Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting
Abstract
:1. Introduction
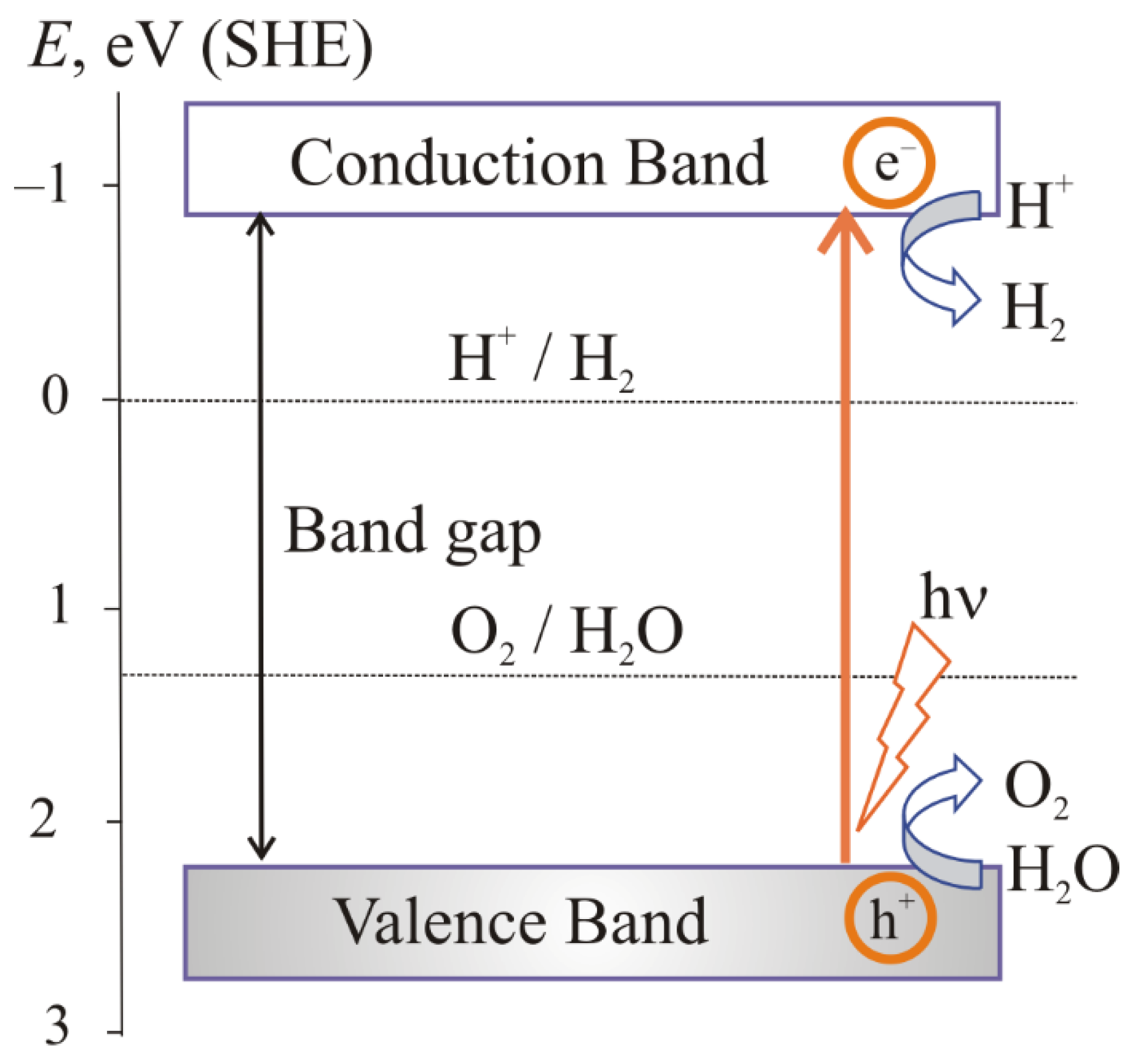
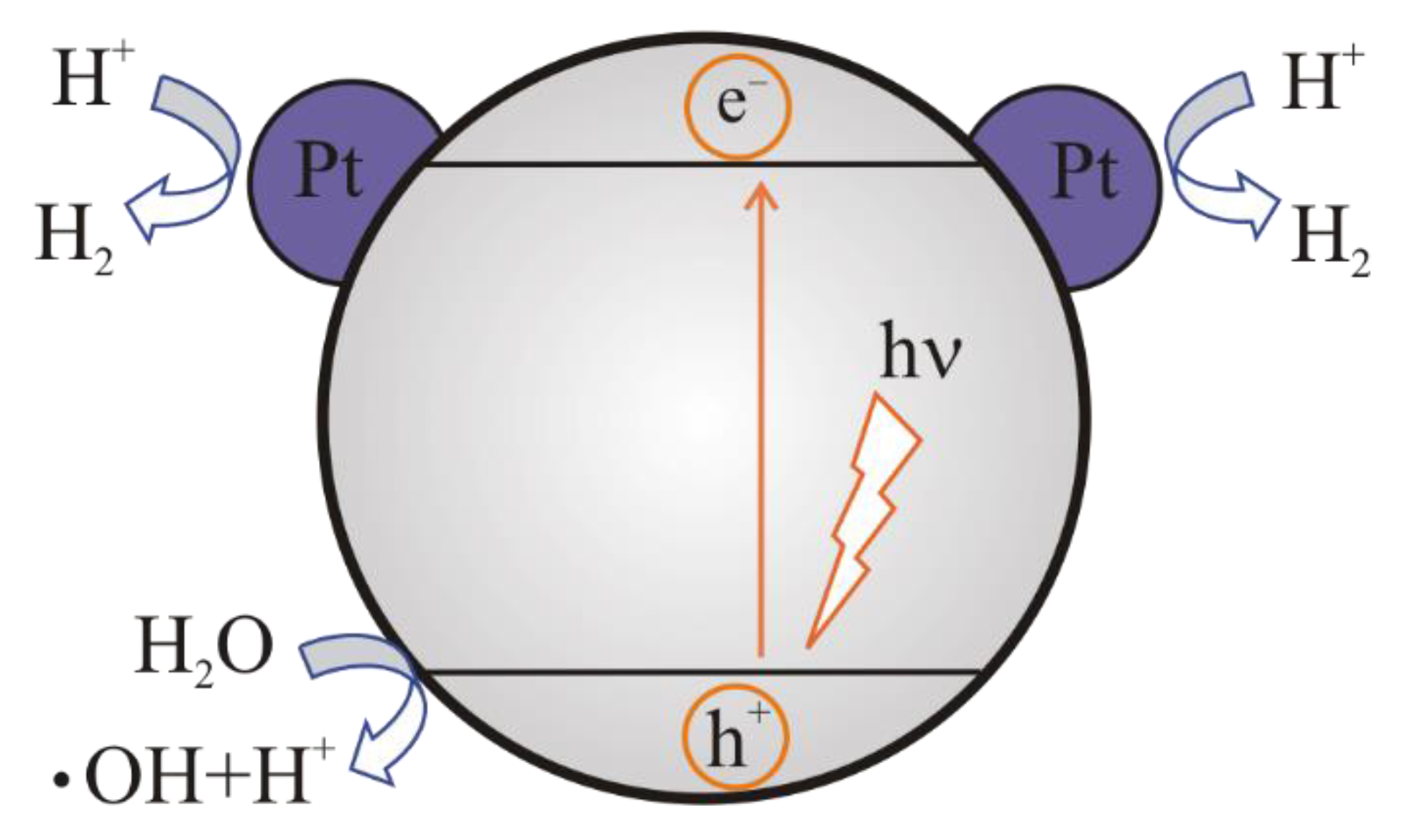
2. Mechanism of Photocatalytic Water Splitting
2.1. The Main Stages of the Photocatalytic Water Splitting
- –
- the absorption of light by a semiconductor;
- –
- excitation of charge carriers;
- –
- separation and transfer of charge carriers;
- –
- surface catalytic reactions.
