Electrochemical Performance of Graphene Oxide/Black Arsenic Phosphorus/Carbon Nanotubes as Anode Material for LIBs
Abstract
:1. Introduction
| Theo. Spec. Cap (mAh g−1) | Theo. Vol. Cap. (mAh g−1) [39] | Av. Charge Potential (V vs. Li+/Li) | Av. Discharge Potential (V vs. Li+/Li) | Vol. Variation (%) | Lithiated Phase | |
|---|---|---|---|---|---|---|
| Graphite | 372 | 837 | 0.1–0.1 | 0.17 | 12 | LiC6 |
| Si [40] | 4200 | 9786 | 0.2 | 0.45 | 420 | Li4.4Si |
| Ge [41] | 1600 | 8645 | 0.4 | 0.65 | 370 | Li4.4Ge |
| Sn [15] | 994 | 7216 | 0.4 | 0.6 | 257 | Li4.4Sn |
| BP [37] | 2596 | 2266 | 0.45 | 0.9 | 300 | Li3BP |
| As [36] | 1073 | 2057 | 0.9 | 1.1 | 182 | Li3As |
| Sb [42] | 660 | 1750 | 0.8 | 1 | 150 | Li3Sb |
| Bi [43] | 386 | 3765 | 0.7 | 0.9 | 215 | Li3Bi |
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Electrochemical Tests
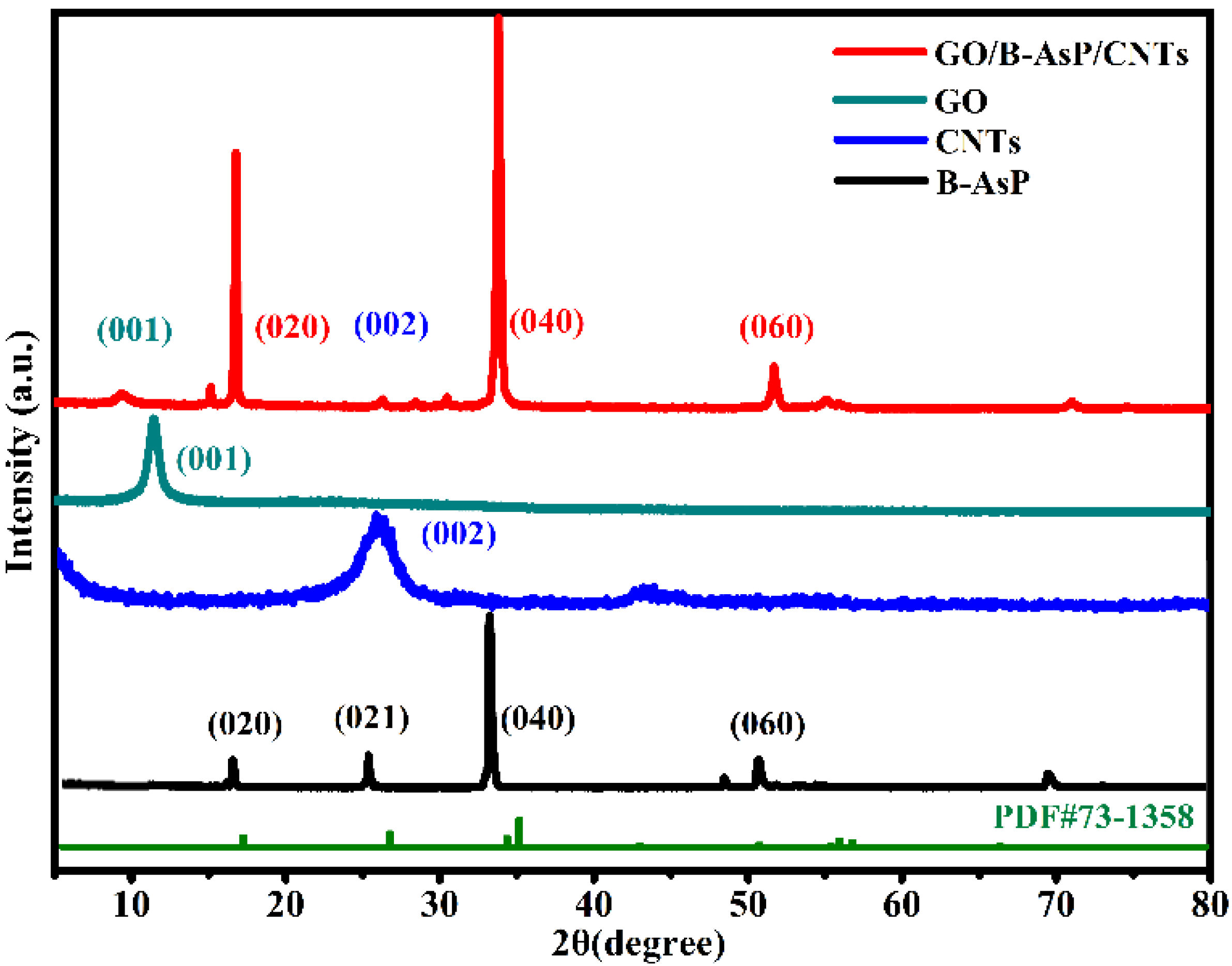
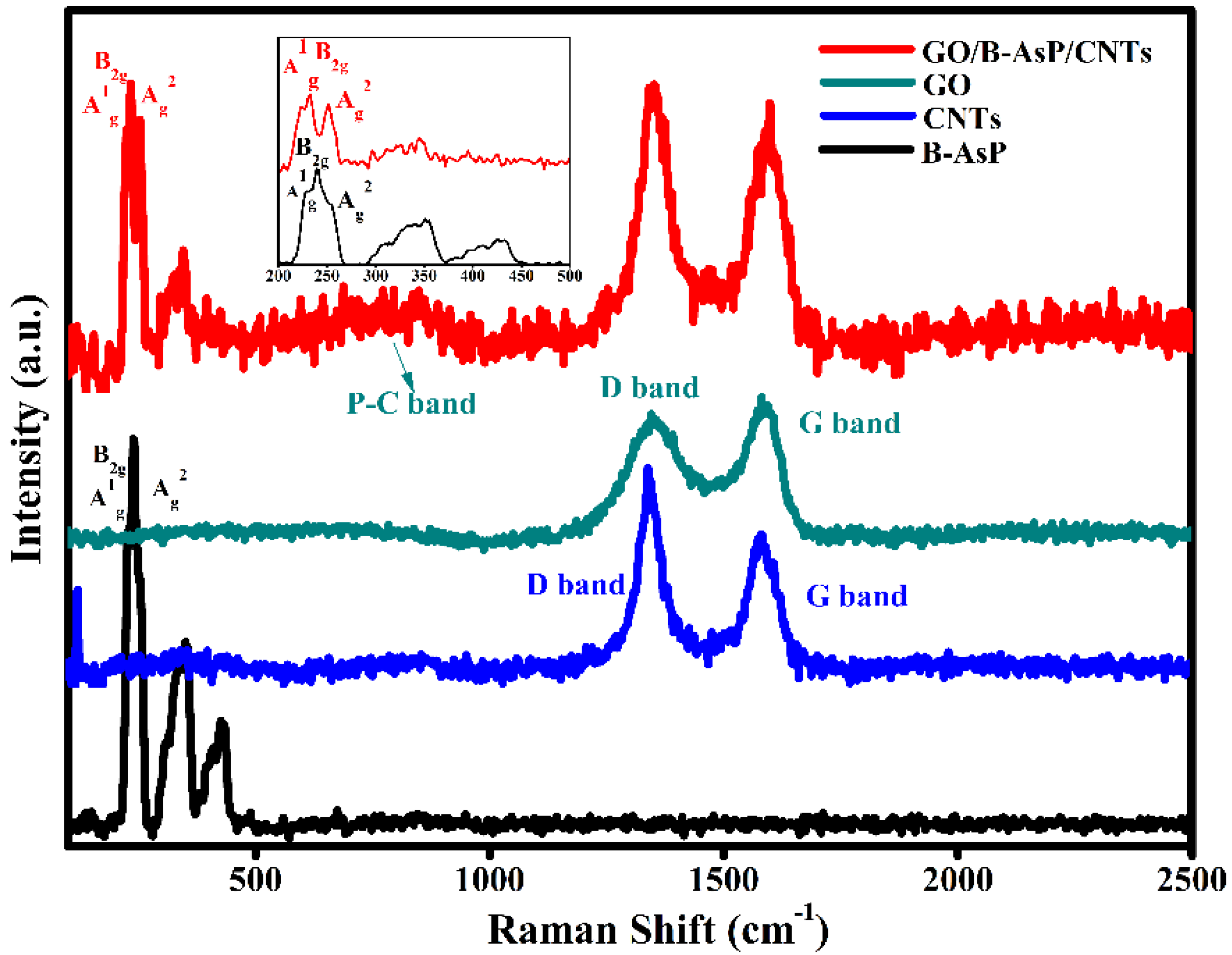
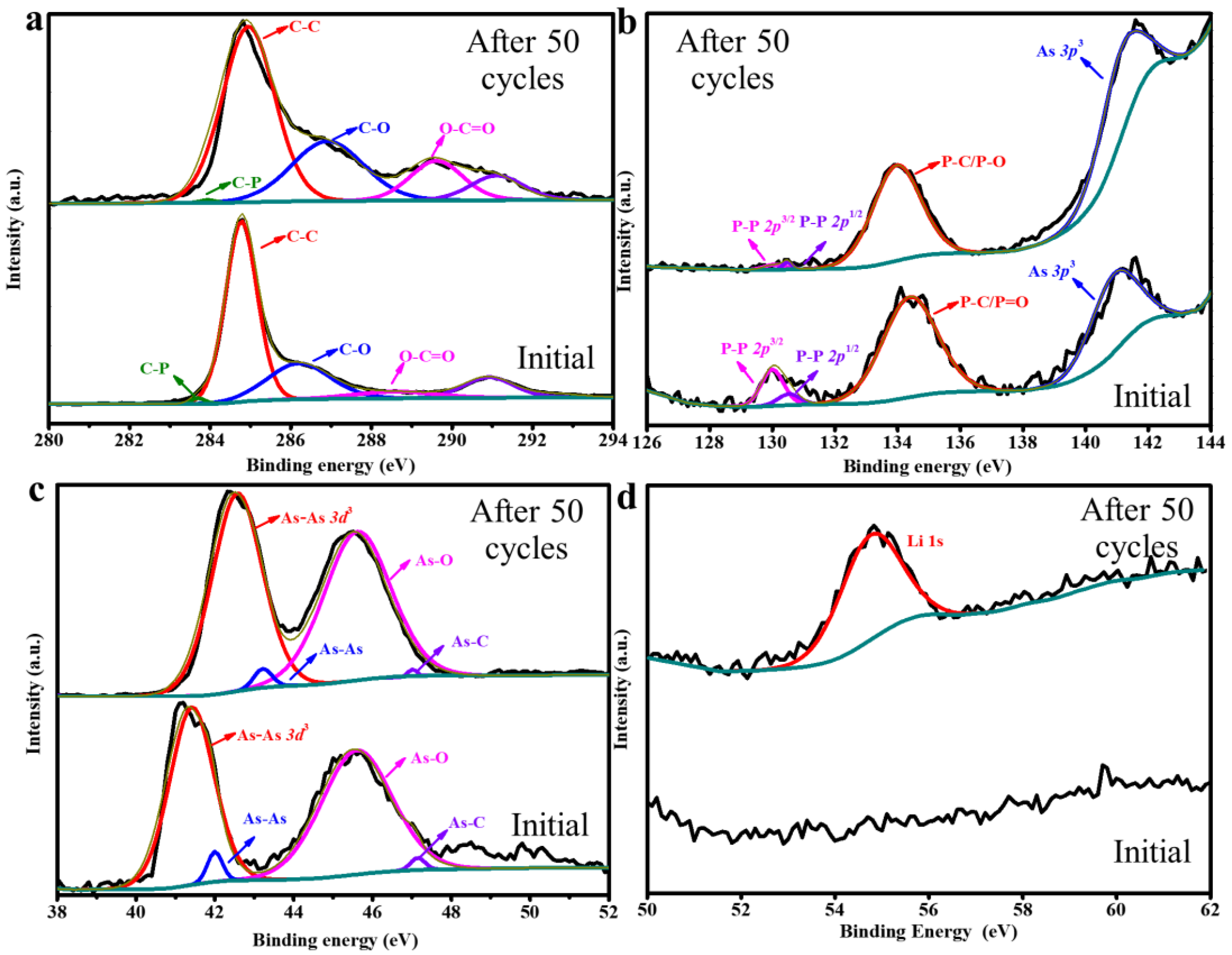
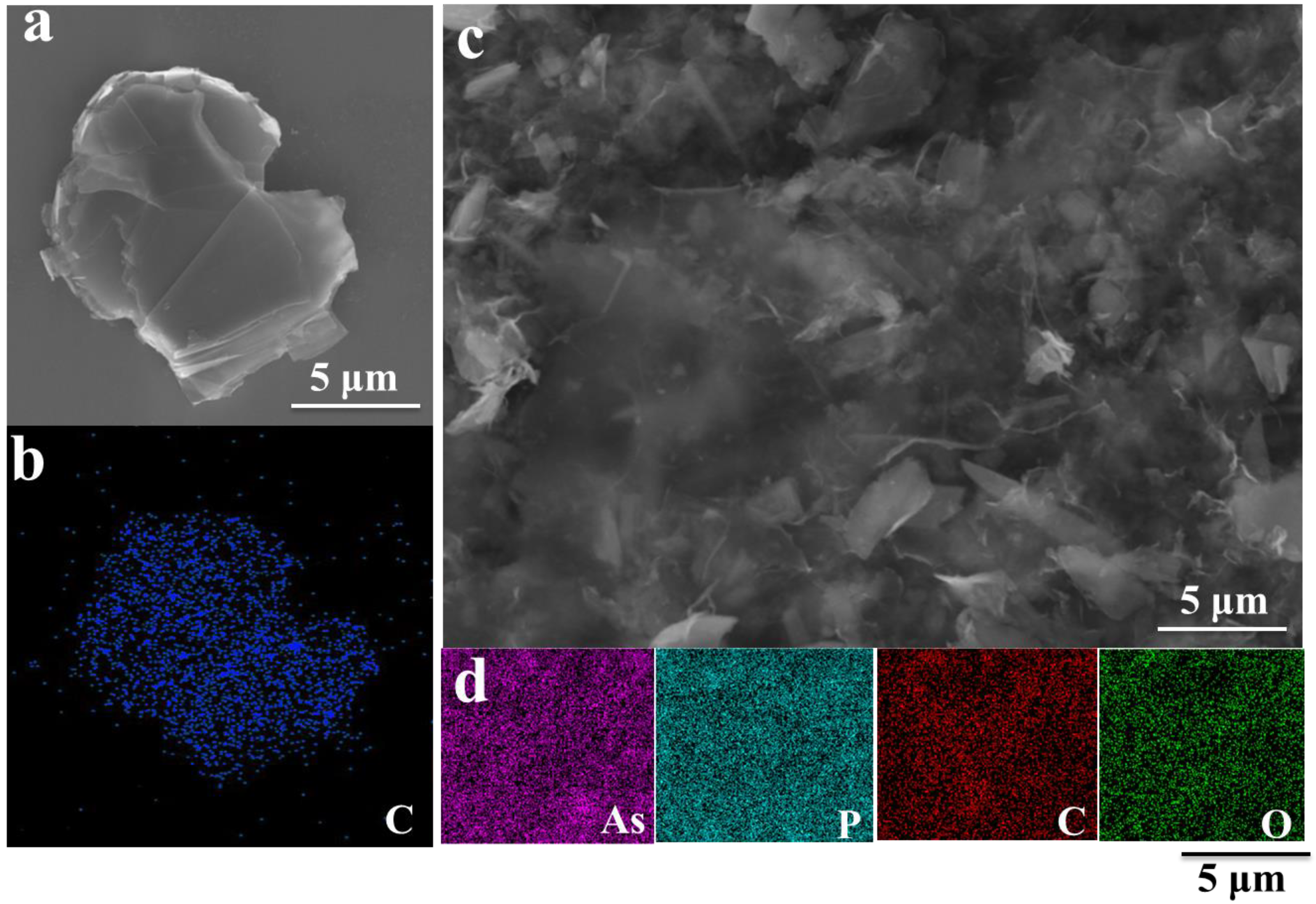
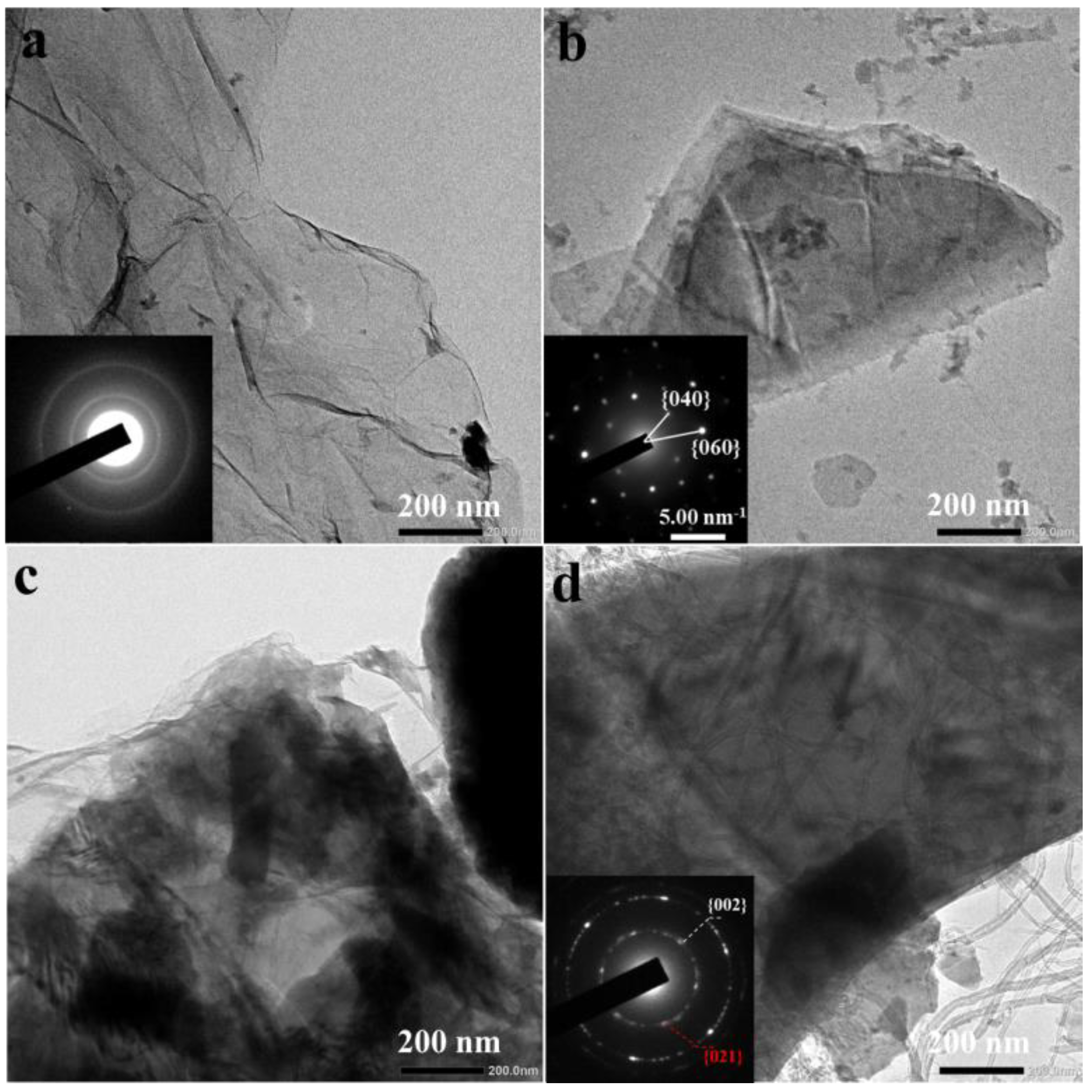
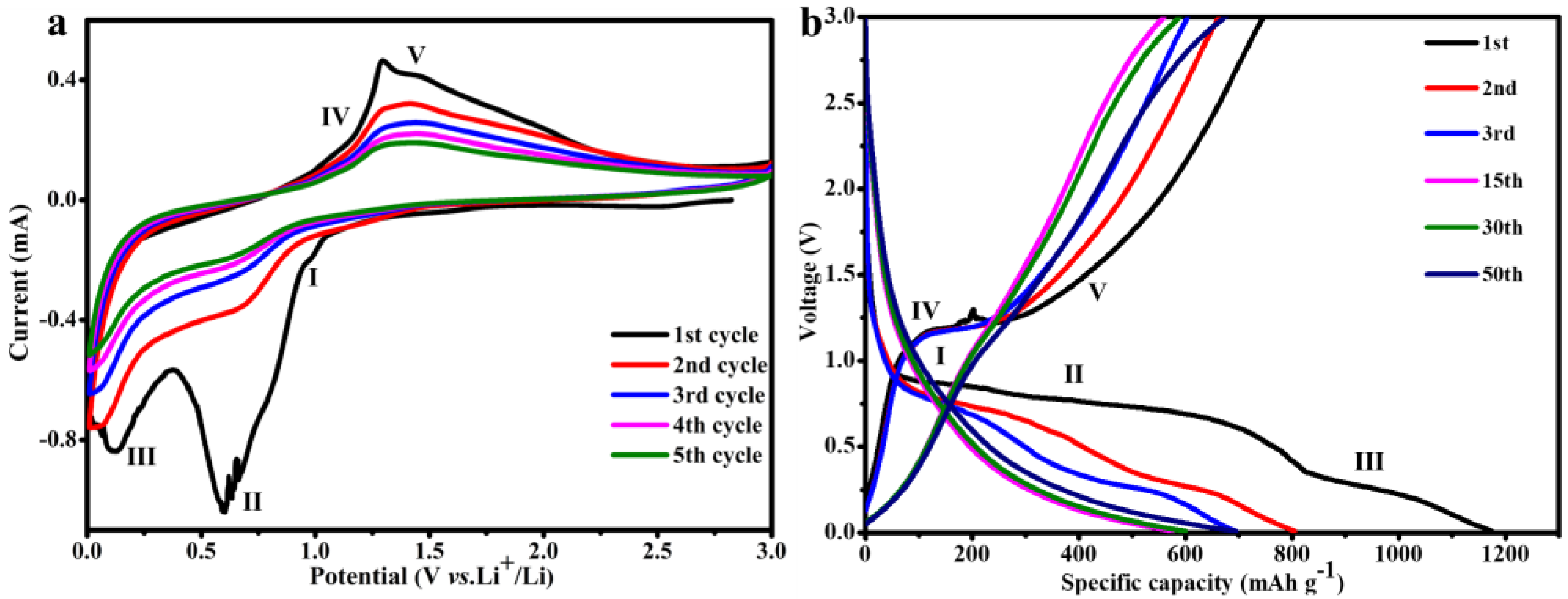
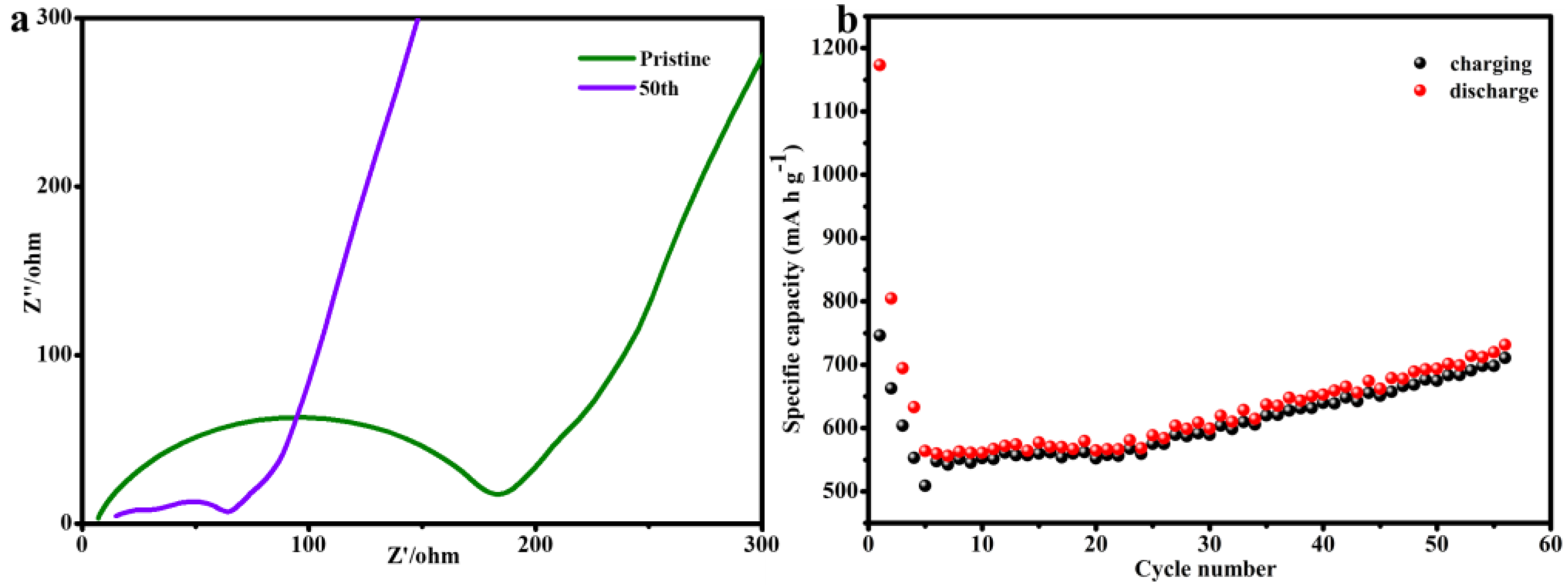
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, W.; Fang, B.; Ignaszak, A.; Wu, Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Heubner, C.; Liebmann, T.; Schneider, M.; Michaelis, A. Recent insights into the electrochemical behavior of blended lithium insertion cathodes: A review. Electrochim. Acta 2018, 269, 745–760. [Google Scholar] [CrossRef]
