Flake Graphene as an Efficient Agent Governing Cellular Fate and Antimicrobial Properties of Fibrous Tissue Engineering Scaffolds—A Review
Abstract
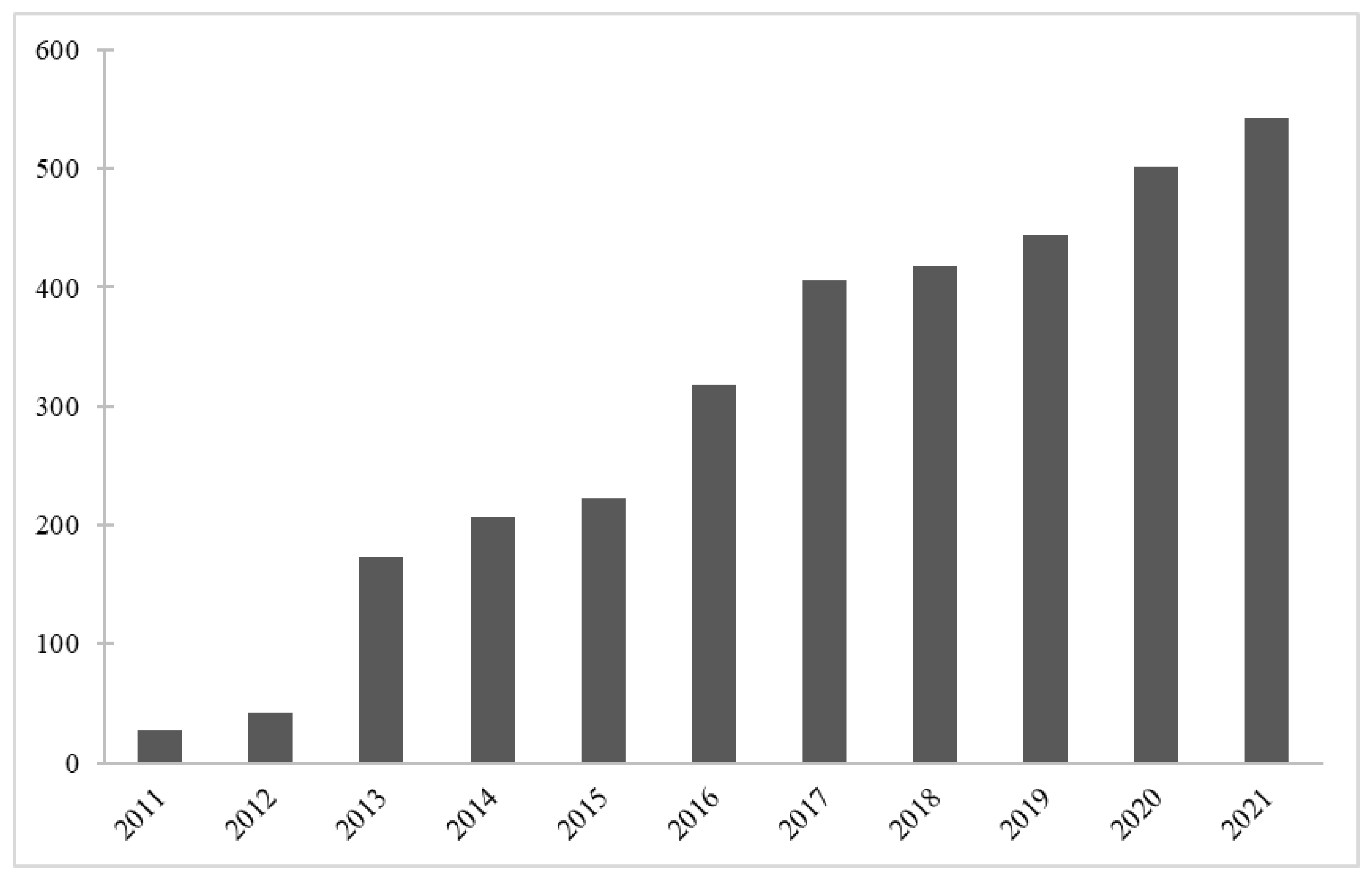
:1. Introduction
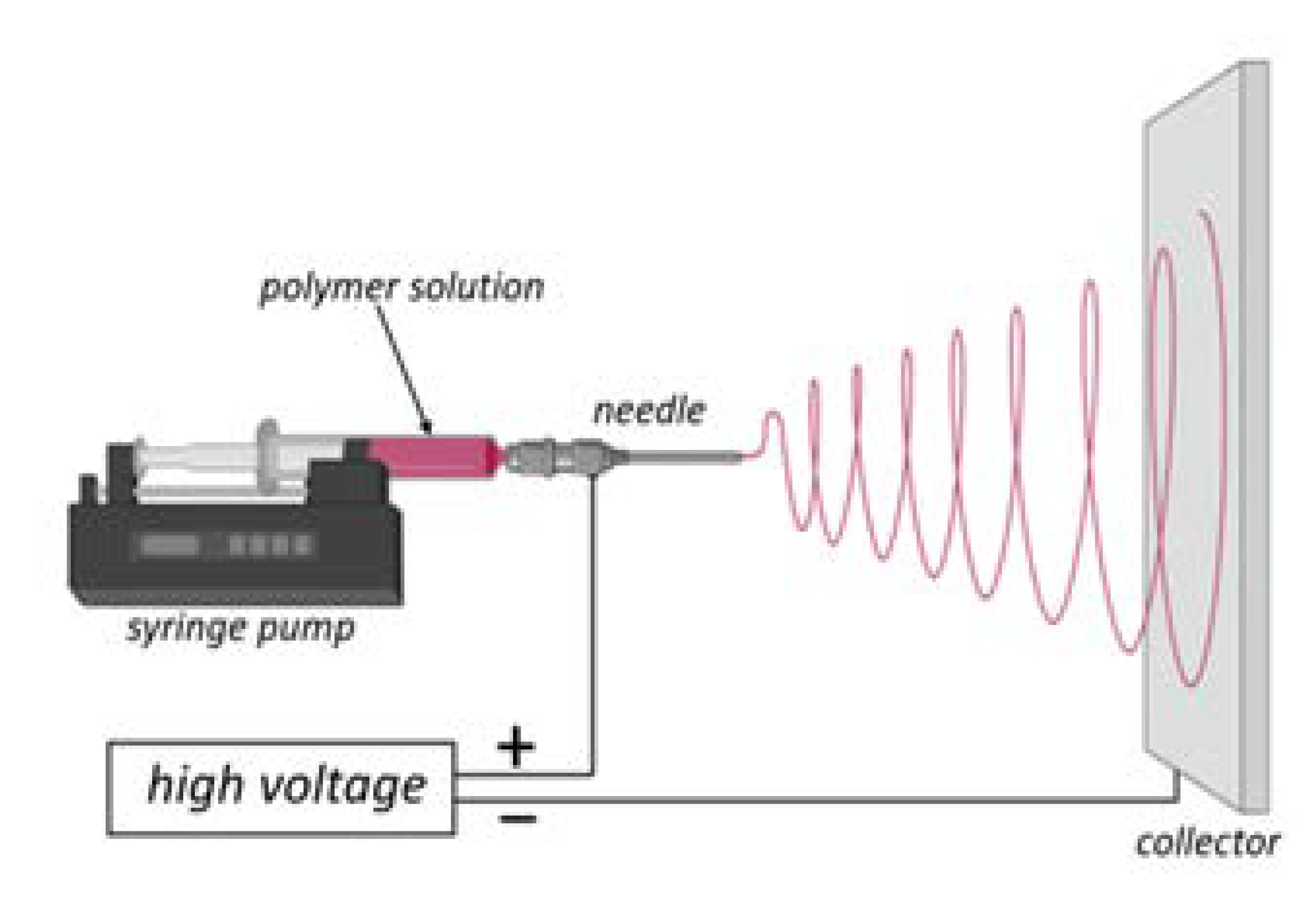
2. Electrospinning as a Satisfactory Method for Producing Micro- and Nano-Sized Fibers
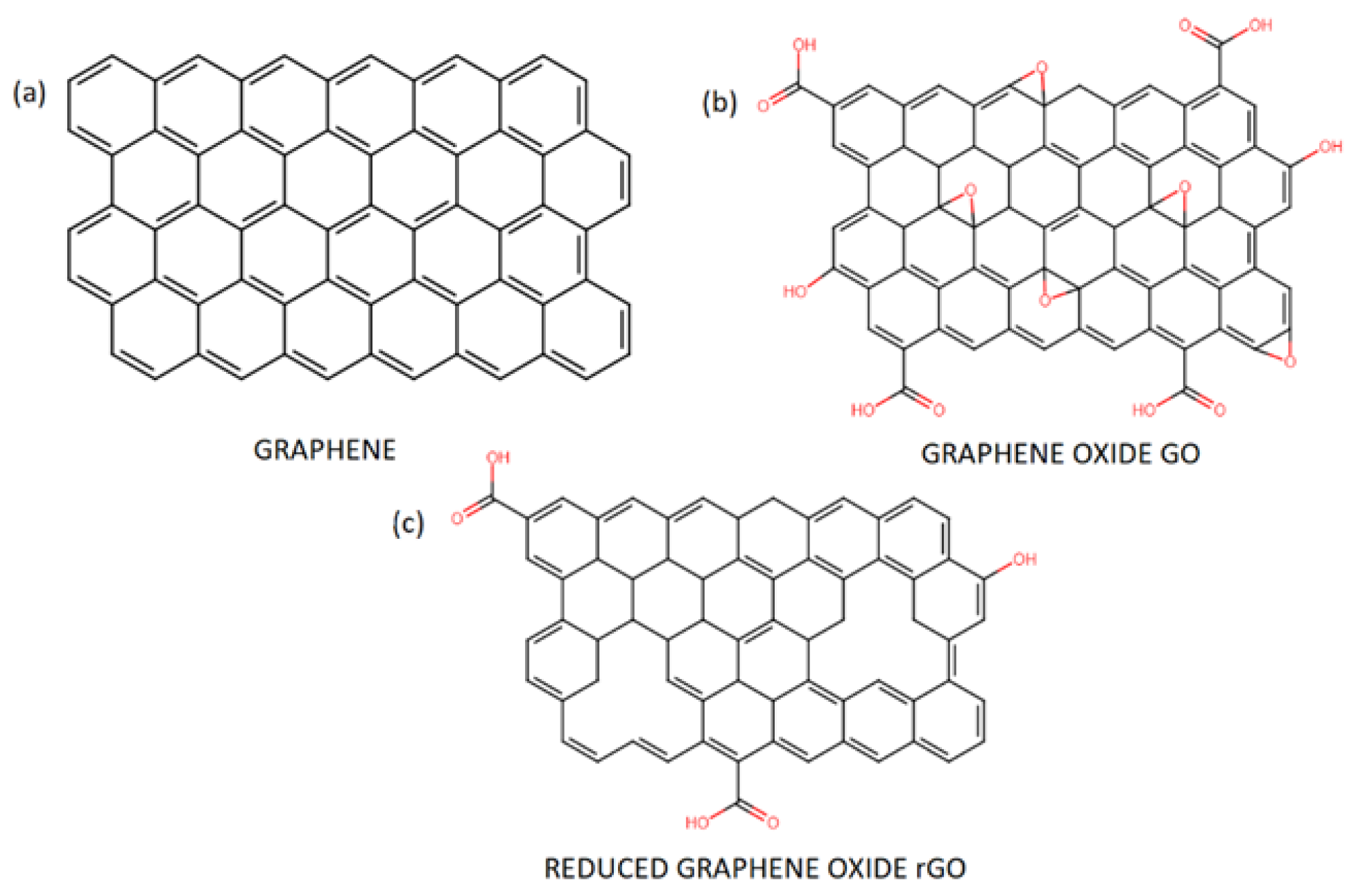
3. Graphene—Properties, Synthesis, and Applications
4. Antimicrobial and Antiviral Activity of Graphene Oxide GO and Reduced Graphene Oxide rGO
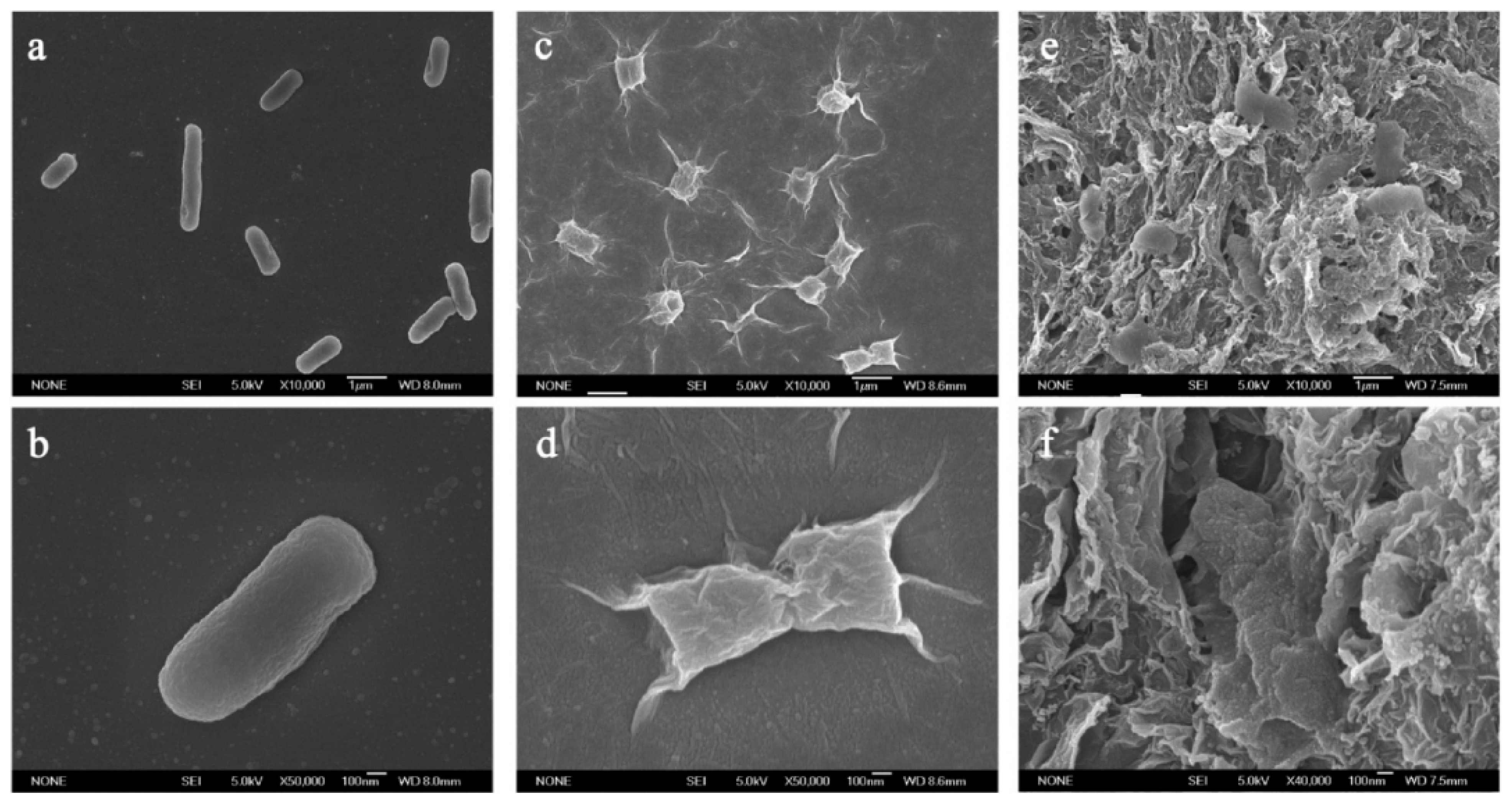
4.1. Toxicity towards Bacteria and Fungi
4.2. Cytotoxicity of Graphene Derivatives
4.3. The Antimicrobial Mechanisms of GO and rGO
4.4. Antiviral Properties of Graphene Derivatives
5. Effects of Modifications by Graphene Derivatives
5.1. Efect of Modifications by GO/rGO on the Cellular Response of Mammalian Cells
5.2. Effects of Modifications by GO/rGO on Antibacterial Properties
6. Conclusions and Future Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bleeker, E.A.J.; de Jong, W.H.; Geertsma, R.E.; Groenewold, M.; Heugens, E.H.W.; Koers-Jacquemijns, M.; van de Meent, D.; Popma, J.R.; Rietveld, A.G.; Wijnhoven, S.W.P.; et al. Considerations on the EU Definition of a Nanomaterial: Science to Support Policy Making. Regul. Toxicol. Pharmacol. 2013, 65, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Chaudhry, Q. A Complementary Definition of Nanomaterial. Nano Today 2010, 5, 165–168. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer Based Nanomaterials in Drug Delivery Systems: A Review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-Based Nanomaterials for Drug Delivery and Tissue Engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Teleanu, R.I. Nanomaterials for Drug Delivery to the Central Nervous System. Nanomaterials 2019, 9, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Peng, N.; He, M.; Teramoto, Y.; Nishio, Y.; Zhang, L. Fabrication and Properties of Chitin/Hydroxyapatite Hybrid Hydrogels as Scaffold Nano-Materials. Carbohydr. Polym. 2013, 91, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating Biologically Inspired Nanomaterials and Table-Top Stereolithography for 3D Printed Biomimetic Osteochondral Scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef]
