Gel Evolution of Copper Tailing-Based Green Geopolymers in Marine Related Environments
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Characterizations
3. Results and Discussion
3.1. Compressive Strength
3.2. Microstructure Characterizations
3.2.1. Phase Transformation
3.2.2. FTIR Analysis
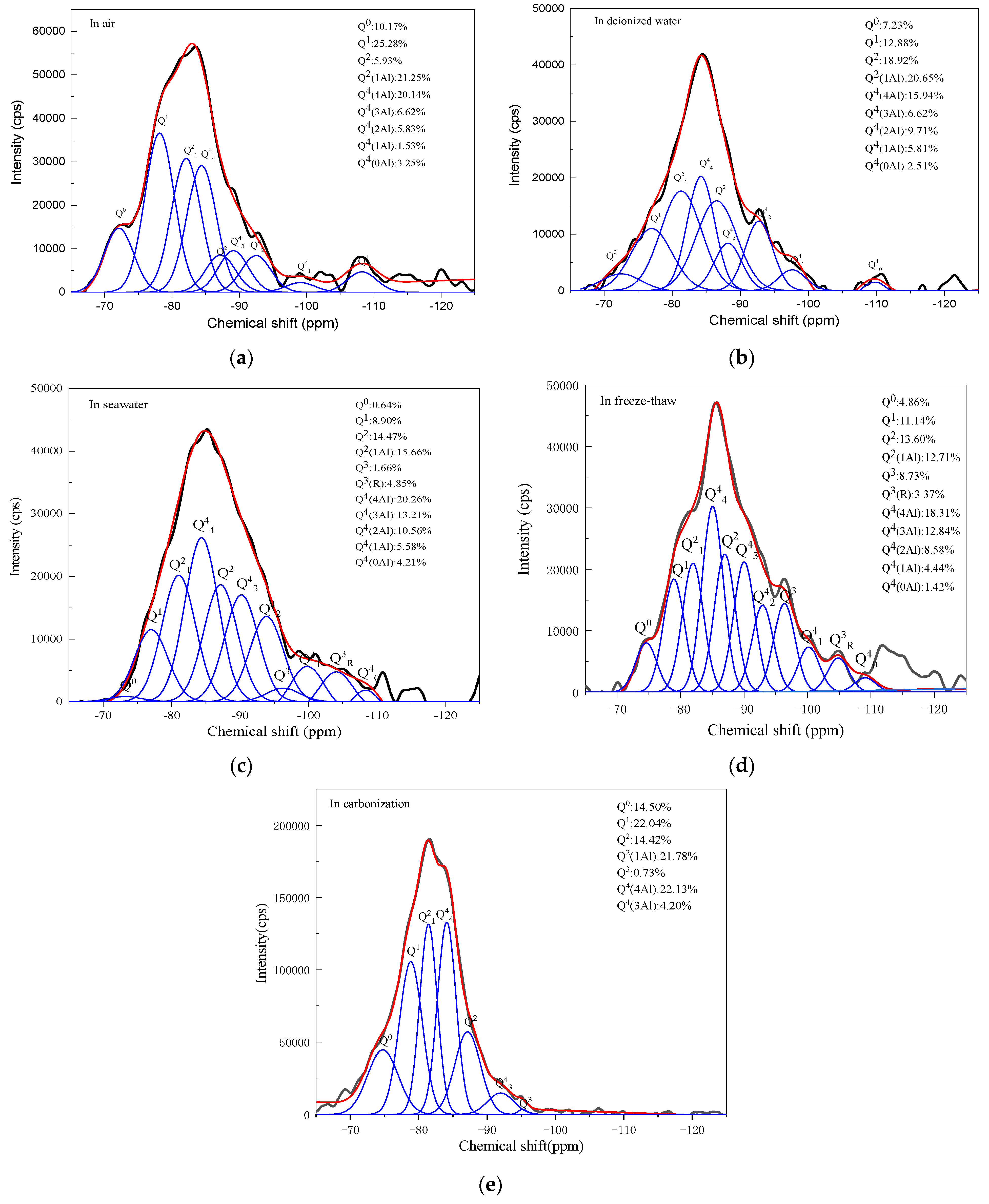
3.2.3. NMR Analysis
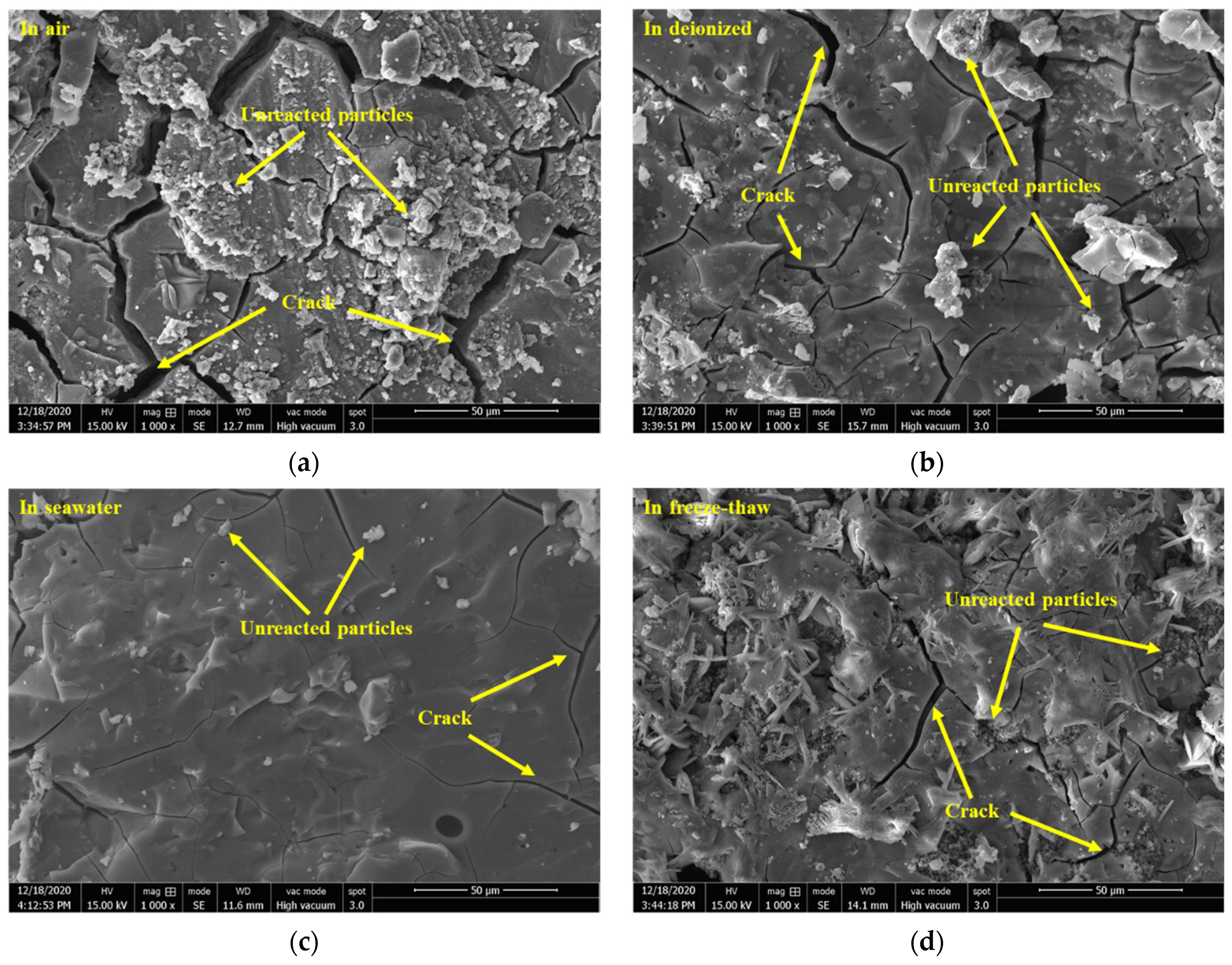
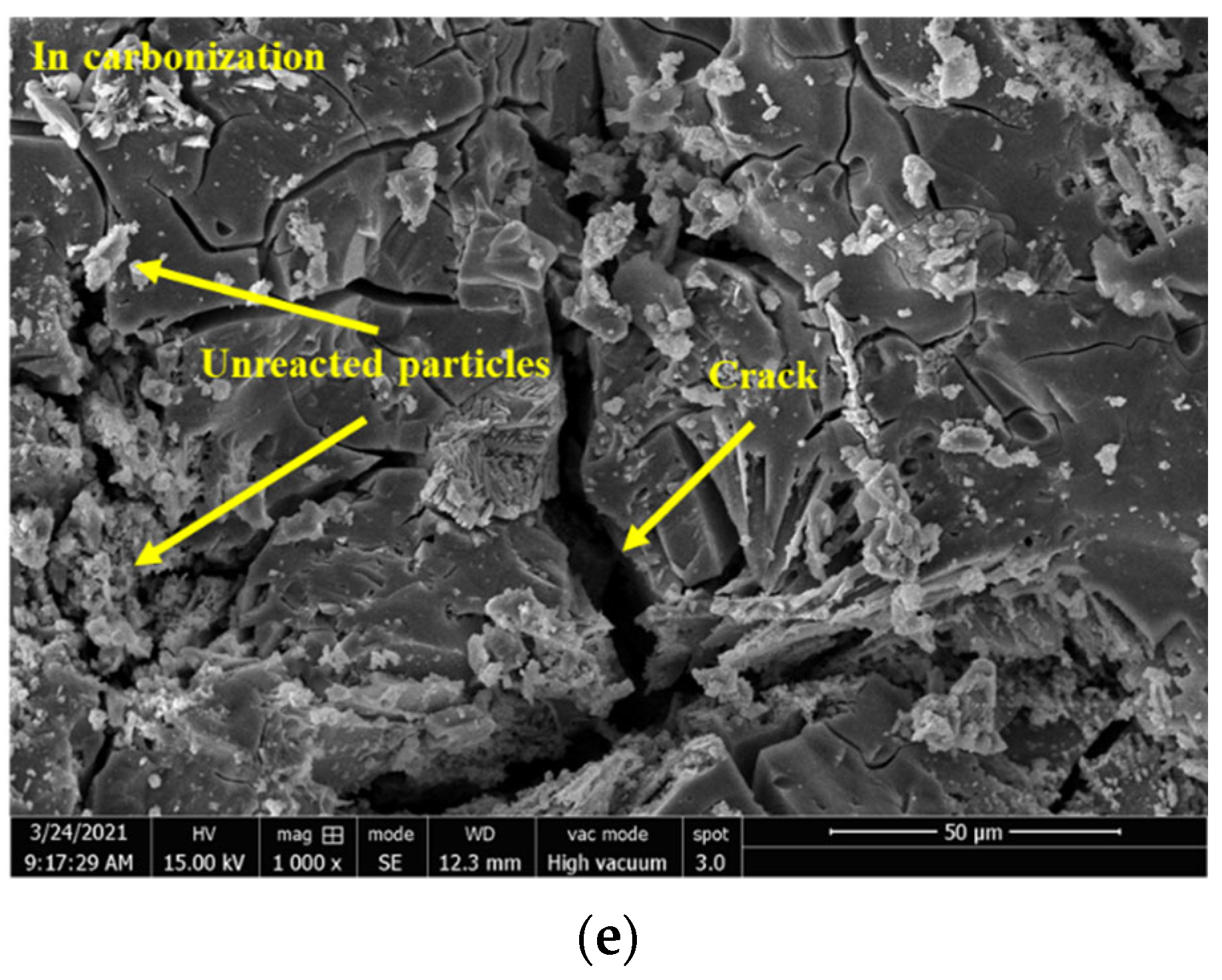
3.2.4. Morphological Analysis by SEM
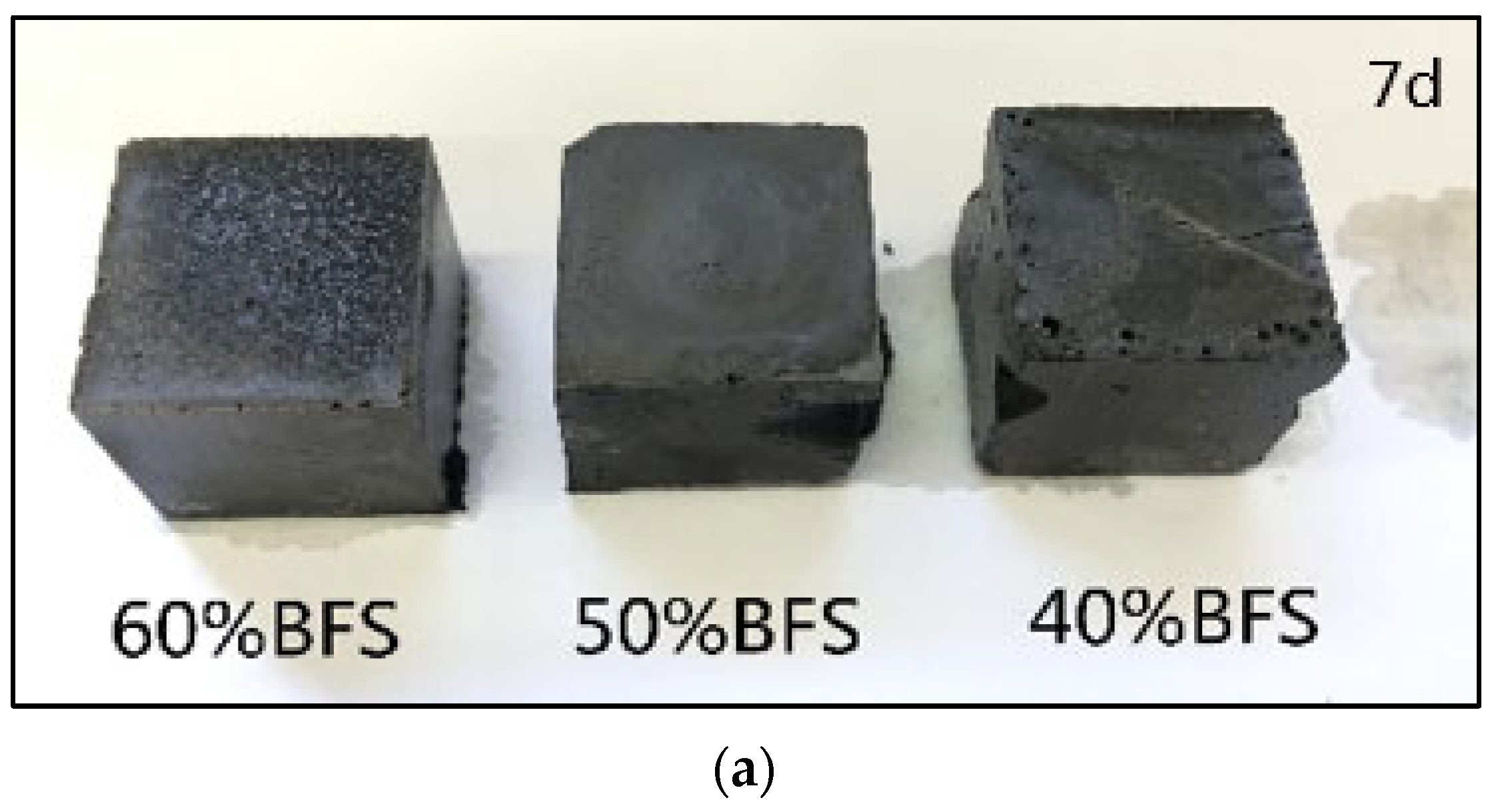
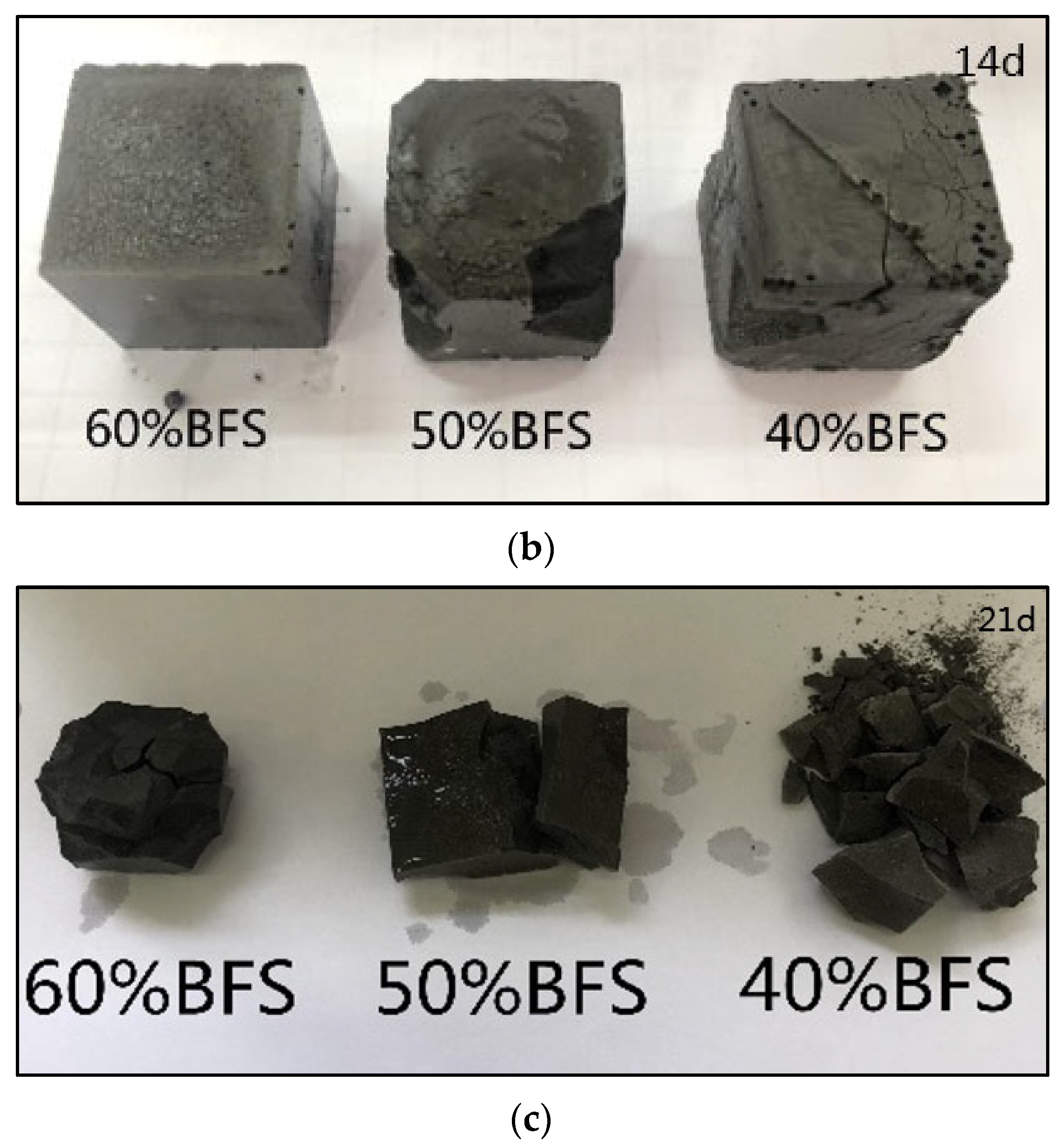
3.2.5. Macromorphology Analysis of Geopolymer Exposed to Freeze–Thaw Cycle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization Reaction, Microstructure and Simulation of Metakaolin-Based Geopolymers at Extended Si/Al Ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Cement. A Rev. Geopolymer. Inst. Tech. Pap. 2013, 21, 1–11. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The Role of Inorganic Polymer Technology in the Development of ‘Green Concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; Estrella, R.M.; Song, S. Effects of Aluminum Dosage on Gel Formation and Heavy Metals Immobilization in Alkali-Activated MSWI Fly Ash. Energy Fuels 2020, 34, 4727–4733. [Google Scholar] [CrossRef]
