Vacancy Energetics and Diffusivities in the Equiatomic Multielement Nb-Mo-Ta-W Alloy
Abstract
:1. Introduction
2. Methods
2.1. Molecular Statics Calculations
2.2. Metropolis Monte-Carlo Method
- 1.
- Randomly choose a type of atom-pair with different atom species (e.g., Nb and Ta, Nb and Mo), and attempt swaps for 100 times for candidates that belong to the chosen type.
- 2.
- Run a conjugate gradient (CG) minimization of the structure at that point.
- 3.
- Repeat steps 1 and 2 until the total energy of the supercell converges with the number of iterations.
2.3. Vacancy Formation and Migration Energies
3. Results
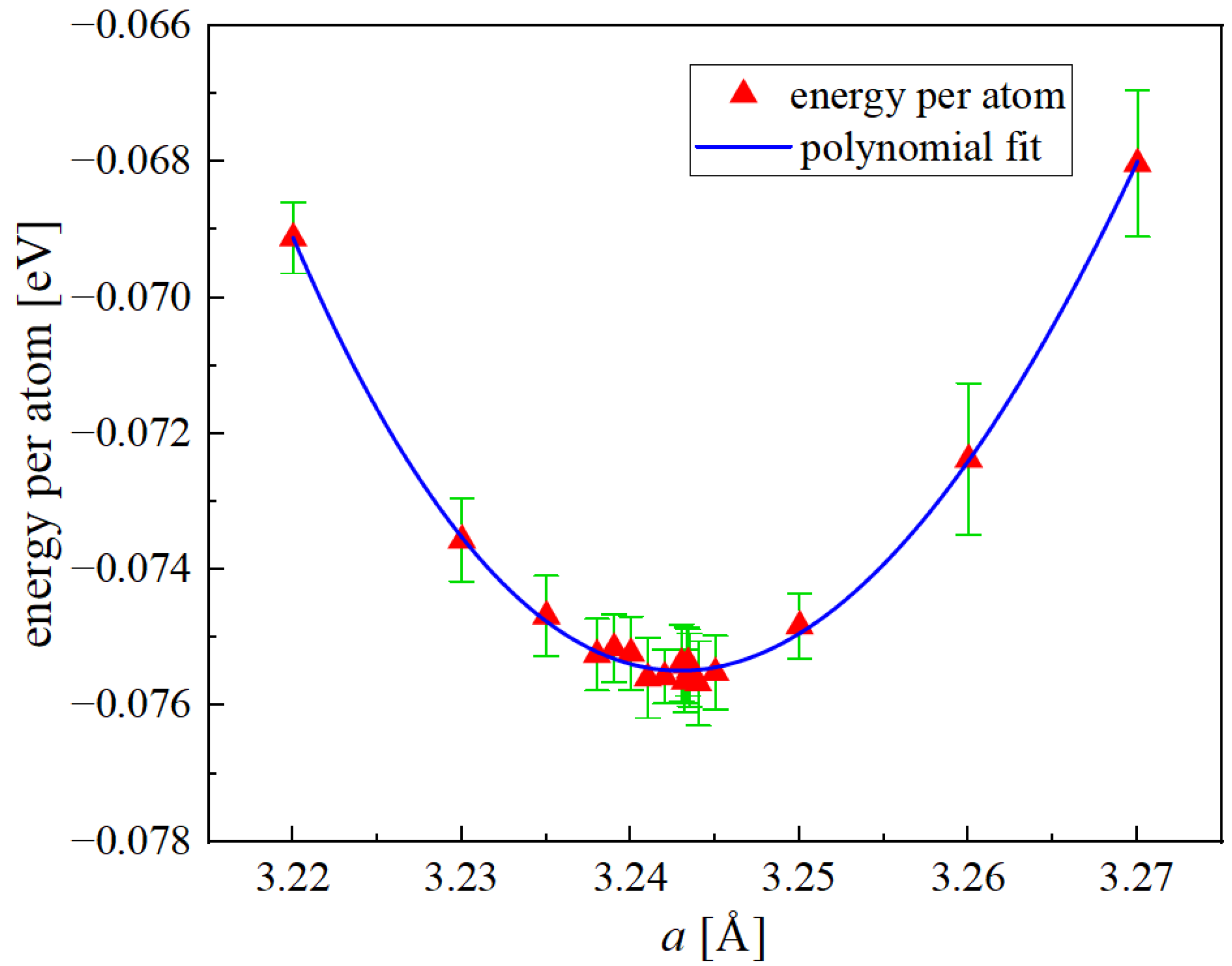
3.1. Equilibrium Lattice Parameter
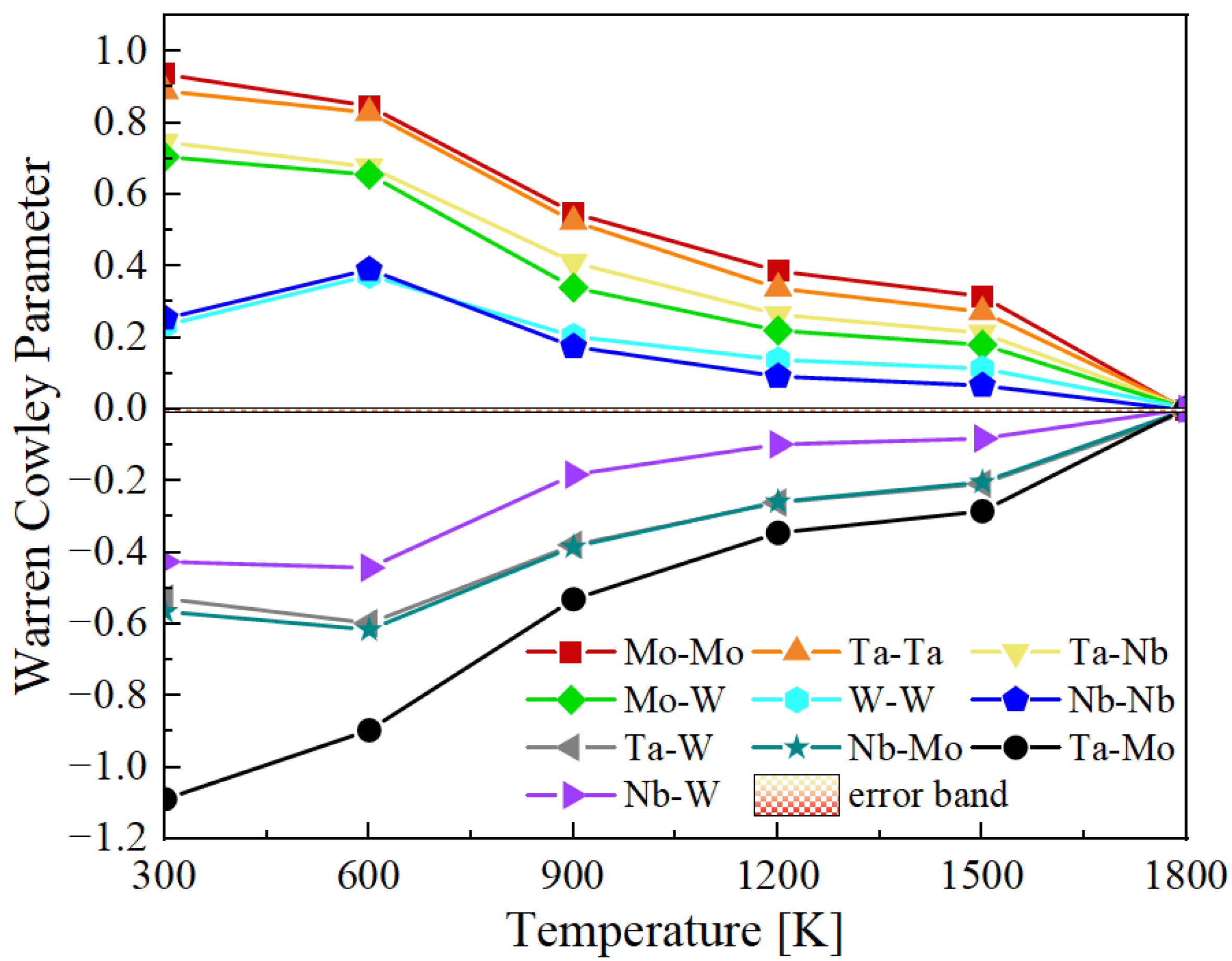
3.2. Short Range Order Calculation
3.3. Vacancy Energetics in Bulk Systems
3.3.1. Vacancy Formation Energies in Bulk Nb-Mo-Ta-W
3.3.2. Effective Vacancy Formation Energy in Bulk
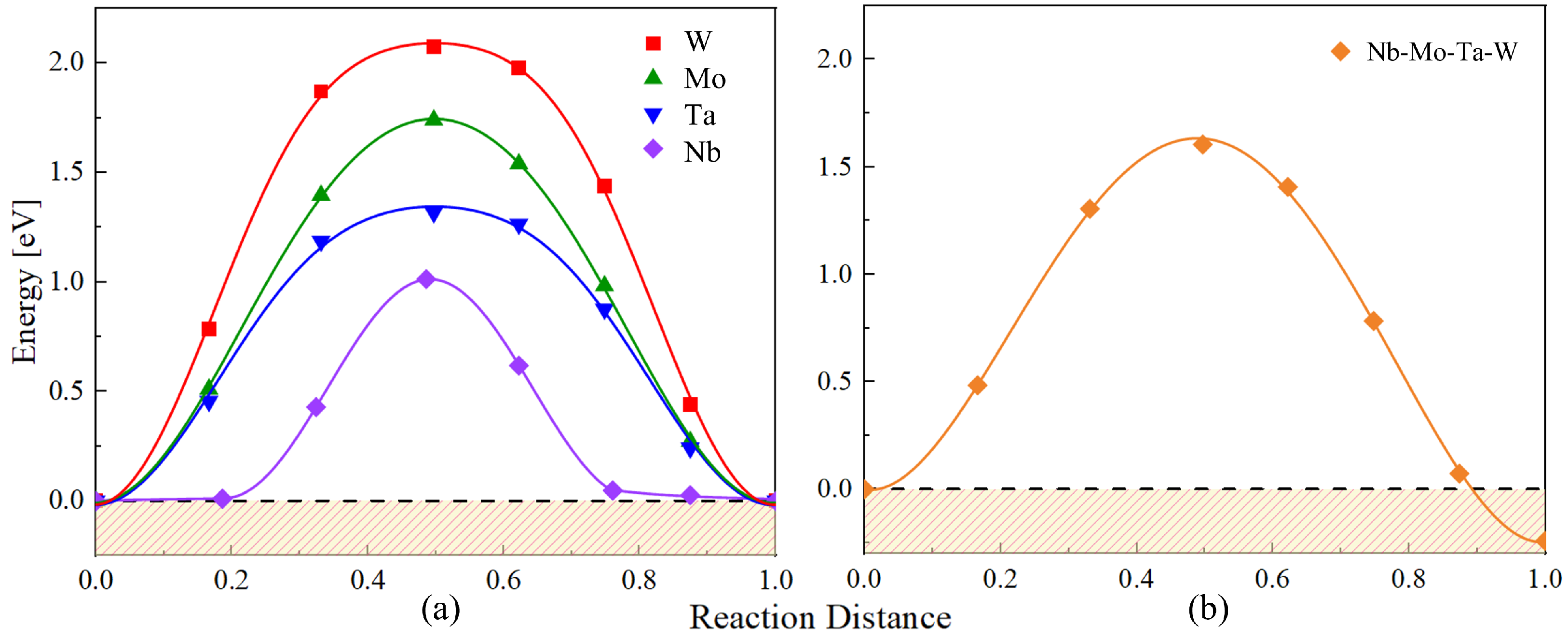
