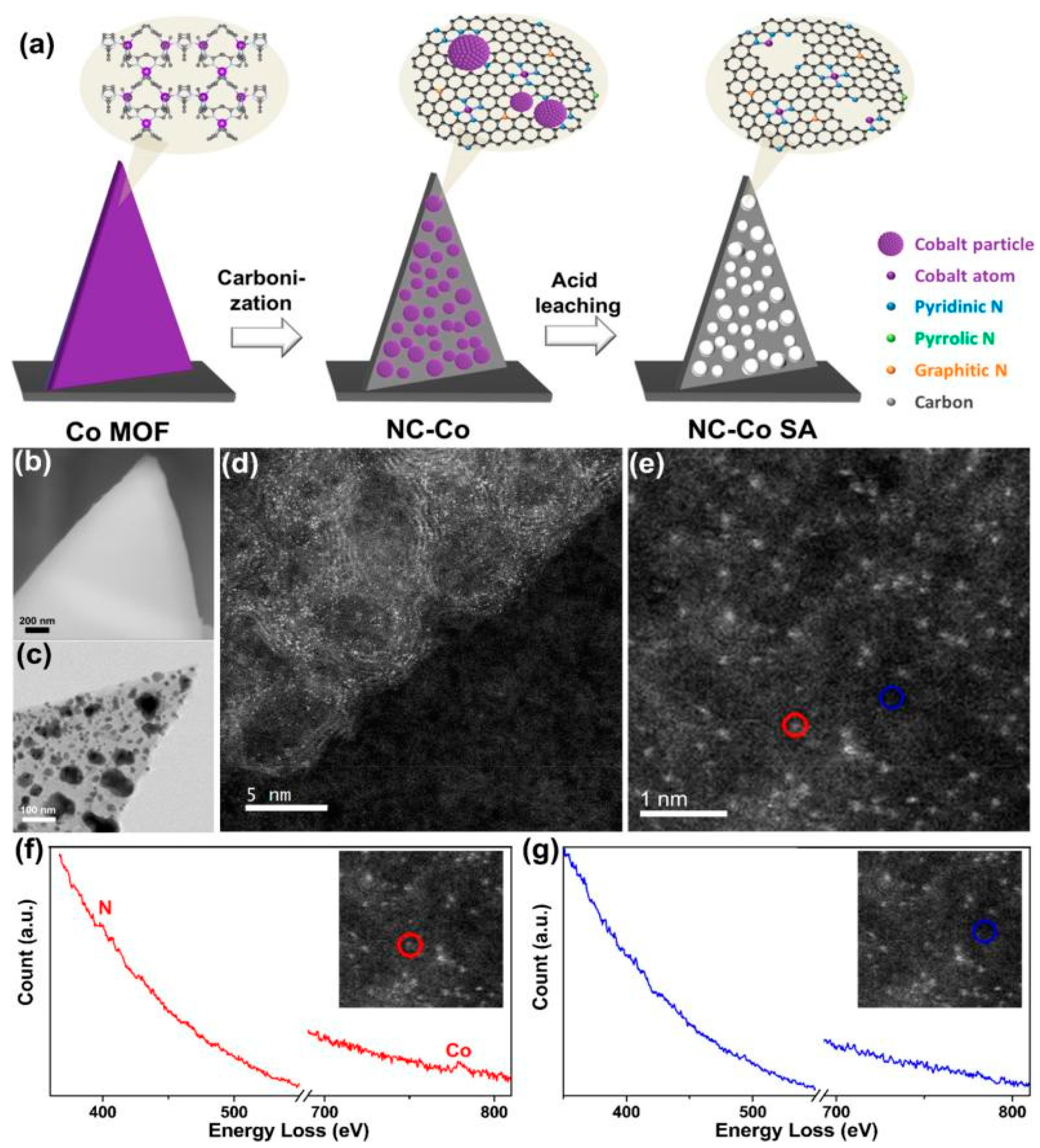
3.1.1. MOF-Derived Co-Based Catalysts
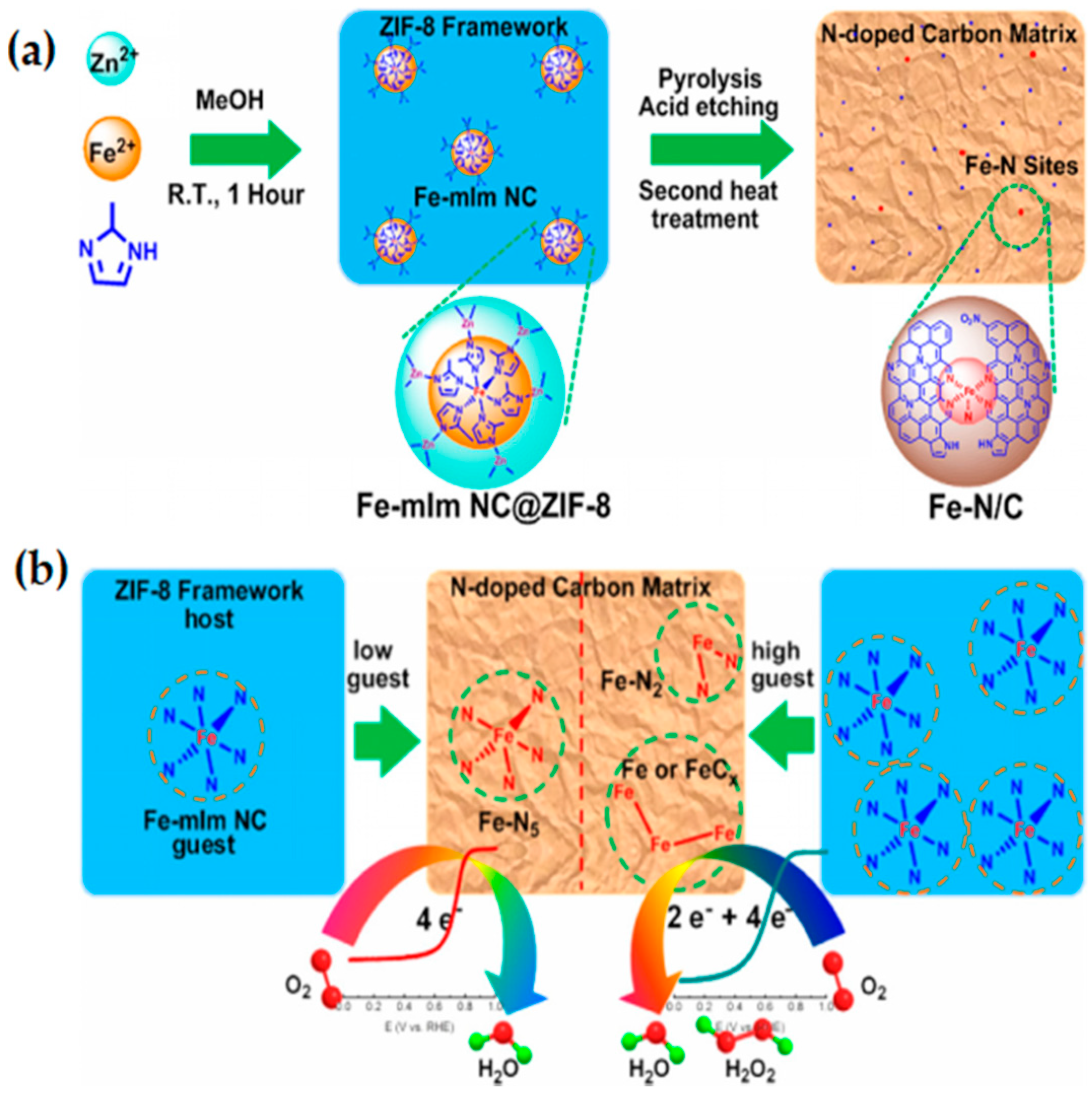
3.1.2. MOF-Derived Fe-Based Catalysts
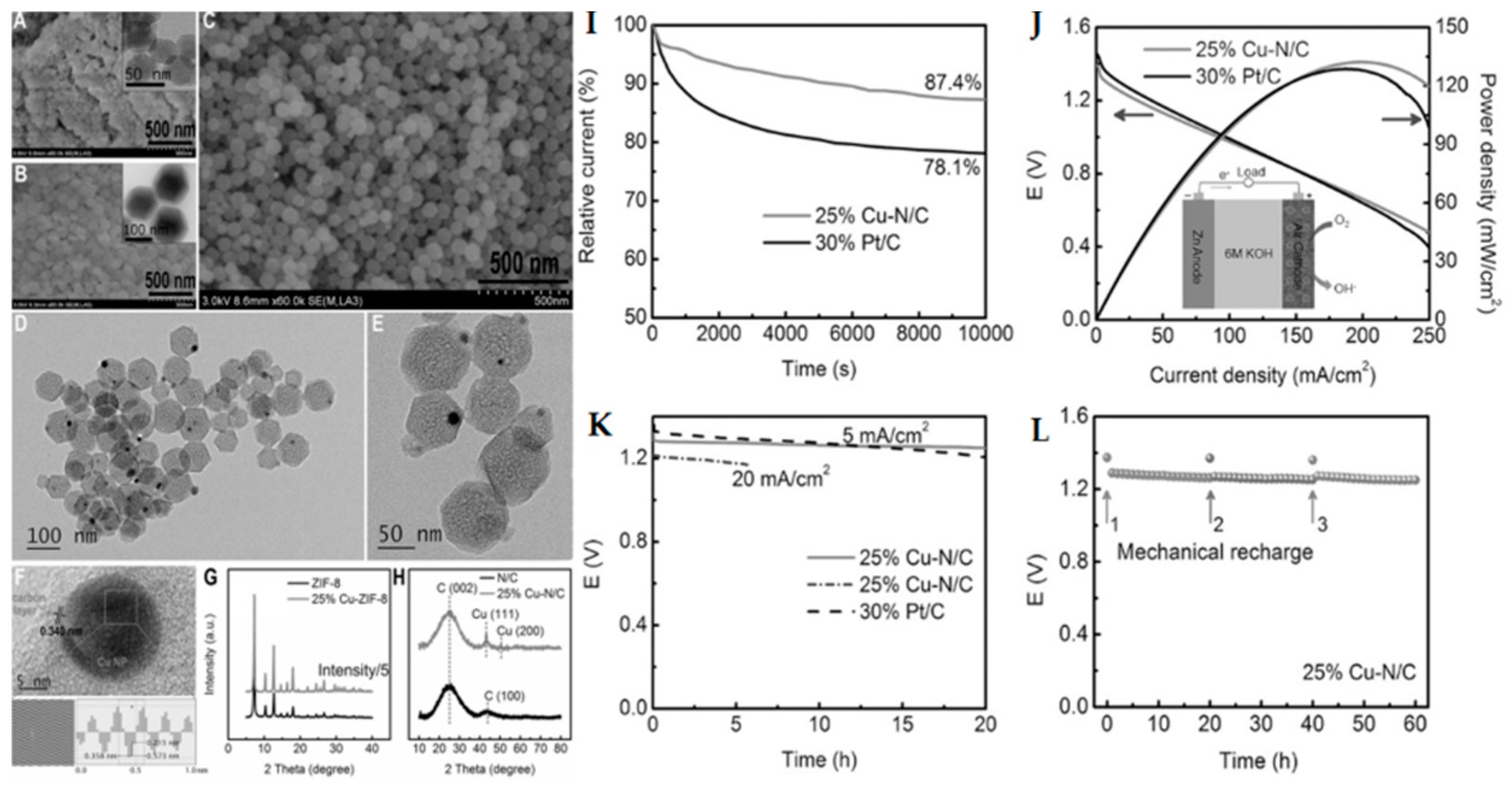
3.1.3. MOF-Derived Other Catalysts
3.2. MOF-Derived Bimetallic Catalyst
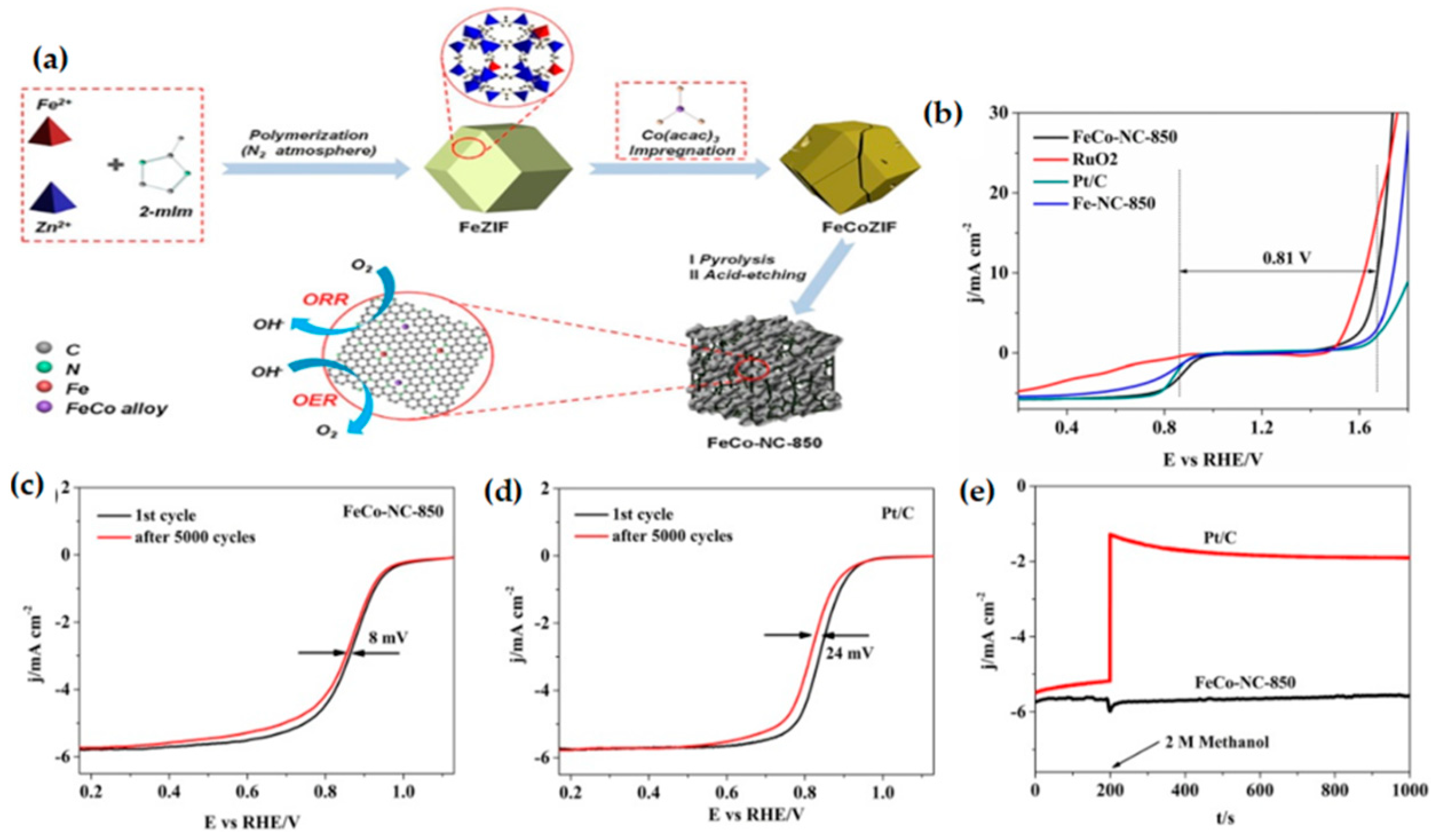
3.2.1. MOF-Derived Iron-Containing Bimetallic Catalysts
3.2.2. MOF-Derived Non-Iron Bimetallic Catalysts
4. MOF-Derived Non-Metallic Catalysts
5. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Xu, Q. Materials design for rechargeable metal-air batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- McLarnon, F.R.; Cairns, E.J. The secondary alkaline zinc electrode. J. Electrochem. Soc. 1991, 138, 645. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, P.; Liu, X.; Kai, W.; Liu, Y.; Wang, Y.; Xia, Y.Y.; Zhang, J. Challenges, Mitigation strategies and perspectives in development of zinc-electrode materials/fabrications for rechargeable zinc-air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc-air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- See, D.M.; White, R.E. Temperature and concentration dependence of the specific conductivity of concentrated solutions of potassium hydroxide. J. Chem. Eng. Data 1997, 42, 1266–1268. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-air batteries: Will they be the future electrochemical energy storage device of choice. ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Wu, M.; Prakash, J.; Sun, S. Rational design of multifunctional air electrodes for rechargeable Zn-air batteries: Recent progress and future perspectives. Energy Storage Mater. 2019, 21, 253–286. [Google Scholar] [CrossRef]
- Guan, Y.; Li, Y.; Luo, S.; Ren, X.; Deng, L.; Sun, L.; Mi, H.; Zhang, P.; Liu, J. Rational design of positive-hexagon-shaped two-dimensional ZIF-derived materials as improved bifunctional oxygen electrocatalysts for use as long-lasting rechargeable Zn-air batteries. Appl. Catal. B Environ. 2019, 256, 117871. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Chen, W.; Lu, Z.; Xu, J.; Pei, A.; Peng, Y.; Zheng, X.; Zhang, Z.; Chu, S.; et al. Breathing-mimicking electrocatalysis for oxygen evolution and reduction. Joule 2019, 3, 557–569. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Karahan, H.E.; Shao, Q.; Wei, L.; Chen, Y. Recent advances in materials and design of electrochemically rechargeable zinc-air batteries. Small 2018, 14, 1801929. [Google Scholar] [CrossRef] [PubMed]
- Holade, Y.; da Silva, R.G.; Servat, K.; Napporn, T.W.; Canaff, C.; de Andrade, A.R.; Kokoh, K.B. Facile synthesis of highly active and durable PdM/C (M = Fe, Mn) nanocatalysts for the oxygen reduction reaction in an alkaline medium. J. Mater. Chem. A 2016, 4, 8337–8349. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, X.; Liu, S.; Han, Y.; Meng, H.; Jiang, Q.; Zhao, S.; Wei, F.; Sun, J.; Tan, T.; et al. Superdurable bifunctional oxygen electrocatalyst for high-performance zinc-air batteries. J. Am. Chem. Soc. 2022, 144, 2694–2704. [Google Scholar] [CrossRef]
- Kuo, D.Y.; Paik, H.; Kloppenburg, J.; Faeth, B.; Shen, K.M.; Schlom, D.G.; Hautier, G.; Suntivich, J. Measurements of oxygen electroadsorption energies and oxygen evolution reaction on RuO2(110): A discussion of the sabatier principle and its role in electrocatalysis. J. Am. Chem. Soc. 2018, 140, 17597–17605. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Chen, Y.; Wang, S.; Gao, S.; Lou, X.W. Interfacing manganese oxide and cobalt in porous graphitic carbon polyhedrons boosts oxygen electrocatalysis for Zn–air batteries. Adv. Mater. 2019, 31, 1902339. [Google Scholar] [CrossRef]
- Fu, G.; Wang, J.; Chen, Y.; Liu, Y.; Tang, Y.; Goodenough, J.B.; Lee, J.M. Exploring indium-based ternary thiospinel as conceivable high-potential air-cathode for rechargeable Zn-air batteries. Adv. Energy Mater. 2018, 8, 1802263. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Zhang, Q. A review of precious-metal-free bifunctional oxygen electrocatalysts: Rational design and applications in Zn−air batteries. Adv. Funct. Mater. 2018, 28, 1803329. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, H.; Niu, T.; Wang, S.; Fu, G.; Jin, W.; Ma, T. Sulfurated metal–organic framework-derived nanocomposites for efficient bifunctional oxygen electrocatalysis and rechargeable Zn–air battery. ACS Sustain. Chem. Eng. 2020, 8, 9226–9234. [Google Scholar] [CrossRef]
- Xue, D.X.; Wang, Q.; Bai, J. Amide-functionalized metal–organic frameworks: Syntheses, structures and improved gas storage and separation properties. Coord. Chem. Rev. 2019, 378, 2–16. [Google Scholar] [CrossRef]
