Facile Synthesis of Diatomite/β-Cyclodextrin Composite and Application for the Adsorption of Diphenolic Acid from Wastewater
Abstract
:1. Introduction
2. Experimental Sections
2.1. Chemicals and Reagents
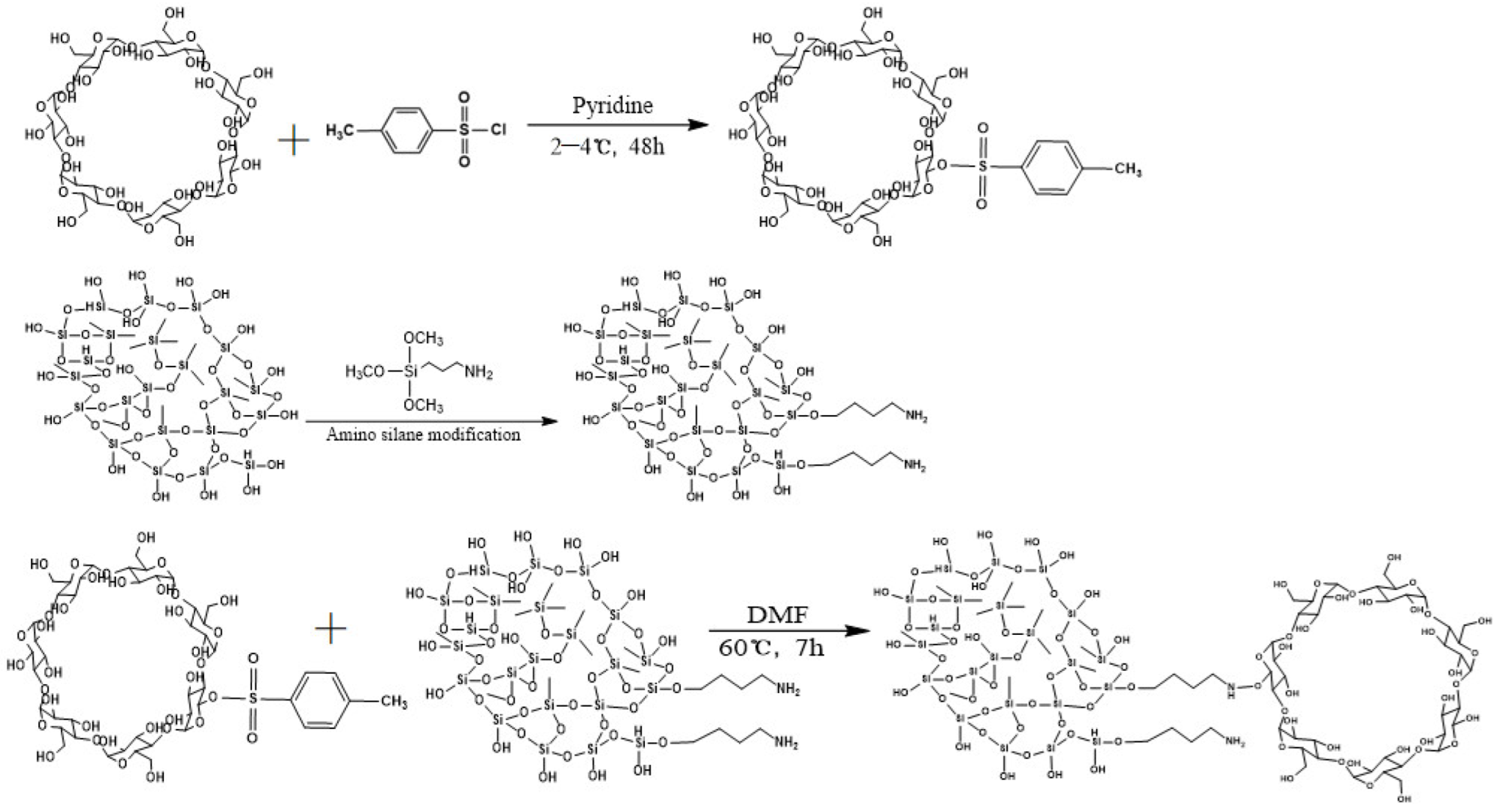
2.2. Synthesis of Ts-β-CD
2.3. Synthesis of DA/β-CD
2.4. Characterizations
2.5. Adsorption Experiments
3. Results and Discussion
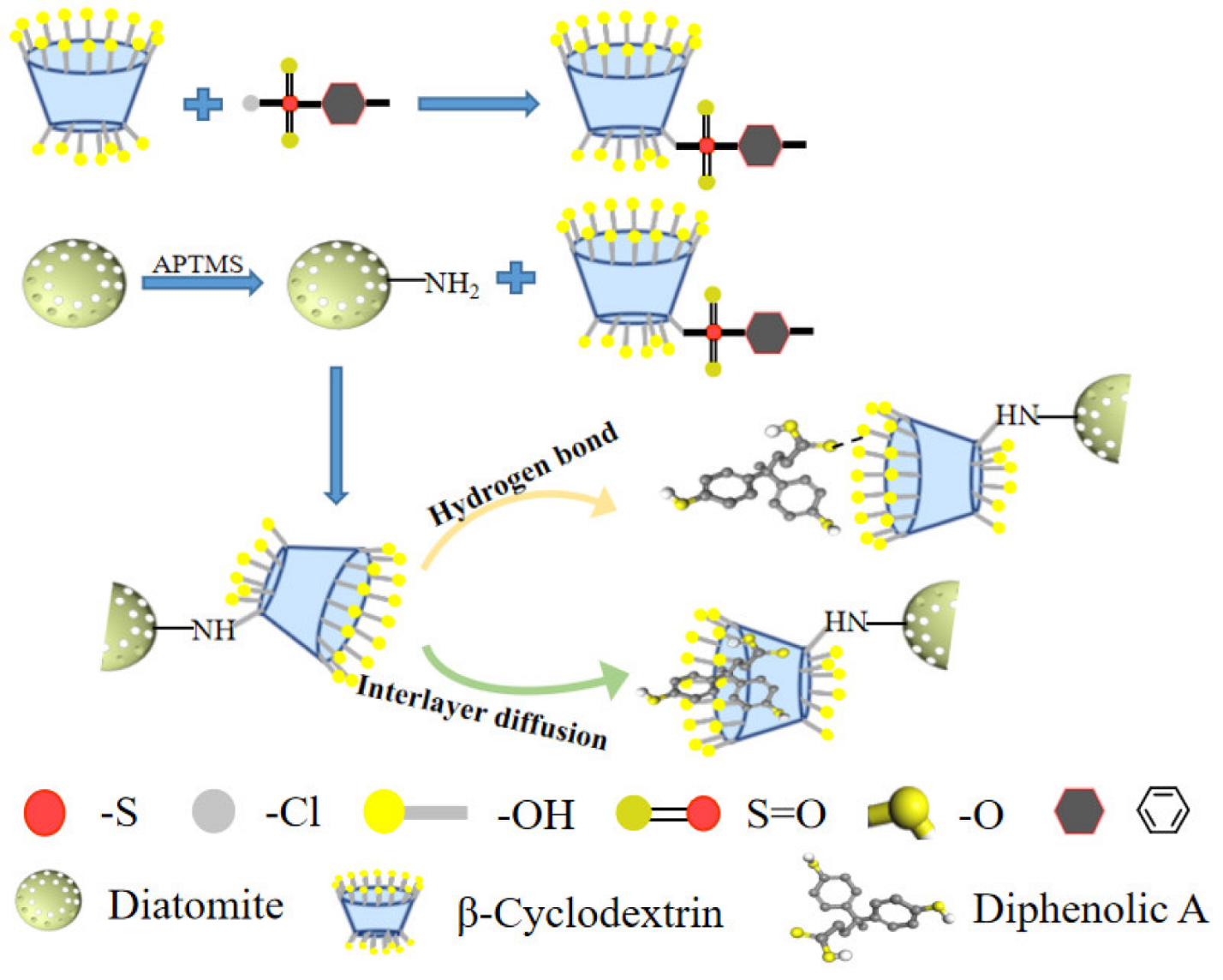
3.1. Reaction Mechanism and Discussion
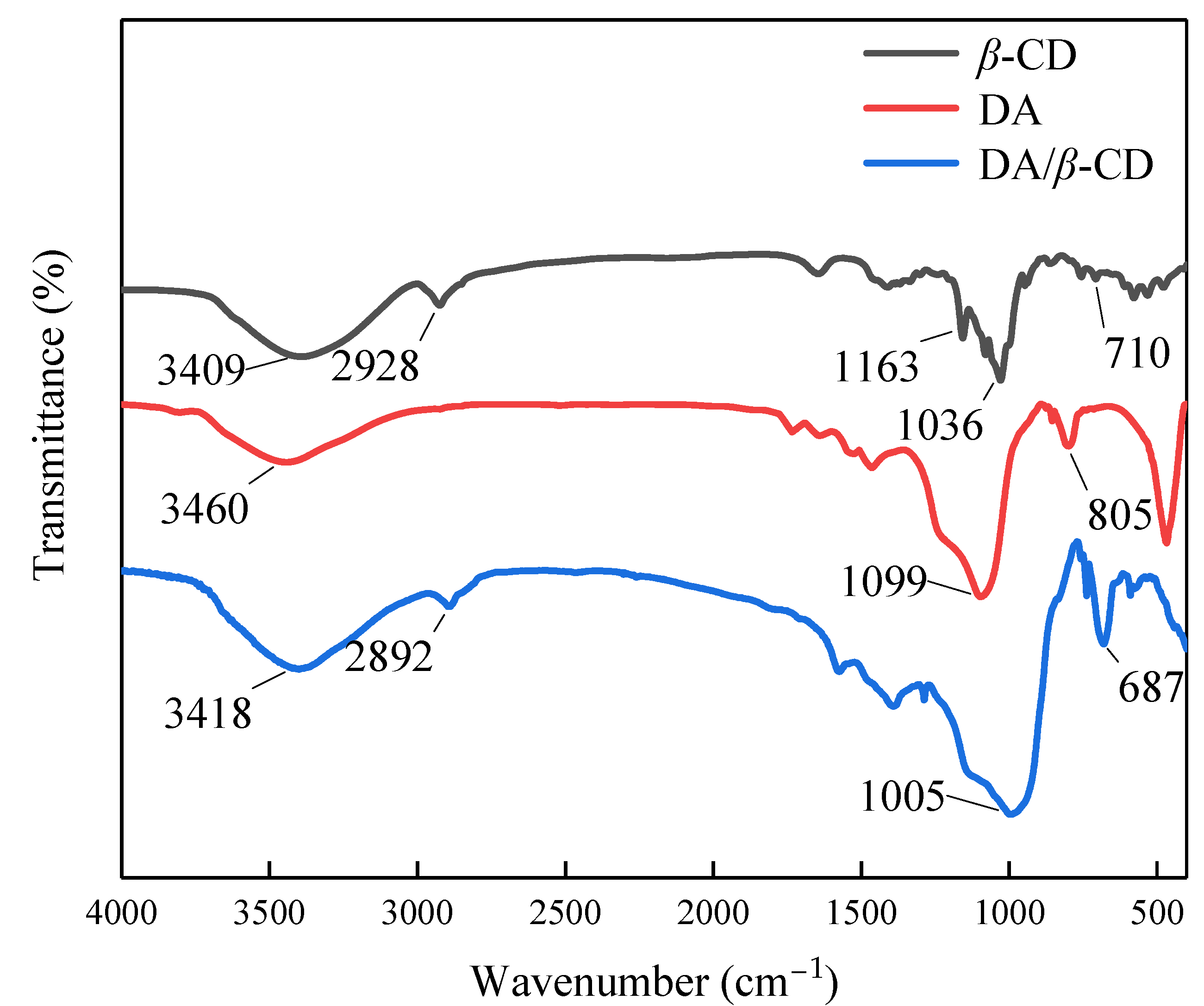
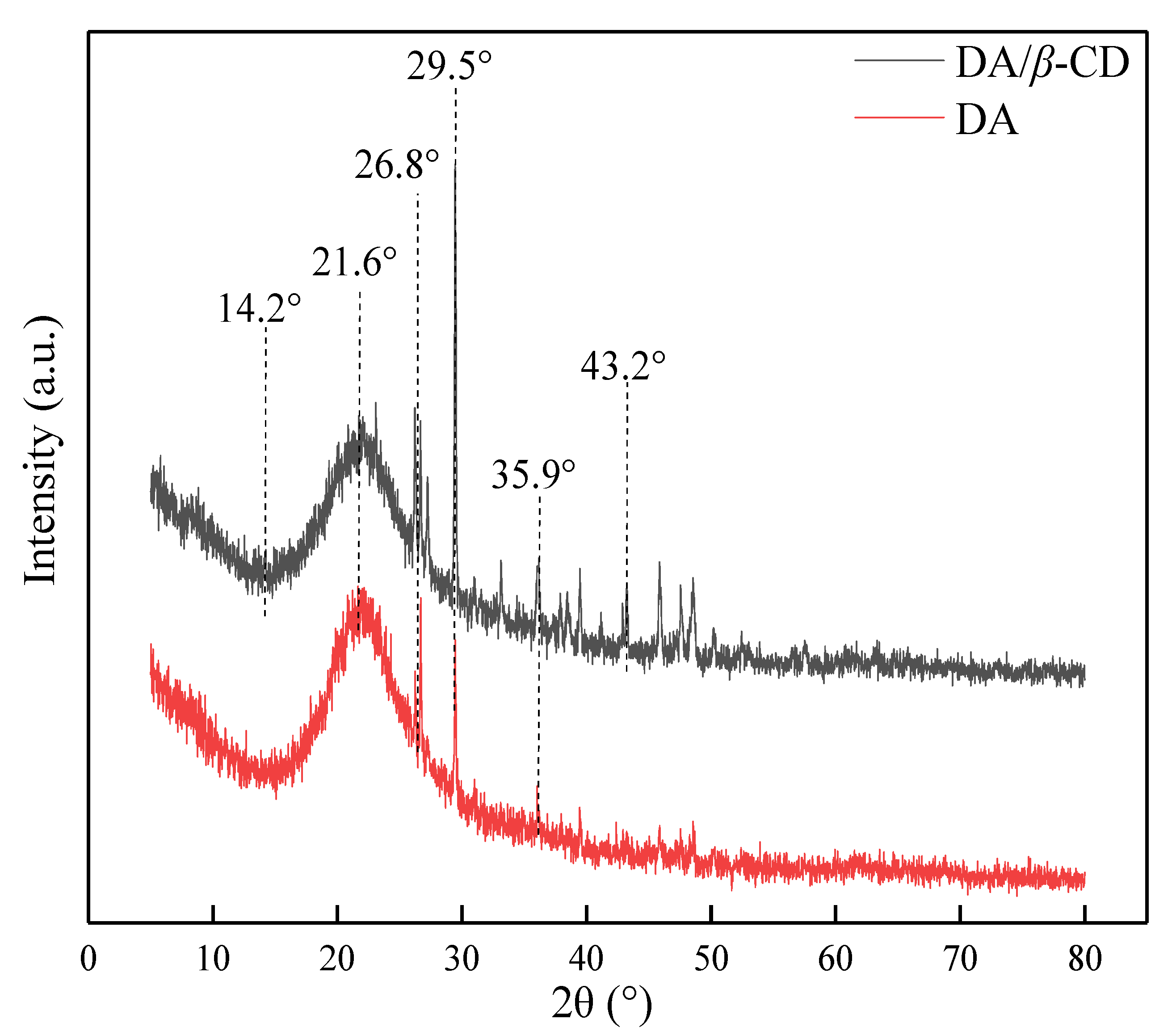
3.2. Characterization
3.3. Adsorption Properties
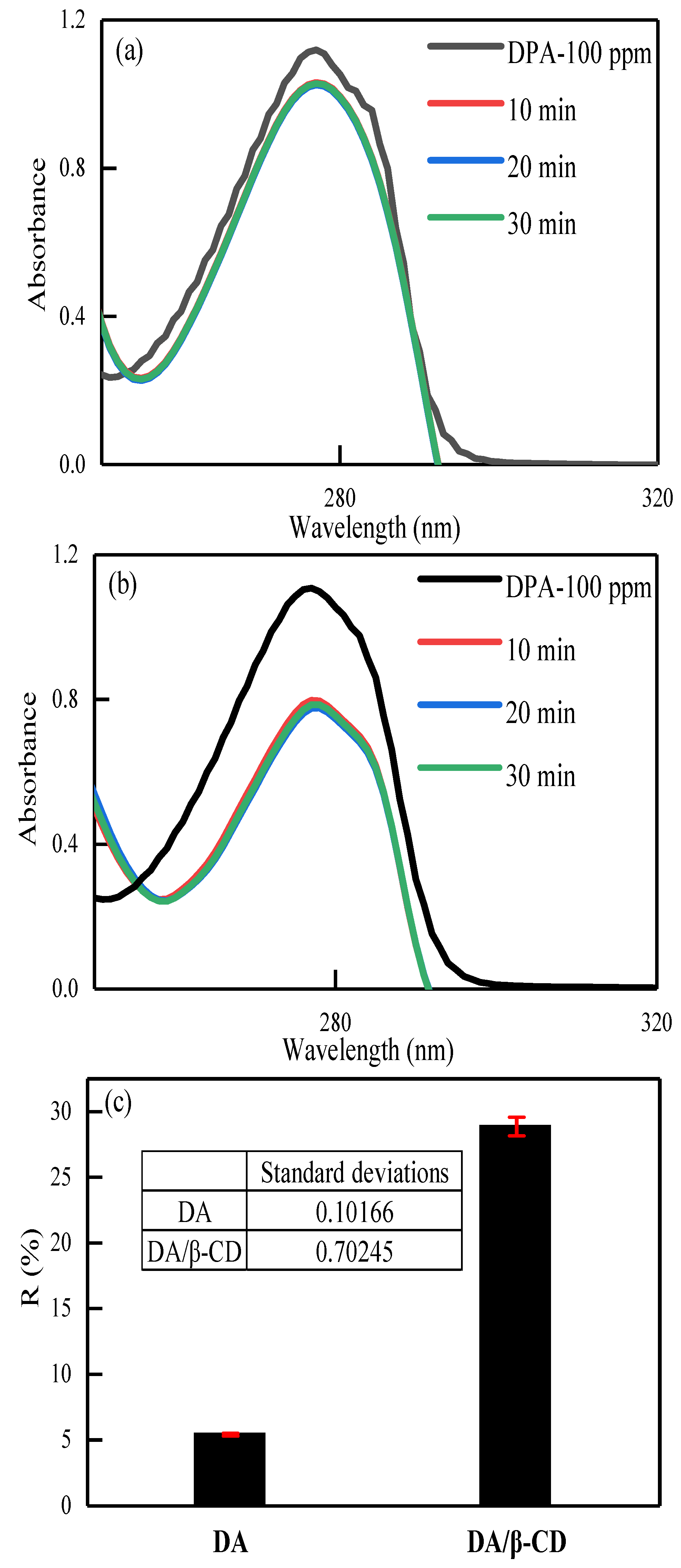
3.3.1. Standard Curve Plotting
3.3.2. Adsorption Performance of DA/β-CD
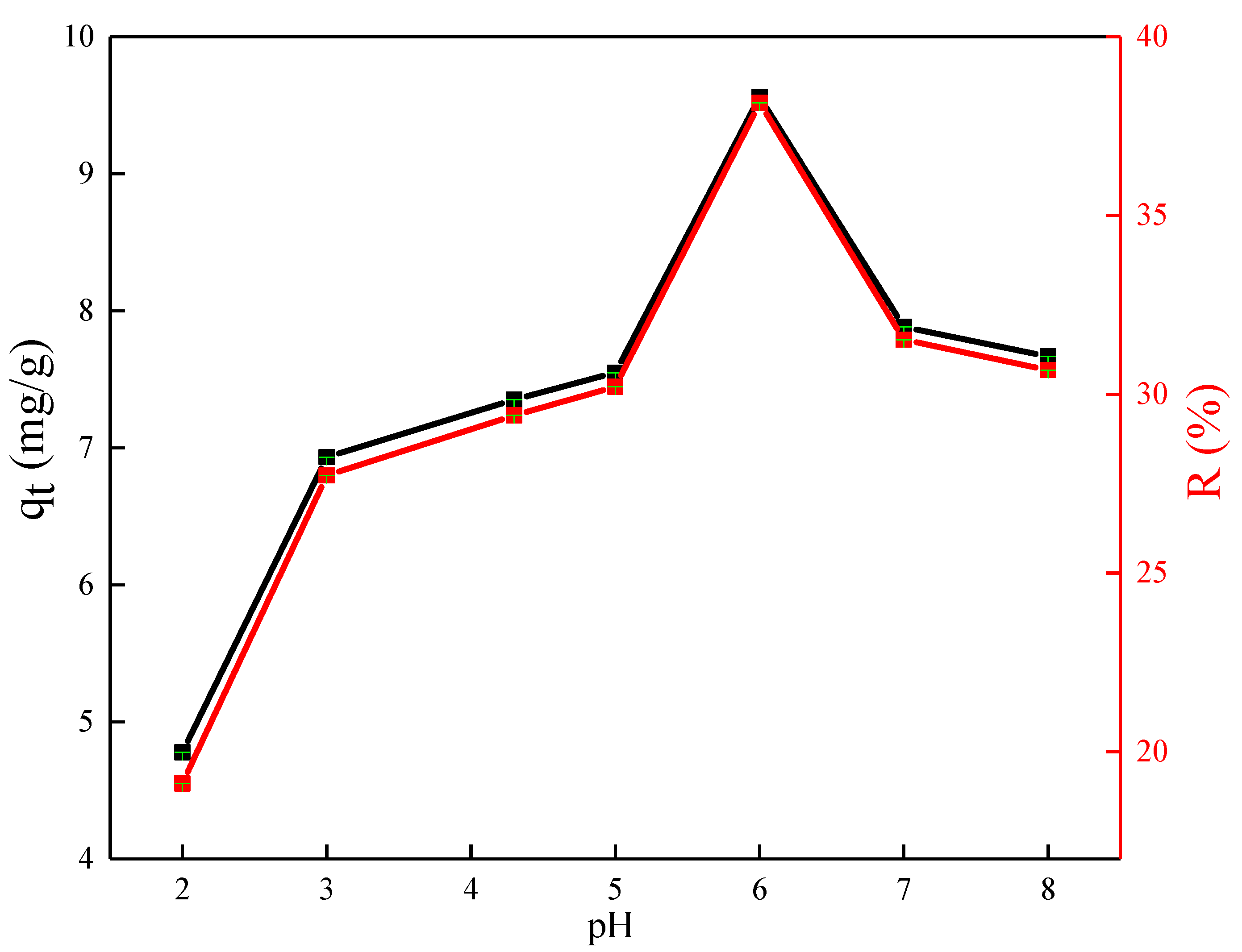
3.3.3. Effect of pH
3.4. Isotherm Models
3.5. Thermodynamic Analysis
3.6. Stability and Reusability of the DA/β-CD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhadra, B.N.; Lee, J.K.; Cho, C.W.; Jhung, S.H. Remarkably efficient adsorbent for the removal of bisphenol A from water: Bio-MOF-1-derived porous carbon. Chem. Eng. J. 2018, 343, 225–234. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, L. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, R.; Chen, K.; Zhao, X.; Gu, X.; Lu, J. Enhanced adsorption and photo-degradation of bisphenol A by β-cyclodextrin modified pine sawdust in an aquatic environment. J. Taiwan Inst. Chem. Eng. 2017, 78, 510–516. [Google Scholar] [CrossRef]
