pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MoSe2-mQDs and Dispersion
2.3. Characterization
3. Results
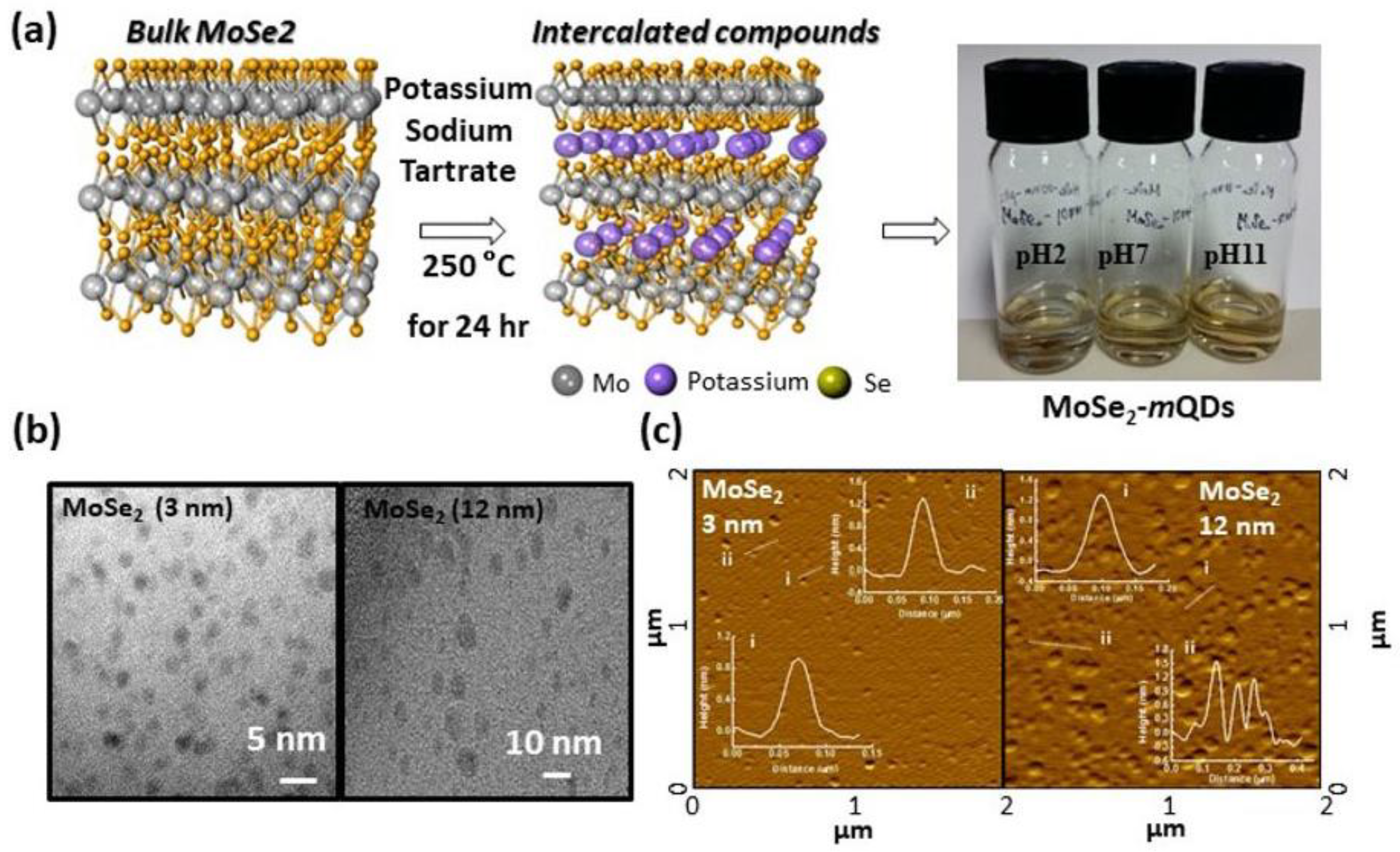
3.1. Preparation and Structural Characterization
3.2. Photoluminescence (PL) and UV Absorbance
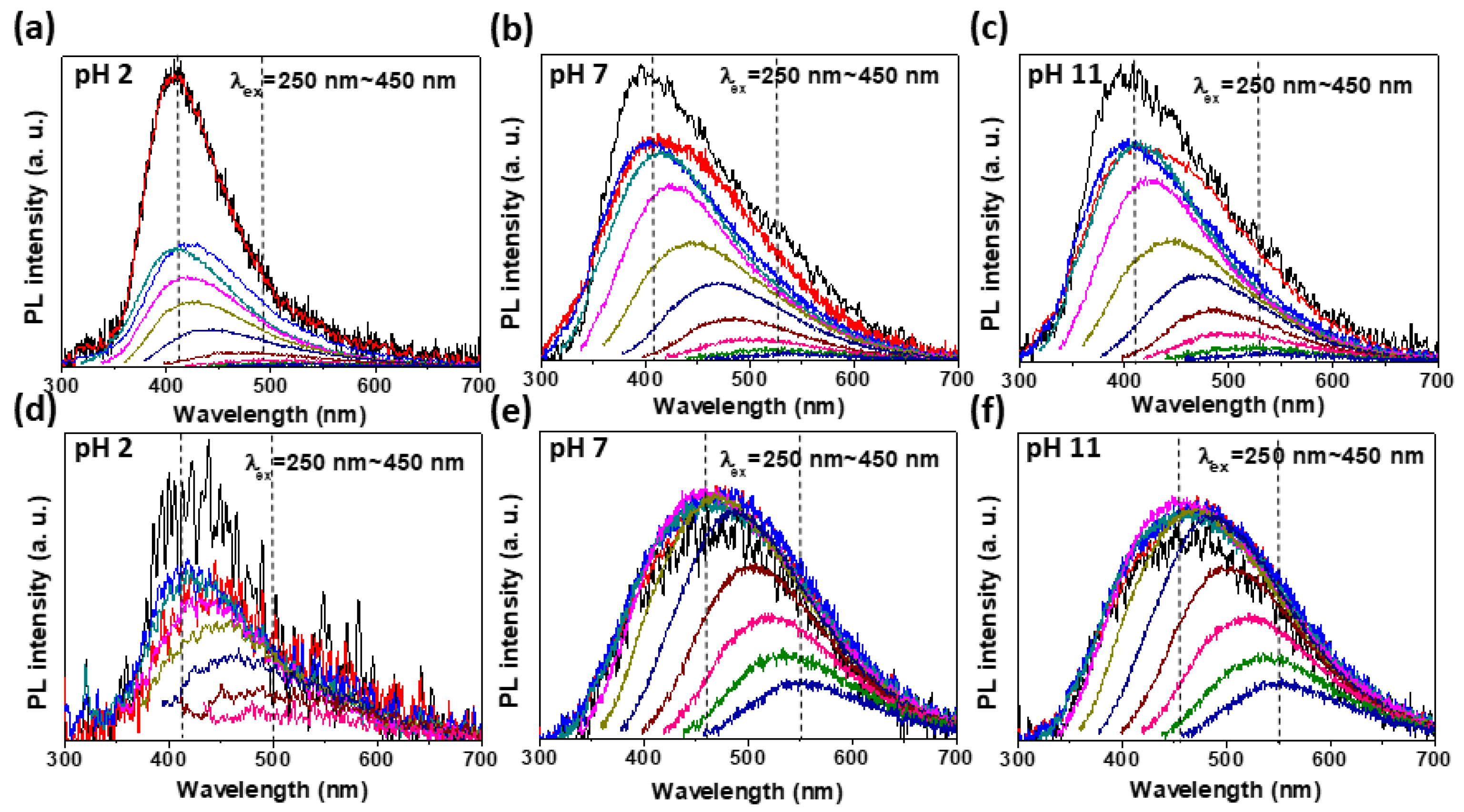
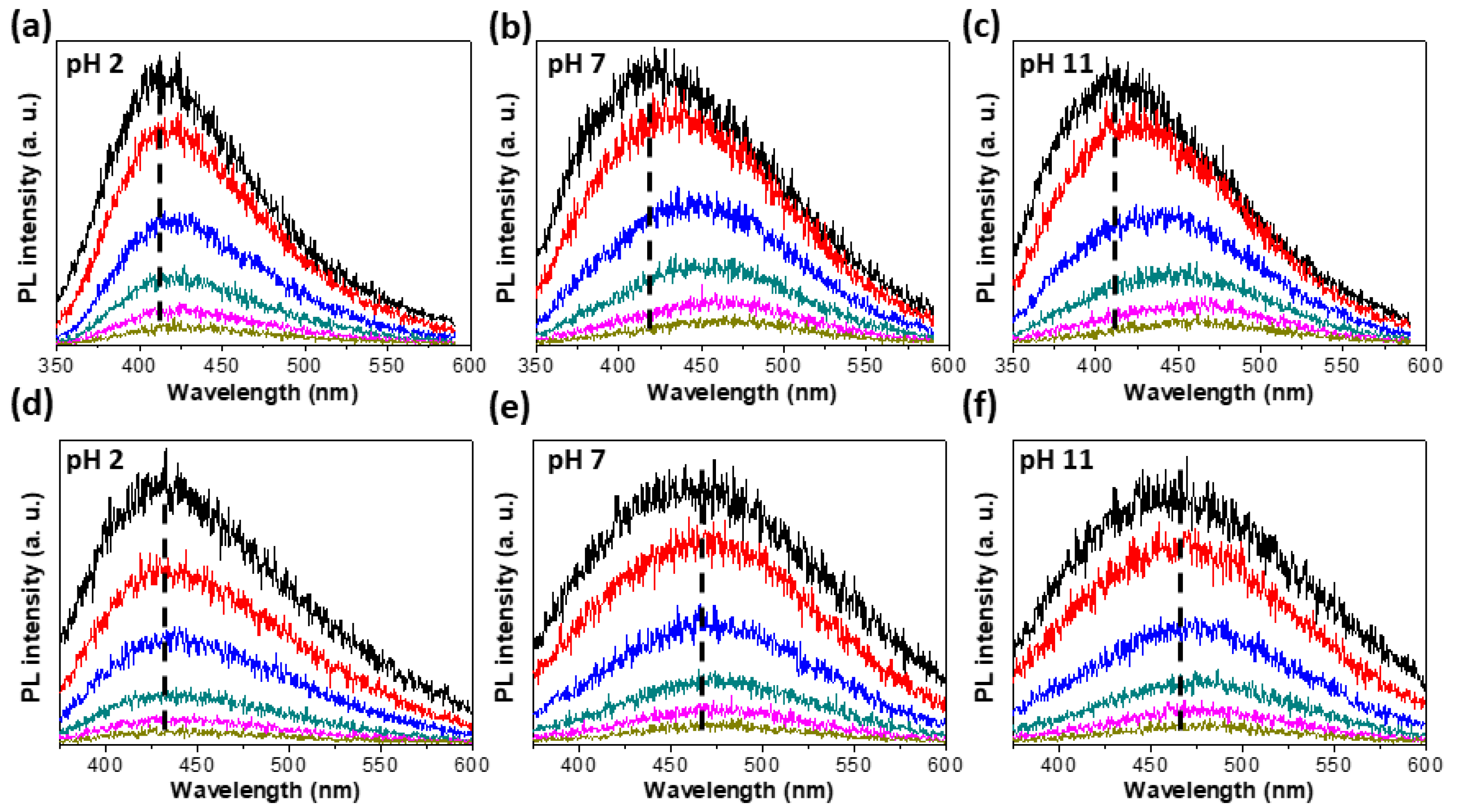
3.3. Excitation Wavelength Dependent PL (PLE)
3.4. Time Resolved PL (TRPL)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Xu, Y.; Zhang, S.; Ross, I.M.; Ong, A.C.; Allwood, D.A. Fabrication of luminescent monolayered tungsten dichalcogenides quantum dots with giant spin-valley coupling. ACS Nano 2013, 7, 8214–8223. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Najmaei, S.; Liu, Z.; Bao, Y.; Wang, Y.; Zhu, X.; Halas, N.J.; Nordlander, P.; Ajayan, P.M.; Lou, J. Plasmonic hot electron induced structural phase transition in a MoS2 monolayer. Adv. Mater. 2014, 26, 6467–6471. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Dumcenco, D.O.; Huang, Y.-S.; Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS 2. Nat. Nanotechnol. 2014, 9, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Liu, L.; Wu, H.; Hao, Y.; Shan, Y.; Wu, X.; Chu, P.K. Quantum confinement effects across two-dimensional planes in MoS2 quantum dots. Appl. Phys. Lett. 2015, 106, 233113. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, B.H.; Choe, D.H.; Kim, J.; Kim, D.C.; Lee, D.J.; Kim, J.M.; Chang, K.J.; Jeon, S. Bandgap widening of phase quilted, 2D MoS2 by oxidative intercalation. Adv. Mater. 2015, 27, 3152–3158. [Google Scholar] [CrossRef]
