Excellent Photonic and Mechanical Properties of Macromorphic Fibers Formed by Eu3+-Complex-Anchored, Unzipped, Multiwalled Carbon Nanotubes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
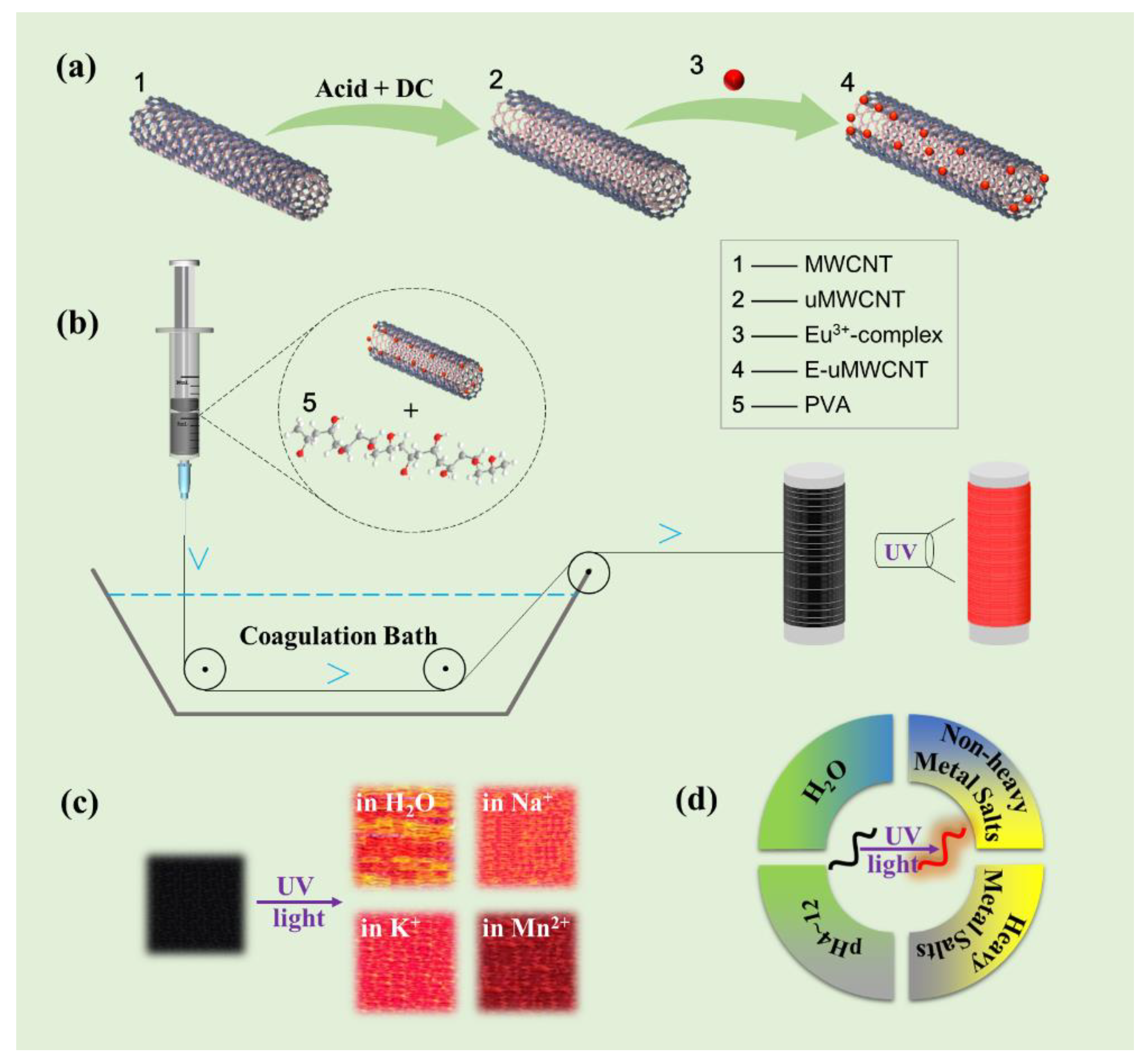
2.2. Electrochemical Method of Preparation of uMWCNTs
2.3. Preparation of Macromorphic Fibers by Wet-Spinning Method
2.4. Characterizations
3. Results and Discussion
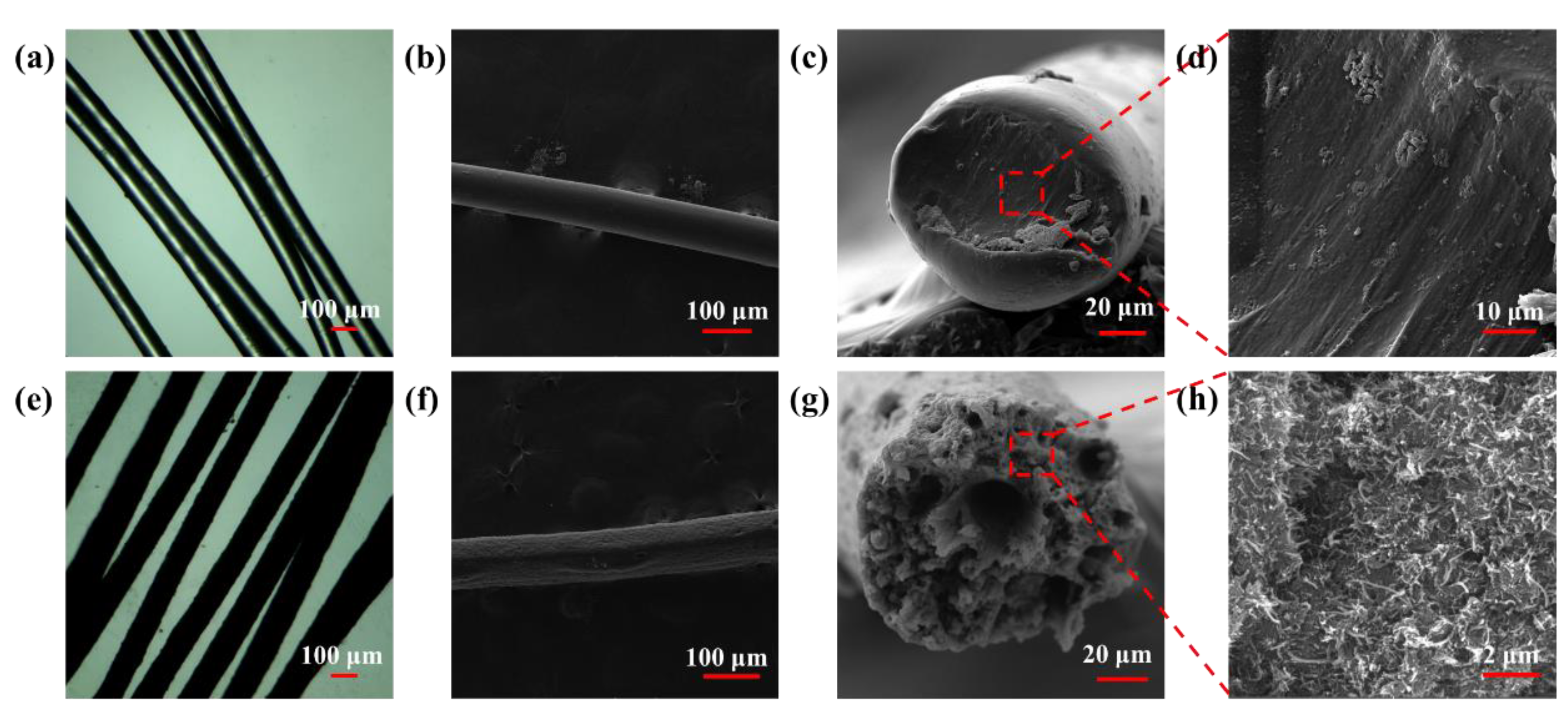
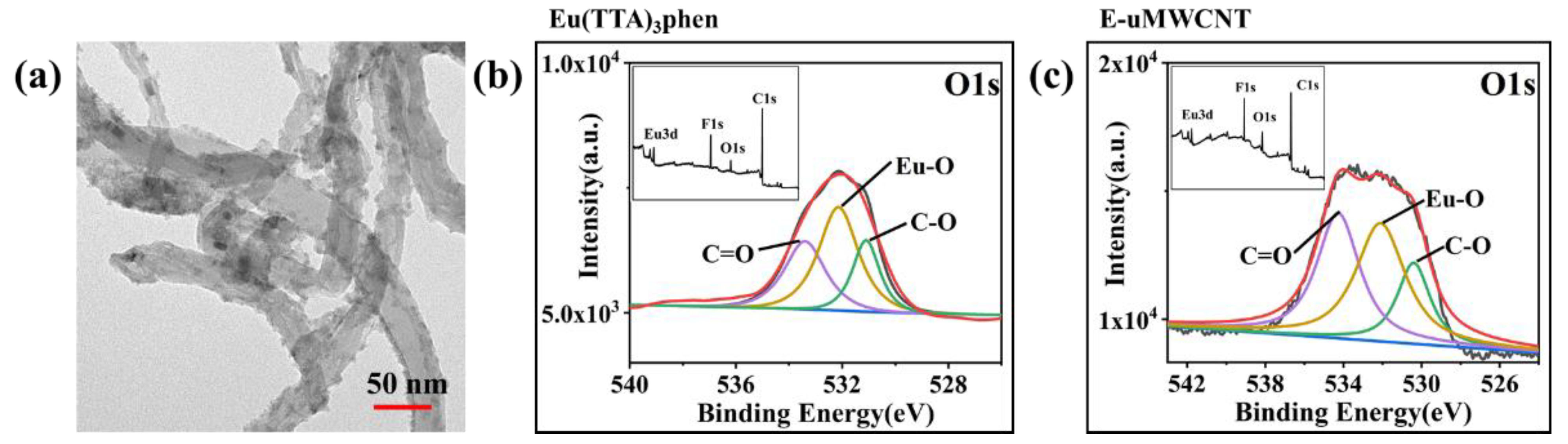
3.1. Morphology and Structure of uMWCNTs
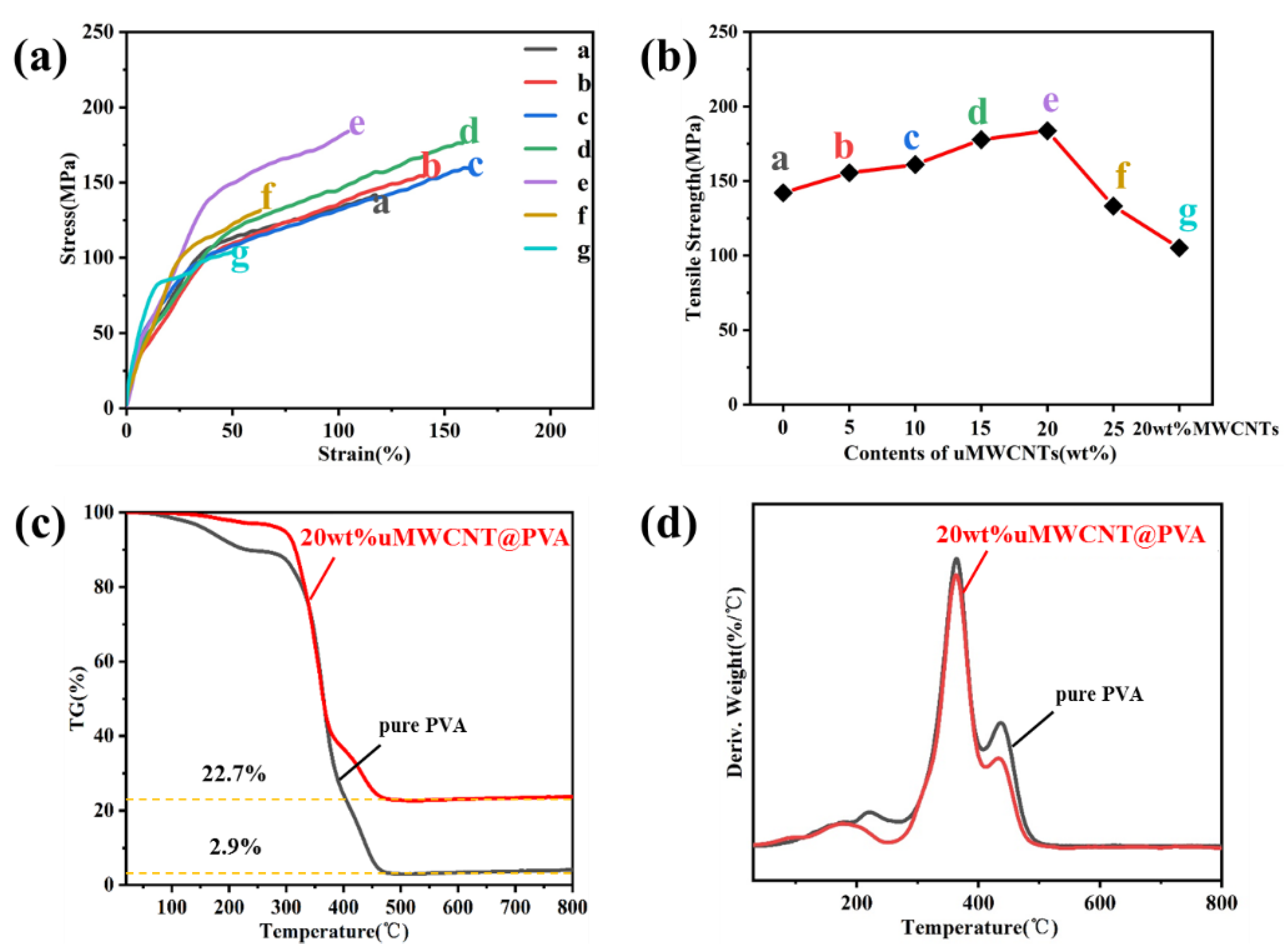
3.2. Structures and Performances of uMWCNT-Fs
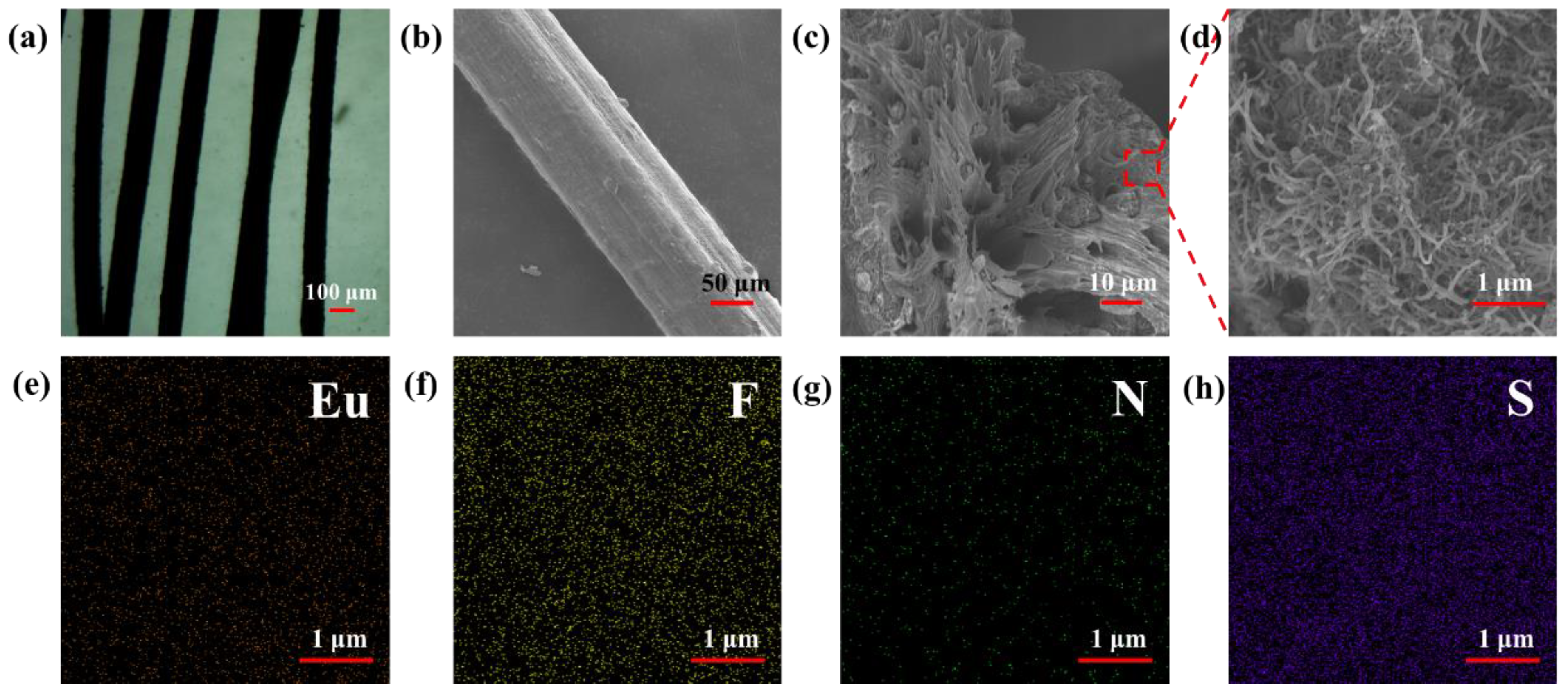
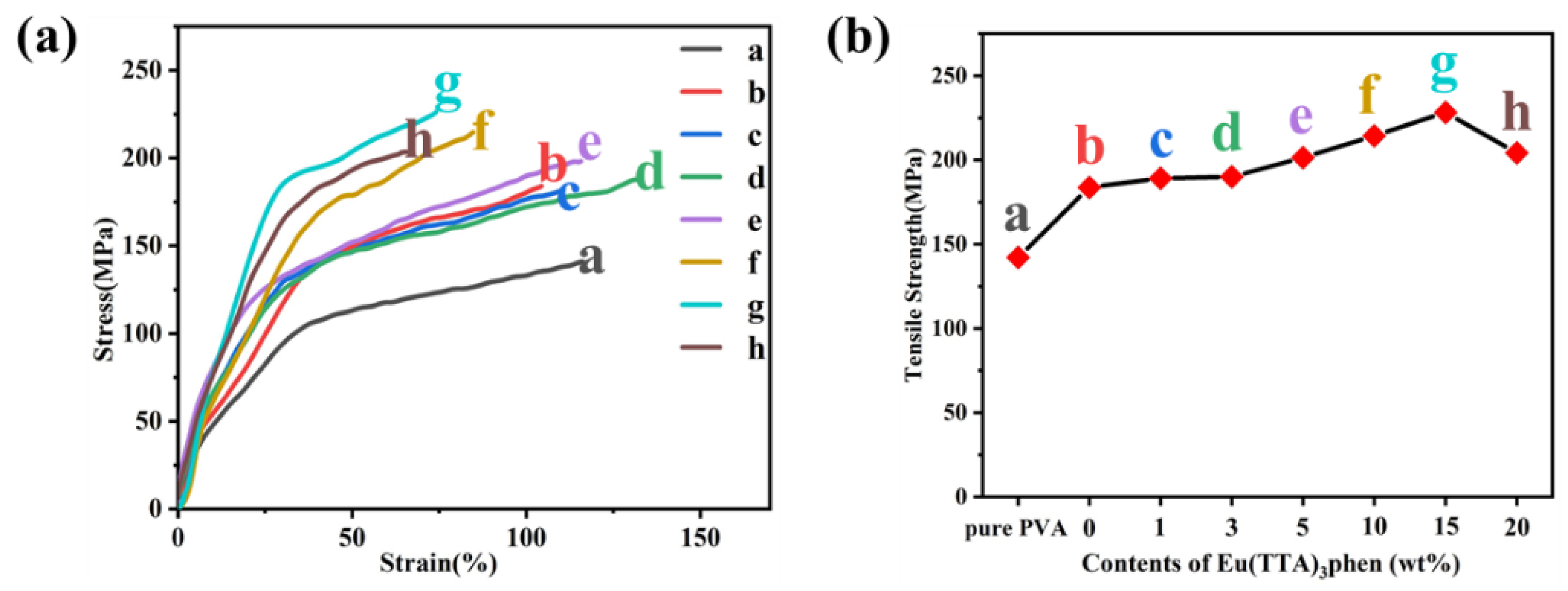
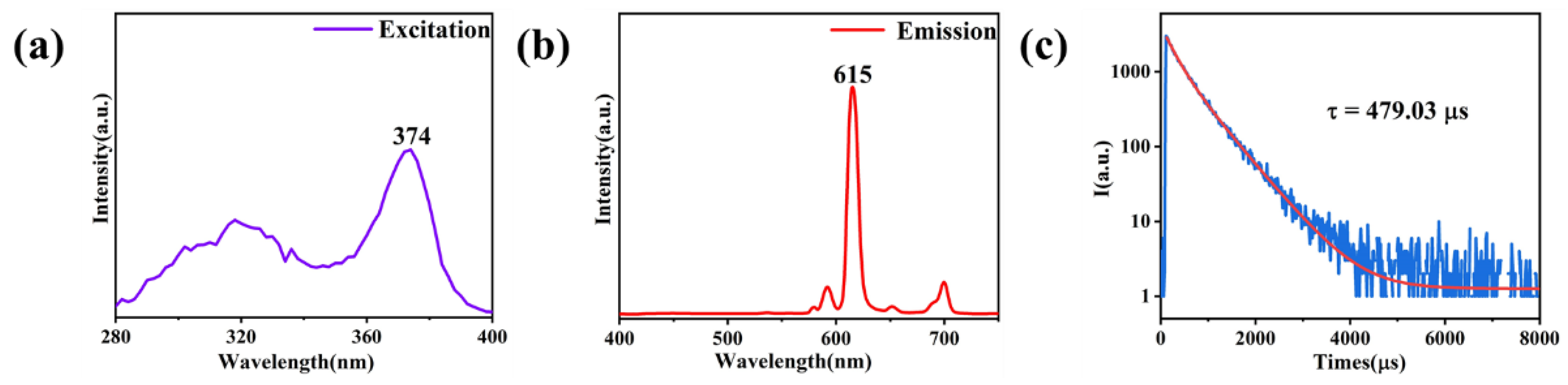
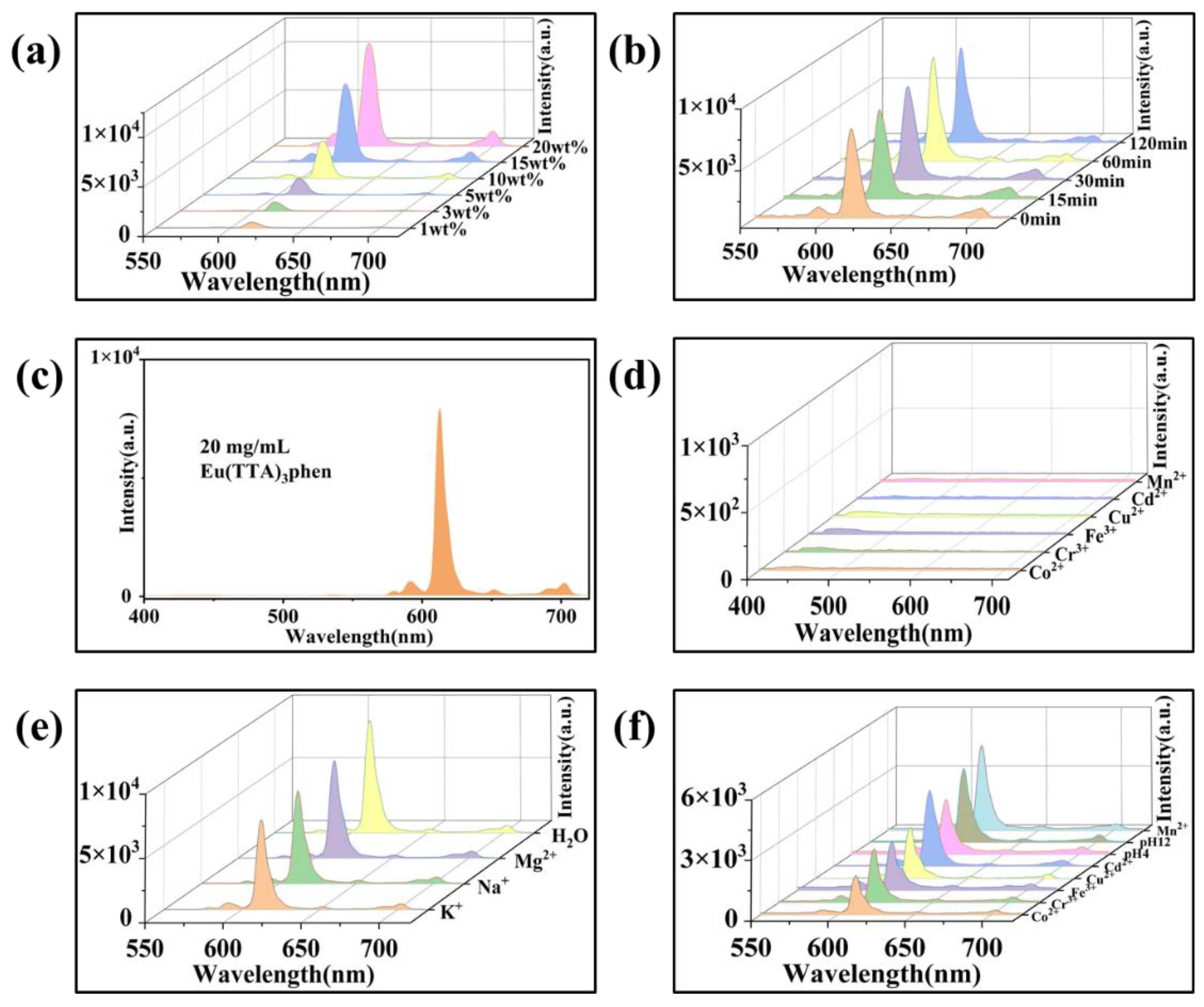
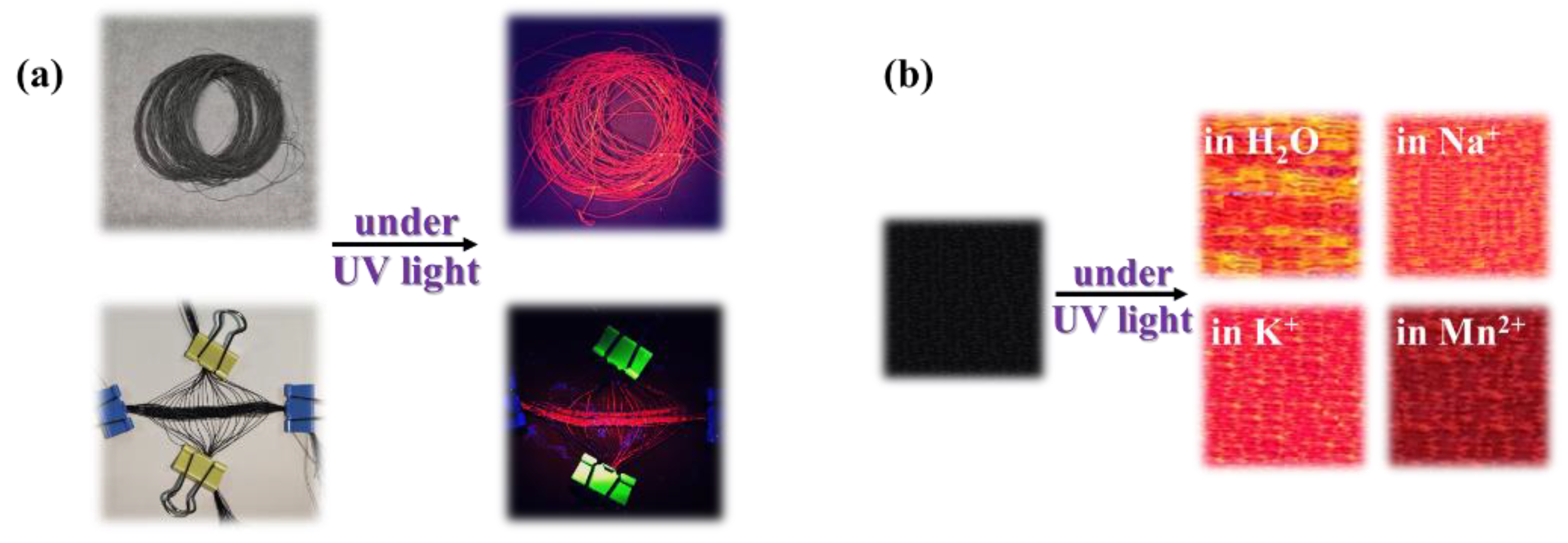
3.3. Structures and Performances of E-uMWCNT-Fs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MWCNTs | Multiwalled Carbon Nanotubes |
| uMWCNTs | Unzipped, Multiwalled Carbon Nanotubes |
| E-uWMCNTs | Eu(TTA)3phen-complex-anchored uMWCNTs |
| uMWCNT-Fs | Macromorphic Fibers of uMWCNT |
| E-uMWCNT-Fs | Macromorphic Fibers of E-uMWCNT |
References