- Ilie, I.; Ilie, R.; Mocan, T.; Bartos, D.; Mocan, L. Influence of Nanomaterials on Stem Cell Differentiation: Designing an Appropriate Nanobiointerface. Int. J. Nanomed. 2012, 7, 2211–2225. [Google Scholar] [CrossRef] [Green Version]
- Azizi, M.; Navidbakhsh, M.; Hosseinzadeh, S.; Sajjadi, M. Cardiac Cell Differentiation of Muscle Satellite Cells on Aligned Composite Electrospun Polyurethane with Reduced Graphene Oxide. J. Polym. Res. 2019, 26, 258. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene Quantum Dots-Band-Aids Used for Wound Disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A.; de la Guardia, M.; Bani, M.S.; Chenab, K.K.; Pashazadeh-Panahi, P.; Baradaran, B.; Mokhtarzadeh, A.; Hamblin, M.R. Carbon Based Nanomaterials for Tissue Engineering of Bone: Building New Bone on Small Black Scaffolds: A Review. J. Adv. Res. 2019, 18, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene Nanomaterials as Biocompatible and Conductive Scaffolds for Stem Cells: Impact for Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef] [PubMed]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Mahmood Dar, A.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. Biomed. Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Shaji, A.; Zachariah, A.K. Surface Area Analysis of Nanomaterials. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 197–231. [Google Scholar]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive Review on Electrospinning Techniques as Versatile Approaches toward Antimicrobial Biopolymeric Composite Fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, C.; Du, D.; Zhu, J.; Lin, Y. Graphene-like Two-Dimensional Layered Nanomaterials: Applications in Biosensors and Nanomedicine. Nanoscale 2015, 7, 14217–14231. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Q.; Li, Y.; Cai, Y.; Zhu, X.; Zhang, T.; Liu, Y. High Antibacterial Performance of Electrospinning Silk Fibroin/Gelatin Film Modified with Graphene Oxide-sliver Nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47904. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Yin, T.; Yu, Q. Applications of electrospinning technique in drug delivery. Chem. Eng. Commun. 