- Zúñiga, C.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polybenzoxazines based in diphenolic acid. Polymer 2012, 53, 1617–1623. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Wierckx, N.J.P.; Ballerstedt, H.; de Bont, J.A.M.; Wery, J. Engineering of solvent-tolerant Pseudomonas putida S12 for bioproduction of phenol from glucose. Appl. Environ. Microbiol. 2005, 71, 8221–8227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.F.; Moore, J.A. Synthesis, characterization and properties of polycarbonate containing carboxyl side groups. Macromol. Symp. 2003, 199, 375–390. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, L.B.; Li, B.G. Thermal stability of aromatic polyesters prepared from diphenolic acid and its esters. Polym. Degrad. Stabil. 2009, 94, 1261–1266. [Google Scholar] [CrossRef]
- Yan, H.Q.; Li, N.N.; Fang, Z.P.; Wang, H. Application of poly(diphenolic acid-phenyl phosphate)-based layer by layer nanocoating in flame retardant ramie fabrics. J. Appl. Polym. Sci. 2017, 134, 13. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Y.; Fang, Z.P. Diphenolic acid based biphosphate on the properties of polylactic acid: Synthesis, fire behavior and flame retardant mechanism. Polymer 2017, 108, 29–37. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, L.B.; Bu, Z.Y.; Li, B.G. Interfacial polycondensation of diphenolic acid and isophthaloyl chloride. J. Appl. Polym. Sci. 2008, 108, 3586–3592. [Google Scholar] [CrossRef]
- Guo, L.; Wang, B.; Huang, W.; Wu, F.; Huang, J. Photocatalytic degradation of diphenolic acid in the presence of beta-cyclodextrin under UV light. In Proceedings of the 2009 International Conference on Environmental Science and Information Application Technology, Wuhan, China, 4–5 July 2009; pp. 322–324. [Google Scholar] [CrossRef]