- Denning, S.; Majid, A.A.A.; Lucero, J.M.; Crawford, J.M.; Carreon, M.A.; Koh, C.A. Metal–organic framework HKUST-1 promotes methane hydrate formation for improved gas storage capacity. ACS Appl. Mater. Interfaces 2020, 12, 53510–53518. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.A.H. Implementing metal-organic frameworks for naturalgas storage. Crystals 2019, 9, 406. [Google Scholar] [CrossRef]
- Feng, X.; Chen, C.; He, C.; Chai, S.; Yu, Y.; Cheng, J. Non-thermal plasma coupled with MOF-74 derived Mn-Co-Ni-O porous composite oxide for toluene efficient degradation. J. Hazard. Mater. 2020, 383, 121143. [Google Scholar] [CrossRef]
- Lei, Z.; Deng, Y.; Wang, C. Multiphase surface growth of hydrophobic ZIF-8 on melamine sponge for excellent oil/water separation and effective catalysis in a Knoevenagel reaction. J. Mater. Chem. A 2018, 6, 3258–3263. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Liu, X.; Liang, T.; Zhang, R.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Iron-based metal–organic frameworks in drug delivery and biomedicine. ACS Appl. Mater. Interfaces 2021, 13, 9643–9655. [Google Scholar] [CrossRef]
- Chedid, G.; Yassin, A. Recent trends in covalent and metal organic frameworks for biomedical Applications. Nanomaterials 2018, 8, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Qu, C.; Guo, W.; Zou, R.; Xu, Q. Pristine metal–organic frameworks and their composites for energy storage and conversion. Adv. Mater. 2018, 30, 1702891. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xu, L.; Wang, D.; Li, Y. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 2019, 12, 2067–2080. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, L.; Sun, W.; Wang, Y. Two-dimensional metal-organic framework materials for energy conversion and storage. J. Power Sources 2020, 477, 228919. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Sapkota, P.; Kim, H. Zinc–air fuel cell, a potential candidate for alternative energy. J. Ind. Eng. Chem. 2009, 15, 445–450. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Antoine, O.; Durand, R. RRDE study of oxygen reduction on Pt nanoparticles inside Nafion®: H2O2 production in PEMFC cathode conditions. J. Appl. Electrochem. 2000, 30, 839–844. [Google Scholar] [CrossRef]
- Li, C.; Zhao, D.H.; Long, H.L.; Li, M. Recent advances in carbonized non-noble metal–organic frameworks for electrochemical catalyst of oxygen reduction reaction. Rare Met. 2021, 40, 2657–2689. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.N.; Cui, H.; Zhou, Z. Bifunctional electrocatalysts for rechargeable Zn-air batteries. Chin. J. Catal. 2019, 40, 1298–1310. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, Z.; Xu, X.; Zhang, Z.; Li, P.; Fang, X.; Zhang, K.; Yuan, K.; Liu, K.; Ran, G.; et al. Engineering active edge sites of fractal-shaped single-layer MoS2 catalysts for high-efficiency hydrogen evolution. Nano Energy 2018, 51, 786–792. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.P. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Deria, P.; Gómez-Gualdrón, D.A.; Hod, I.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Framework-topology-dependent catalytic activity of zirconium-based (porphinato)zinc(II) MOFs. J. Am. Chem. Soc. 2016, 138, 14449–14457. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Kanchanakungwankul, S.; Lu, Z.; Noh, H.; Syed, Z.H.; Farha, O.K.; Truhlar, D.G.; Hupp, J.T. Unexpected “spontaneous” evolution of catalytic, MOF-supported single Cu(II) cations to catalytic, MOF-supported Cu(0) nanoparticles. J. Am. Chem. Soc. 2020, 142, 21169–21177. [Google Scholar] [CrossRef]
- Yi, X.; He, X.; Yin, F.; Chen, B.; Li, G.; Yin, H. Co-CoO-Co3O4/N-doped carbon derived from metal-organic framework: The addition of carbon black for boosting oxygen electrocatalysis and Zn-air battery. Electrochim. Acta 2019, 295, 966–977. [Google Scholar] [CrossRef]
- Cho, K.; Han, S.H.; Suh, M.P. Copper-organic framework fabricated with CuS nanoparticles: Synthesis, electrical conductivity, and electrocatalytic activities for oxygen reduction reaction. Angew. Chem. Int. Ed. 2016, 55, 15301–15305. [Google Scholar] [CrossRef]
- Xiong, J.; Zhao, J.; Xiang, Z.; Li, C.; Wu, M.; Wang, X.; Pan, Y.; Lu, W.; Liu, R. Carbon nanotube@ZIF–derived Fe-N-doped carbon electrocatalysts for oxygen reduction and evolution reactions. J. Solid State Electrochem. 2019, 23, 2225–2232. [Google Scholar] [CrossRef]
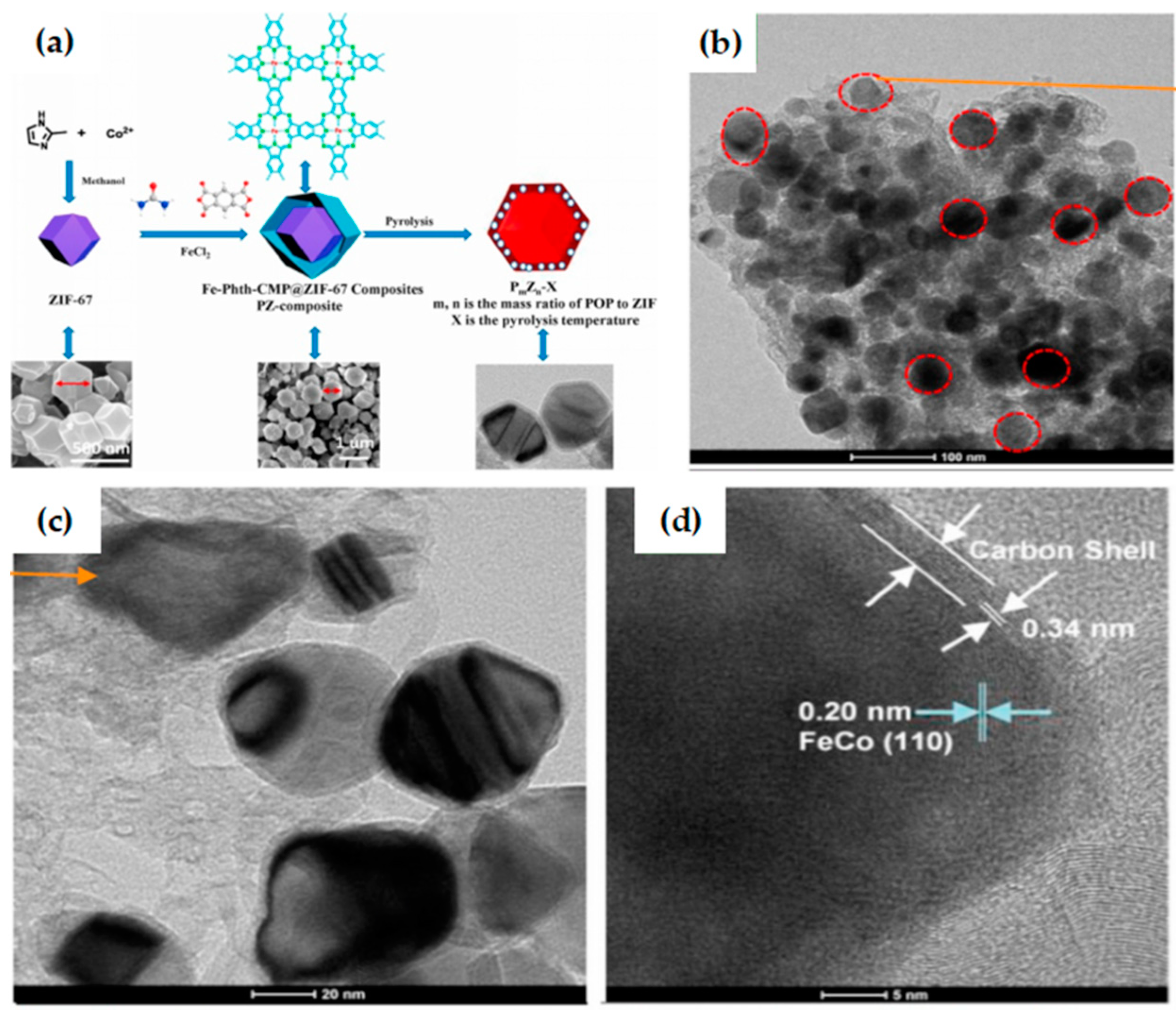