- Chen, L.F.; Feng, Y.; Liang, H.W.; Wu, Z.Y.; Yu, S.H. Macroscopic-scale three-dimensional carbon nanofiber architectures for electrochemical energy storage devices. Adv. Energy Mater. 2017, 7, 1700826. [Google Scholar] [CrossRef] [Green Version]
- Park, C.M.; Kim, J.H.; Kim, H.; Sohn, H.J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Zhou, M.; Xu, T.; Wang, D. Micro-sized silicon-carbon composites composed of carbon-coated sub-10 nm Si primary particles as high-performance anode materials for lithium-ion batteries. J. Mater. Chem. A 2013, 2, 1257–1262. [Google Scholar] [CrossRef]
- Cui, L.F.; Yang, Y.; Hsu, C.M.; Cui, Y. Carbon-silicon core-shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett. 2009, 9, 3370–3374. [Google Scholar] [CrossRef]
- Larcher, D.; Mudalige, C.; George, A.E.; Porter, V.; Gharghouri, M.; Dahn, J.R. Si-containing disordered carbons prepared by pyrolysis of pitch polysilane blends: Effect of oxygen and sulfur. Solid State Ion. 1999, 122, 71–83. [Google Scholar] [CrossRef]
- Wen, Z.S.; Yang, J.; Wang, B.F.; Wang, K.; Liu, Y. High capacity silicon/carbon composite anode materials for lithium ion batteries. Electrochem. Commun. 2003, 5, 165–168. [Google Scholar] [CrossRef]
- Tobishima, S.; Shodai, T.; Yamaki, J.; Okada, S. Study of Li3xMxN (M: Co, Ni or Cu) system for use as anode material in lithium rechargeable cells. Solid State Ion. Diffus. React. 1996, 86, 785–789. [Google Scholar]