2010, 197, 1315–1338. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive Polymer Ultrafine Fibers via Electrospinning: Preparation, Physical Properties and Applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Nasajpour, A.; Mostafavi, A.; Chlanda, A.; Rinoldi, C.; Sharifi, S.; Ji, M.S.; Ye, M.; Jonas, S.J.; Swieszkowski, W.; Weiss, P.S.; et al. Cholesteryl Ester Liquid Crystal Nanofibers for Tissue Engineering Applications. ACS Mater. Lett. 2020, 2, 1067–1073. [Google Scholar] [CrossRef]
- Chlanda, A.; Kijeńska-Gawrońska, E.; Zdunek, J.; Swieszkowski, W. Internal Nanocrystalline Structure and Stiffness Alterations of Electrospun Polycaprolactone-Based Mats after Six Months of in Vitro Degradation. An Atomic Force Microscopy Assay. J. Mech. Behav. Biomed. Mater. 2020, 101, 103437. [Google Scholar] [CrossRef] [PubMed]
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-Jet Electrospinning Methods for Nanofiber Processing: A Review. Mater. Manuf. Process. 2018, 33, 479–498. [Google Scholar] [CrossRef]
- Teo, W.E.; Kotaki, M.; Mo, X.M.; Ramakrishna, S. Porous Tubular Structures with Controlled Fibre Orientation Using a Modified Electrospinning Method. Nanotechnology 2005, 16, 918–924. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Adapted from “FullTemplateName”, by BioRender.com. 2022. Available online: https://app.biorender.com/biorender-templates (accessed on 13 March 2022).
- Teo, W.E.; Ramakrishna, S. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Orlita, M.; Faugeras, C.; Plochocka, P.; Neugebauer, P.; Martinez, G.; Maude, D.K.; Barra, A.L.; Sprinkle, M.; Berger, C.; De Heer, W.A.; et al. Approaching the Dirac Point in High-Mobility Multilayer Epitaxial Graphene. Phys. Rev. Lett. 2008, 101, 267601. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.S.; Ahn, S.; Sung, Y.K.; Kim, H. Emerging Analysis on the Preparation and Application of Graphene by Bibliometry. J. Mater. Sci. Eng. 2015, 4, 5. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-Efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef]
- “V2 Graphene Mask—Flextrapower”. Available online: https://www.flextrapower.com/graphene-mask/p/mask-v2 (accessed on 24 February 2022).