- Zhang, M.; Yan, F.; Bai, J.; Li, X.; Zheng, X.; Dou, J.; Wang, X.; Zhang, W.; Zhou, B. ZIF-67-templated synthesis of core-shell-structured POP@MOF composite derived porous carbon with embedding FeCo alloy nanoparticles as high-performance bifunctional oxygen electrocatalysts. Microporous Mesoporous Mater. 2021, 312, 110627. [Google Scholar] [CrossRef]
- Yang, L.; Xu, G.; Ban, J.; Zhang, L.; Xu, G.; Lv, Y.; Jia, D. Metal-organic framework-derived metal-free highly graphitized nitrogen-doped porous carbon with a hierarchical porous structure as an efficient and stable electrocatalyst for oxygen reduction reaction. J. Colloid Interface Sci. 2019, 535, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Zhao, Q.; Zhang, X.; Liu, R.; Ge, X.; Wang, G.; Zhao, H.; Cai, W. Metal-organic framework derived nitrogen-doped porous carbon@graphene sandwich-like structured composites as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Carbon 2016, 106, 74–83. [Google Scholar] [CrossRef]
- Tao, L.; Lin, C.Y.; Dou, S.; Feng, S.; Chen, D.; Liu, D.; Huo, J.; Xia, Z.; Wang, S. Creating coordinatively unsaturated metal sites in metal-organic-frameworks as efficient electrocatalysts for the oxygen evolution reaction: Insights into the active centers. Nano Energy 2017, 41, 417–425. [Google Scholar] [CrossRef]
- Zhao, W.; Wan, G.; Peng, C.; Sheng, H.; Wen, J.; Chen, H. Key Single-atom electrocatalysis in metal-organic framework (MOF)-derived bifunctional catalysts. ChemSusChem 2018, 11, 3473–3479. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W.; et al. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Ma, S.; Goenaga, G.A.; Call, A.V.; Liu, D.J. Cobalt imidazolate framework as precursor for oxygen reduction reaction electrocatalysts. Chem. A Eur. J. 2011, 17, 2063–2067. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF nanocrystals to Co–Nx/C nanorod array electrocatalysts for ORR, OER, and Zn-air batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Duan, X.; Pan, N.; Sun, C.; Zhang, K.; Zhu, X.; Zhang, M.; Song, L.; Zheng, H. MOF-derived Co-MOF,O-doped carbon as trifunctional electrocatalysts to enable highly efficient Zn–air batteries and water-splitting. J. Energy Chem. 2021, 56, 290–298. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Arnal, P.M.; Comotti, M.; Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 2006, 45, 8224–8227. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Zhou, S.; Yu, F.; Yu, M.; Chiang, C.Y.; Zhou, W.; Zhao, J.; Qiu, J. Metal–organic-framework-derived hybrid carbon nanocages as a bifunctional electrocatalyst for oxygen reduction and evolution. Adv. Mater. 2017, 29, 1700874. [Google Scholar] [CrossRef]
- Zhou, D.; Fu, H.; Long, J.; Shen, K.; Gou, X. Novel fusiform core-shell-MOF derived intact metal@carbon composite: An efficient cathode catalyst for aqueous and solid-state Zn-air batteries. J. Energy Chem. 2022, 64, 385–394. [Google Scholar] [CrossRef]
- Zhao, Q.; Xu, X.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H. Carbon-coated Co3O4 with porosity derived from zeolite imidazole framework-67 as a bi-functional electrocatalyst for rechargeable zinc air batteries. J. Nanoparticle Res. 2020, 22, 299. [Google Scholar] [CrossRef]
- Ding, D.; Shen, K.; Chen, X.; Chen, H.; Chen, J.; Fan, T.; Wu, R.; Li, Y. Multi-level architecture optimization of MOF-templated Co-based nanoparticles embedded in hollow N-doped carbon polyhedra for efficient OER and ORR. ACS Catal. 2018, 8, 7879–7888. [Google Scholar] [CrossRef]
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. A metal–organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576. [Google Scholar] [CrossRef]
- Wu, G.; Mack, N.H.; Gao, W.; Ma, S.; Zhong, R.; Han, J.; Baldwin, J.K.; Zelenay, P. Nitrogen-doped graphene-rich catalysts derived from heteroatom polymers for oxygen reduction in nonaqueous lithium–O2 battery cathodes. ACS Nano 2012, 6, 9764–9776. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Lin, X.; Tian, M.; An, X.; Fu, Y.; Li, R.; Jin, J.; Ma, J. MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: Extraordinary bi-functional electrocatalysts for OER and ORR. J. Mater. Chem. A 2015, 3, 17392–17402. [Google Scholar] [CrossRef]
- Zang, W.; Sumboja, A.; Ma, Y.; Zhang, H.; Wu, Y.; Wu, S.; Wu, H.; Liu, Z.; Guan, C.; Wang, J.; et al. Single Co atoms anchored in porous N-doped carbon for efficient zinc-air battery cathodes. ACS Catal. 2018, 8, 8961–8969. [Google Scholar] [CrossRef]
- Liu, K.; Kattel, S.; Mao, V.; Wang, G. Electrochemical and computational study of oxygen reduction reaction on nonprecious transition metal/nitrogen doped carbon nanofibers in acid medium. J. Phys. Chem. C 2016, 120, 1586–1596. [Google Scholar] [CrossRef]