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host-Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Qu, J.; Tian, M.; Han, X.; Zhang, R.; Wang, Q. Photo-thermal conversion characteristics of MWCNT-H2O nanofluids for direct solar thermal energy absorption applications. Appl. Therm. Eng. 2017, 124, 486–493. [Google Scholar] [CrossRef]
- Qin, Z.; Sun, X.; Yu, Q.; Zhang, H.; Wu, X.; Yao, M.; Liu, W.; Yao, F.; Li, J. Carbon Nanotubes/Hydrophobically Associated Hydrogels as Ultrastretchable, Highly Sensitive, Stable Strain, and Pressure Sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Sambyal, P.; Iqbal, A.; Hong, J.; Kim, H.; Kim, M.K.; Hong, S.M.; Han, M.K.; Gogotsi, Y.; Koo, C.M. Ultralight and Mechanically Robust Ti3C2Tx Hybrid Aerogel Reinforced by Carbon Nanotubes for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2019, 11, 38046–38054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Ye, J.; Shi, L.; Feng, X. Magnetic CoFe alloy@C nanocomposites derived from ZnCo-MOF for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 123096. [Google Scholar] [CrossRef]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef]
- Avouris, P.; Freitag, M.; Perebeinos, V. Carbon-nanotube photonics and optoelectronics. Nat. Photonics 2008, 2, 341–350. [Google Scholar] [CrossRef]
- Headrick, R.J.; Tsentalovich, D.E.; Berdegue, J.; Bengio, E.A.; Liberman, L.; Kleinerman, O.; Lucas, M.S.; Talmon, Y.; Pasquali, M. Structure-Property Relations in Carbon Nanotube Fibers by Downscaling Solution Processing. Adv. Mater. 2018, 30, 1704482. [Google Scholar] [CrossRef]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R-Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Lago, R.M.; Tsang, S.C.; Lu, K.L.; Chen, Y.K.; Green, M.L.H. Filling carbon nanotubes with small palladium metal crystallites: The effect of surface acid groups. J. Chem. Soc. Chem. Commun. 1995, 13, 1355–1356. [Google Scholar] [CrossRef]
- Tsang, S.C.; Chen, Y.K.; Harris, P.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, Z.; Gu, Z.; Iijima, S. Structure modification of single-wall carbon nanotubes. Carbon 2000, 38, 2055–2059. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H. Chemical modification of multiwalled carbon nanotubes for sorption of Zn2+ from aqueous solution. Chem. Eng. J. 2008, 139, 462–468. [Google Scholar] [CrossRef]