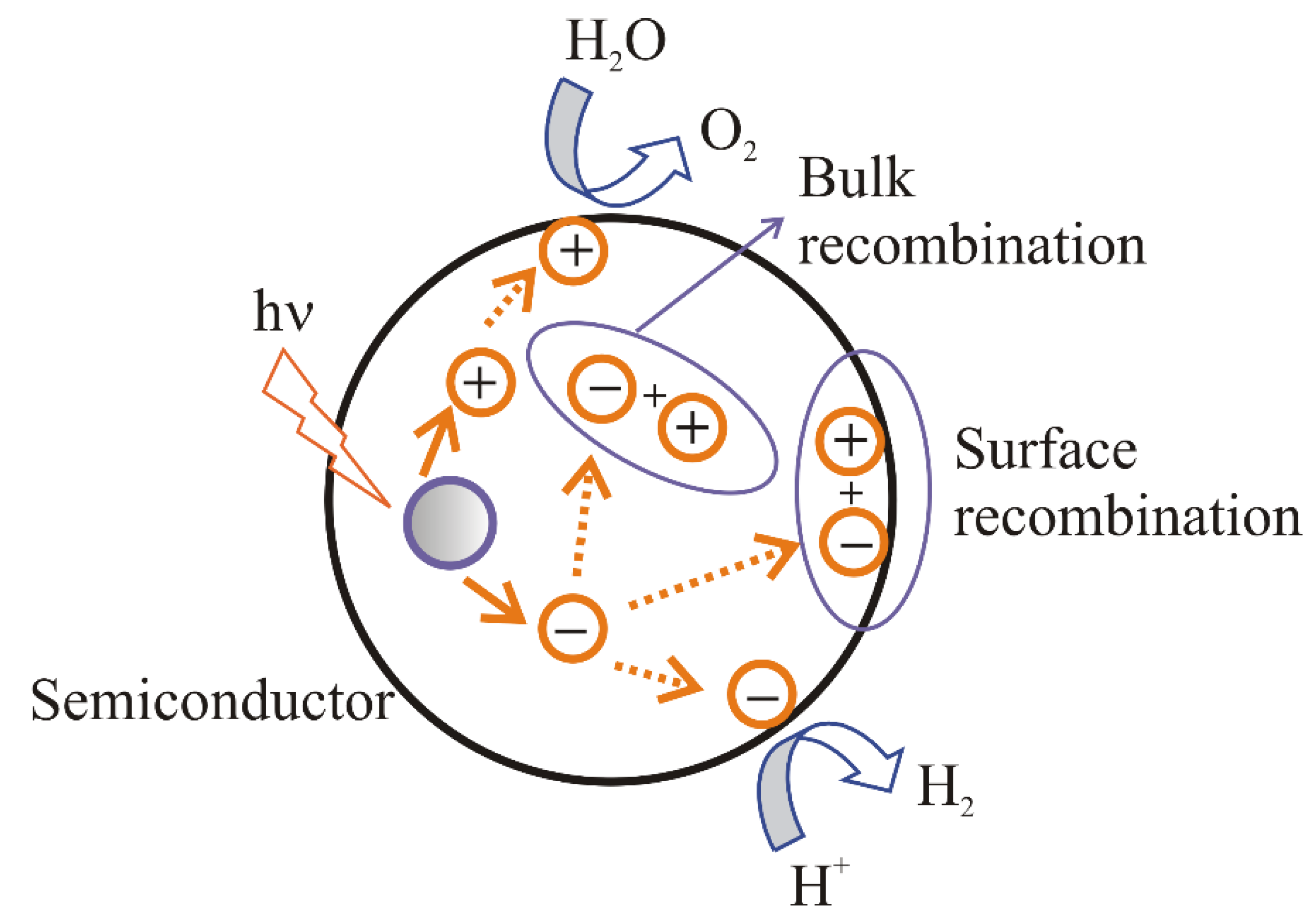
2.2. The Main Conditions for Water Splitting
- (i).
- recombination of electrons and holes on the surface of a semiconductor;
- (ii).
- recombination of electrons and holes in the bulk of a semiconductor;
- (iii).
- transport of the electrons to the surface and participation in the reduction reaction;
- (iv).
- transport of the holes to the surface and participation in the oxidation reaction.