- Ding, X.; Peng, F.; Zhou, J.; Gong, W.; Slaven, G.; Loh, K.P.; Lim, C.T.; Leong, D.T. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Li, N.; Chen, P. Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016, 45, 2239–2262. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, X.; Zhang, W.L.; Lv, F.; Guo, S. Recent progress in two-dimensional inorganic quantum dots. Chem. Soc. Rev. 2018, 47, 586–625. [Google Scholar] [CrossRef]
- Xu, Q.; Cai, W.; Li, W.; Sreeprasad, T.S.; He, Z.; Ong, W.-J.; Li, N. Two-dimensional quantum dots: Fundamentals, photoluminescence mechanism and their energy and environmental applications. Mater. Today Energy 2018, 10, 222–240. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Abdelwahab, I.; Ding, Z.; Zhao, X.; Yang, T.; Loke, G.Z.; Lin, H.; Verzhbitskiy, I.; Poh, S.M.; Xu, H. Chemical stabilization of 1T′ phase transition metal dichalcogenides with giant optical Kerr nonlinearity. J. Am. Chem. Soc. 2017, 139, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fan, X.; Singh, D.J.; Zheng, W.T. Recent progress of TMD nanomaterials: Phase transitions and applications. Nanoscale 2020, 12, 1247–1268. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X.; Zheng, L. Preparation of High-Percentage 1T-Phase Transition Metal Dichalcogenide Nanodots for Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Jang, M.-H.; Yoon, H.; Kim, H.J.; Cho, Y.-H.; Jeon, S.; Song, S.-H. Metallic phase transition metal dichalcogenide quantum dots showing different optical charge excitation and decay pathways. NPG Asia Mater. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Mishra, H.; Singh, V.K.; Vikram, K.; Srivastava, R.K.; Srivastava, S.; Srivastava, A. pH dependent luminescence switching of tin disulfide quantum dots. J. Lumin. 2019, 213, 401–408. [Google Scholar] [CrossRef]
- Thomas, A.; Jinesh, K.B. Excitons and Trions in MoS2 Quantum Dots: The Influence of the Dispersing Medium. ACS Omega 2022, 7, 6531–6538. [Google Scholar] [CrossRef] [PubMed]
- Komsa, H.-P.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A.V. Two-dimensional transition metal dichalcogenides under electron irradiation: Defect production and doping. Phys. Rev. Lett. 2012, 109, 035503. [Google Scholar] [CrossRef]
- Lin, Y.; Ling, X.; Yu, L.; Huang, S.; Hsu, A.L.; Lee, Y.-H.; Kong, J.; Dresselhaus, M.S.; Palacios, T. Dielectric screening of excitons and trions in single-layer MoS2. Nano Lett. 2014, 14, 5569–5576. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, H.; Du, H.; Choi, J.H. Understanding solvent effects on the properties of two-dimensional transition metal dichalcogenides. ACS Appl. Mater. Interfaces 2016, 8, 8864–8869. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Synthesis of blue photoluminescent WS2 quantum dots via ultrasonic cavitation. J. Lumin. 2017, 185, 236–240. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, L.; Zhang, W.; Pan, H.; Sun, Y.; Ge, C.; Du, Y.; Tang, N. Ultrasmall and Monolayered Tungsten Dichalcogenide Quantum Dots with Giant Spin–Valley Coupling and Purple Luminescence. ACS Omega 2018, 3, 12188–12194. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ahn, M.; Jeong, S.; Han, J.H.; Yoo, D.; Son, D.H.; Cheon, J. Colloidal single-layer quantum dots with lateral confinement effects on 2D exciton. J. Am. Chem. Soc. 2016, 138, 13253–13259. [Google Scholar] [CrossRef] [PubMed]
- Loh, G.; Pandey, R.; Yap, Y.K.; Karna, S.P. MoS2 quantum dot: Effects of passivation, additional layer, and h-BN substrate on its stability and electronic properties. J. Phys. Chem. C 2015, 119, 1565–1574. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Ponraj, J.S.; Bao, Q.; Zhang, H. 2D–Materials-Based Quantum Dots: Gateway Towards Next-Generation Optical Devices. Adv. Opt. Mater. 2017, 5, 1700257. [Google Scholar] [CrossRef]
- Luan, C.-Y.; Xie, S.; Ma, C.; Wang, S.; Kong, Y.; Xu, M. Elucidation of luminescent mechanisms of size-controllable MoSe2 quantum dots. Appl. Phys. Lett. 2017, 111, 073105. [Google Scholar] [CrossRef]
- Mishra, H.; Umrao, S.; Singh, J.; Srivastava, R.K.; Ali, R.; Misra, A.; Srivastava, A. pH dependent optical switching and fluorescence modulation of molybdenum sulfide quantum dots. Adv. Opt. Mater. 2017, 5, 1601021. [Google Scholar] [CrossRef]
- Van Tuan, D.; Yang, M.; Dery, H. Coulomb interaction in monolayer transition-metal dichalcogenides. Phys. Rev. B 2018, 98, 125308. [Google Scholar] [CrossRef] [Green Version]
- Caigas, S.P.; Santiago SR, M.; Lin, T.-N.; Lin, C.-A.J.; Yuan, C.-T.; Shen, J.-L.; Lin, T.-Y. Origins of excitation-wavelength-dependent photoluminescence in WS2 quantum dots. Appl. Phys. Lett. 2018, 112, 092106. [Google Scholar] [CrossRef]