- Shinde, D.B.; Debgupta, J.; Kushwaha, A.; Aslam, M.; Pillai, V.K. Electrochemical Unzipping of Multi-walled Carbon Nanotubes for Facile Synthesis of High-Quality Graphene Nanoribbons. J. Am. Chem. Soc. 2011, 133, 4168–4171. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Nie, G.; Zhang, H.; Duan, X.; Wang, S. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, S.M.; Spinks, G.M.; Kim, S.J. Carbon Nanotube Yarn for Fiber-Shaped Electrical Sensors, Actuators, and Energy Storage for Smart Systems. Adv. Mater. 2020, 32, 1902670. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-Driven Carbon Nanotube Functional Materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef]
- Accorsi, G.; Armaroli, N.; Parisini, A.; Meneghetti, M.; Marega, R.; Prato, M.; Bonifazi, D. Wet adsorption of a luminescent EuIII complex on carbon nanotubes sidewalls. Adv. Funct. Mater. 2007, 17, 2975–2982. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Forrest, S.R. Electroluminescence mechanisms in organic light emitting devices employing a europium chelate doped in a wide energy gap bipolar conducting host. J. Appl. Phys. 2000, 87, 8049–8055. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Lehn, J.M. luminescent lanthanide complexes as photochemical supramolecular devices. Coord. Chem. Rev. 1993, 123, 201–228. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, S.; Yang, S.; Chen, W.; Weng, W.; Cheng, Y.; Zhu, M. Flexible all-solid-state asymmetric supercapacitor based on transition metal oxide nanorods/reduced graphene oxide hybrid fibers with high energy density. Carbon 2017, 113, 151–158. [Google Scholar] [CrossRef]
- Zhou, G.; Byun, J.-H.; Oh, Y.; Jung, B.-M.; Cha, H.-J.; Seong, D.-G.; Um, M.-K.; Hyun, S.; Chou, T.-W. Highly sensitive wearable textile-based humidity sensor made of high-strength, single-walled carbon nanotube/poly (vinyl alcohol) filaments. ACS Appl. Mater. Interfaces 2017, 9, 4788–4797. [Google Scholar] [CrossRef] [PubMed]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, W.; Yang, N.; Yang, D.; Bychko, I.; Chen, M.; Wang, Y.; Han, D.; Huang, L.; Jiang, H.; et al. Direct anchoring of Eu3+ complex to derivative surfaces of multi-wall carbon nanotubes (Eu@DSCNTs) for linear fluorescence nanomaterials. J. Alloys Compd. 2021, 853, 156880. [Google Scholar] [CrossRef]