- Graphene-Based Concrete Used in a Commercial Setting for the First Time|Graphene-Info. Available online: https://www.graphene-info.com/graphene-based-concrete-used-commercial-setting-first-time (accessed on 24 February 2022).
- Korkmaz, S.; Kariper, A. Graphene and Graphene Oxide Based Aerogels: Synthesis, Characteristics and Supercapacitor Applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene Oxide as a Scaffold for Bone Regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, A.; Walejewska, E.; Kowiorski, K.; Heljak, M.; Swieszkowski, W.; Lipińska, L. Investigation into Morphological and Electromechanical Surface Properties of Reduced-Graphene-Oxide-Loaded Composite Fibers for Bone Tissue Engineering Applications: A Comprehensive Nanoscale Study Using Atomic Force Microscopy Approach. Micron 2021, 146, 103072. [Google Scholar] [CrossRef] [PubMed]
- Desante, G.; Labude, N.; Rütten, S.; Römer, S.; Kaufmann, R.; Zybała, R.; Jagiełło, J.; Lipińska, L.; Chlanda, A.; Telle, R.; et al. Graphene Oxide Nanofilm to Functionalize Bioinert High Strength Ceramics. Appl. Surf. Sci. 2021, 566, 150670. [Google Scholar] [CrossRef]
- Tian, Z.; Cao, K.; Bai, S.; He, G.; Li, J. One-Pot Transformation of Waste Toner Powder into 3D Graphene Oxide Hydrogel. ACS Sustain. Chem. Eng. 2019, 7, 496–501. [Google Scholar] [CrossRef]
- Chen, H.; Meng, Y.; Jia, S.; Hua, W.; Cheng, Y.; Lu, J.; Wang, H. Graphene Oxide Modified Waste Newspaper for Removal of Heavy Metal Ions and Its Application in Industrial Wastewater. Mater. Chem. Phys. 2020, 244, 122692. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Yusof, N.A.; Zainal, Z. Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterials 2017, 7, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Chlanda, A.; Kowiorski, K.; Małek, M.; Kijeńska-Gawrońska, E.; Bil, M.; Djas, M.; Strachowski, T.; Swieszkowski, W.; Lipińska, L. Morphology and Chemical Purity of Water Suspension of Graphene Oxide FLAKES Aged for 14 Months in Ambient Conditions. A Preliminary Study. Materials 2021, 14, 4108. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.M. The Reduction of Graphene Oxide. Carbon N. Y. 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Lee, J.U.; Yoon, D.; Kim, H.; Lee, S.W.; Cheong, H. Thermal Conductivity of Suspended Pristine Graphene Measured by Raman Spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 081419. [Google Scholar] [CrossRef] [Green Version]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V. Hybridized Graphene for Chemical Sensing. Funct. Graphene Nanocompos. Deriv. Synth. Process. Appl. 2018, 16, 323–338. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Sedaghat, A.; Ram, M.K.; Zayed, A.; Kamal, R.; Shanahan, N. Investigation of Physical Properties of Graphene-Cement Composite for Structural Applications. Open J. Compos. Mater. 2014, 2014, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Mahanta, N.K.; Abramson, A.R. Thermal Conductivity of Graphene and Graphene Oxide Nanoplatelets. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2012; pp. 1–6. [Google Scholar] [CrossRef]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D. An Electrical Method for the Measurement of the Thermal and Electrical Conductivity ofreduced Graphene Oxide Nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene Oxide Powders with Different Oxidation Degree, Prepared by Synthesis Variations of the Hummers Method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.C.; Cheng, Y.W.; Lam, M.K.; Setiabudi, H.D.; Vo, D.V.N. State-of-the-Art of the Synthesis and Applications of Sulfonated Carbon-Based Catalysts for Biodiesel Production: A Review. Energy Technol. 2021, 9, 2100303. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, G.; Chen, H.; Wang, S.; Zhang, G.; Zhang, F.; Fan, X. Sulfonated Graphene as Water-Tolerant Solid Acid Catalyst. Chem. Sci. 2011, 2, 484–487. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Masteri-Farahani, M.; Shahsavarifar, S. Chemical Modification of Reduced Graphene Oxide with Sulfonic Acid Groups: Efficient Solid Acids for Acetalization and Esterification Reactions. J. Taiwan Inst. Chem. Eng. 2019, 102, 34–43. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, S.; Yan, L.; Zhao, F.; Zhao, Y. Graphene-Based Smart Platforms for Combined Cancer Therapy. Adv. Mater. 2019, 31, 1800662. [Google Scholar] [CrossRef] [PubMed]
- Pancewicz, J.; Niklińska, W.E.; Chlanda, A. Flake Graphene-Based Nanomaterial Approach for Triggering a Ferroptosis as an Attractive Theranostic Outlook for Tackling Non-Small Lung Cancer: A Mini Review. Materials 2022, 15, 3456. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Amirkhanian, S. Investigation of the Graphene Oxide and Asphalt Interaction and Its Effect on Asphalt Pavement Performance. Constr. Build. Mater. 2018, 165, 572–584. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Song, L.; Song, Y.; Dai, G.; Liu, J. An Intensive Review on the Role of Graphene Oxide in Cement-Based Materials. Constr. Build. Mater. 2020, 241, 117939. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-Reduction of Graphite- and Graphene Oxide. Carbon N. Y. 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of Graphene by the Rapid and Mild Thermal Reduction of Graphene Oxide Induced by Microwaves. Carbon N. Y. 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced Graphene Oxide Today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Guo, S.; Zhao, W.; Xu, P.; Yu, H.; Xu, T.; Li, X. Carboxyl Functionalized Gold Nanoparticles in Situ Grown on Reduced Graphene Oxide for Micro-Gravimetric Ammonia Sensing. Sens. Actuators B. Chem. 2014, 202, 846–853. [Google Scholar] [CrossRef]
- Ellis, J.E.; Sorescu, D.C.; Burkert, S.C.; White, D.L.; Star, A. Uncondensed Graphitic Carbon Nitride on Reduced Graphene Oxide for Oxygen Sensing via a Photoredox Mechanism. ACS Appl. Mater. Interfaces 2017, 9, 27142–27151. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Cole, M.; De Luca, A.; Torrisi, F.; Ferrari, A.C.; Udrea, F.; Gardner, J.W. Graphene-Coated Rayleigh SAW Resonators for NO2 Detection. Procedia Eng. 2014, 87, 999–1002. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for Energy Storage: Review of Electrode Materials and Methods of Increasing Capacitance for Supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Abdal Dayem, A.; Eppakayala, V.; Kim, J.H. Oxidative Stress-Mediated Antibacterial Activity of Graphene Oxide and Reduced Graphene Oxide in Pseudomonas Aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, X.; Han, H. Evaluation of Antibacterial Effects of Carbon Nanomaterials against Copper-Resistant Ralstonia Solanacearum. Colloids Surf. B. Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Han, H. A New Function of Graphene Oxide Emerges: Inactivating Phytopathogenic Bacterium Xanthomonas Oryzae Pv. Oryzae. J. Nanoparticle Res. 2013, 15, 1658. [Google Scholar] [CrossRef]
- Sawangphruk, M.; Srimuk, P.; Chiochan, P.; Sangsri, T.; Siwayaprahm, P. Synthesis and Antifungal Activity of Reduced Graphene Oxide Nanosheets. Carbon N. Y. 2012, 50, 5156–5161. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef]
- Al-Thani, R.F.; Patan, N.K.; Al-Maadeed, M.A. Graphene Oxide as Antimicrobial Against Two Gram-Positive and Two Gram-Negative Bacteria in Addition to One Fungus. Online J. Biol. Sci. 2014, 14, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of Graphene-Based Nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and Toxicity of Graphene and Its Functionalized Derivatives in Biological Systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef] [PubMed]