- Salomatova, V.A.; Pozdnyakov, I.P.; Yanshole, V.V.; Wu, F.; Grivin, V.P.; Bazhin, N.M.; Plyusnin, V.F. Photodegradation of 4, 4-Bis (4-hydroxyphenyl) valeric acid and its inclusion complex with β-cyclodextrin in aqueous solution. J. Photochem. Photobiol. A Chem. 2014, 274, 27–32. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Ocampo-Pérez, R.; Sánchez-Polo, M.; López-Peñalver, J.J. Treatment of water contaminated with diphenolic acid by gamma radiation in the presence of different compounds. Chem. Eng. J. 2013, 219, 371–379. [Google Scholar] [CrossRef]
- Moslemi, M.; Davies, S.H.; Masten, S.J. Empirical modeling of bromate formation during drinking water treatment using hybrid ozonation membrane filtration. Desalination 2012, 292, 113–118. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Graham, N.; Gao, N. The aqueous degradation of bisphenol A and steroid estrogens by ferrate. Water Res. 2008, 42, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ding, Y.; Tang, H.; Han, X.; Zhu, L.; Wang, N. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism. Chem. Eng. J. 2014, 236, 251–262. [Google Scholar] [CrossRef]
- Yang, X.; Tian, P.-F.; Zhang, C.; Deng, Y.-Q.; Xu, J.; Gong, J.; Han, Y.-F. Au/carbon as Fenton-like catalysts for the oxidative degradation of bisphenol A. Appl. Catal. B Environ. 2013, 134, 145–152. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Fu, Z.; Shen, Z.; Fan, Q.; Hao, S.; Wang, Y.; Liu, X.; Tong, X.; Kong, X.; Yang, Z. Preparation of multi-functional magnetic–plasmonic nanocomposite for adsorption and detection of thiram using SERS. J. Hazard. Mater. 