- Dorenbos, P. The Eu3+ charge transfer energy and the relation with the band gap of compounds. J. Lumin. 2005, 111, 89–104. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.H. Recent Advances in Luminescence Imaging of Biological Systems Using Lanthanide(III) Luminescent Complexes. Molecules 2020, 25, 2089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.Y.; Liu, Y. Investigation on fluorescence quenching of dyes by graphite oxide and graphene. Appl. Surf. Sci. 2011, 257, 5513–5518. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Wei, J.; Yuan, Y.; Li, H.; Hao, D.; Sun, C.; Zheng, G.; Wang, R. A novel fluorescent sensor for water in organic solvents based on dynamic quenching of carbon quantum dots. New J. Chem. 2018, 42, 18787–18793. [Google Scholar] [CrossRef]
- Aleem, A.R.; Liu, J.; Wang, J.; Zhao, Y.; Wang, Y.; Wang, Y.; Wang, W.; Rehman, F.U.; Kipper, M.J.; Tang, J. Selective Sensing of Cu2+ and Fe3+ Ions with Vis-Excitation using Fluorescent Eu3+-Induced Aggregates of Polysaccharides (EIAP) in Mammalian Cells and Aqueous Systems. J. Hazard. Mater. 2020, 399, 122991. [Google Scholar] [CrossRef]

| Sample | C (%) | O (%) |
|---|---|---|