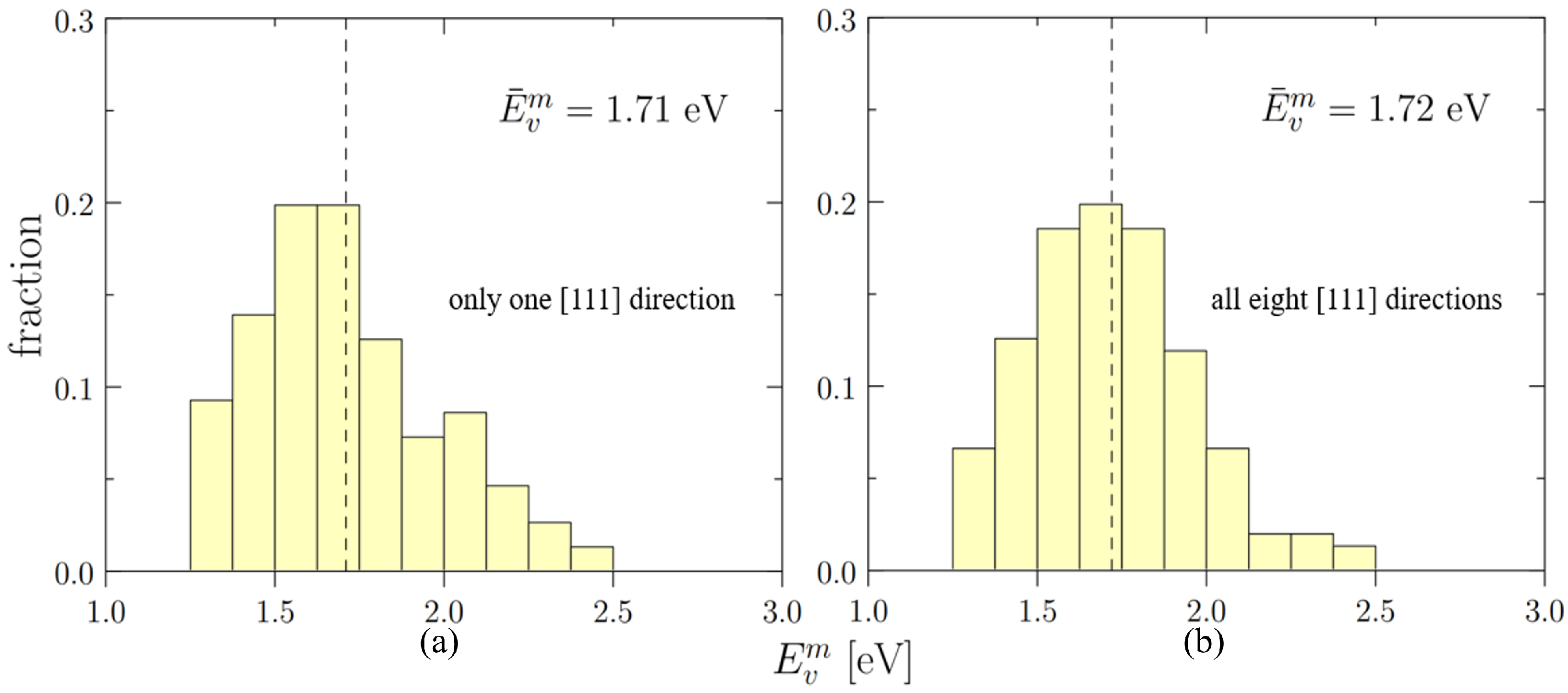
3.3.3. Vacancy Migration Energies in the Bulk
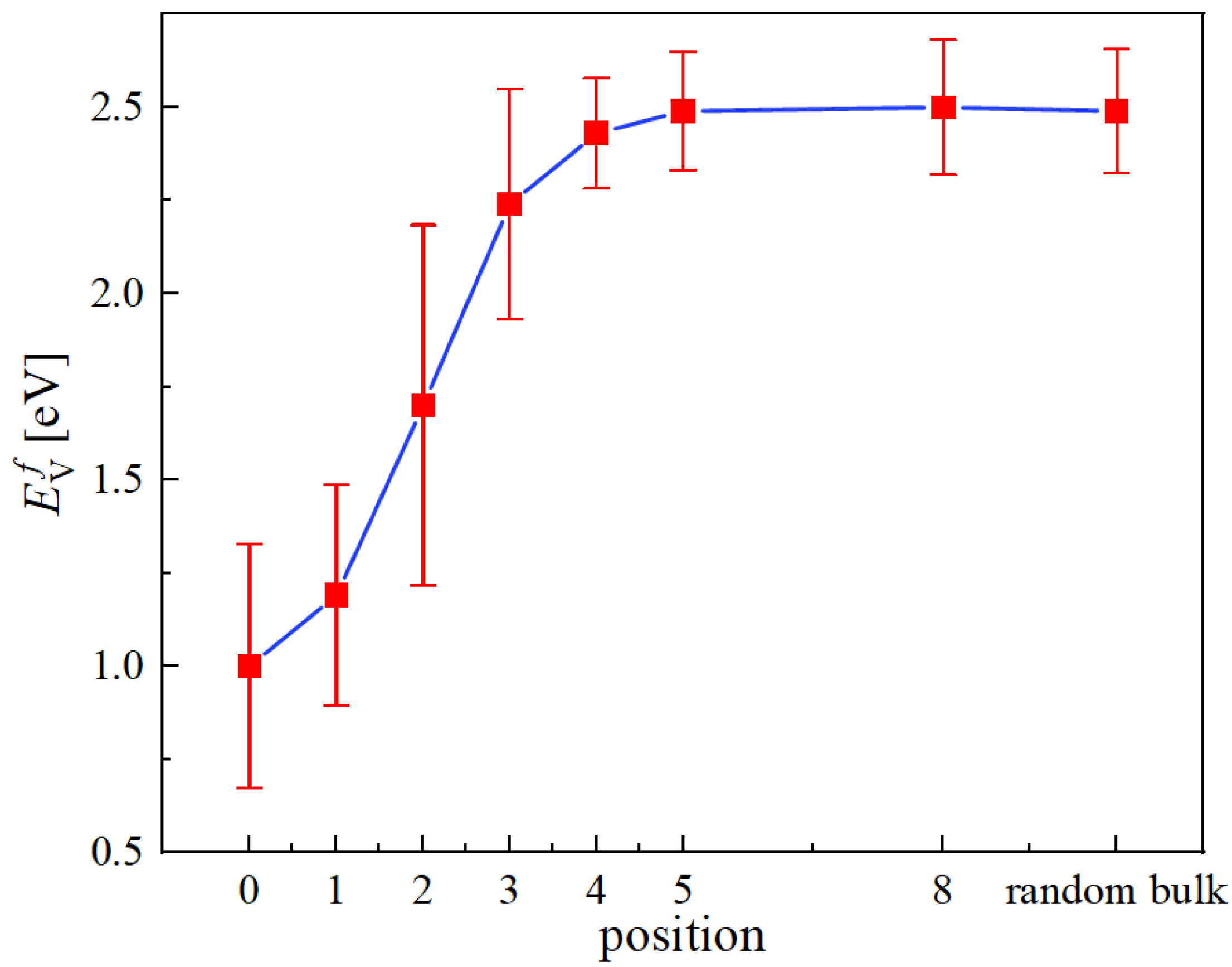
3.4. Vacancy Energetics at Edge Dislocation Cores
Formation Energies
4. Discussion
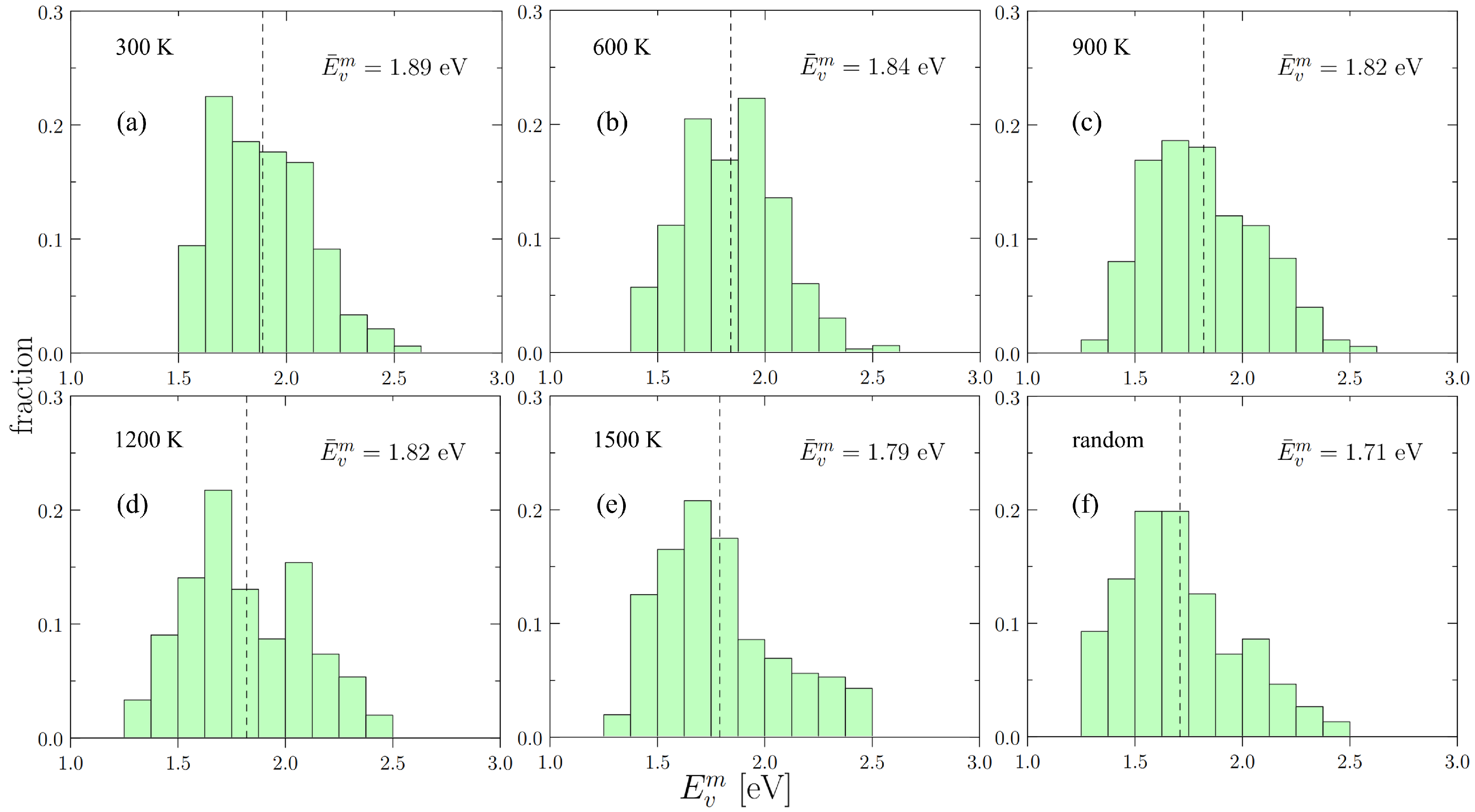
4.1. Statistical Distribution of Vacancy Energetics
4.2. Analysis of Vacancy Formation Energy and Migration Energy
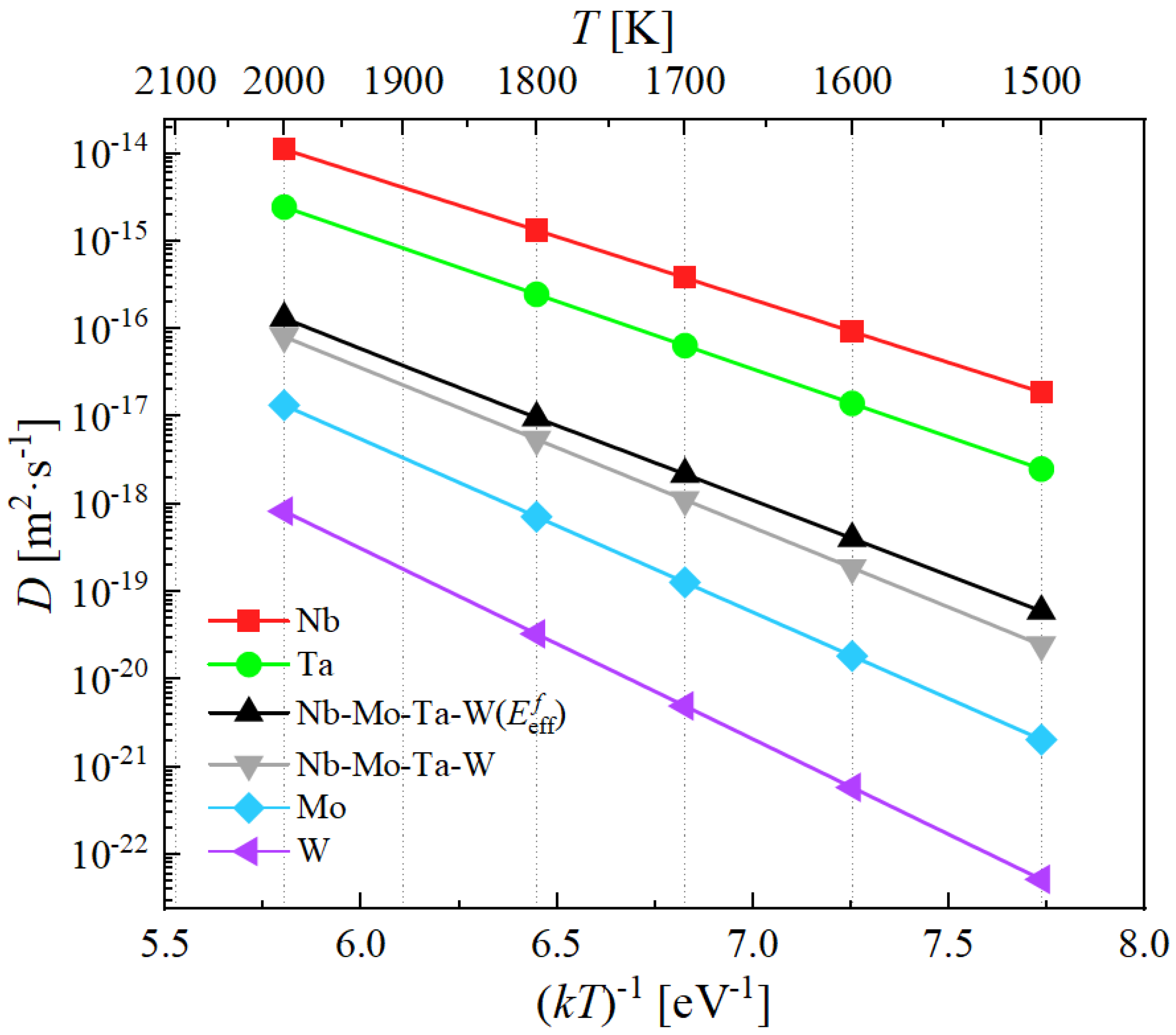
4.3. Self-Diffusion Coefficients of Nb-Mo-Ta-W
4.4. Comparison with Other Works
5. Conclusions
- 1.
- Both vacancy formation and migration energies in Nb-Ta-Mo-W are defined by statistical distributions with a wide spread, on the order of 1.0 eV in some cases.
- 2.
- Vacancy energetics in Nb-Mo-Ta-W display a non-negligible dependence on SRO, which is reflected by the decrease in from 2.54 eV to 2.48 eV and from 1.89 eV to 1.71 eV as SRO weakens with increasing temperature.
- 3.
- The vacancy formation energies are reduced by 1.4 eV on average as they approach an edge dislocation core from the bulk. Vacancies with low energies near zero can be found at core positions, from which we hypothesize that the formation of ‘superjogs’ on edge dislocation lines would be easy.
- 4.
- Due to the widespread distribution of vacancy formation energies, its thermal sampling becomes asymmetric towards lower values, resulting in lowering in effective vacancy formation energies compared to statistical averages in formation energy distributions.