- Yan, X.; Liu, K.; Wang, T.; You, Y.; Liu, J.; Wang, P.; Pan, X.; Wang, G.; Luo, J.; Zhu, J. Atomic interpretation of high activity on transition metal and nitrogen-doped carbon nanofibers for catalyzing oxygen reduction. J. Mater. Chem. A 2017, 5, 3336–3345. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, H.; Chen, Y.; Zhu, J.; Gao, L.; Jin, Z.; Ge, J.; Jiang, Z.; Chen, S.; Liu, C.; et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 site. Nano Energy 2018, 46, 396–403. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Xu, B.Z.; Fu, S.; Wan, G.; Yang, C.; Yao, S.; Song, J.; Zhou, H.; Du, D.; et al. Hierarchically porous M–N–C (M = Co and Fe) single-atom electrocatalysts with robust MNx active moieties enable enhanced ORR performance. Adv. Energy Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]
- Xu, M.; Ge, J.; Liu, C.; Xing, W. Recent advances in active sites identification and new M−N−C catalysts development towards ORR. J. Phys. Mater. 2021, 4, 044008. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.; Chu, Y.; Gao, N.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Construction and regulation of a surface protophilic environment to enhance oxygen reduction reaction electrocatalytic activity. ACS Appl. Mater. Interfaces 2020, 12, 41269–41276. [Google Scholar] [CrossRef]
- Yang, L.; Larouche, N.; Chenitz, R.; Zhang, G.; Lefèvre, M.; Dodelet, J.P. Activity, performance, and durability for the reduction of oxygen in PEM fuel cells, of Fe/N/C electrocatalysts obtained from the pyrolysis of metal-organic-framework and iron porphyrin precursors. Electrochim. Acta 2015, 159, 184–197. [Google Scholar] [CrossRef]
- Zhang, G.; Chenitz, R.; Lefèvre, M.; Sun, S.; Dodelet, J.P. Is iron involved in the lack of stability of Fe/N/C electrocatalysts used to reduce oxygen at the cathode of PEM fuel cells. Nano Energy 2016, 29, 111–125. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, L.; Liang, Y.; He, J.; Zhao, J.; Chen, J. Metal–organic-framework-derived Fe-N/C rlectrocatalyst with five-coordinated Fe-Nx sites for advanced oxygen reduction in acid media. ACS Catal. 2017, 7, 1655–1663. [Google Scholar] [CrossRef]
- Hijazi, I.; Bourgeteau, T.; Cornut, R.; Morozan, A.; Filoramo, A.; Leroy, J.; Derycke, V.; Jousselme, B.; Campidelli, S. Carbon nanotube-templated synthesis of covalent porphyrin network for oxygen reduction reaction. J. Am. Chem. Soc. 2014, 136, 6348–6354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Gyu Kim, M.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jia, N.; Yang, L.; Su, D.; Park, J.; Choi, Y.; Gong, K. Heterojunction nanowires having high activity and stability for the reduction of oxygen: Formation by self-assembly of iron phthalocyanine with single walled carbon nanotubes (FePc/SWNTs). J. Colloid Interface Sci. 2014, 419, 61–67. [Google Scholar] [CrossRef]
- Cheng, W.Z.; Liang, J.L.; Yin, H.B.; Wang, Y.J.; Yan, W.F.; Zhang, J.N. Bifunctional iron-phtalocyanine metal-organic framework catalyst for ORR, OER and rechargeable zinc–air battery. Rare Met. 2020, 39, 815–823. [Google Scholar] [CrossRef]
- Wen, Z.; Ci, S.; Hou, Y.; Chen, J. Facile one-pot, one-step synthesis of a carbon nanoarchitecture for an advanced multifunctonal electrocatalyst. Angew. Chem. Int. Ed. 2014, 53, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.; Yue, X.; Jia, J.; Guo, S. Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.X.; Wang, J.; Zhang, Y.W.; Xu, W.L.; Xing, W.; Xu, D.; Zhang, Y.F.; Zhang, X.B. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem. Int. Ed. 2014, 53, 14235–14239. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.; Liang, Y.; Kong, B.; Zhang, J.; Song, J.; Bao, Q.; Simon, G.P.; Jiang, S.P.; Wang, H. Nitrogen-doped nanoporous carbon/graphene nano-sandwiches: Synthesis and application for efficient oxygen reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. [Google Scholar] [CrossRef]
- Zhao, P.; Hua, X.; Xu, W.; Luo, W.; Chen, S.; Cheng, G. Metal–organic framework-derived hybrid of Fe3C nanorod-encapsulated, N-doped CNTs on porous carbon sheets for highly efficient oxygen reduction and water oxidation. Catal. Sci. Technol. 2016, 6, 6365–6371. [Google Scholar] [CrossRef]
- Wang, X.X.; Prabhakaran, V.; He, Y.; Shao, Y.; Wu, G. Iron-free cathode catalysts for proton-exchange-membrane fuel cells: Cobalt catalysts and the peroxide mitigation approach. Adv. Mater. 2019, 31, 1805126. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Cullen, D.A.; Hwang, S.; Wang, M.; Li, B.; Liu, K.; Karakalos, S.; Lucero, M.; Zhang, H.; et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 2018, 1, 935–945. [Google Scholar] [CrossRef]