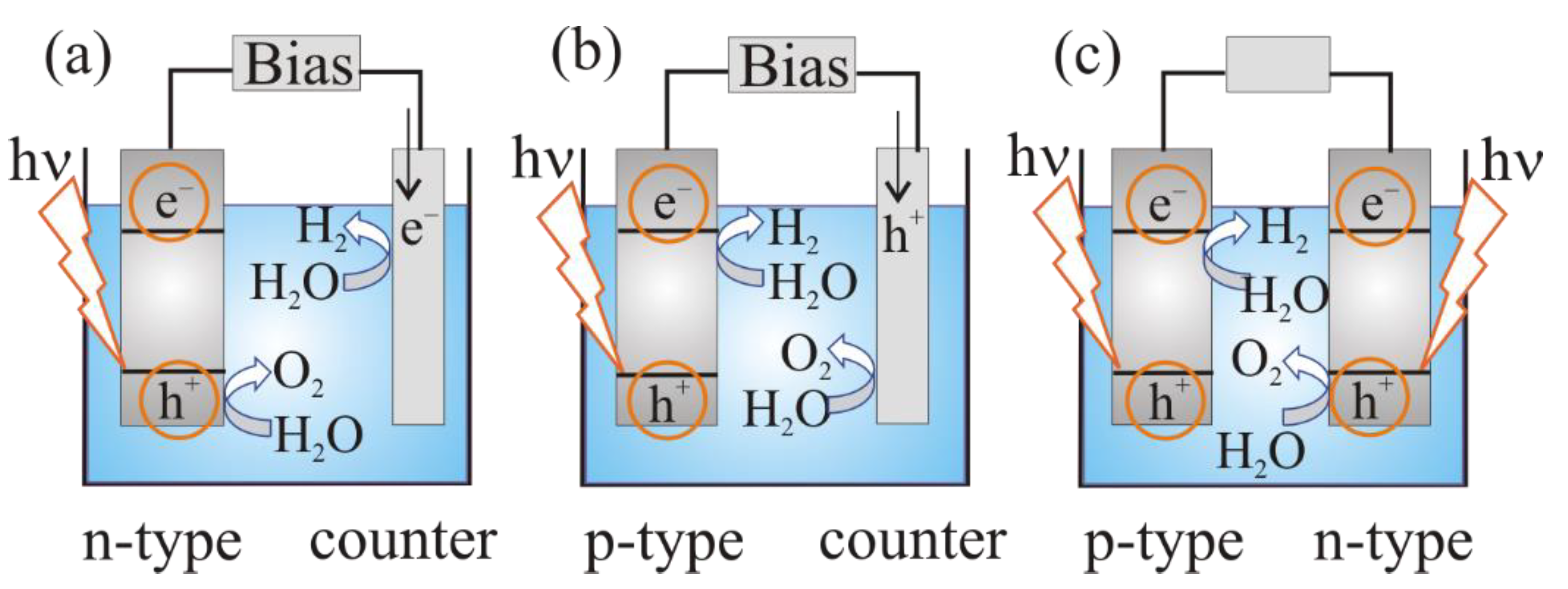
2.3. The Mechanism of Photoelectrochemical Water Splitting
3. Requirements for Photocatalysts
- (i).
- Bandgap ensures the most effective light absorption of a wide spectrum;
- (ii).
- The lower edge of the conduction band and the upper edge of the valence band are more negative than the hydrogen evolution potential, and more positive than the oxygen evolution potential, respectively;
- (iii).
- A low number of defects for efficient charge transfer and reduced possibility of charge carriers’ recombination;
- (iv).
- High corrosion resistance and photochemical stability;
- (v).
- Low cost.
3.1. The Value of Bandgap
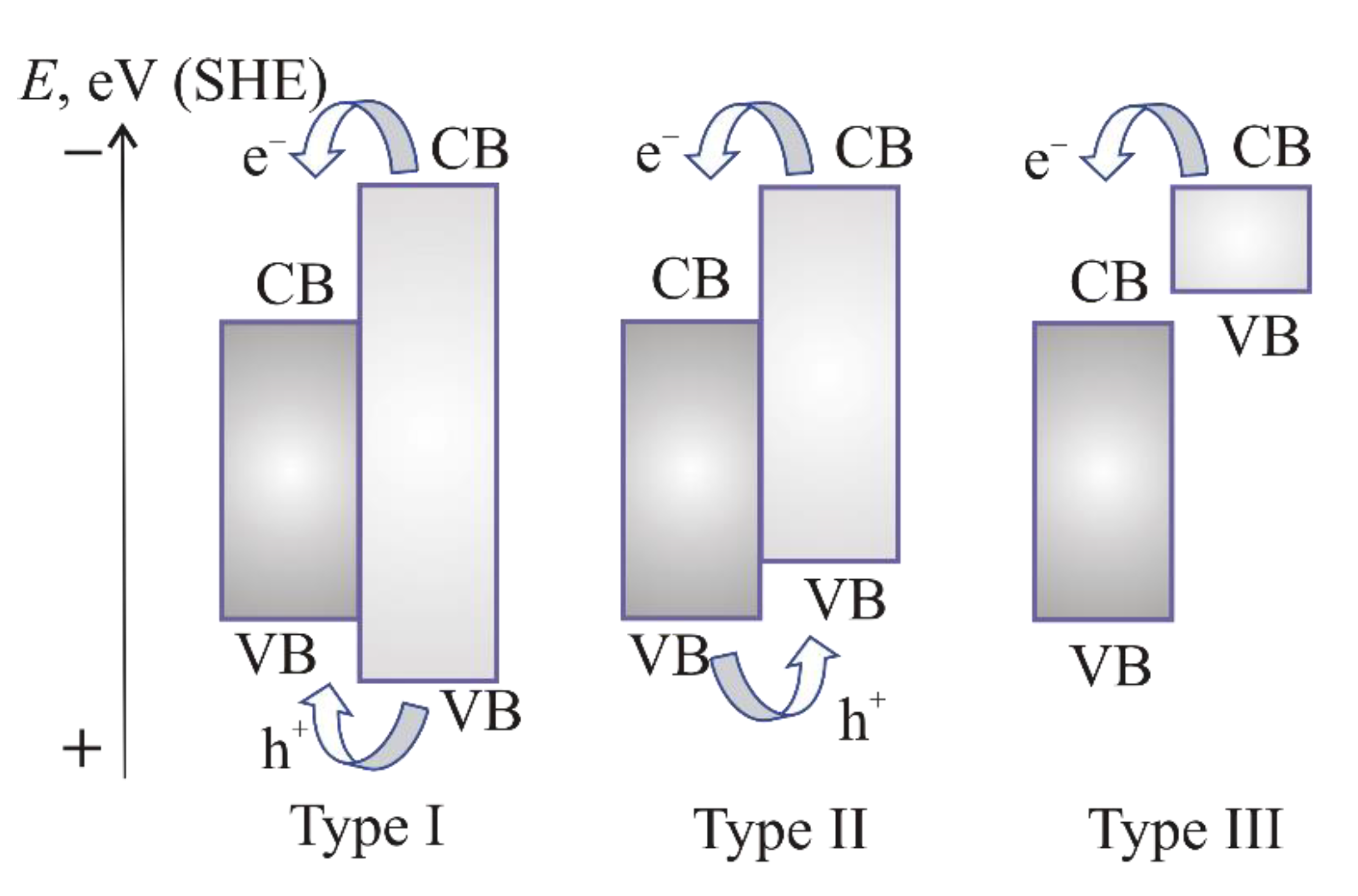
3.2. The Arrangement of Band Edges
3.3. The Number of Defects
3.4. Corrosion Resistance and Photochemical Stability
4. Strategies of the Metal Oxide Photocatalyst Modification
- (i).