- 5.
- The effective diffusivity in Nb-Mo-Ta-W using is smaller than the diffusivities of Nb and Ta, however it is larger than those of Nb-Mo-Ta-W using the statistical average as well as Mo and W, the value of which starts from m s at 1500 K to m s at 2000 K, confirming the sluggish diffusion reported in the equimolar Nb-Ta-Mo-W alloy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEA | High Entropy Alloy |
| RMEA | Refractory Multi-element Alloy |
| LAMMPS | Large-scale Atomic/Molecular Massively Parallel Simulator |
| NEB | Nudged Elastic Band |
| MEP | Minimum Energy Path |
| CG | Conjugate Gradient |
| SRO | Short-range Order |
| 1NN | 1st-nearest Neighbor |
References
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Kozak, R.; Sologubenko, A.; Steurer, W. Single-phase high-entropy alloys: An overview. Z. Krist.-Cryst. Mater. 2015, 230, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.C.; Jablonski, P.D.; Hawk, J.A.; Alman, D.E. High-entropy alloys: Formation and properties. In Proceedings of the ASME 2018 Symposium on Elevated Temperature Application of Materials for Fossil, Nuclear, and Petrochemical Industries, Seattle, DC, USA, 3–5 April 2018; p. V001T01A004. [Google Scholar]
- Senkov, O.; Miller, J.; Miracle, D.; Woodward, C. Accelerated exploration of multi-principal element alloys for structural applications. Calphad 2015, 50, 32–48. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Xia, S.Q.; Zhen, W.; Yang, T.F.; Zhang, Y. Irradiation behavior in high entropy alloys. J. Iron Steel Res. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Xia, S.; Gao, M.C.; Yang, T.; Liaw, P.K.; Zhang, Y. Phase stability and microstructures of high entropy alloys ion irradiated to high doses. J. Nucl. Mater. 2016, 480, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.K.; Li, C.; Leonard, K.; Bei, H.; Zinkle, S. Microstructural stability and mechanical behavior of fenimncr high entropy alloy under ion irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Egami, T.; Guo, W.; Rack, P.; Nagase, T. Irradiation resistance of multicomponent alloys. Metall. Mater. Trans. 2014, 45, 180–183. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Woodward, C.J.A.M. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Juan, C.C.; Tsai, M.H.; Tsai, C.W.; Lin, C.M.; Wang, W.R.; Yang, C.C.; Chen, S.K.; Lin, S.J.; Yeh, J.W. Enhanced mechanical properties of HfMoTaTiZr and HfMoNbTaTiZr refractory high-entropy alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and properties of aluminum-containing refractory high-entropy alloys. Jom 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Rizi, M.S.; Minouei, H.; Lee, B.J.; Pouraliakbar, H.; Toroghinejad, M.R.; Hong, S.I. Hierarchically activated deformation mechanisms to form ultra-fine grain microstructure in carbon containing FeMnCoCr twinning induced plasticity high entropy alloy. Mater. Sci. Eng. A 2021, 824, 141803. [Google Scholar] [CrossRef]
- Shim, S.H.; Pouraliakbar, H.; Hong, S.I. High strength dual fcc phase CoCuFeMnNi high-entropy alloy wires with dislocation wall boundaries stabilized by phase boundaries. Mater. Sci. Eng. A 2021, 825, 141875. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Zou, Y.; Maiti, S.; Steurer, W.; Spolenak, R. Size-dependent plasticity in an nb25mo25ta25w25 refractory high-entropy alloy. Acta Mater. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Yao, H.; Qiao, J.-W.; Gao, M.C.; Hawk, J.A.; Ma, S.-G.; Zhou, H. MoNbTaV medium-entropy alloy. Entropy 2016, 18, 189. [Google Scholar] [CrossRef] [Green Version]
- Körmann, F.; Sluiter, M.H.F. Interplay between lattice distortions, vibrations and phase stability in NbMoTaW high entropy alloys. Entropy 2016, 18, 403. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.W.; Qiao, J.W.; Hawk, J.A.; Zhou, H.F.; Chen, M.W.; Gao, M.C. Mechanical properties of refractory high-entropy alloys: Experiments and modeling. J. Alloy. Compd. 2017, 696, 1139–1150. [Google Scholar] [CrossRef]
- Dobbelstein, H.; Thiele, M.; Gurevich, E.L.; George, E.P.; Ostendorf, A. Direct metal deposition of refractory high entropy alloy MoNbTaW. Phys. Procedia 2016, 83, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Kube, S.A.; Sohn, S.; Uhl, D.; Datye, A.; Mehta, A.; Schroers, J. Phase selection motifs in high entropy alloys revealed through combinatorial methods: Large atomic size difference favors BCC over FCC. Acta Mater. 2019, 166, 677–686. [Google Scholar] [CrossRef]
- Xu, Q.; Guan, H.Q.; Zhong, Z.H.; Huang, S.S.; Zhao, J.J. Irradiation resistance mechanism of the CoCrFeMnNi equiatomic high-entropy alloy. Sci. Rep. 2021, 11, 608. [Google Scholar] [CrossRef]