- Romero-Vargas Castrillón, S.; Perreault, F.; De Faria, A.F.; Elimelech, M. Interaction of Graphene Oxide with Bacterial Cell Membranes: Insights from Force Spectroscopy. Environ. Sci. Technol. Lett. 2015, 2, 112–117. [Google Scholar] [CrossRef]
- Mao, J.; Guo, R.; Yan, L.T. Simulation and Analysis of Cellular Internalization Pathways and Membrane Perturbation for Graphene Nanosheets. Biomaterials 2014, 35, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21578–21579. [Google Scholar] [CrossRef] [PubMed]
- Sametband, M.; Kalt, I.; Gedanken, A.; Sarid, R. Herpes Simplex Virus Type-1 Attachment Inhibition by Functionalized Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 1228–1235. [Google Scholar] [CrossRef]
- Chen, Y.N.; Hsueh, Y.H.; Hsieh, C.T.; Tzou, D.Y.; Chang, P.L. Antiviral Activity of Graphene–Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baheiraei, N.; Yeganeh, H.; Ai, J.; Gharibi, R.; Ebrahimi-Barough, S.; Azami, M.; Vahdat, S.; Baharvand, H. Preparation of a Porous Conductive Scaffold from Aniline Pentamer-Modified Polyurethane/PCL Blend for Cardiac Tissue Engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3179–3187. [Google Scholar] [CrossRef]
- Guan, J.; Wang, F.; Li, Z.; Chen, J.; Guo, X.; Liao, J.; Moldovan, N.I. The Stimulation of the Cardiac Differentiation of Mesenchymal Stem Cells in Tissue Constructs That Mimic Myocardium Structure and Biomechanics. Biomaterials 2011, 32, 5568–5580. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudifard, M.; Soleimani, M.; Hatamie, S.; Zamanlui, S.; Ranjbarvan, P.; Vossoughi, M.; Hosseinzadeh, S. The Different Fate of Satellite Cells on Conductive Composite Electrospun Nanofibers with Graphene and Graphene Oxide Nanosheets. Biomed. Mater. 2016, 11, 025006. [Google Scholar] [CrossRef] [PubMed]
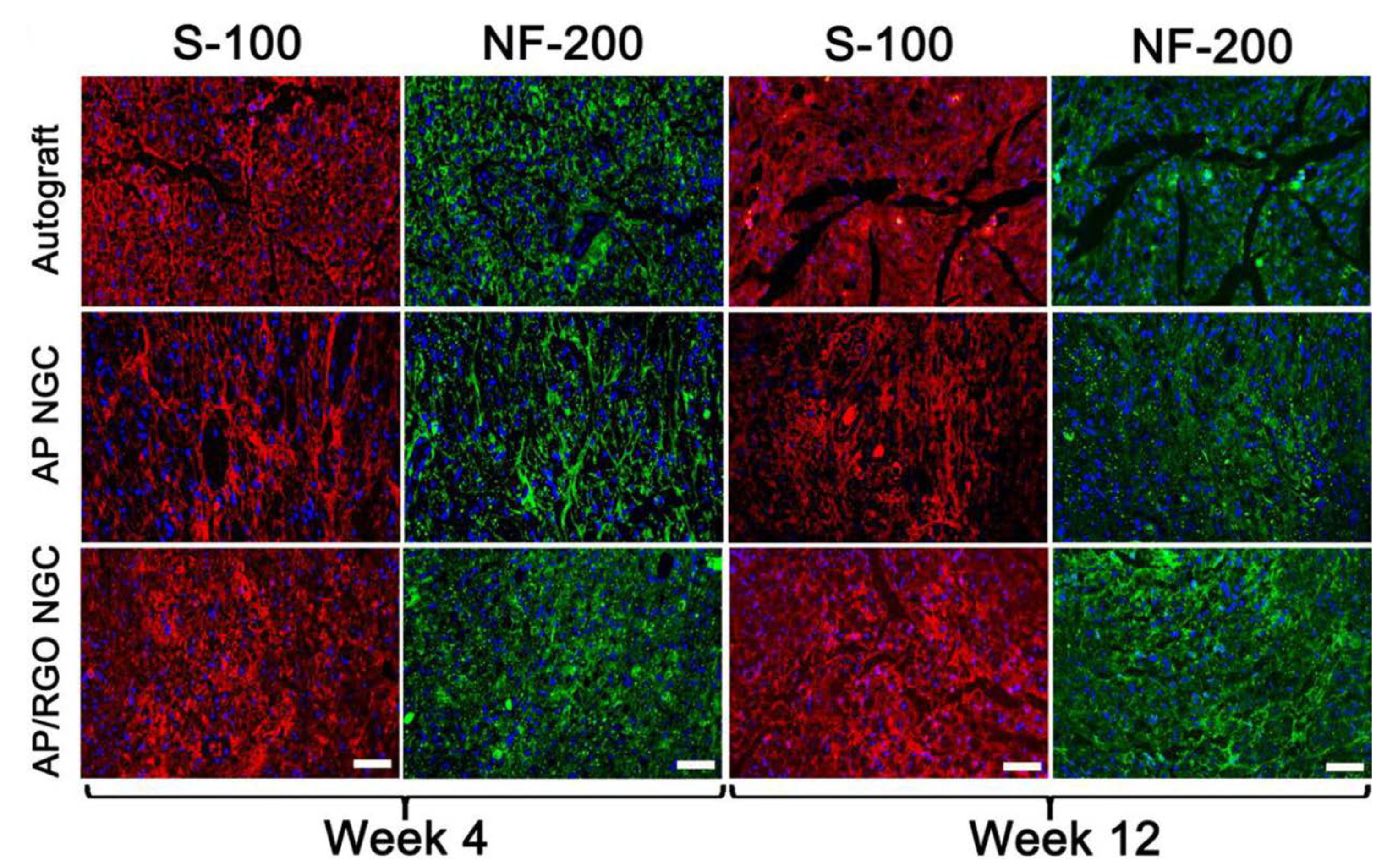
- Liu, Q.; Liu, G.; Liu, X.; Yang, M.; Xing, S.; Du, Y.; Xiong, X. Synthesis of an Electrospun PHA/RGO/Au Scaffold for Peripheral Nerve Regeneration: An In Vitro Study. Appl. Nanosci. 2020, 10, 687–694. [Google Scholar] [CrossRef]
- Heo, C.; Yoo, J.; Lee, S.; Jo, A.; Jung, S.; Yoo, H.; Lee, Y.H.; Suh, M. The Control of Neural Cell-to-Cell Interactions through Non-Contact Electrical Field Stimulation Using Graphene Electrodes. Biomaterials 2011, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Lin, S.; Mequanint, K. Preparation and Characterization of Electrospun RGO-Poly(Ester Amide) Conductive Scaffolds. Mater. Sci. Eng. C 2019, 98, 324–332. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Chen, Y.-H.; Pal, A.; Chou, S.-H.; Chang, S.-J.; Huang, E.-W.; Lin, Z.-H.; Chen, S.-Y. Regulation of Cell Differentiation via Synergistic Self-Powered Stimulation and Degradation Behavior of a Biodegradable Composite Piezoelectric Scaffold for Cartilage Tissue. Nano Energy 2021, 90, 106545. [Google Scholar] [CrossRef]
- Girão, A.F.; Sousa, J.; Domínguez-Bajo, A.; González-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casañ-Pastor, N.; Hortigüela, M.J.; Otero-Irurueta, G.; Completo, A.; et al. 3D Reduced Graphene Oxide Scaffolds with a Combinatorial Fibrous-Porous Architecture for Neural Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The Preparation and Characterization of Polycaprolactone/Graphene Oxide Biocomposite Nanofiber Scaffolds and Their Application for Directing Cell Behaviors. Carbon N. Y. 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In Vitro and In Vivo Studies of Electroactive Reduced Graphene Oxide-Modified Nanofiber Scaffolds for Peripheral Nerve Regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef]