- Modification of the crystal structure and morphology;
- (ii).
- Doping of a semiconductor with metal and/or nonmetal ions;
- (iii).
- Sensitization of semiconductors by quantum dots;
- (iv).
- Formation of solid solutions or heterojunctions;
- (v).
- Application of a cocatalyst.
4.1. Modification of the Crystal Structure and Morphology
4.1.1. Modification of the Crystal Structure
4.1.2. Modification of Size and Morphology
4.2. Doping of an Oxide Semiconductor with Metal and/or Nonmetal Ions
4.3. Sensitization of Oxide Semiconductors by Quantum Dots
4.4. Formation of Solid Solutions or Heterojunctions
- –
- with shuttle redox mediator;
- –
- with solid-state electronic linker;
- –
- direct systems.
4.5. Application of a Cocatalyst
5. Common Factors Affecting the Efficiency of Photocatalytic Water Splitting
5.1. Irradiation Intensity
5.2. pH of the Solution
5.3. Temperature
5.4. Photocatalyst Dosage
5.5. Surface Effects
6. Conclusions
Funding
Conflicts of Interest
References
- Wang, J. Barriers of scaling-up fuel cells: Cost, durability and reliability. Energy 2015, 80, 509–521. [Google Scholar] [CrossRef]
- Lund, H.; Østergaard, P.A.; Stadler, I. Towards 100% renewable energy systems. Appl. Energy 2011, 88, 419–421. [Google Scholar] [CrossRef]
- Fang, Y.; Hou, Y.; Fu, X.; Wang, X. Semiconducting Polymers for Oxygen Evolution Reaction under Light Illumination. Chem. Rev. 2022, 122, 4204–4256. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liao, A.; Guo, W.; Luo, W.; Zhou, Y.; Zou, Z. State-of-the-art progress in the use of ternary metal oxides as photoelectrode materials for water splitting and organic synthesis. Nano Today 2019, 28, 100763. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Chen, J.; Yang, D.; Song, D.; Jiang, J.; Ma, A.; Hu, M.Z.; Ni, C. Recent progress in enhancing solar-to-hydrogen efficiency. J. Power Source 2015, 280, 649–666. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Masudy-Panah, S.; Siavash Moakhar, R.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef]
- Singla, A.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic water splitting hydrogen production via environmental benign carbon based nanomaterials. Int. J. Hydrog. Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Lee, K.-H.; Lee, J.S. Research Update: Strategies for efficient photoelectrochemical water splitting using metal oxide photoanodes. APL Mater. 2014, 2, 010703. [Google Scholar] [CrossRef] [Green Version]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrog. Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Chouhan, N.; Ameta, R.; Meena, R.K.; Mandawat, N.; Ghildiyal, R. Visible light harvesting Pt/CdS/Co-doped ZnO nanorods molecular device for hydrogen generation. Int. J. Hydrog. Energy 2016, 41, 2298–2306. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, Y.; Wang, X. Efficient development of Type-II TiO2 heterojunction using electrochemical approach for an enhanced photoelectrochemical water splitting performance. Chin. J. Catal. 2018, 39, 438–445. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. Part B 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Rongé, J.; Bosserez, T.; Huguenin, L.; Dumortier, M.; Haussener, S.; Martens, J.A. Solar hydrogen reaching maturity. Oil Gas Sci. Technol. Rev. D’ifp Energ. Nouv. 2015, 70, 863–876. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Ortega Méndez, J.A.; López, C.R.; Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Fernández Hevia, D.; Macías, M. Production of hydrogen by water photo-splitting over commercial and synthesised Au/TiO2 catalysts. Appl. Catal. B Environ. 2014, 147, 439–452. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Al-Azri, Z.H.N.; Dosado, A.G.; Nadeem, M.A.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Effect of TiO2 polymorph and alcohol sacrificial agent on the activity of Au/TiO2 photocatalysts for H2 production in alcohole-water mixtures. J. Catal. 2015, 329, 499–513. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5167. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M. Morphological effect of 1D/1D In2O3/TiO2 NRs/NWs heterojunction photo-embedded with Cu-NPs for enhanced photocatalytic H2 evolution under visible light. Appl. Surf. Sci. 2020, 506, 145034. [Google Scholar] [CrossRef]
- Tahir, B.; Er, P.W.; Tahir, M.; Nawawi, M.G.M.; Siraj, M.; Alias, H.; Fatehmulla, A. Tailoring metal/support interaction in 0D TiO2 NPs/MPs embedded 2D MAX composite with boosted interfacial charge carrier separation for stimulating photocatalytic H2 production. J. Environ. Chem. Eng. 2020, 8, 104529. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Zamfirescu, C. A review on selected heterogeneous photocatalysts for hydrogen production. Int. J. Energy Res. 2014, 38, 1903–1920. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M.; Zakaria, Z.Y. Fabricating structured 2D Ti3AlC2 MAX dispersed TiO2 heterostructure with Ni2P as a cocatalyst for efficient photocatalytic H2 production. J. Alloys Compd. 2020, 842, 155752. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G. Visible light driven photocatalytic hydrogen evolution using different sacrificial reagents. Chem. Eng. Trans. 2019, 74, 547–552. [Google Scholar] [CrossRef]
- Yang, R.; Song, K.; He, J.; Fan, Y.; Zhu, R. Photocatalytic hydrogen production by RGO/ZnIn2S4 under visible light with simultaneous organic amine degradation. ACS Omega 2019, 4, 11135–11140. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, X.; Yu, J. Surface and interface modification strategies of CdS-based photocatalysts. In Interface Science and Technology, 1st ed.; Yu, J., Jaroniec, M., Jiang, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 313–348. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, W.H.; Chen, C.C.; Zhao, J.C.; Shuai, Z.G. Efficient degradation of toxic organic pollutants with Ni2O3/TiO2−xBx under visible irradiation. J. Am. Chem. Soc. 2004, 126, e4783. [Google Scholar] [CrossRef]