| MWCNT | 97.144 | 2.856 |
| uMWCNT | 93.226 | 6.774 |
| Sample | Tensile Strength (MPa) | Strain (%) |
|---|---|---|
| (a) pure PVA fiber | 142.1 | 111.8 |
| (b) 5 wt% uMWCNT-F | 155.5 | 140.7 |
| (c) 10 wt% uMWCNT-F | 161.0 | 170.0 |
| (d) 15 wt% uMWCNT-F | 177.7 | 159.2 |
| (e) 20 wt% uMWCNT-F | 183.7 | 104.2 |
| (f) 25 wt% uMWCNT-F | 133.2 | 64.4 |
| (g) 20 wt% MWCNT-F | 105.1 | 51.9 |
| Sample | Tensile Strength (MPa) | Strain (%) |
|---|---|---|
| (a) pure PVA fiber | 142.1 | 111.8 |
| (b) 20 wt% uMWCNT-F | 183.7 | 104.2 |
| (c) 1 wt% E-uMWCNT-F | 189.2 | 109.2 |
| (d) 3 wt% E-uMWCNT-F | 190.1 | 130.5 |
| (e) 5 wt% E-uMWCNT-F | 201.4 | 117.2 |
| (f) 10 wt% E-uMWCNT-F | 214.4 | 84.7 |
| (g) 15 wt% E-uMWCNT-F | 228.2 | 74.2 |
| (h) 20 wt% E-uMWCNT-F | 204.2 | 66.6 |
| Sample | QY (%) |
|---|---|
| 1 wt% E-uMWCNT-F | 11.7 |
| 3 wt% E-uMWCNT-F | 12.3 |
| 5 wt% E-uMWCNT-F | 12.6 |
| 10 wt% E-uMWCNT-F | 12.9 |
| 15 wt% E-uMWCNT-F | 14.1 |
| 20 wt% E-uMWCNT-F | 14.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Wang, H.; Liu, G.; Wei, H.; Hu, J.; Wang, Y.; Gong, X.; Mao, S.; Danilov, M.; Rusetskyi, I.; et al. Excellent Photonic and Mechanical Properties of Macromorphic Fibers Formed by Eu3+-Complex-Anchored, Unzipped, Multiwalled Carbon Nanotubes. Materials 2022, 15, 4933. https://doi.org/10.3390/ma15144933