- Ma, H.; Su, W.; Tai, Z.; Sun, D.; Yan, X.; Liu, B.; Xue, Q. Preparation and Cytocompatibility of Polylactic Acid/Hydroxyapatite/Graphene Oxide Nanocomposite Fibrous Membrane. Chin. Sci. Bull. 2012, 57, 3051–3058. [Google Scholar] [CrossRef] [Green Version]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene Oxide and Electroactive Reduced Graphene Oxide-Based Composite Fibrous Scaffolds for Engineering Excitable Nerve Tissue. Mater. Sci. Eng. C 2021, 119, 111632. [Google Scholar] [CrossRef]
- Cojocaru, E.; Ghitman, J.; Pircalabioru, G.G.; Stavarache, C.; Serafim, A.; Vasile, E.; Iovu, H. Electrospun Nanofibrous Membranes Based on Citric Acid-Functionalized Chitosan Containing RGO-TEPA with Potential Application in Wound Dressings. Polymer 2022, 14, 294. [Google Scholar] [CrossRef]
- Pan, N.; Wei, Y.; Zuo, M.; Li, R.; Ren, X.; Huang, T.-S. Antibacterial Poly (ε-Caprolactone) Fibrous Membranes Filled with Reduced Graphene Oxide-Silver. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125186. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Jain, S.; Chatterjee, K. Multifunctional Biodegradable Polymer Nanocomposite Incorporating Graphene-Silver Hybrid for Biomedical Applications. Mater. Des. 2016, 108, 319–332. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide–Silver Nanocomposite as a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and Characterization of an Electrospun PHA/Graphene Silver Nanocomposite Scaffold for Antibacterial Applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared By Electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and Characterization of Antibacterial Electrospun Chitosan/Poly (Vinyl Alcohol)/Graphene Oxide Composite Nanofibrous Membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Ahmadi Majd, S.; Rabbani Khorasgani, M.; Moshtaghian, S.J.; Talebi, A.; Khezri, M. Application of Chitosan/PVA Nano Fiber as a Potential Wound Dressing for Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Macromol. 2016, 92, 1162–1168. [Google Scholar] [CrossRef]

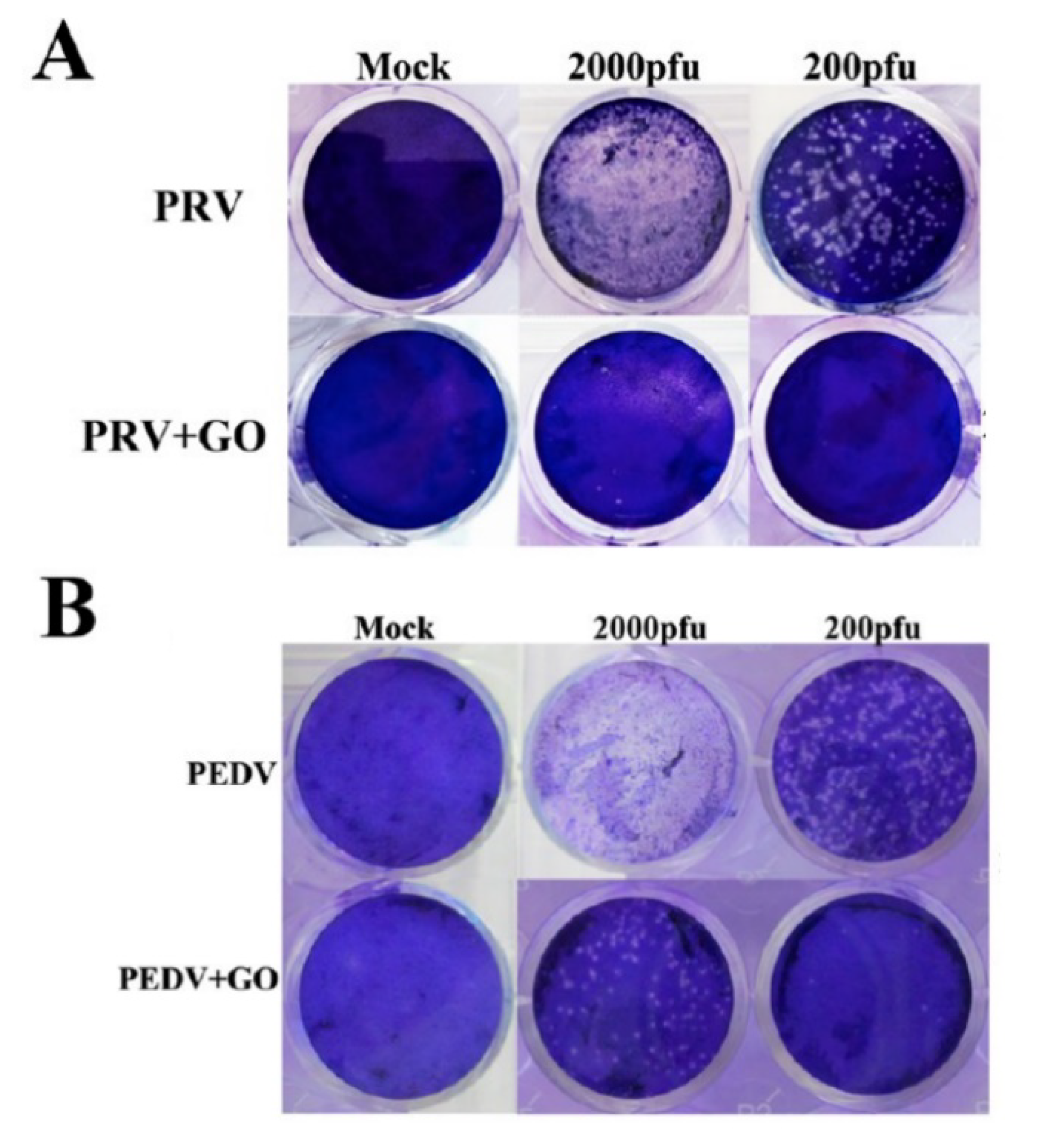
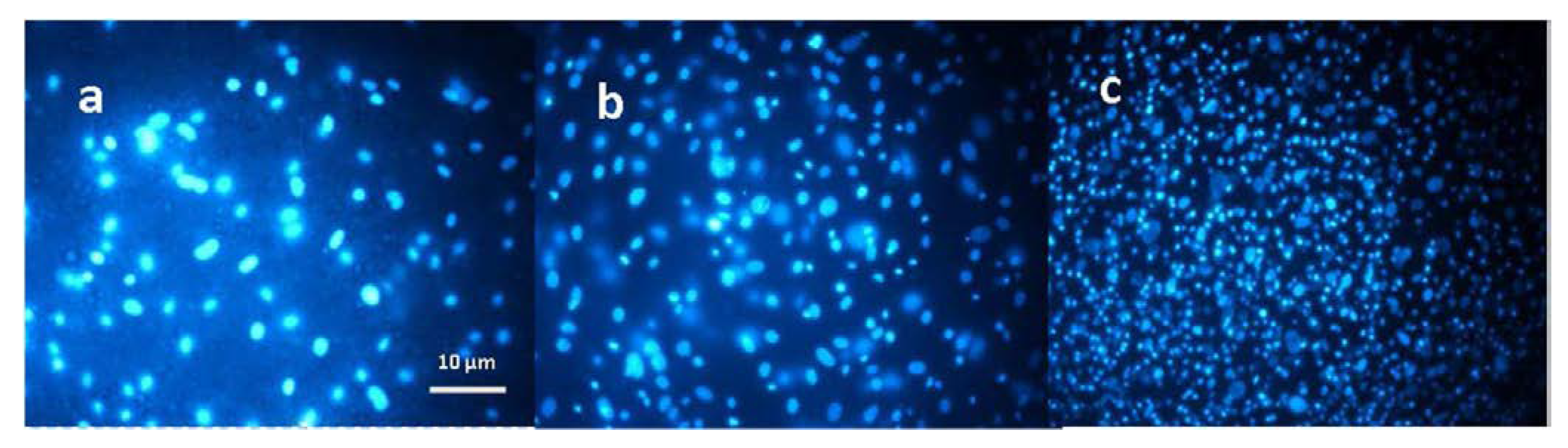
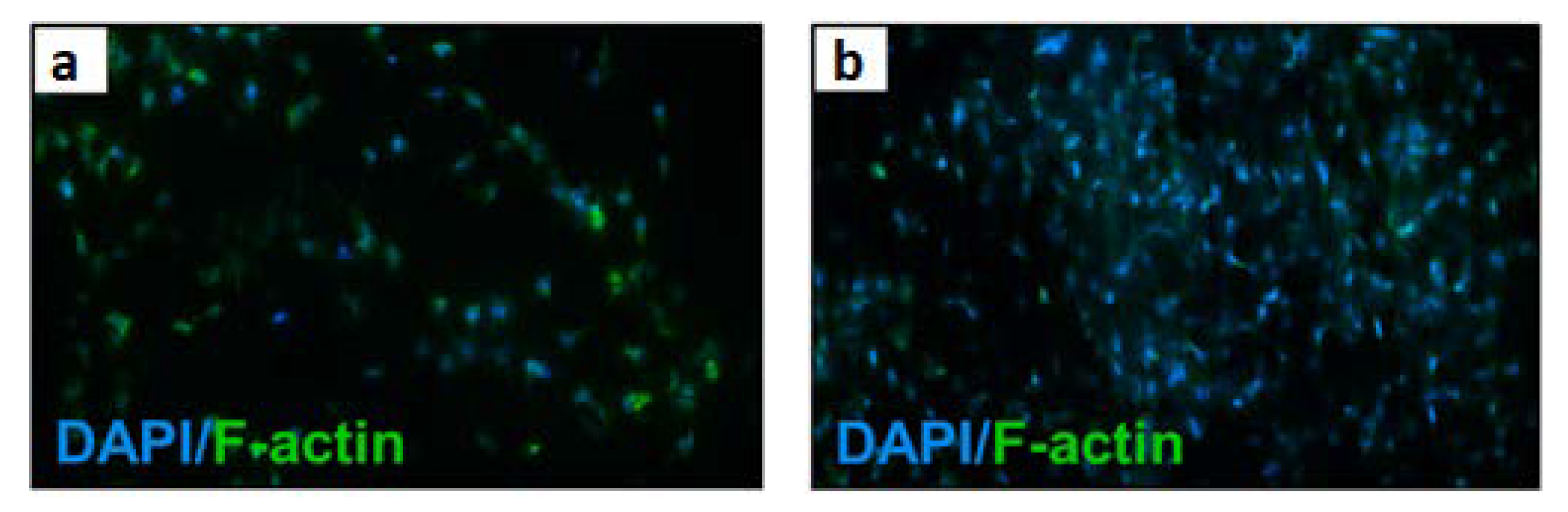
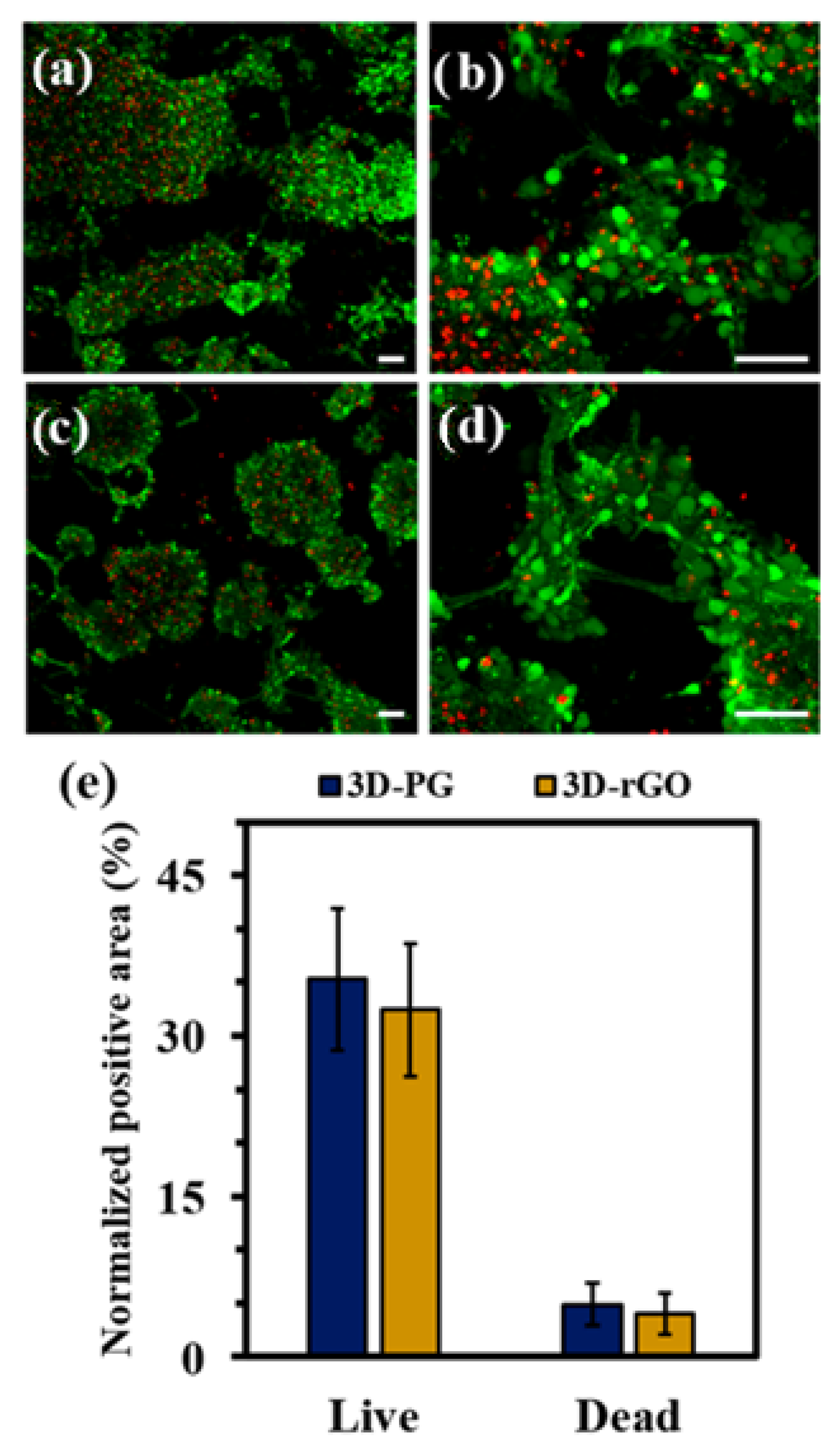

| Material | Studied Organism | Results | Reference |
|---|---|---|---|
| GO | E. coli | The loss of viability at: 69.3% after 1 h to 89.7% after 4 h [40 µg/mL]; 91.6% at 1 h [80 µg/mL] | [75] |
| rGO | E. coli | The loss of viability at: 47.4% after 1 h to 74.9% after 4 h [40 µg/mL]; 76.8% after 1 h [80 µg/mL] | [75] |
| GO/rGO | E. coli | The loss of viability at: above 98.5% after 2 h [85 µg/mL] | [76] |
| GO/rGO paper | E. coli | No cell growth on GO paper, some number of E. coli colonies on rGO paper compared to control group | [76] |
| GO | E. coli | The loss of viability is depending on the size of GO sheets; i.e., the smaller the GO sheet, the higher is the loss of viability | [77] |
| GO | Mammalian cell line—A549 | The loss of viability at: 30% after 2 h and 50% after 24 h [85 µg/mL], (mild cytotoxicity) | [76] |
| rGO | Mammalian cell line—A549 | The loss of viability at: above 75% after 2 h [85 µg/mL] (high cytotoxicity) | [76] |
| GO | Pseudomonas aeruginosa | The loss of viability at: 70% after 2 h to 85% after 4 h [100 µg/mL], above 90% after 2 h [150 µg/mL] | [78] |
| rGO | Pseudomonas aeruginosa | The loss of viability at: 60% after 2 h to 85% after 4 h [100 µg/mL], above 90% after 2 h [150 µg/mL] | [78] |
| GO | Ralstonia solanacearum | The loss of viability at: 60% after 2 h [100 µg/mL], above 90% after 2 h [250 µg/mL] | [79] |