2020, 392, 122356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Wang, F.; Ou, P.; Zhu, H.; Lai, Y.X.; Zhao, Y.L.; Shi, W.L.; Chen, Z.; Li, S.; Wang, T. High efficiency and rapid degradation of bisphenol A by the synergy between adsorption and oxidization on the MnO2@nano hollow carbon sphere. J. Hazard. Mater. 2018, 360, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zou, C.; Liang, H.; Peng, H.; Liao, Y. The effective removal of nickel ions from aqueous solution onto magnetic multi-walled carbon nanotubes modified by β-cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126544. [Google Scholar] [CrossRef]
- Peng, H.; Zou, C.; Wang, C.; Tang, W.; Zhou, J. The effective removal of phenol from aqueous solution via adsorption on CS/β-CD/CTA multicomponent adsorbent and its application for COD degradation of drilling wastewater. Environ. Sci. Pollut. Res. 2020, 27, 33668–33680. [Google Scholar] [CrossRef]
- Devaraj, M.; Saravanan, R.; Deivasigamani, R.; Gupta, V.K.; Gracia, F.; Jayadevan, S. Fabrication of novel shape Cu and Cu/Cu2O nanoparticles modified electrode for the determination of dopamine and paracetamol. J. Mol. Liq. 2016, 221, 930–941. [Google Scholar] [CrossRef]
- Saravanan, R.; Gupta, V.K.; Narayanan, V.; Stephen, A. Comparative study on photocatalytic activity of ZnO prepared by different methods. J. Mol. Liq. 2013, 181, 133–141. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Liu, M.; Zhao, Y.; Ma, X.; Gan, L.; Noonan, O.; Yu, C. Enlargement of uniform micropores in hierarchically ordered micro–mesoporous carbon for high level decontamination of bisphenol A. J. Mater. Chem. A 2014, 2, 8534–8544. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Wang, H.; Guo, L.; Zhai, Y.; Zhang, J.; Yang, Y.; Wang, H.; Yin, Z.; Lu, Y. Preparation of molecularly imprinted ordered mesoporous silica for rapid and selective separation of trace bisphenol A from water samples. Appl. Surf. Sci. 2018, 448, 380–388. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.L.; Li, S.; Qin, G.W.; Yang, L. Natural diatomite particles: Size-, dose- and shape- dependent cytotoxicity and reinforcing effect on injectable bone cement. J. Mater. Sci. Technol. 2018, 34, 1044–1053. [Google Scholar] [CrossRef]
- Shen, Z.; Fan, Q.; Yu, Q.; Wang, R.; Wang, H.; Kong, X. Facile detection of carbendazim in food using TLC-SERS on diatomite thin layer chromatography. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119037. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Darezereshki, E.; khodadadi Darban, A.; Abdollahy, M.; Jamshidi-Zanjani, A. Influence of heavy metals on the adsorption of arsenate by magnetite nanoparticles: Kinetics and thermodynamic. Environ. Nanotechnol. Monit. Manag. 2018, 10, 51–62. [Google Scholar] [CrossRef]
- Gong, X.; Chen, J.; Li, X.; Xie, W.; Wu, J. Sulfonylation of Benzylic C− H Bonds through the Reaction of Aryl (o-tolyl) methanones with Sulfonyl Hydrazides or Sulfonyl Chlorides. Chem. Asian J. 2018, 13, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Bresson, C.; Menu, M.