- Li, X.; Rao, F.; Song, S.; Ma, Q. Deterioration in the Microstructure of Metakaolin-Based Geopolymers in Marine Environment. J. Mater. Res. Technol. 2019, 8, 2747–2752. [Google Scholar] [CrossRef]
- Li, X.; Rao, F.; Song, S.; Ma, Q. Effect of Cristobalite on the Mechanical Behaviour of Metakaolin-Based Geopolymer in Artificial Seawater. Adv. Appl. Ceram. 2020, 119, 29–36. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Rao, F.; Yin, W. Characterization of Slag Reprocessing Tailings-Based Geopolymers in Marine Environment. Minerals 2020, 10, 832. [Google Scholar] [CrossRef]
- De Weerdt, K.; Justnes, H.; Geiker, M.R. Changes in the Phase Assemblage of Concrete Exposed to Sea Water. Cem. Concr. Compos. 2014, 47, 53–63. [Google Scholar] [CrossRef]
- Tennakoon, C.; Shayan, A.; Sanjayan, J.G.; Xu, A. Chloride Ingress and Steel Corrosion in Geopolymer Concrete Based on Long Term Tests. Mater. Des. 2017, 116, 287–299. [Google Scholar] [CrossRef]
- Xie, L.; Zhan, Z.; Li, Z.; She, J.; Peng, H. Effect of Sulfate on Rheological Properties of Inorganic Polymers. Guangdong Build. Mater. 2017, 33, 6–9. [Google Scholar]
- She, J.; Zhan, Z.; Li, Z.; Yang, Y.; Xie, L.; Peng, H. Effect of Sodium Chloride on Rheological Properties of Silicate Cement Slurry. Guangdong Build. Mater. 2017, 33, 3–6. [Google Scholar]
- Xie, L.; Zhan, Z.; Li, Z.; Yang, Y.; She, J.; Peng, H. Effect of Chloride Ion on Hydration Properties and Hardening Properties of Inorganic Polymers and Portland Cement. Concrete 2017, 5, 86–89, 93. [Google Scholar] [CrossRef]
- Zhao, R.; Yuan, Y.; Cheng, Z.; Wen, T.; Li, J.; Li, F.; Ma, Z.J. Freeze-Thaw Resistance of Class F Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2019, 222, 474–483. [Google Scholar] [CrossRef]
- Yi, L.; Mi, H.; Wu, Q.; Xia, J.; Zhang, B. Current Situation of Comprehensive Utilization of Tailings Resources in China. Conserv. Util. Miner. Resour. 2020, 40, 79–84. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A Review of Recent Strategies for Acid Mine Drainage Prevention and Mine Tailings Recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.; Song, S.; Rao, F.; Xia, L. Effects of Curing Temperature on the Compressive Strength and Microstructure of Copper Tailing-Based Geopolymers. Chemosphere 2020, 253, 126754. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z. Quantification of Chloride Diffusion in Fly Ash–Slag-Based Geopolymers by X-ray Fluorescence (XRF). Constr. Build. Mater. 2014, 69, 109–115. [Google Scholar] [CrossRef]
- Nawab, L.; Ghani, U. Synthesis and Characterization of Chloride Resistant Cement from Industrial Waste through Geopolymerization. J. Clean. Prod. 2017, 156, 577–580. [Google Scholar] [CrossRef]
- Ma, Q.; Nanukuttan, S.V.; Basheer, P.A.M.; Bai, Y.; Yang, C. Chloride Transport and the Resulting Corrosion of Steel Bars in Alkali Activated Slag Concretes. Mater. Struct. 2016, 49, 3663–3677. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Zhang, H.; Zhang, T.; Fernández, C.A. Alkali-Activated Copper Tailings-Based Pastes: Compressive Strength and Microstructural Characterization. J. Mater. Res. Technol. 2020, 9, 6557–6567. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, X.; Zhu, H. Potential Application of Geopolymers as Protection Coatings for Marine Concrete: I.Basic Properties. Appl. Clay Sci. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; Li, C.; Ge, W.; Lara, N.O.; Song, S.; Xia, L. Solidification of Municipal Solid Waste Incineration Fly Ash and Immobilization of Heavy Metals Using Waste Glass in Alkaline Activation System. Chemosphere 2021, 283, 131240. [Google Scholar] [CrossRef]
- Tian, X.; Rao, F.; León-Patiño, C.A.; Song, S. Co-Disposal of MSWI Fly Ash and Spent Caustic through Alkaline-Activation Consolidation. Cem. Concr. Compos. 2021, 116, 103888. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Meheron, S.J. Deterioration of Marine Concrete Exposed to Wetting-Drying Action. J. Clean. Prod. 2021, 278, 123383. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s Salt Formation in Cements Rich in Tri-Calcium Aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s Salt, Ca2Al (OH) 6 (Cl, OH)·2H2O: Its Solid Solutions and Their Role in Chloride Binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- De Weerdt, K.; Lothenbach, B.; Geiker, M.R. Comparing Chloride Ingress from Seawater and NaCl Solution in Portland Cement Mortar. Cem. Concr. Res. 2019, 115, 80–89. [Google Scholar] [CrossRef]