- Nozik, A.J.; Memming, R. Physical chemistry of semiconductor—Liquid interfaces. J. Phys. Chem. 1996, 100, 13061–13078. [Google Scholar] [CrossRef]
- Meng, X.-D.; Wang, D.-Z.; Liu, J.-H.; Zhang, S.-Y. Preparation and characterization of sodium titanate nanowires from brookite nanocrystallites. Mater. Res. Bull. 2004, 39, 2163–2170. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Grewe, T.; Meggouh, M.; Tüysüz, H. Nanocatalysts for solar water splitting and a perspective on hydrogen economy. Chem. Asian J. 2016, 11, 22–42. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Sivula, K. Solar-to-chemical energy conversion with photoelectrochemical tandem cells. Chimia 2013, 67, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tasleem, S.; Tahir, M.; Khalifa, W.A. Current trends in structural development and modification strategies for metal-organic frameworks (MOFs) towards photocatalytic H2 production: A review. Int. J. Hydrog. Energy 2021, 46, 14148–14189. [Google Scholar] [CrossRef]
- Medina, M.; Corradini, P.G.; Mascaro, L.H. Facile one-step electrodeposition fabrication of amorphous MoS2 catalysts in titanium for hydrogen evolution reaction. J. Braz. Chem. Soc. 2019, 30, 2210–2218. [Google Scholar] [CrossRef]
- Petala, A.; Kondarides, D.I. Photocatalytic hydrogen production over mixed Cd-Zn sulfide catalysts promoted with nickel or nickel phosphide. Catal. Today 2020, 355, 851–859. [Google Scholar] [CrossRef]
- Toroker, M.C.; Kanan, D.K.; Alidoust, N.; Isseroff, L.Y.; Liao, P.; Carter, E.A. First principles scheme to evaluate band edge positions in potential transition metal oxide photocatalysts and photoelectrodes. Phys. Chem. Chem. Phys. 2011, 13, 16644–16654. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.F.; Berglund, S.P. Recent developments in complex metal oxide photoelectrodes. J. Phys. D Appl. Phys. 2017, 50, 193002. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, D.; Lumley, M.A.; Choi, K.-S. Progress on ternary oxide-based photoanodes for use in photoelectrochemical cells for solar water splitting. Chem. Soc. Rev. 2019, 48, 2126–2157. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.K.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Xiong, A.; Yoshinaga, T.; Maeda, K.; Domen, K.; Teranishi, T. Polyol synthesis of size-controlled Rh nanoparticles and their application to photocatalytic overall water splitting under visible light. J. Phys. Chem. C 2013, 117, 2467–2473. [Google Scholar] [CrossRef]
- Méndez-Ramos, J.; Ruiz-Morales, J.C.; Acosta-Mora, P.; del-Castillo, J.; Yanes, A.C. Rare-earth doped nano-glass-ceramics for extending spectral response of water-splitting semiconductor electrodes by high intense UV-blue up-conversion: Turning the sun in to blue. J. Power Source 2013, 238, 217–313. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Feng, P. Self-doped Ti3+@TiO2 visible light photocatalyst: Influence of synthetic parameters on the H2 production activity. Int. J. Hydrog. Energy 2014, 39, 711–717. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Lin, J.; Li, X.; Peng, C.; Gao, X.; Huang, Q.; Wang, J.; Xu, H.; Fan, C. Lattice defect-enhanced hydrogen production in nanostructured hematite-based photoelectrochemical device. ACS Appl. Mater. Interfaces 2012, 4, 2295–2302. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, G.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett. 2011, 11, 2119–2125. [Google Scholar] [CrossRef]
- Zhan, X.; Luo, Y.; Wang, Z.; Xiang, Y.; Peng, Z.; Han, Y.; Zhang, H.; Chen, R.; Zhou, Q.; Peng, H.; et al. Formation of multifaceted nano-groove structure on rutile TiO2 photoanode for efficient electron-hole separation and water splitting. J. Energy Chem. 2022, 65, 19–25. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Wang, J.; Peng, C.; Huang, Q.; Su, S.; Wang, L.; Huang, W.; Fan, C. Template-free synthesis of hematite photoanodes with nanostructured ATO conductive underlayer for PEC water splitting. ACS Appl. Mater. Interfaces 2014, 6, 36–40. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Hahn, C.; Liu, B.; Yang, P. Photoelectrochemical properties of TiO2 nanowire arrays: A study of the dependence on length and atomic layer deposition coating. ACS Nano 2012, 6, 5060–5069. [Google Scholar] [CrossRef]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Jankulovska, M.; Barceló, I.; Lana-Villarreal, T.; Gómez, R. Improving the photoelectrochemical response of TiO2 nanotubes upon decoration with quantum-sized anatase nanowires. J. Phys. Chem. C 2013, 117, 4024–4031. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-S.; Liao, W.-P.; Wu, J.-J. Morphology and interfacial energetics controls for hierarchical anatase/rutile TiO2 nanostructured array for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2013, 5, 7425–7431. [Google Scholar] [CrossRef]
- Kong, J.; Wei, Y.; Yang, L.; Yee, W.A.; Dong, Y.; Zhou, R.; Wong, S.Y.; Ke, L.; Sun, X.W.; Du, H.; et al. Electrospinning-derived "Hairy Seaweed" and its photoelectrochemical properties. J. Phys. Chem. C 2013, 117, 10106–10113. [Google Scholar] [CrossRef]
- Kargar, A.; Jing, Y.; Kim, S.J.; Riley, C.T.; Pan, X.; Wang, D. ZnO/CuO heterojunction branched nanowires for photoelectrochemical hydrogen generation. ACS Nano 2013, 7, 11112–11120. [Google Scholar] [CrossRef]