- McAlpine, S.W.; Logan, J.V.; Short, M.P. Predicting single phase stability and segregation in the NbMoTaTi–(W,V) high entropy alloy system with the vacancy exchange potential. Scr. Mater. 2021, 191, 29–33. [Google Scholar] [CrossRef]
- Chokshi, A.H. High temperature deformation in fine grained high entropy alloys. Mater. Chem. Phys. 2018, 210, 152–161. [Google Scholar] [CrossRef]
- Chen, W.; Ding, X.; Feng, Y.; Liu, X.; Liu, K.; Lu, Z.P.; Li, D.; Li, Y.; Liu, C.T.; Chen, X.Q. Vacancy formation enthalpies of high-entropy FeCoCrNi alloy via first-principles calculations and possible implications to its superior radiation tolerance. J. Mater. Sci. Technol. 2018, 34, 355–364. [Google Scholar] [CrossRef]
- Roy, A.; Munshi, J.; Balasubramanian, G. Low energy atomic traps sluggardize the diffusion in compositionally complex refractory alloys. Intermetallics 2021, 131, 107106. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lai, Y.C.; Lin, Y.H.; Wu, S.K. A study on the severely cold-rolled and annealed quaternary equiatomic derivatives from quinary HfNbTaTiZr refractory high entropy alloy. J. Alloy. Compd. 2021, 855, 157404. [Google Scholar] [CrossRef]
- Cao, P.P.; Huang, H.L.; Jiang, S.H.; Liu, X.J.; Wang, H.; Wu, Y.; Lu, Z.P. Microstructural stability and aging behavior of refractory high entropy alloys at intermediate temperatures. J. Mater. Sci. Technol. 2022, 122, 243–254. [Google Scholar] [CrossRef]
- Antillon, E.; Woodward, C.; Rao, S.I.; Akdim, B.; Parthasarathy, T.A. Chemical short range order strengthening in a model FCC high entropy alloy. Acta Mater. 2020, 190, 29–42. [Google Scholar] [CrossRef]
- Ma, E. Unusual dislocation behavior in high-entropy alloys. Scr. Mater. 2020, 181, 127–133. [Google Scholar] [CrossRef]
- Lu, C.; Yang, T.; Jin, K.; Gao, N.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; William, J.; Weber, K.S.; et al. Radiation-induced segregation on defect clusters in single-phase concentrated solid-solution alloys. Acta Mater. 2017, 127, 98–107. [Google Scholar] [CrossRef]
- Jin, K.; Sales, B.C.; Stocks, G.M.; Samolyuk, G.D.; Daene, M.; Weber, W.J.; Bei, H. Tailoring the physical properties of Ni-based single-phase equiatomic alloys by modifying the chemical complexity. Sci. Rep. 2016, 6, 20159. [Google Scholar] [CrossRef]
- Sales, B.C.; Jin, K.; Bei, H.; Stocks, G.M.; Samolyuk, G.D.; May, A.F. Quantum critical behavior in a concentrated ternary solid solution. Sci. Rep. 2016, 6, 26179. [Google Scholar] [CrossRef] [Green Version]
- Tulip, P.R.; Staunton, J.B.; Lowitzer, S.; Ködderitzsch, D.; Ebert, H. Theory of electronic transport in random alloys with short-range order: Korringa-Kohn-Rostoker nonlocal coherent potential approximation. Phys. Rev. B 2008, 77, 165116. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Du, J.P.; Shinzato, S.; Sato, Y.; Yu, P.; Ogata, S. Kinetic Monte Carlo simulation framework for chemical short-range order formation kinetics in a multi-principal-element alloy. Comput. Mater. Sci. 2021, 198, 110670. [Google Scholar] [CrossRef]
- Zhao, S. Role of chemical disorder and local ordering on defect evolution in high-entropy alloys. Phys. Rev. Mater. 2021, 5, 103604. [Google Scholar] [CrossRef]
- Zhao, S. Effects of local elemental ordering on defect-grain boundary interactions in high-entropy alloys. J. Alloys Compd. 2021, 887, 161314. [Google Scholar] [CrossRef]
- Barr, C.M.; Nathaniel, J.E., II; Unocic, K.A.; Liu, J.; Zhang, Y.; Wang, Y.; Taheri, M.L. Exploring radiation induced segregation mechanisms at grain boundaries in equiatomic CoCrFeNiMn high entropy alloy under heavy ion irradiation. Scr. Mater. 2018, 156, 80–84. [Google Scholar] [CrossRef]
- Esfandiarpour, A.; Nasrabadi, M.N. Vacancy formation energy in CuNiCo equimolar alloy and CuNiCoFe high entropy alloy: Ab initio based study. Calphad 2019, 66, 101634. [Google Scholar] [CrossRef]
- Zhang, X.; Divinski, S.V.; Grabowski, B. Ab initio prediction of vacancy energetics in HCP Al-Hf-Sc-Ti-Zr high entropy alloys and the subsystems. Acta Mater. 2022, 227, 117677. [Google Scholar] [CrossRef]
- Li, C.; Yin, J.; Odbadrakh, K.; Sales, B.C.; Zinkle, S.J.; Stocks, G.M.; Wirth, B.D. First principle study of magnetism and vacancy energetics in a near equimolar NiFeMnCr high entropy alloy. J. Appl. Phys. 2019, 125, 155103. [Google Scholar] [CrossRef]
- Xing, B.; Wang, X.; Bowman, W.J.; Cao, P. Short-range order localizing diffusion in multi-principal element alloys. Scr. Mater. 2022, 210, 114450. [Google Scholar] [CrossRef]
- Zhao, S. Defect properties in a VTaCrW equiatomic high entropy alloy (HEA) with the body centered cubic (bcc) structure. J. Mater. Sci. Technol. 2020, 44, 133–139. [Google Scholar] [CrossRef]