- Nishijima, M.; Tadokoro, N.; Takeda, Y.; Imanishi, N.; Yamamoto, O. Li deintercalation-intercalation reaction and structural change in lithium transition metal nitride, Li7MnN4. J. Electrochem. Soc. 1994, 141, 2966–2971. [Google Scholar] [CrossRef]
- Idota, Y.; Kubota, T.; Matsufuji, A.; Maekawa, Y.; Miyasaka, T. Tin-based amorphous oxide: A high-capacity lithium-ion-storage material. Science 1997, 276, 1395–1397. [Google Scholar] [CrossRef] [Green Version]
- Lou, X.W.; Wang, Y.; Yuan, C.; Lee, J.Y.; Archer, L.A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329. [Google Scholar] [CrossRef]
- Courtney, I.A.; Dahn, J.R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide composites. J. Electrochem. Soc. 1997, 144, 2045–2052. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Legut, D.; Zhang, Q. Modeling and theoretical design of next-generation lithium metal batteries. Energy Storage Mater. 2019, 16, 169–193. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Obergfell, D.; Roth, S.; Girit, C.; Zettl, A. On the roughness of single-and bi-layer graphene membranes. Solid State Commun. 2007, 143, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Marino, C.; Debenedetti, A.; Fraisse, B.; Favier, F.; Monconduit, L. Activated-phosphorus as new electrode material for Li-ion batteries. Electrochem. Commun. 2011, 13, 346–349. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Lee, H.W.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of stable phosphorus-carbon bond for enhanced performance in black phosphorus nanoparticle-graphite composite battery anodes. Nano Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem. 2013, 125, 4731–4734. [Google Scholar] [CrossRef]
- Qian, J.; Qiao, D.; Ai, X.; Cao, Y.; Yang, H. Reversible 3-Li storage reactions of amorphous phosphorus as high capacity and cycling-stable anodes for Li-ion batteries. Chem. Commun. 2012, 48, 8931–8933. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Yoon, S.; Lee, S.I.; Kim, J.H.; Jung, J.H.; Sohn, H.J. Erratum: High-rate capability and enhanced cyclability of antimony-based composites for lithium rechargeable batteries. J. Electrochem. Soc. 2007, 154, S19. [Google Scholar] [CrossRef]
- He, M.; Kravchyk, K.; Walter, M.; Kovalenko, M.V. Monodisperse antimony nanocrystals for high-rate Li-ion and Na-ion battery anodes: Nano versus bulk. Nano Lett. 2014, 14, 1255–1262. [Google Scholar] [CrossRef]
- Park, C.M.; Yoon, S.; Lee, S.I.; Sohn, H.J. Enhanced electrochemical properties of nanostructured bismuth-based composites for rechargeable lithium batteries. J. Power Sources 2009, 186, 206–210. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Kim, J.; Lee, J.; Ji, H.R.; Oh, S.M.; Lee, K.T. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv. Mater. 2013, 25, 3045–3049. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Fritz, H.P. Reversibles elektrochemisches legieren von metallen der V. hauptgruppe in organischen Li+-Lsungen. Electrochim. Acta 1975, 20, 513–517. [Google Scholar] [CrossRef]
- Park, C.M. Electrochemical lithium quasi-intercalation with arsenic. J. Solid State Electrochem. 2016, 20, 517–523. [Google Scholar] [CrossRef]
- Loebenstein, J.R. Materials Flow of Arsenic in the United States. Inf. Circ. 1994, 9382, 1–12. [Google Scholar]
- U.S. EPA. National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminants Monitoring; Final Rule; EPA: Washington, DC, USA, 2001.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, V.; Peralta, J.E. Magnetic boron nitride nanoribbons with tunable electronic properties. Nano Lett. 2008, 8, 2210–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, G.; Guo, M.; Zhang, Y.W. Strain-tunable electronic and transport properties of MoS2 nanotubes. Nano Res. 2014, 7, 518–527. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, L.; Yao, Z.; Li, F.; Li, S.; Ye, S. Enhanced reversibility of red phosphorus/active carbon composite as anode for lithium ion batteries. Electrochim. Acta 2015, 163, 71–76. [Google Scholar] [CrossRef]
- Hays, K.A.; Banek, N.A.; Wagner, M.J. High performance arsenic: Multiwall carbon nanotube composite anodes for Li-ion batteries. J. Electrochem. Soc. 2017, 164, A1635–A1643. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Z.; Zhong, G.; Liu, Y.; Yang, Y.; Ma, T.; Ren, Y.; Zuo, X.; Wu, X.; Zhang, X. Nanostructured black phosphorus/ketjenblack-MWCNTs composite as high performance anode material for sodium-ion batteries. Nano Lett. 2016, 16, 3955–3965. [Google Scholar] [CrossRef]
- Lim, Y.R.; Shojaei, F.; Park, K.; Jung, C.S.; Park, J.; Cho, W.I.; Kang, H.S. Arsenic for high-capacity lithium-and sodium-ion batteries. Nanoscale 2018, 10, 7047–7057. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High capacity Li-ion battery anodes using Ge nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wu, C.; Wen, Y.; Yin, K.; Chiang, F.-K.; Hu, R.; Liu, J.; Sun, L.; Gu, L. New nanoconfined galvanic replacement synthesis of hollow Sb@ C yolk–shell spheres constituting a stable anode for high-rate Li/Na-ion batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, F.; Zhang, Z.; Zhang, K.; Lai, Y.; Li, J. Bismuth nanoparticles embedded in carbon spheres as anode materials for sodium/lithium-ion batteries. Chem. Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ma, S.; Liu, Q.; Zhang, S.; Chu, Y.; Hao, X.; Han, B.; Xu, B. 2D black arsenic phosphorus and its application for anodes of lithium ion batteries. CrystEngComm 2020, 22, 8228–8235. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. Acs Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, T.