-J.; Dartiguenave, M.; Dartiguenave, Y. Triethoxysilyl-substituted aminoethanethiol ligands for zinc and cadmium complexes and aminoethanethiol-modified silica gel. Evaluation of the corresponding supported molecular trap for metallic pollutant uptake (Cd2+, Hg2+ and Pb2+). J. Environ. Monit. 2000, 2, 240–247. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Caporaso, M.; Tuza, K.; Martina, K.; Calcio Gaudino, E.; Cravotto, G. Nucleophilic substitutions of 6I-O-Monotosyl-β-cyclodextrin in a planetary ball mill. ACS Sustain. Chem. Eng. 2016, 4, 919–929. [Google Scholar] [CrossRef]
- Alzate-Sánchez, D.M.; Smith, B.J.; Alsbaiee, A.; Hinestroza, J.P.; Dichtel, W.R. Cotton fabric functionalized with a β-cyclodextrin polymer captures organic pollutants from contaminated air and water. Chem. Mater. 2016, 28, 8340–8346. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Shi, S.; Ocampo-Pérez, R.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S.; Feng, J. Diatomite cross-linked β-Cyclodextrin polymers: A novel vision of diatomite adsorbent for the removal of bisphenol A. Environ. Technol. Innov. 2021, 23, 101602. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Z.; Lu, Y.-Y.; Shen, B.; Guan, Y.; Wang, X.-X.; Yin, Y.-C.; Zhu, B.-S.; Lu, L.-L.; Ni, Y.; et al. Diatomite derived hierarchical hybrid anode for high performance all-solid-state lithium metal batteries. Nat. Commun. 2019, 10, 2482. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A. Analysis and modeling of fixed bed column operations on As (V) removal by adsorption onto iron oxide-coated cement (IOCC). J. Colloid Interface Sci. 2005, 290, 52–60. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Sun, Z.; Bian, R.; Dong, X.; Zhang, X.; Zheng, S. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate. Chem. Eng. J. 2020, 388, 124386. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Zhang, H.-H.; Zou, Y.-W.; Yang, G.-P. Distribution and ecotoxicological state of phthalate esters in the sea-surface microlayer, seawater and sediment of the Bohai Sea and the Yellow Sea. Environ. Pollut. 2018, 240, 235–247. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, Y.; Song, L.; Shi, X.; Chen, S.-W.; Wu, W. Dihydroxy bezladely derivatives functionalized mesoporous silica SBA-15 for the sorption of U (VI). J. Radioanal. Nucl. Chem. 2016, 310, 125–137. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Çelik, G.; Arica, M.Y. Studies on accumulation of uranium by fungus Lentinus sajor-caju. J. Hazard. Mater. 2006, 136, 345–353. [Google Scholar] [CrossRef]
- Hritcu, D.; Humelnicu, D.; Dodi, G.; Popa, M.I. Magnetic chitosan composite particles: Evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr. Polym. 2012, 87, 1185–1191. [Google Scholar] [CrossRef]
- Von Oepen, B.; Kördel, W.; Klein, W. Sorption of nonpolar and polar compounds to soils: Processes, measurements and experience with the applicability of the modified OECD-Guideline 106. Chemosphere 1991, 22, 285–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Zhou, L.; Zhao, Y.; Chen, J.; Chen, Z.; Wang, F. Polypyrrole/reduced graphene oxide aerogel particle electrodes for high-efficiency electro-catalytic synergistic removal of Cr (VI) and bisphenol A. Chem. Eng. J. 2018, 336, 690–700. [Google Scholar] [CrossRef]