- Han, X.; Zhang, T.; Chen, W.; Dong, B.; Meng, G.; Zheng, L.; Yang, C.; Sun, X.; Zhuang, Z.; Wang, D.; et al. Mn-N4 oxygen reduction electrocatalyst: Operando investigation of active sites and high performance in zinc–air battery. Adv. Energy Mater. 2021, 11, 2002753. [Google Scholar] [CrossRef]
- Li, F.; Qin, T.; Sun, Y.; Jiang, R.; Yuan, J.; Liu, X.; O’Mullane, A.P. Preparation of a one-dimensional hierarchical MnO@CNT@Co–N/C ternary nanostructure as a high-performance bifunctional electrocatalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2021, 9, 22533–22543. [Google Scholar] [CrossRef]
- Najam, T.; Cai, X.; Aslam, M.K.; Tufail, M.K.; Shah, S.S.A. Nano-engineered directed growth of Mn3O4 quasi-nanocubes on N-doped polyhedrons: Efficient electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2020, 45, 12903–12910. [Google Scholar] [CrossRef]
- Wahab, A.; Iqbal, N.; Noor, T.; Ashraf, S.; Raza, M.A.; Ahmad, A.; Khan, U.A. Thermally reduced mesoporous manganese MOF@reduced graphene oxide nanocomposite as bifunctional electrocatalyst for oxygen reduction and evolution. RSC Adv. 2020, 10, 27728–27742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, S.; Dou, M.; Liu, H.; Gu, L.; Wang, F. Systematic study of the transition-metal (Fe, Co, Ni, Cu) phthalocyanine as electrocatalyst for oxygen reduction and its evaluation by DFT. RSC Adv. 2016, 6, 67049–67056. [Google Scholar] [CrossRef]
- Iwase, K.; Yoshioka, T.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Copper-modified covalent triazine frameworks as non-noble-metal electrocatalysts for oxygen reduction. Angew. Chem. Int. Ed. 2015, 54, 11068–11072. [Google Scholar] [CrossRef]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu complexes that catalyze the oxygen reduction reaction. Coord. Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Lai, Q.; Zhu, J.; Zhao, Y.; Liang, Y.; He, J.; Chen, J. MOF-based metal-doping-induced synthesis of hierarchical porous Cu-N/C oxygen reduction electrocatalysts for Zn–air batteries. Small 2017, 13, 1700740. [Google Scholar] [CrossRef]
- Ge, F.; Qiao, Q.; Chen, X.; Wu, Y. Probing the catalytic activity of M-N4−xOx embedded graphene for the oxygen reduction reaction by density functional theory. Front. Chem. Sci. Eng. 2021, 15, 1206–1216. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, J.; Wang, H.; Shao, M. Carbon-based electrocatalysts for hydrogen and oxygen evolution reactions. ACS Catal. 2017, 7, 7855–7865. [Google Scholar] [CrossRef]
- Peng, H.; Liu, F.; Liu, X.; Liao, S.; You, C.; Tian, X.; Nan, H.; Luo, F.; Song, H.; Fu, Z.; et al. Effect of transition metals on the structure and performance of the doped carbon catalysts derived from polyaniline and melamine for ORR application. ACS Catal. 2014, 4, 3797–3805. [Google Scholar] [CrossRef]
- Zeng, H.; Liu, X.; Chen, F.; Chen, Z.; Fan, X.; Lau, W. Single atoms on a nitrogen-doped boron phosphide monolayer: A new promising bifunctional electrocatalyst for ORR and OER. ACS Appl. Mater. Interfaces 2020, 12, 52549–52559. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Lu, M.; Yang, S.; Xu, C.; Liu, Z.; Lee, J.Y. Activity of transition-metal (Manganese, Iron, Cobalt, and Nickel) phosphates for oxygen electrocatalysis in alkaline solution. ChemCatChem 2016, 8, 372–379. [Google Scholar] [CrossRef]
- Singh, A.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem 2013, 5, 652–676. [Google Scholar] [CrossRef]
- Wang, C.; Li, D.; Chi, M.; Pearson, J.; Rankin, R.B.; Greeley, J.; Duan, Z.; Wang, G.; van der Vliet, D.; More, K.L.; et al. Rational development of ternary alloy electrocatalysts. J. Phys. Chem. Lett. 2012, 3, 1668–1673. [Google Scholar] [CrossRef]
- Li, G.; Zheng, K.; Xu, C. An ingenious approach for ZIFs derived N-doped hierarchical porous carbon hybrids with FeCo alloy nanoparticles as efficient bifunctional oxygen electrocatalysts. Appl. Surf. Sci. 2019, 487, 496–502. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Chen, J.; Chen, Y.; Zhang, C.; Luo, Y.; Wang, G.; Wang, R. Bi-functional electrocatalysis through synergetic coupling strategy of atomically dispersed Fe and Co active sites anchored on 3D nitrogen-doped carbon sheets for Zn-air battery. J. Catal. 2021, 397, 223–232. [Google Scholar] [CrossRef]
- Duan, X.; Ren, S.; Pan, N.; Zhang, M.; Zheng, H. MOF-derived Fe,Co@N–C bifunctional oxygen electrocatalysts for Zn–air batteries. J. Mater. Chem. A 2020, 8, 9355–9363. [Google Scholar] [CrossRef]