Huang M, Wang H, Liu G, Wei H, Hu J, Wang Y, Gong X, Mao S, Danilov M, Rusetskyi I, et al. Excellent Photonic and Mechanical Properties of Macromorphic Fibers Formed by Eu3+-Complex-Anchored, Unzipped, Multiwalled Carbon Nanotubes. Materials. 2022; 15(14):4933. https://doi.org/10.3390/ma15144933
Chicago/Turabian StyleHuang, Mengjie, Haihang Wang, Gaohan Liu, Heng Wei, Jie Hu, Yao Wang, Xuezhong Gong, Sui Mao, Michail Danilov, Ihor Rusetskyi, and et al. 2022. "Excellent Photonic and Mechanical Properties of Macromorphic Fibers Formed by Eu3+-Complex-Anchored, Unzipped, Multiwalled Carbon Nanotubes" Materials 15, no. 14: 4933. https://doi.org/10.3390/ma15144933
APA StyleHuang, M., Wang, H., Liu, G., Wei, H., Hu, J., Wang, Y., Gong, X., Mao, S., Danilov, M., Rusetskyi, I., & Tang, J. (2022). Excellent Photonic and Mechanical Properties of Macromorphic Fibers Formed by Eu3+-Complex-Anchored, Unzipped, Multiwalled Carbon Nanotubes. Materials, 15(14), 4933. https://doi.org/10.3390/ma15144933