- Farnam, Y.; Dick, S.; Wiese, A.; Davis, J.; Bentz, D.; Weiss, J. The Influence of Calcium Chloride Deicing Salt on Phase Changes and Damage Development in Cementitious Materials. Cem. Concr. Compos. 2015, 64, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Tumidajski, P.J.; Chan, G.W. Durability of High Performance Concrete in Magnesium Brine. Cem. Concr. Res. 1996, 26, 557–565. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of Binder Structure in Sodium Silicate-Activated Slag-Metakaolin Blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Yang, T.; Yao, X.; Zhang, Z.; Zhu, H. Effects of NaOH Solution Concentration and Reaction Time on Metakaolin Geopolymerization. J. Nanjing Univ. Technol. 2013, 35, 21–25. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; Cholico-González, D.F.; Ortiz, N.L. Combination Formation in the Reinforcement of Metakaolin Geopolymers with Quartz Sand. Cem. Concr. Compos. 2017, 80, 115–122. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-Varela, M.T.; Granizo, M.L.; Puertas, F.; Vazquez, T.; Grutzeck, M.W. Chemical Stability of Cementitious Materials Based on Metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Klinowski, J. Nuclear Magnetic Resonance Studies of Zeolites. Prog. Nucl. Magn. Reson. Spectrosc. 1984, 16, 237–309. [Google Scholar] [CrossRef]
- Singh, P.S.; Bastow, T.; Trigg, M. Structural Studies of Geopolymers by 29Si and 27Al MAS-NMR. J. Mater. Sci. 2005, 40, 3951–3961. [Google Scholar] [CrossRef]
- Engelhardt, G.; Michel, D. High-Resolution Solid-State NMR of Silicates and Zeolites; John Wiley and Sons: New York, NY, USA, 1987. [Google Scholar]
- Duxson, P.; Lukey, G.C.; Separovic, F.; Van Deventer, J.S.J. Effect of Alkali Cations on Aluminum Incorporation in Geopolymeric Gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and Subflorescence Induced Microstructural and Mechanical Evolution in Fly Ash-Based Geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
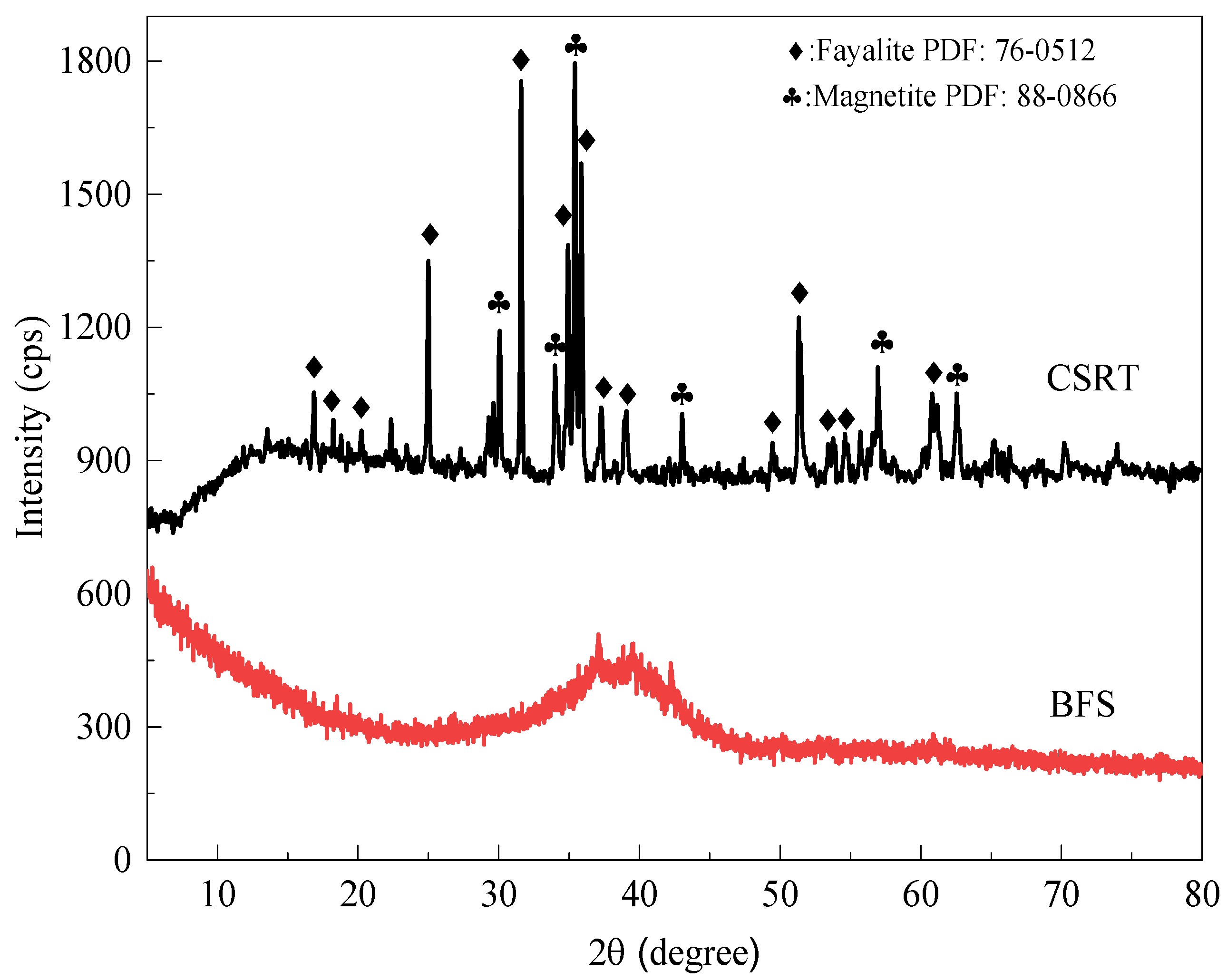
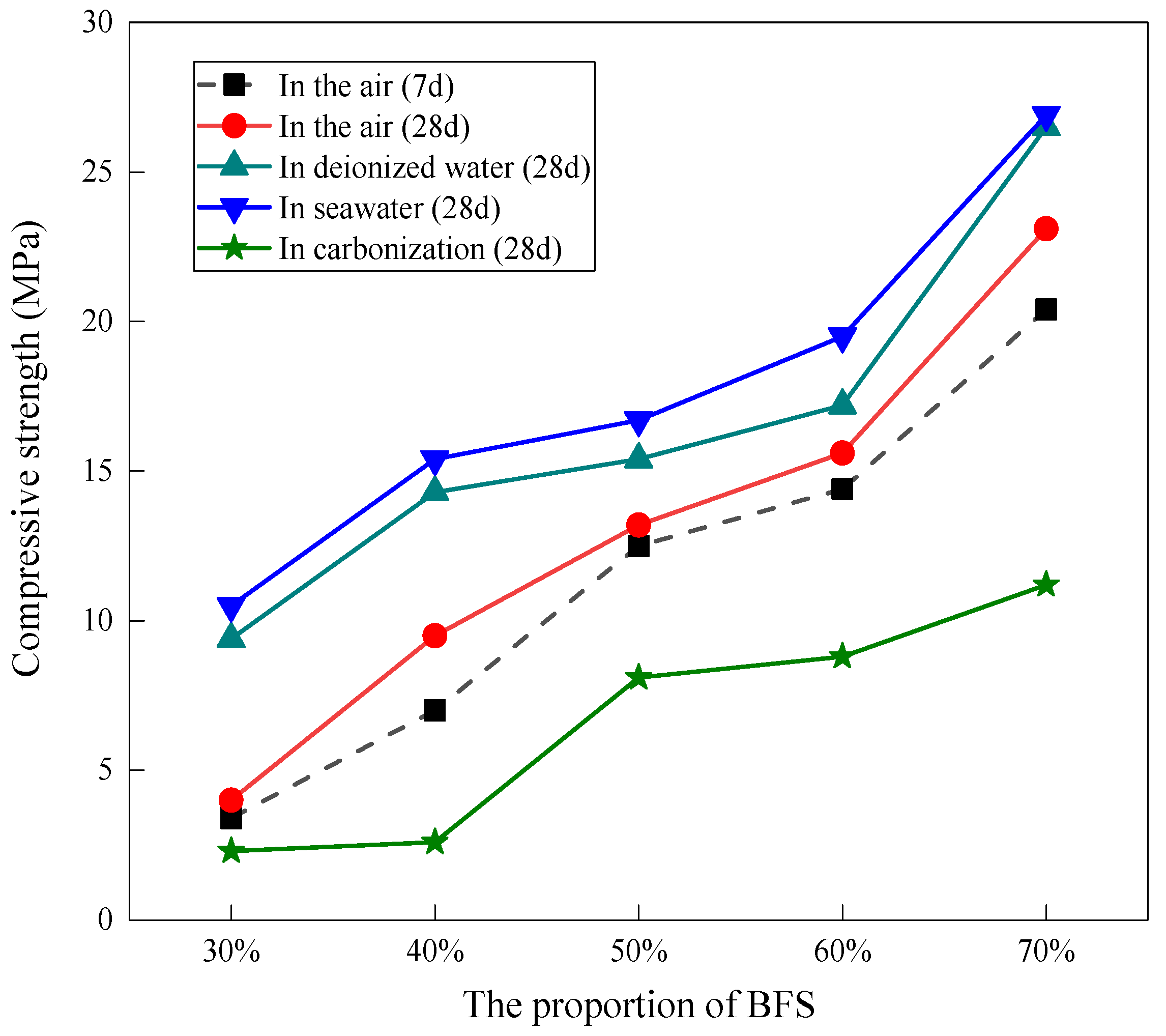
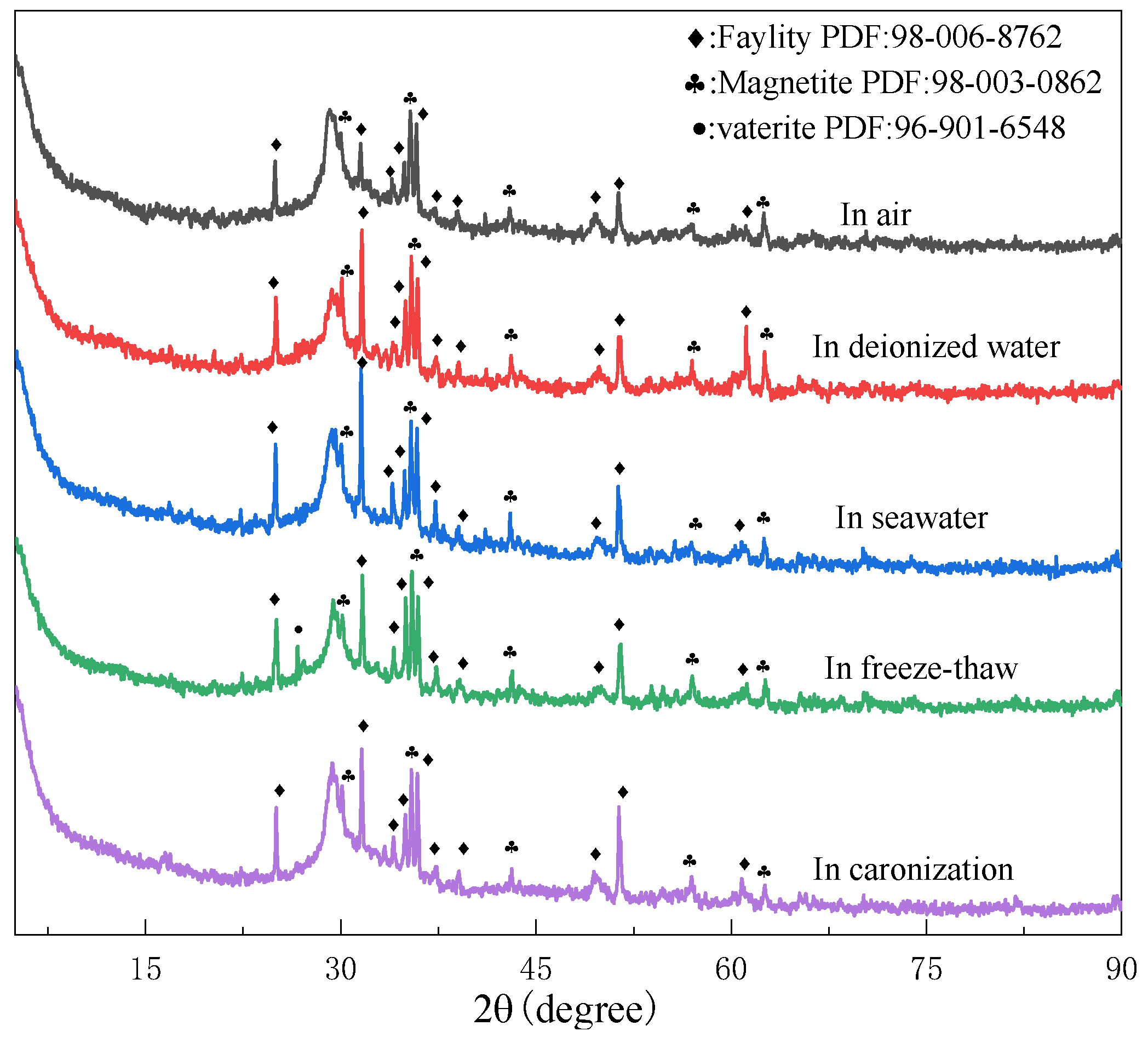
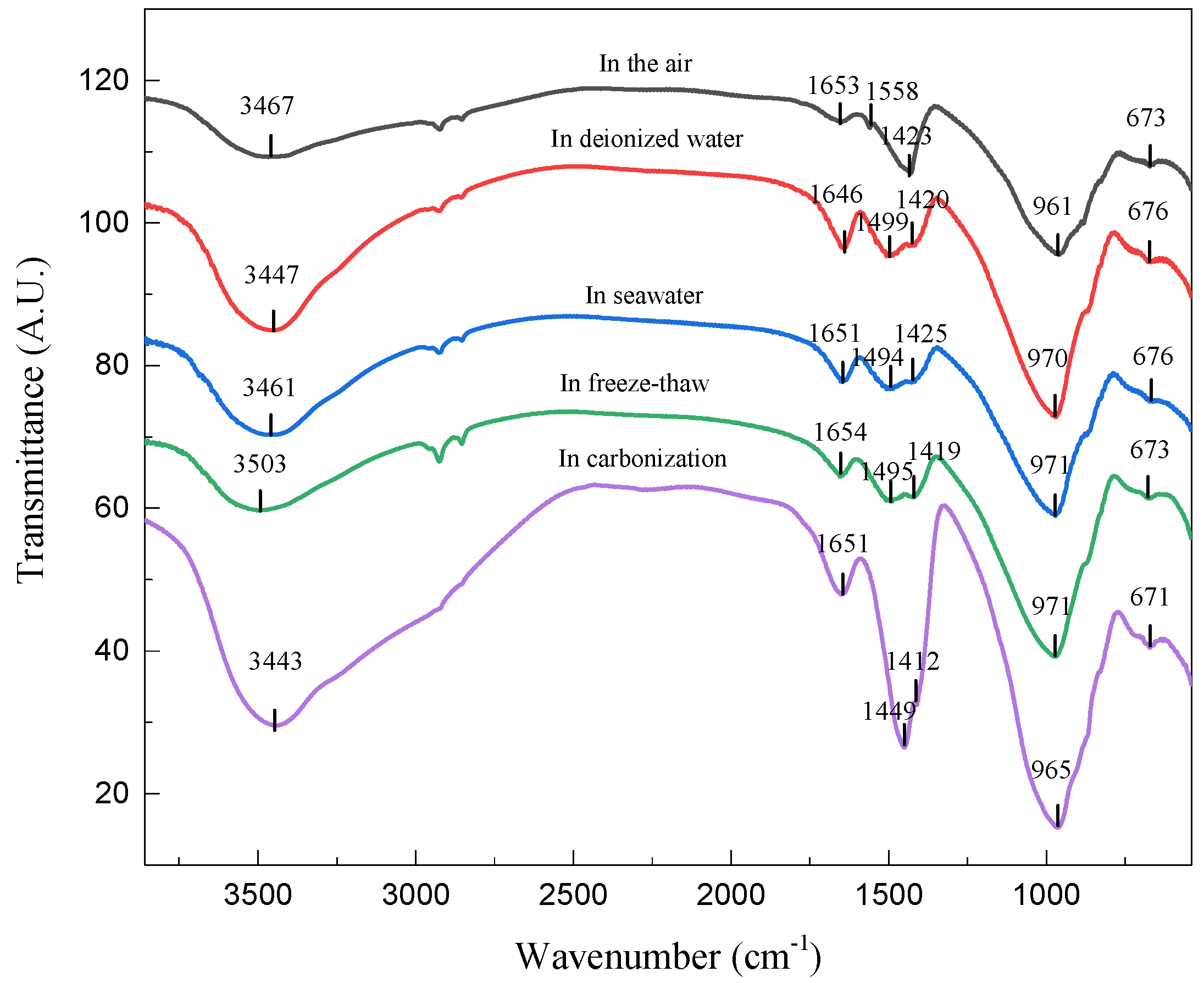

| Components (%) | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | TiO2 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| CT | 28.19 | 4.24 | 57.83 | 1.101 | 1.906 | 0.303 | 0.525 | 0.81 |
| BFS | 30.57 | 15.09 | 0.33 | 1.305 | 38.55 | 1.60 | 0.50 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yang, L.; Rao, F.; Tian, X. Gel Evolution of Copper Tailing-Based Green Geopolymers in Marine Related Environments. Materials 2022, 15, 4599. https://doi.org/10.3390/ma15134599
Li J, Yang L, Rao F, Tian X. Gel Evolution of Copper Tailing-Based Green Geopolymers in Marine Related Environments. Materials. 2022; 15(13):4599. https://doi.org/10.3390/ma15134599
Chicago/Turabian StyleLi, Jing, Lang Yang, Feng Rao, and Xiang Tian. 2022. "Gel Evolution of Copper Tailing-Based Green Geopolymers in Marine Related Environments" Materials 15, no. 13: 4599. https://doi.org/10.3390/ma15134599
APA StyleLi, J., Yang, L., Rao, F., & Tian, X. (2022). Gel Evolution of Copper Tailing-Based Green Geopolymers in Marine Related Environments. Materials, 15(13), 4599. https://doi.org/10.3390/ma15134599