- Fang, H.; Huang, T.; Sun, Y.; Kang, B.; Liang, D.; Yao, S.; Yu, J.; Dinesh, M.M.; Wu, S.; Lee, J.Y.; et al. Metal-organic framework-derived core-shell-structured nitrogen-doped CoCx/FeCo@C hybrid supported by reduced graphene oxide sheets as high performance bifunctional electrocatalysts for ORR and OER. J. Catal. 2019, 371, 185–195. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Rähn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional oxygen electrocatalysis on mixed metal phthalocyanine-modified carbon nanotubes prepared via pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Hu, F.; Yu, D.; Han, S.; Li, L.; Peng, S. Hierarchical FeC/MnO2 composite with in-situ grown CNTs as an advanced trifunctional catalyst for water splitting and metal-air batteries. Ceram. Int. 2021, 47, 18424–18432. [Google Scholar] [CrossRef]
- Lin, S.Y.; Xia, L.X.; Zhang, L.; Feng, J.J.; Zhao, Y.; Wang, A.J. Highly active Fe centered FeM-N-doped carbon (M = Co/Ni/Mn): A general strategy for efficient oxygen conversion in Zn–air battery. Chem. Eng. J. 2021, 424, 130559. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, C.; Gao, Y.; Gong, Y.; Liu, H.; He, J. FeNi Nanoparticles coated on N-doped ultrathin graphene-like nanosheets as stable bifunctional catalyst for Zn-air batteries. Chem. Asian J. 2021, 16, 1592–1602. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.; Liu, J.; Ma, X.Y.D.; Kong, J.; Zhang, Y.; Yan, T.; Lu, X. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, X.; Zeng, K.; Yan, J.; Sun, Z.; Rümmeli, M.H.; Yang, R. A self-jet vapor-phase growth of 3D FeNi@NCNT clusters as efficient oxygen electrocatalysts for zinc-air batteries. Small 2021, 17, 2006183. [Google Scholar] [CrossRef]
- Li, G.L.; Yang, B.B.; Xu, X.C.; Cao, S.; Shi, Y.; Yan, Y.; Song, X.; Hao, C. FeNi alloy nanoparticles encapsulated in carbon shells supported on N-doped graphene-like carbon as efficient and stable bifunctional oxygen electrocatalysts. Chem. A Eur. J. 2020, 26, 2890–2896. [Google Scholar] [CrossRef]
- Chen, K.; Kim, S.; Rajendiran, R.; Prabakar, K.; Li, G.; Shi, Z.; Jeong, C.; Kang, J.; Li, O.L. Enhancing ORR/OER active sites through lattice distortion of Fe-enriched FeNi3 intermetallic nanoparticles doped N-doped carbon for high-performance rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 582, 977–990. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Liu, G.; Ye, Y.; Quan, Y.; Wang, C.; Wei, W.; Zhu, W.; Xu, C.; Li, H.; et al. In situ confinement growth of peasecod-like N-doped carbon nanotubes encapsulate bimetallic FeCu alloy as a bifunctional oxygen reaction cathode electrocatalyst for sustainable energy batteries. J. Alloy. Compd. 2020, 826, 154152. [Google Scholar] [CrossRef]
- Yao, Z.; Chen, D.; Li, Y.; Lyu, Q.; Wang, J.; Zhong, Q. MOF-derived multi-metal embedded N-doped carbon sheets rich in CNTs as efficient bifunctional oxygen electrocatalysts for rechargeable ZABs. Int. J. Hydrogen Energy 2022, 47, 984–992. [Google Scholar] [CrossRef]
- Wu, M.; Guo, B.; Nie, A.; Liu, R. Tailored architectures of FeNi alloy embedded in N-doped carbon as bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. J. Colloid Interface Sci. 2020, 561, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Leng, Y.; Xiang, Z. FeNi co-doped electrocatalyst synthesized via binary ligand strategy as a bifunctional catalyst for Zn-air flow battery. Chem. Eng. Sci. 2022, 247, 117038. [Google Scholar] [CrossRef]
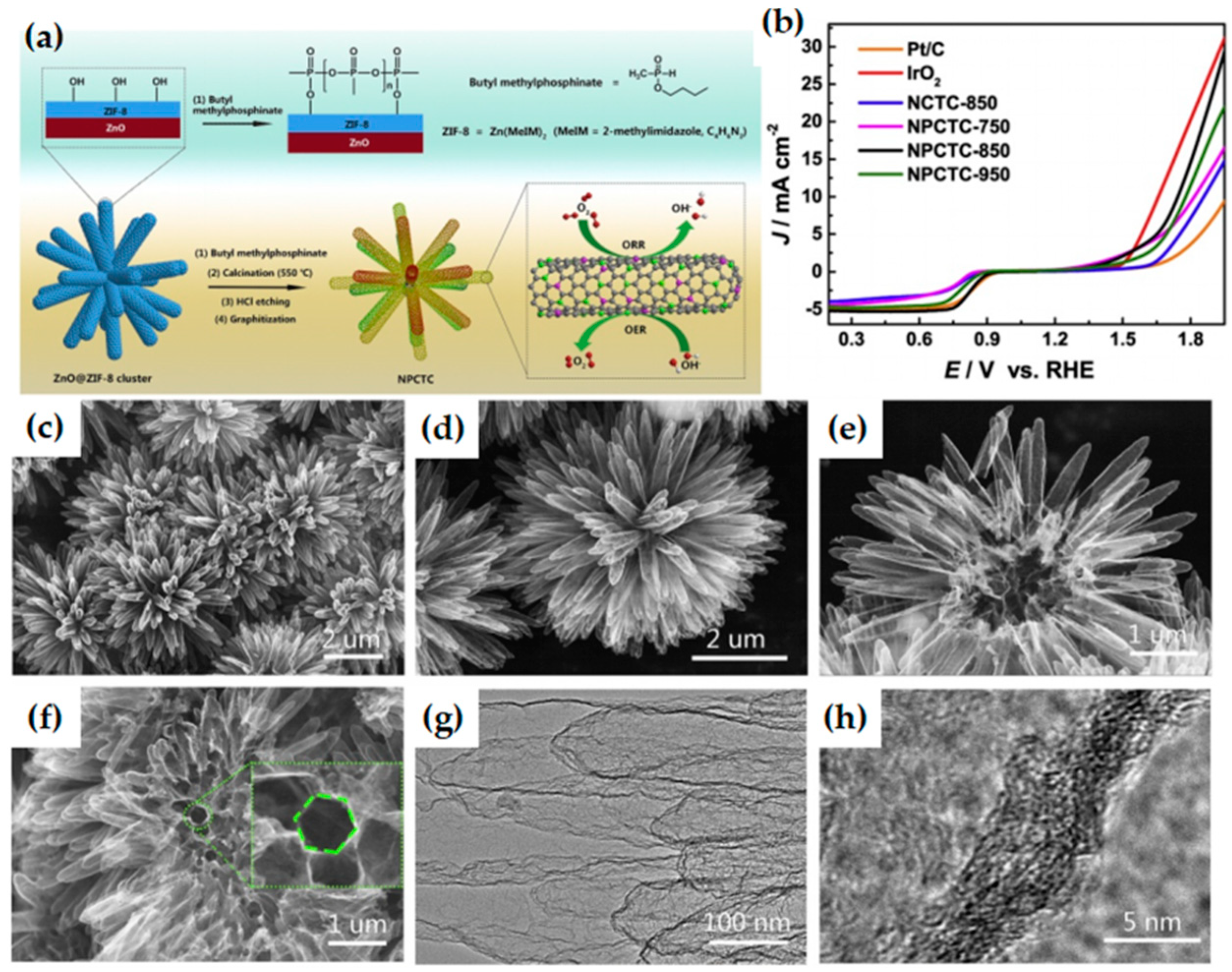
- Zhao, S.; Wang, Y.; Dong, J.; He, C.T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Huang, K.; Guo, S.; Wang, R.; Lin, S.; Hussain, N.; Wei, H.; Deng, B.; Long, Y.; Lei, M.; Tang, H.; et al. Two-dimensional MOF/MOF derivative arrays on nickel foam as efficient bifunctional coupled oxygen electrodes. Chin. J. Catal. 2020, 41, 1754–1760. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, S.; Xue, H.; Pang, H. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Zheng, X.; Cao, Y.; Liu, D.; Cai, M.; Ding, J.; Liu, X.; Wang, J.; Hu, W.; Zhong, C. Bimetallic metal–organic-framework/reduced graphene oxide composites as bifunctional electrocatalysts for rechargeable Zn–air batteries. ACS Appl. Mater. Interfaces 2019, 11, 15662–15669. [Google Scholar] [CrossRef]
- Li, Z.; He, H.; Cao, H.; Sun, S.; Diao, W.; Gao, D.; Lu, P.; Zhang, S.; Guo, Z.; Li, M.; et al. Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl. Catal. B Environ. 2019, 240, 112–121. [Google Scholar] [CrossRef]