- Kargar, A.; Sun, K.; Jing, Y.; Choi, C.; Jeong, H.; Jung, G.Y.; Jin, S.; Wang, D. 3D branched nanowire photoelectrochemical electrodes for efficient solar water splitting. ACS Nano 2013, 7, 9407–9415. [Google Scholar] [CrossRef]
- Kargar, A.; Sun, K.; Jing, Y.; Choi, C.; Jeong, H.; Zhou, Y.; Madsen, K.; Naughton, P.; Jin, S.; Jung, G.Y.; et al. Tailoring n-ZnO/p-Si branched nanowire heterostructures for selective photoelectrochemical water oxidation or reduction. Nano Lett. 2013, 13, 3017–3022. [Google Scholar] [CrossRef]
- Liu, C.; Dasgupta, N.P.; Yang, P. Semiconductor nanowires for artificial photosynthesis. Chem. Mater. 2014, 26, 415–422. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Liu, R.-H.; Zhang, L.; Zhang, J.; Wilkinson, D.P. Nano- architecture and material designs for water splitting photoelectrodes. Chem. Soc. Rev. 2012, 41, 5654–5671. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Cao, S.; Wu, Z.; Ji, Y.; Mi, Y.; Wu, X.; Liu, X.; Piao, L. The effect of directed photogenerated carrier separation on photocatalytic hydrogen production. Nano Energy 2017, 41, 488–493. [Google Scholar] [CrossRef]
- Leng, W.H.; Barnes, P.R.F.; Juozapavicius, M.; O’Regan, B.C.; Durrant, J.R. Electron diffusion length in mesoporous nanocrystalline TiO2 photoelectrodes during water oxidation. J. Phys. Chem. Lett. 2010, 1, 967–972. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Basu, S.; Shetti, N.P.; Reddy, K.R. 2D materials and its heterostructured photocatalysts: Synthesis, properties, functionalization and applications in environmental remediation. J. Environ. Chem. Eng. 2021, 9, 106408. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Z.; Du, Y.; Qi, X.; Huang, Y.; Xue, C.; Zhang, H. Full solution-processed synthesis of all metal oxide-based tree-like heterostructures on fluorine-doped tin oxide for water splitting. Adv. Mater. 2012, 24, 5374–5378. [Google Scholar] [CrossRef]
- Tezcan, F.; Mahmood, A.; Kardaş, G. The investigation of Cu2O electrochemical deposition time effect on ZnO for water splitting. J. Mol. Struct. 2019, 1193, 342–347. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.; Gao, H.; Xie, T.; Wang, D. Photocatalytic property of ZnO microrods modified by Cu2O nanocrystals. J. Alloys Compd. 2013, 552, 299–303. [Google Scholar] [CrossRef]
- Li, J.; Cui, M.; Guo, Z.; Liu, Z.; Zhu, Z. Synthesis of dumbbell-like CuO–BiVO4 heterogeneous nanostructures with enhanced visible-light photocatalytic activity. Mater. Lett. 2014, 130, 36–39. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Hassan, M.A.; Waseem, A.; Johar, M.A.; Kim, D.-H.; Ryu, S.-W. Cu2O as an emerging photocathode for solar water splitting—A status review. Int. J. Hydrog. Energy 2019, 44, 21351–21378. [Google Scholar] [CrossRef]
- Dubale, A.A.; Tamirat, A.G.; Chen, H.-M.; Berhe, T.A.; Pan, C.-J.; Su, W.-N.; Hwang, B.-J. A highly stable CuS and CuS-Pt modified Cu2O/CuO heterostructure as an efficient photocathode for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 2205–2216. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lai, Y.-H.; Mersch, D.; Reisner, E. Cu2O|NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem. Sci. 2012, 3, 3482–3487. [Google Scholar] [CrossRef]
- Septina, W.; Prabhakar, R.R.; Wick, R.; Moehl, T.; Tilley, S.D. Stabilized solar hydrogen production with CuO/CdS heterojunction thin film photocathodes. Chem. Mater. 2017, 29, 1735–1743. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Tilley, S.D.; Vrubel, H.; Grätzel, M.; Hu, X. Hydrogen evolution from a copper(I) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat. Commun. 2014, 5, 3059. [Google Scholar] [CrossRef]
- Santos, H.L.S.; Corradini, P.G.; Andrade, M.A.S., Jr.; Mascaro, L.H. CuO/NiOx thin film–based photocathodes for photoelectrochemical water splitting. J. Solid State Electrochem. 2020, 24, 1899–1908. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrog. Energy 2020, 45, 19078–19111. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Nishikawa, T.; Shinohara, Y.; Nakajima, T.; Fujita, M.; Mishima, S. Prospect of Activating a Photocatalyst by Sunlight—A Quantum Chemical Study of Isomorphically Substituted Titania. Chem. Lett. 1999, 28, 1133–1134. [Google Scholar] [CrossRef]
- Yang, X.; Wolcott, A.; Wang, G.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-doped ZnO nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef]
- Zong, X.; Sun, C.; Yu, H.; Chen, Z.G.; Xing, Z.; Ye, D.; Lu, G.Q.; Li, X.; Wang, L. Activation of photocatalytic water oxidation on N-doped ZnO bundle-like nanoparticles under visible light. J. Phys. Chem. C 2013, 117, 4937–4942. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, K.; Deng, H.; Yang, S. Secondary branching and nitrogen doping of ZnO nanotetrapods: Building a highly active network for photoelectrochemical water splitting. Nano Lett. 2012, 12, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Husin, H.; Chen, H.-M.; Su, W.-N.; Pan, C.-J.; Chuang, W.-T.; Sheu, H.S.; Hwang, B.-J. Green fabrication of La-doped NaTaO3 via H2O2 assisted solegel route for photocatalytic hydrogen production. Appl. Catal. B Environ. 2011, 102, 343–351. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S.; Thushari, I.; Laohhasurayotin, K. Stability of La dopants in NaTaO3 photocatalysts. J. Alloys Compd. 2019, 775, 1277–1285. [Google Scholar] [CrossRef]
- Cong, Y.; Tian, B.; Zhang, J. Improving the thermal stability and photocatalytic activity of nanosized titanium dioxide via La3+ and N co-doping. Appl. Catal. B Environ. 2011, 101, 376–381. [Google Scholar] [CrossRef]