- Luke, N. Simulating Vacancy Formation and Diffusion in NbMoTaW; University of California: San Diego, CA, USA, 2020. [Google Scholar]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; Shan, R.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Appl. Phys. Lett. 2015, 106, 161906. [Google Scholar] [CrossRef]
- Li, X.-G.; Chen, C.; Zheng, H.; Zuo, Y.; Ong, S.P. Complex strengthening mechanisms in the NbMoTaW multi-principal element alloy. npj Comput. Mater. 2020, 6, 70. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- de Fontaine, D. The number of independent pair-correlation functions in multi-component systems. J. Appl. Crystallogr. 1971, 4, 15–19. [Google Scholar] [CrossRef]
- Fernández-Caballero, A.; Wróbel, J.S.; Mummery, P.M.; Nguyen-Manh, D. Short-range order in high entropy alloys: Theoretical formulation and application to Mo-Nb-Ta-V-W system. J. Phase Equilibria Diffus. 2017, 38, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Byggmästar, J.; Nordlund, K.; Djurabekova, F. Modeling refractory high-entropy alloys with efficient machine-learned interatomic potentials: Defects and segregation. Phys. Rev. B 2021, 104, 104101. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Marian, J. Stress-dependent solute energetics in W–Re alloys from first-principles calculations. Acta Mater. 2014, 80, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Matsuoka, N.; Mizuno, M.; Araki, H. Vacancy formation enthalpy in CoCrFeMnNi high-entropy alloy. Scr. Mater. 2020, 176, 32–35. [Google Scholar] [CrossRef]
- Huang, E.W.; Chou, H.S.; Tu, K.N.; Hung, W.S.; Lam, T.N.; Tsai, C.W. Element effects on high-entropy alloy vacancy and heterogeneous lattice distortion subjected to quasi-equilibrium heating. Sci. Rep. 2019, 9, 14788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleti, R.R.; Bhattacharjee, T.; Shibata, A.; Tsuji, N. Unique deformation behavior and microstructure evolution in high temperature processing of HfNbTaTiZr refractory high entropy alloy. Acta Mater. 2019, 171, 132–145. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, G.; Liu, Y.; Sui, X.; Chen, Y.; Luo, S. Hot deformation behaviors of an ultrafine-grained MoNbTaTiV refractory high-entropy alloy fabricated by powder metallurgy. Mater. Sci. Eng. A 2021, 809, 140922. [Google Scholar] [CrossRef]
- Prohaska, T.; Irrgeher, J.; Benefield, J.; Böhlke, J.K.; Chesson, L.A.; Coplen, T.B.; Ding, T.; Dunn, P.J.H.; Gröning, M.; Holden, N.E.; et al. Standard atomic weights of the elements 2021 (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 573–600. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, Y.; Ma, S.; Zhang, J.; Xu, B.; Kai, J.J. Defect accumulation and evolution in refractory multi-principal element alloys. Acta Mater. 2021, 219, 117233. [Google Scholar] [CrossRef]
- Roy, A.; Singh, P.; Balasubramanian, G.; Johnson, D.D. Vacancy formation energies and migration barriers in multi-principal element alloys. Scr. Mater. 2022, 226, 117611. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.T.; Dou, P. Thermodynamics of vacancies and clusters in high-entropy alloys. Phys. Rev. Mater. 2017, 1, 043601. [Google Scholar] [CrossRef]




| Symbol | [Å] |
|---|---|
| Nb-Mo-Ta-W | 3.24 |
| Nb | 3.33 |
| Ta | 3.32 |
| Mo | 3.17 |
| W | 3.18 |
| ‘average’ | 3.25 |
| Symbol | [meV] |
|---|---|
| Nb | 6.1 |
| Ta | 13.4 |
| Mo | 22.3 |
| W | 21.3 |
| ‘average’ | 15.8 |
| Alloy Condition | [eV] |
|---|---|
| 300 K | 2.54 |
| 600 K | 2.53 |
| 900 K | 2.53 |
| 1200 K | 2.49 |
| 1500 K | 2.49 |
| random | 2.48 |
| Nb | 2.32 |
| Ta | 2.24 |
| Mo | 2.81 |
| W | 2.93 |
| ‘average’ | 2.57 |
| T [K] | [eV] |
|---|---|
| 1500 | 2.367 |
| 1600 | 2.375 |
| 1700 | 2.383 |
| 1800 | 2.393 |
| 2000 | 2.398 |
| Alloy Condition | [eV] |
|---|---|
| 300 K | 1.89 |
| 600 K | 1.84 |
| 900 K | 1.82 |
| 1200 K | 1.82 |
| 1500 K | 1.79 |
| random | 1.71 |
| Nb | 0.99 |
| Ta | 1.32 |
| Mo | 1.74 |
| W | 2.07 |
| ‘average’ | 1.53 |
| Alloy Condition | [eV] |
|---|---|
| 300 K | 1.11 |
| 600 K | 1.12 |
| 900 K | 1.05 |
| 1200 K | 1.09 |
| 1500 K | 1.10 |
| random | 1.00 |
| Nb | 0.86 |
| Ta | 0.81 |
| Mo | 0.94 |
| W | 0.97 |
| ‘average’ | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; He, S.; Marian, J. Vacancy Energetics and Diffusivities in the Equiatomic Multielement Nb-Mo-Ta-W Alloy. Materials 2022, 15, 5468. https://doi.org/10.3390/ma15155468
Zhou X, He S, Marian J. Vacancy Energetics and Diffusivities in the Equiatomic Multielement Nb-Mo-Ta-W Alloy. Materials. 2022; 15(15):5468. https://doi.org/10.3390/ma15155468
Chicago/Turabian StyleZhou, Xinran, Sicong He, and Jaime Marian. 2022. "Vacancy Energetics and Diffusivities in the Equiatomic Multielement Nb-Mo-Ta-W Alloy" Materials 15, no. 15: 5468. https://doi.org/10.3390/ma15155468