- Zhang, G.; Xing, J.; Zhao, Y.; Yang, F. Hierarchical N,P co-doped graphene aerogels framework assembling vertically grown CoMn-LDH nanosheets as efficient bifunctional electrocatalyst for rechargeable zinc-air battery. J. Colloid Interface Sci. 2021, 590, 476–486. [Google Scholar] [CrossRef]
- Deng, C.; Wu, K.H.; Scott, J.; Zhu, S.; Amal, R.; Wang, D.W. Ternary MnO/CoMn alloy@N-doped graphitic composites derived from a bi-metallic pigment as bi-functional electrocatalysts. J. Mater. Chem. A 2019, 7, 20649–20657. [Google Scholar] [CrossRef]
- Duarte, M.F.P.; Rocha, I.M.; Figueiredo, J.L.; Freire, C.; Pereira, M.F.R. CoMn-LDH@carbon nanotube composites: Bifunctional electrocatalysts for oxygen reactions. Catal. Today 2018, 301, 17–24. [Google Scholar] [CrossRef]
- Du, J.; Chen, C.; Cheng, F.; Chen, J. Rapid synthesis and efficient electrocatalytic oxygen reduction/evolution reaction of CoMn2O4 nanodots supported on graphene. Inorg. Chem. 2015, 54, 5467–5474. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, D.; Lv, Y.; Cao, D. Nitrogen-doped graphitic carbons with encapsulated CoNi bimetallic nanoparticles as bifunctional electrocatalysts for rechargeable Zn-air batteries. Carbon 2019, 144, 8–14. [Google Scholar] [CrossRef]
- Hou, J.J.; Lu, L.; Shen, Z.Y.; Gao, H.; Liu, H. Rational design of CoNi alloy and atomic Co/Ni composite as an efficient electrocatalyst. Surf. Innov. 2021, 9, 37–48. [Google Scholar]
- Wan, W.; Liu, X.; Li, H.; Peng, X.; Xi, D.; Luo, J. 3D carbon framework-supported CoNi nanoparticles as bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries. Appl. Catal. B Environ. 2019, 240, 193–200. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, W.; Di, J.; Zeng, S.; Qiao, J.; Wang, X.; Lv, B.; Li, Q. CoNi nanoparticles anchored inside carbon nanotube networks by transient heating: Low loading and high activity for oxygen reduction and evolution. J. Energy Chem. 2021, 54, 63–71. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. Acs Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Gu, J.; Magagula, S.; Zhao, J.; Chen, Z. Boosting ORR/OER activity of graphdiyne by simple heteroatom doping. Small Methods 2019, 3, 1800550. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, H.; Chen, S.; Yu, X.; Zhu, J.; Xu, J.; Zhang, K.A.I.; Zhang, C.; Liu, T. Metal-free multi-heteroatom-doped carbon bifunctional electrocatalysts derived from a covalent triazine polymer. Small 2020, 16, 2004342. [Google Scholar] [CrossRef]
- Lee, C.H.; Jun, B.; Lee, S.U. Metal-free oxygen evolution and oxygen reduction reaction bifunctional electrocatalyst in alkaline media: From mechanisms to structure-catalytic activity relationship. ACS Sustain. Chem. Eng. 2018, 6, 4973–4980. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Lim, J.; Jung, J.W.; Kim, N.Y.; Lee, G.Y.; Lee, H.J.; Lee, Y.; Choi, D.S.; Yoon, K.R.; Kim, Y.H.; Kim, I.D.; et al. N2-dopant of graphene with electrochemically switchable bifunctional ORR/OER catalysis for Zn-air battery. Energy Storage Mater. 2020, 32, 517–524. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, G.L.; Yang, S.; Spivey, J.J. Nitrogen-doped fullerene as a potential catalyst for hydrogen fuel cells. J. Am. Chem. Soc. 2013, 135, 3315–3318. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of active sites for oxygen reduction reactions by transformation of nitrogen functionalities in nitrogen-doped carbon nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jiang, C.; Wang, J.; Lu, L. High-rate oxygen electroreduction over graphitic-N species exposed on 3D hierarchically porous nitrogen-doped carbons. Angew. Chem. Int. Ed. 2014, 53, 9503–9507. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liang, H.; Parvez, K.; Zhuang, X.; Feng, X.; Müllen, K. Nitrogen-doped carbon nanosheets with size-defined mesopores as highly efficient metal-free catalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2014, 53, 1570–1574. [Google Scholar] [CrossRef]