- Holmes, M.A.; Townsend, T.K.; Osterloh, F.E. Quantum confinement controlled photocatalytic water splitting by suspended CdSe nanocrystals. Chem. Commun. 2012, 48, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Emin, S.; Fanetti, M.; Abdi, F.F.; Lisjak, D.; Valant, M.; van de Krol, R.; Dam, B. Photoelectrochemical properties of cadmium chalcogenide-sensitized textured porous zinc oxide plate electrodes. ACS Appl. Mater. Interfaces 2013, 5, 1113–1121. [Google Scholar] [CrossRef]
- Chouhan, N.; Yeh, C.L.; Hu, S.F.; Huang, J.H.; Tsai, C.W.; Liu, R.S.; Chang, W.S.; Chen, K.H. Array of CdSe QD-sensitized ZnO nanorods serves as photoanode for water splitting. J. Electrochem. Soc. 2010, 157, B1430–B1433. [Google Scholar] [CrossRef]
- Chouhan, N.; Yeh, C.L.; Hu, S.-F.; Liu, R.-S.; Chang, W.-S.; Chen, K.-H. Photocatalytic CdSe QDs-decorated ZnO nanotubes: An effective photoelectrode for splitting water. Chem. Commun. 2011, 47, 3493–3495. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W.; Yu, J. High-efficiency photoelectrochemical properties by a highly crystalline CdS-sensitized ZnO nanorod array. ACS Appl. Mater. Interfaces 2013, 5, 5097–5104. [Google Scholar] [CrossRef]
- Seol, M.; Jang, J.-W.; Cho, S.; Lee, J.S.; Yong, K. Highly efficient and stable cadmium chalcogenide quantum dot/ZnO nanowires for photoelectrochemical hydrogen generation. Chem. Mater. 2013, 25, 184–189. [Google Scholar] [CrossRef]
- Wang, G.; Yang, X.; Qian, F.; Zhang, J.Z.; Li, Y. Double-sided CdS and CdSe quantum dot Co-sensitized ZnO nanowire arrays for photoelectrochemical hydrogen generation. Nano Lett. 2010, 10, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yong, K. Highly efficient photoelectrochemical hydrogen generation using a quantum dot coupled hierarchical ZnO nanowires array. ACS Appl. Mater. Interfaces 2013, 5, 13258–13264. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seol, M.; Lee, J.; Yong, K. Highly efficient photoelectrochemical hydrogen generation using hierarchical ZnO/WOx nanowires cosensitized with CdSe/CdS. J. Phys. Chem. C 2011, 115, 25429–25436. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chang, Y.-C.; Tsai, C.-W.; Liu, R.-S.; Hu, S.-F.; Chang, W.-S.; Chen, K.-H. Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: True efficiency for water splitting. Angew. Chem. 2010, 122, 6102–6105. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Takata, T.; Hara, M.; Saito, N.; Toda, K.; Inoue, Y.; Kobayashi, H.; Domen, K. Overall water splitting on (Ga1−xZnx)(N1−xOx) solid solution photocatalyst: Relationship between physical properties and photocatalytic activity. J. Phys. Chem. B 2005, 109, 20504–20510. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Solid solution of GaN and ZnO as a stable photocatalyst for overall water splitting under visible light. Chem. Mater. 2010, 22, 612–623. [Google Scholar] [CrossRef]
- Reinert, A.A.; Payne, C.; Wang, L.; Ciston, J.; Zhu, Y.; Khalifah, P.G. Synthesis and characterization of visible light absorbing (GaN)1–x(ZnO)x semiconductor nanorods. Inorg. Chem. 2013, 52, 8389–8398. [Google Scholar] [CrossRef]
- Yan, X.; Pu, R.; Xie, R.; Zhang, B.; Shi, Y.; Liu, W.; Ma, G.; Yang, N. Design and fabrication of Bi2O3/BiFeO3 heterojunction film with improved photoelectrochemical performance. Appl. Surf. Sci. 2021, 552, 149442. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-L.; Du, H.; Jiang, Y.-F.; Jiang, N.; Shen, C.-C.; Zhou, X.; Liu, Y.-N.; Xu, A.-W. Artificial photosynthetic Z-scheme photocatalyst for hydrogen evolution with high quantum efficiency. J. Phys. Chem. C 2017, 121, 107–114. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Chen, Z.-G.; Li, F.; Wang, L.; Lu, G.Q.; Cheng, H.-M. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures. Chem. Commun. 2009, 3452–3454. [Google Scholar] [CrossRef]
- Yoo, H.; Kahng, S.; Kim, J.H. Z-scheme assisted ZnO/Cu2O-CuO photocatalysts to increase photoactive electrons in hydrogen evolution by water splitting. Sol. Energy Mater. Sol. Cells 2020, 204, 110211. [Google Scholar] [CrossRef]
- Linic, S.; Christofer, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Yang, G.; Li, S.; Xiao, N.; Xu, B.; Liu, S.; Qiu, P.; Hao, S.; Ge, L. Fe2TiO5 as an efficient co-catalyst to improve the photoelectrochemical water splitting performance of BiVO4. ACS Appl. Mater. Interfaces 2018, 10, 39713–39722. [Google Scholar] [CrossRef]
- Shao, M.; Shao, Y.; Ding, S.; Tong, R.; Zhong, X.; Yao, L.; Ip, W.F.; Xu, B.; Shi, X.-Q.; Sun, Y.-Y.; et al. Carbonized MoS2: Super-active co-catalyst for highly efficient water splitting on CdS. ACS Sustain. Chem. Eng. 2019, 7, 4220–4229. [Google Scholar] [CrossRef]
- Cai, Q.; Liu, Z.; Han, C.; Tong, Z.; Ma, C. CuInS2/Sb2S3 heterostructure modified with noble metal co-catalyst for efficient photoelectrochemical water splitting. J. Alloys Compd. 2019, 795, 319–326. [Google Scholar] [CrossRef]
- Yalavarthi, R.; Zbořil, R.; Schmuki, P.; Naldoni, A.; Kment, Š. Elucidating the role of surface states of BiVO4 with Mo doping and a CoOOH co-catalyst for photoelectrochemical water splitting. J. Power Source 2021, 483, 229080. [Google Scholar] [CrossRef]
- Qian, H.; Liu, Z.; Ya, J.; Xin, Y.; Ma, J.; Wu, X. Construction homojunction and co-catalyst in ZnIn2S4 photoelectrode by Co ion doping for efficient photoelectrochemical water splitting. J. Alloys Compd. 2021, 867, 159028. [Google Scholar] [CrossRef]