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N. Uniaxial strain in graphene by raman spectroscopy: G peak splitting, Gruneisen parameters and sample orientation. Phys. Rev. B 2008, 79, 205433. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Zhang, A.; Cai, J.; Cao, X.; Li, Z.; Asimow, P.D.; Zhou, C. Room-temperature pressure synthesis of layered black phosphorus graphene composite for sodium-ion battery anodes. ACS Nano 2018, 12, 8323–8329. [Google Scholar] [CrossRef]
- Gu, J.; Du, Z.; Zhang, C.; Ma, J.; Li, B.; Yang, S.; Gu, J.N.; Du, Z.G.; Zhang, C.; Ma, J. Liquid-phase exfoliated metallic antimony nanosheets toward high volumetric sodium storage. Adv. Energy Mater. 2017, 7, 1700447. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon Am. Carbon Comm. 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Cai, W.; Zhu, Y.; Liang, J.; Zhang, K.; Lan, Y.; Jiang, Z.; Wang, G.; Qian, Y. Wet-chemical synthesis of hollow red-phosphorus nanospheres with porous shells as anodes for high-performance lithium-ion and sodium-ion batteries. Adv. Mater. 2017, 29, 1700214.1–1700214.7. [Google Scholar] [CrossRef]
- Luo, W.; Zemlyanov, D.Y.; Milligan, C.A.; Du, Y.; Yang, L.; Wu, Y.; Ye, P.D. Surface chemistry of black phosphorus under a controlled oxidative environment. Nanotechnology 2016, 27, 434002. [Google Scholar] [CrossRef]
- Subbaiah, Y.; Saji, K.J.; Tiwari, A. Atomically thin MoS2: A versatile nongraphene 2D material. Adv. Funct. Mater. 2016, 26, 2046–2069. [Google Scholar] [CrossRef]