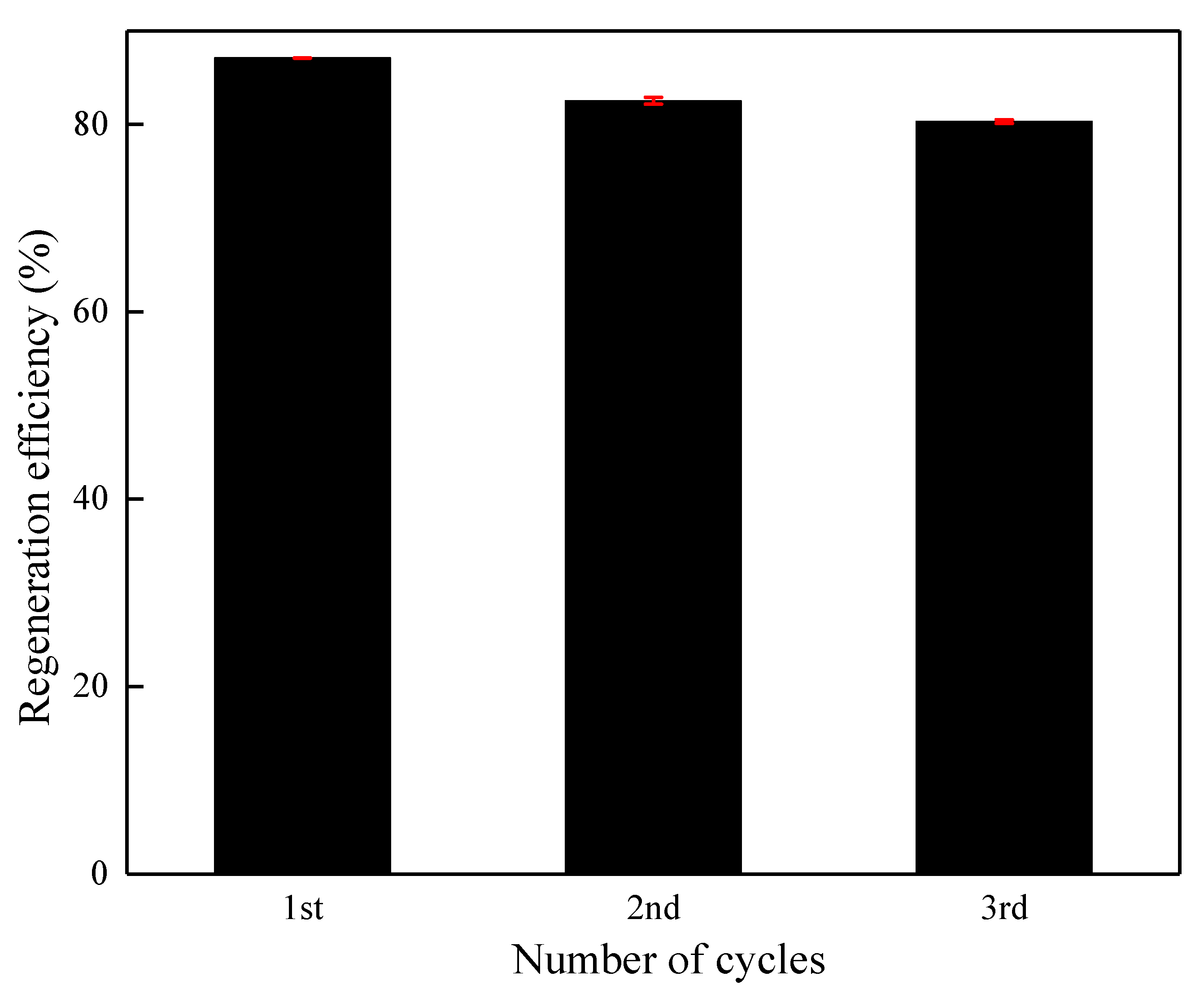
| Models | DA/β-CD |
|---|---|
| DPA | |
| Langmuir | qm = 2.0235 mg g−1 |
| b = 2.8067 L mg−1 | |
| R2 = 0.9867 | |
| Freundlich | kF = 1.5941 L mg−1 |
| n = 0.8427 | |
| R2 = 0.9748 |
| C0 (ppm) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1) | ΔG0 (Kj mol−1) | ||
|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | |||
| 40 | −13.5 | −32.4 | −3.8 | −3.7 | −3.4 |
| 80 | −12.8 | −29.9 | −3.9 | −3.8 | −3.5 |
| 100 | −12.3 | −27.8 | −4.1 | −3.9 | −3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Wang, Z.; Yu, Q.; Kong, X.; Zhang, M. Facile Synthesis of Diatomite/β-Cyclodextrin Composite and Application for the Adsorption of Diphenolic Acid from Wastewater. Materials 2022, 15, 4588. https://doi.org/10.3390/ma15134588
Hou M, Wang Z, Yu Q, Kong X, Zhang M. Facile Synthesis of Diatomite/β-Cyclodextrin Composite and Application for the Adsorption of Diphenolic Acid from Wastewater. Materials. 2022; 15(13):4588. https://doi.org/10.3390/ma15134588
Chicago/Turabian StyleHou, Min, Zhiyi Wang, Qian Yu, Xianming Kong, and Miao Zhang. 2022. "Facile Synthesis of Diatomite/β-Cyclodextrin Composite and Application for the Adsorption of Diphenolic Acid from Wastewater" Materials 15, no. 13: 4588. https://doi.org/10.3390/ma15134588
APA StyleHou, M., Wang, Z., Yu, Q., Kong, X., & Zhang, M. (2022). Facile Synthesis of Diatomite/β-Cyclodextrin Composite and Application for the Adsorption of Diphenolic Acid from Wastewater. Materials, 15(13), 4588. https://doi.org/10.3390/ma15134588