- Park, K.H.; Yang, J.Y.; Jung, S.; Ko, B.M.; Song, G.; Hong, S.-J.; Kim, N.C.; Lee, D.; Song, S.H. Metallic phase transition metal dichalcogenide quantum dots as promising bio-imaging materials. Nanomaterials 2022, 12, 1645. [Google Scholar] [CrossRef]
- Doolen, R.; Laitinen, R.; Parsapour, F.; Kelley, D. Trap state dynamics in MoS2 nanoclusters. J. Phys. Chem. B 1998, 102, 3906–3911. [Google Scholar] [CrossRef]

| Materials | Size (nm) | pH | Peak (nm/eV) | FWHM (eV) | QY (%) | Abs @ 325 nm |
|---|---|---|---|---|---|---|
| MoSe2 | 3 | 2 | 418.3 | 0.79 | 3.7 | 0.326 |
| 7 | 427.5 | 0.9 | 5.2 | 0.374 | ||
| 11 | 425.3 | 0.9 | 5 | 0.352 | ||
| 12 | 2 | 437.6 | 0.84 | |||
| 7 | 461.3 | 1.07 | 0.4 | 0.689 | ||
| 11 | 461.9 | 1.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, B.; Ahn, J.; Song, S.H. pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots. Materials 2022, 15, 4945. https://doi.org/10.3390/ma15144945
Ko B, Ahn J, Song SH. pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots. Materials. 2022; 15(14):4945. https://doi.org/10.3390/ma15144945
Chicago/Turabian StyleKo, Boemjin, Jaegyu Ahn, and Sung Ho Song. 2022. "pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots" Materials 15, no. 14: 4945. https://doi.org/10.3390/ma15144945
APA StyleKo, B., Ahn, J., & Song, S. H. (2022). pH-Dependent Photophysical Properties of Metallic Phase MoSe2 Quantum Dots. Materials, 15(14), 4945. https://doi.org/10.3390/ma15144945