- Ma, B.; Dang, Y.; Li, D.; Wang, X.; Lin, K.; Wang, W.; Zhou, X.; Chen, Y.; Xie, T.; Zhang, X.; et al. Yin-Yang hybrid co-catalyst (CoOx-Mo2N) for photocatalytic overall water splitting. Appl. Catal. B Environ. 2021, 298, 120491. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Wei, X.; Wang, L.; She, H.; Huang, J.; Wang, Q. Preparation of double-layered Co–Ci/NiFeOOH co-catalyst for highly meliorated PEC performance in water splitting. Adv. Powder Mater. 2022, 1, 100024. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. Fabrication of highly efficient and stable indirect Z-scheme assembly of AgBr/TiO2 via graphene as a solid-state electron mediator for visible light induced enhanced photocatalytic H2 production. Appl. Surf. Sci. 2019, 463, 445–455. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Measured effects of light intensity and catalyst concentration on photocatalytic hydrogen and oxygen production with zinc sulfide suspensions. Int. J. Hydrog. Energy 2013, 38, 9158–9168. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, G.; Li, S. The role of Cu(I) species for photocatalytic hydrogen generation over CuOx/TiO2. Catal. Lett. 2009, 133, 97–105. [Google Scholar] [CrossRef]
- Velazquez, J.J.; Fernandez-Gonzalez, R.; Díaz, L.; Pulido Melian, E.; Rodríguez, V.D.; Núnez, P. Effect of reaction temperature and sacrificial agent on the photocatalytic H2-production of PtTiO2. J. Alloys Compd. 2017, 721, 405–410. [Google Scholar] [CrossRef]
- Huaxu, L.; Fuqiang, W.; Ziming, C.; Shengpeng, H.; Bing, X.; Xiangtao, G.; Lin, b.; Jianyu, T.; Xiangzheng, L.; Ruiyang, C.; et al. Analyzing the effects of reaction temperature on photo-thermo chemical synergetic catalytic water splitting under full-spectrum solar irradiation: An experimental and thermodynamic investigation. Int. J. Hydrogen Energy 2017, 42, 12133–12142. [Google Scholar] [CrossRef]
- Dias, P.; Lopes, T.; Med, L.; Andrade, L.; Mendes, A. Photoelectrochemical water splitting using WO3 photoanodes: The substrate and temperature roles. Phys. Chem. Chem. Phys. 2016, 18, 5232–5243. [Google Scholar] [CrossRef] [Green Version]

| Year, Ref. | Photo-Catalysts | Bandgap, eV | Light Irradiation Parameters | Reagents | Sacrificial Reagent | Hydrogen Production, mmol g−1 h−1 |
|---|---|---|---|---|---|---|
| 2014, [17] | Au/TiO2 | 2.77–3.26 | Set of 3 Solarium Philips HB175 lamps each equipped by 4 15 W Philips CLEO florescent tubes | 1 g L−1 photocatalyst 25 vol.% methanol in water pH ~5 | Methanol | 0.303–1.543 |
| 2015, [18] | Au/TiO2 | 3.03–3.33 | Spectroline model SB-100P/F lamp 100 W 365 nm | 10 vol.% sacrificial reagents | Glycerol | 1.9–27.9 |
| Ethylene glycol | 1.4–20.9 | |||||
| Methanol | 0.9–13.5 | |||||
| Ethanol | 0.4–9.8 | |||||
| 2017, [19] | Zn0.5 Cd0.5S g-C3N4 TiO2 | - | 300 W Xe lamp, wavelength ≥ 420 nm | Aqueous solution 0.2 g L−1 photocatalyst powder + 20 vol.% sacrificial reagents | Triethanol amine | 1.197 |
| Formic acid | 0.845 | |||||
| Methanol | 0.599 | |||||
| Methyl amine | 0.279 | |||||
| Ethylene glycol | 0.116 | |||||
| Ethanol | 0.111 | |||||
| Ethylamine | 0.101 | |||||
| Ethylene diamine | 0.084 | |||||
| 2020, [20] | Cu/In2O3 /TiO2 NPs Cu/In2O3 NRs /TiO2 NWs | 2.69 2.90 | 35 W HID lamp, light intensity 20 mW cm−2, wavelength 450 nm | 0.01 g of photocatalyst was dispersed in 130 mL aqueous solution + 10 vol.% sacrificial reagent | Glycerol | 6.09 |
| Ethylene glycol | 4.85 | |||||
| Methanol | 4.39 | |||||
| Ethanol | 2.84 | |||||
| 2020, [21] | TiO2 NPs TiO2 MPs | 3.20 3.10 | 35 W HID Xenon lamp, 20 mW cm−2, wavelength ~420 nm | 0.1 g of photocatalyst catalyst was dispersed in 100 mL water containing sacrificial reagent | Glycerol | 9.073 |
| Methanol | 4.574 | |||||
| Phenol | 0.146 | |||||
| 0.2 M Na2S/Na2SO3 | 0.508 | |||||
| 0.1 M Na2S/Na2SO3 | 0.124 |
| Year, Ref. | Catalysts | Bandgap, eV | Light Irradiation Parameters | Solution | Cocatalyst | Photocurrent Density, mA cm−2 |
|---|---|---|---|---|---|---|
| 2012, [74] | Cu/nano Cu2O | 2.0 | LED light illumination 26 mW cm−2 λ = 425–660 nm | 0.1 M Na2SO4 pH = 6 | - | –0.140 |
| NiOx | –0.415 | |||||
| 2018, [108] | BiVO4 | 2.4 | 500 W Xe arc lamp, 100 mW cm−2 AM 1.5 G | 0.25 M K2B4O7 + 0.2 M Na2SO4 pH = 9.5 | - | ~1 |
| Fe2TiO5 | 3.23 | |||||
| 2019, [109] | CdS | - | 100 mW cm−2 AM 1.5 G | 0.1 M Na2SO4 + 0.1 M Na2SO3 + 0.01 M Na2S | - | 3.1 |
| MoS2 | 4.8 | |||||
| MoSC | 7.7 | |||||
| 2019, [110] | CuInS2 /Sb2S3 | ~1.5 | 100 W Xe lamp, 100 mW cm−2 AM 1.5 G | 0.1 M Na2SO4 | - | –1.86 |
| Pt | –2.48 | |||||
| 2020, [77] | FTO/CuO | 1.5 | Xe lamp 100 mW cm−2 AM 1.5 G | 0.5 M Na2SO4 (pH = 6) | - | –0.92 |
| NiO | –1.02 | |||||
| 2021, [111] | BiVO4 | 2.45 | 150 W Xe lamp, 100 mW cm−2 AM 1.5 G | 0.1 M PBS solution (pH = 7) | - | 0.03 |
| CoOOH | 1.10 | |||||
| 2021, [112] | ZnIn2S4 | 2.4–2.8 | AM 1.5 G illumination | 0.2 M Na2SO4 | - | 0.12 |
| Mg2+ | 0.38 | |||||
| Co2+ | 0.54 | |||||
| Co2+|Mg2+ | 0.92 | |||||
| 2021, [113] | Ge3N4 | 3.4 | 300 W Hg lamp | 0.5 M Na2SO4 | - | 2.9 |
| Mo2N | 3.7 | |||||
| CoOx | 4.1 | |||||
| CoOxMo2N | 5.6 | |||||
| CoOx-Mo2N | 9.2 | |||||
| 2022, [114] | NiFeOOH/BiVO4 | 2.41 | Xe lamp, 100 mW cm−2 AM 1.5 G | 0.5 M Na2SO4 pH = 7.35 | - | 1.9 |
| Co–Sil * | 2.1 | |||||
| Co–Pi * | 2.2 | |||||
| Co–Ci * | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grushevskaya, S.; Belyanskaya, I.; Kozaderov, O. Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting. Materials 2022, 15, 4915. https://doi.org/10.3390/ma15144915
Grushevskaya S, Belyanskaya I, Kozaderov O. Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting. Materials. 2022; 15(14):4915. https://doi.org/10.3390/ma15144915
Chicago/Turabian StyleGrushevskaya, Svetlana, Irina Belyanskaya, and Oleg Kozaderov. 2022. "Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting" Materials 15, no. 14: 4915. https://doi.org/10.3390/ma15144915
APA StyleGrushevskaya, S., Belyanskaya, I., & Kozaderov, O. (2022). Approaches for Modifying Oxide-Semiconductor Materials to Increase the Efficiency of Photocatalytic Water Splitting. Materials, 15(14), 4915. https://doi.org/10.3390/ma15144915