| rGO | Ralstonia solanacearum | The loss of viability at: 5% after 2 h [100 µg/mL], above 15% after 2 h [250 µg/mL] | [79] |
| GO | Xanthomonas oryzae pv. Oryzae | The loss of viability at: 19.4% after 1 h to 66.1% after 4 h [50 µg/mL]; 47.8% after 1 h to 88.6% after 4 h [250 µg/mL] | [80] |
| rGO | Xanthomonas oryzae pv. Oryzae | The loss of viability at: 10.8% after 1 h to 24.8% after 4 h [50 µg/mL]; 12.9% after 1 h to 30.5% after 4 h [250 µg/mL] | [80] |
| rGO | A. niger, A. oryzae, F. oxysporum (fungi) | Concentrations of rGO above 250 µg/mL almost completely inhibited fungi growth | [81] |
| Field of Regenerative Medicine | Polymer | Cell Type | Modifier Substance | Results | Reference |
|---|---|---|---|---|---|
| myocardial regeneration | polyurethane | stem cells | rGO | increased adhesion to substrate, improved proliferation; differentiation of cells into myocardial cells | [9] |
| skeletal muscle regeneration | polyaniline, polyacrylonitrile | stem cells | GO/rGO | increased adhesion to the substrate, improved cell proliferation, differentiation | [94] |
| regeneration of the nervous system | polyhydroxyalkanolane (PHA) | Schwann cells | rGO/Au | promoting proliferation and migration | [109] |
| myocardial regeneration/regeneration of the nervous system | poly(esteramid) (PEA), chitosan | stem cells | rGO | differentiation | [97] |
| cartilage regeneration | poly(L-lactic acid) (PLLA) | ATDC9 cells | rGO/PDA | improved proliferation, increased cell adhesion and biocompatibility | [98] |
| regeneration of the nervous system | polycaprolactone-gelatin (PG) | ENPC cells | GO/rGO | promoting proliferation, migration and differentiation | [99] |
| regeneration of the nervous system/bone regeneration | polycaprolactone | mMSC cells, PC12-L cells | GO/rGO | promoted adhesion, spreading and maturation, significantly increased differentiation | [100] |
| regeneration of the nervous system | antheraea pernyi silk fibroin (ApF), poly(L-lactic acid-co-caprolactone) (PLCL) | Schwann cells, PC12 cells | GO/rGO | promoted migration and proliferation of Schwann cells and growth and differentiation of PC12 cells | [101] |
| regeneration of the nervous system | poly(L-lactic acid) (PLLA) | MC3T3-E1 cells | HA/GO | promoting proliferation and adhesion | [102] |
| regeneration of the nervous system | silk fibroin (SF) | NG108-15 cells | GO/rGO | promote proliferation and metabolic activity | [103] |
| skin regeneration | chitosan | NCTC fibroblasts | rGO/TEPA | promote cell viability and proliferation | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasiak, A.I.; Racki, A.; Małek, M.; Chlanda, A. Flake Graphene as an Efficient Agent Governing Cellular Fate and Antimicrobial Properties of Fibrous Tissue Engineering Scaffolds—A Review. Materials 2022, 15, 5306. https://doi.org/10.3390/ma15155306
Banasiak AI, Racki A, Małek M, Chlanda A. Flake Graphene as an Efficient Agent Governing Cellular Fate and Antimicrobial Properties of Fibrous Tissue Engineering Scaffolds—A Review. Materials. 2022; 15(15):5306. https://doi.org/10.3390/ma15155306
Chicago/Turabian StyleBanasiak, Aleksandra Izabela, Adrian Racki, Marcin Małek, and Adrian Chlanda. 2022. "Flake Graphene as an Efficient Agent Governing Cellular Fate and Antimicrobial Properties of Fibrous Tissue Engineering Scaffolds—A Review" Materials 15, no. 15: 5306. https://doi.org/10.3390/ma15155306
APA StyleBanasiak, A. I., Racki, A., Małek, M., & Chlanda, A. (2022). Flake Graphene as an Efficient Agent Governing Cellular Fate and Antimicrobial Properties of Fibrous Tissue Engineering Scaffolds—A Review. Materials, 15(15), 5306. https://doi.org/10.3390/ma15155306