- Qian, Y.; Hu, Z.; Ge, X.; Yang, S.; Peng, Y.; Kang, Z.; Liu, Z.; Lee, J.Y.; Zhao, D. A metal-free ORR/OER bifunctional electrocatalyst derived from metal-organic frameworks for rechargeable Zn-air batteries. Carbon 2017, 111, 641–650. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Z.; Wang, Q.; Ye, H.; Li, M.; Zhu, L.; Cao, X. Ultrathin, highly branched carbon nanotube cluster with outstanding oxygen electrocatalytic performance. Electrochim. Acta 2018, 282, 224–232. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.L.; Yang, J.X.; Loh, K.P. Structure-directing role of graphene in the synthesis of metal-organic framework nanowire. J. Am. Chem. Soc. 2010, 132, 14487–14495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jayaramulu, K.; Maji, T.K.; Rao, C.N.R. Hybrid nanocomposites of ZIF-8 with graphene oxide exhibiting tunable morphology, significant CO2 uptake and other novel properties. Chem. Commun. 2013, 49, 4947–4949. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, T.; Wen, Z.; Mao, S.; Cui, S.; Chen, J. Metal-organic framework-derived nitrogen-doped core-shell-structured porous Fe/Fe3C@C nanoboxes supported on graphene sheets for efficient oxygen reduction reactions. Adv. Energy Mater. 2014, 4, 1400337. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.H.; Cui, S.M.; Ci, S.Q.; Mao, S.; Chen, J.H. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2015, 25, 872–882. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Y.X.; Wu, Z.X.; Liang, Y.; Leong, S.; Kong, B.; Zhang, X.Y.; Zhao, D.Y.; Simon, G.P.; Wang, H.T. A graphene-directed assembly route to hierarchically porous Co-Nx/C catalysts for high-performance oxygen reduction. J. Mater. Chem. A 2015, 3, 16867–16873. [Google Scholar] [CrossRef]
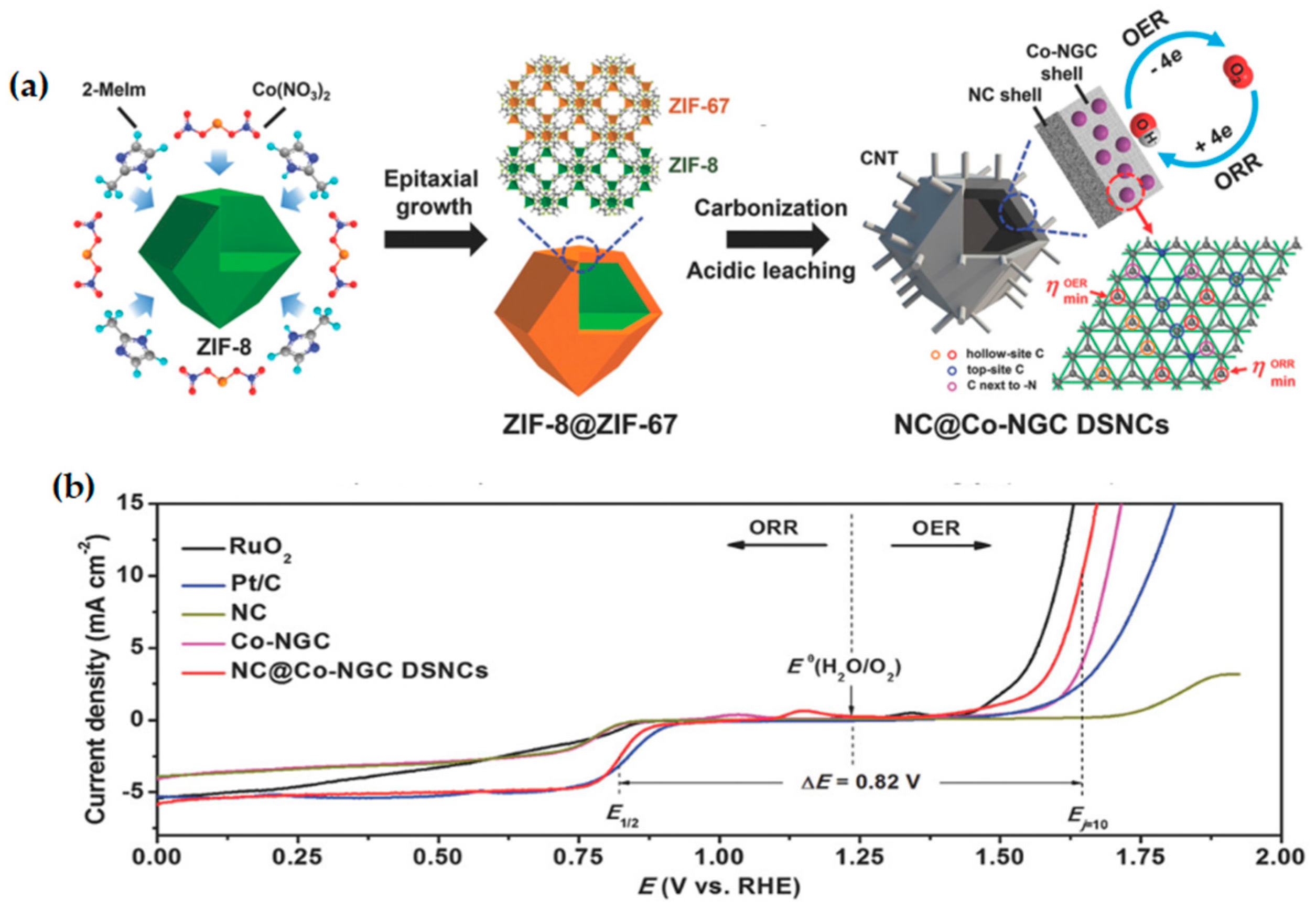





| Catalyst | Electrolyte | ORR | OER | ΔE = Ej=10 − E1/2 | Ref. | |
|---|---|---|---|---|---|---|
| E(onset) V vs. RHE | E(1/2) V vs. RHE | E(overpotential) at 10 mA cm−2 | ||||
| Co-Nx/C | 0.1 M KOH | \ | 0.877 V | 300 mV | 0.653 V | [59] |
| Co-MOF-800 | 0.1 M KOH | \ | 0.84 V | 520 mV | 0.84 V | [60] |
| NC@Co-NGC DSNC | 0.1 M KOH | 0.92 V | 0.82 V | 410 mV | 0.82 V | [63] |
| Co3O4/HNCP-40 | 0.1 M KOH | \ | 0.845 V | 350 mV | 0.729 V | [65] |
| Co/CoO@NSC | 0.1 M KOH | 0.895 V | 0.779 V | 380 mV | 0.775 V | [71] |
| NC-Co SA | 0.1 M KOH | 1.00 V | 0.87 V | 360 mV | 0.72 V | [72] |
| FePPc@CB | 0.1 M KOH | \ | 0.908 V | 358 mV | 0.68 V | [85] |
| Fe3C@NCNT/NPC | 0.1 M KOH | 1.0 V | 0.9 V | 270 mV | \ | [88] |
| Fe-N-HPC-900 | 0.1 M KOH | 1.004 V | 0.886 V | 520 mV | 0.81 V | [51] |
| MnBDC@75% rGO | 0.1 M KOH | 1.09 V | 0.94 V | 610 mV | 0.90 V | [95] |
| Catalyst | Electrolyte | ORR | OER | BET Specific Surface Area | Ref | ||
|---|---|---|---|---|---|---|---|
| E(onset) V vs. RHE | E(1/2) V vs. RHE | E(overpotential) at 10 mA cm−2 | Tafel Slope | ||||
| FeCo-NC-850 | 0.1 M KOH | 0.997 V | 0.864 V | 445 mV | 117 mV dec−1 | 553 m2 g−1 | [107] |
| P2Z3-900 | 0.1 M KOH | 0.950 V | 0.807 V | 370 mV | 57 mV dec−1 | 153.22 m2 g−1 | [52] |
| A-FeCoO@NCNs | 0.1 M KOH | 1.03 V | 0.87 V | 440 mV | 80 mV dec−1 | 809.83 m2 g−1 | [108] |
| FeCo-N-C-700 | 0.1 M KOH | 0.013 V | 0.896 V | 370 mV | 72 mV dec−1 | 332 m2 g−1 | [109] |
| CoCx/FeCo@C | 0.1 M KOH | 1.018 V | 0.965 V | 390 mV | 77.1 mV dec−1 | \ | [110] |
| B-FeNi-N/C-1000 | 0.1 M KOH | \ | 0.9 V | 390 mV | 283 mV dec−1 | 832.7 | [120] |
| 1.5FeNi@NCNT | 0.1 M KOH | 0.95 V | 0.86 V | 230 mV | 55 mV dec−1 | 870.99 m2 g−1 | [121] |
| FeNi-NCS-2 | 0.1 M KOH | \ | 0.867 V | 395 mV | 82.3 mV dec−1 | 454.77 m2 g−1 | [122] |
| R-NCM | 1 M KOH | 0.90 V | \ | 319 mV | 78.2 mV dec−1 | \ | [124] |
| CoN-MOF/rGO | 1 M KOH | 0.88 V | \ | 318 mV | 48 mV dec−1 | \ | [126] |
| CoPNi-N/C | 0.1 M KOH | 0.93 V | 0.84 V | 310 mV | 72 mV dec−1 | 446.8 m2 g−1 | [127] |
| Catalyst | Electrolyte | ORR | OER | BET Specific Surface Area | ΔE = Ej=10 − E1/2 | Ref. | |
|---|---|---|---|---|---|---|---|
| E(onset) V vs. RHE | E(1/2) V vs. RHE | E(j=10 mA cm−2) V vs. RHE | |||||
| BNPC-1100 | 0.1 M KOH | 0.894 V | 0.793 V | 1.38 V | 1348 m2 g−1 | 0.587 V | [146] |
| NPCTC-850 | 0.1 M KOH | 0.92 V | 0.83 V | 1.74 V | 912 m2 g−1 | 0.90 V | [147] |
| N-PC@G-0.02 | 0.1 M KOH | 1.01 V | 0.80 V | 1.63 V | 1094.03 m2 g−1 | 0.83 V | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, D.; Hu, C.; Gao, Z.; Yang, B.; Li, Q.; Zhan, X.; Tong, X.; Tian, J. Metal–Organic Frameworks (MOFs) Derived Materials Used in Zn–Air Battery. Materials 2022, 15, 5837. https://doi.org/10.3390/ma15175837
Song D, Hu C, Gao Z, Yang B, Li Q, Zhan X, Tong X, Tian J. Metal–Organic Frameworks (MOFs) Derived Materials Used in Zn–Air Battery. Materials. 2022; 15(17):5837. https://doi.org/10.3390/ma15175837
Chicago/Turabian StyleSong, Dongmei, Changgang Hu, Zijian Gao, Bo Yang, Qingxia Li, Xinxing Zhan, Xin Tong, and Juan Tian. 2022. "Metal–Organic Frameworks (MOFs) Derived Materials Used in Zn–Air Battery" Materials 15, no. 17: 5837. https://doi.org/10.3390/ma15175837
APA StyleSong, D., Hu, C., Gao, Z., Yang, B., Li, Q., Zhan, X., Tong, X., & Tian, J. (2022). Metal–Organic Frameworks (MOFs) Derived Materials Used in Zn–Air Battery. Materials, 15(17), 5837. https://doi.org/10.3390/ma15175837