- Mohsen, B.M.S.; Martin, P. 2D-pnictogens: Alloy-based anode battery materials with ultrahigh cycling stability. Chem. Soc. Rev. 2018, 47, 6964–6989. [Google Scholar]
- Jin, H.; Wang, H.; Qi, Z.; Bin, D.S.; Zhang, T.; Wan, Y.; Chen, J.; Chuang, C.; Lu, Y.R.; Chan, T.S. A black phosphorus-graphite composite anode for Li-/Na-/K-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 2318–2322. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Ma, S.; Xu, Y.; Zhang, S.; Hao, X.; Xu, B. Electrochemical Performance of Graphene Oxide/Black Arsenic Phosphorus/Carbon Nanotubes as Anode Material for LIBs. Materials 2022, 15, 4576. https://doi.org/10.3390/ma15134576
Hou Y, Ma S, Xu Y, Zhang S, Hao X, Xu B. Electrochemical Performance of Graphene Oxide/Black Arsenic Phosphorus/Carbon Nanotubes as Anode Material for LIBs. Materials. 2022; 15(13):4576. https://doi.org/10.3390/ma15134576
Chicago/Turabian StyleHou, Yanyan, Shufang Ma, Yang Xu, Shuai Zhang, Xiaodong Hao, and Bingshe Xu. 2022. "Electrochemical Performance of Graphene Oxide/Black Arsenic Phosphorus/Carbon Nanotubes as Anode Material for LIBs" Materials 15, no. 13: 4576. https://doi.org/10.3390/ma15134576
APA StyleHou, Y., Ma, S., Xu, Y., Zhang, S., Hao, X., & Xu, B. (2022). Electrochemical Performance of Graphene Oxide/Black Arsenic Phosphorus/Carbon Nanotubes as Anode Material for LIBs. Materials, 15(13), 4576. https://doi.org/10.3390/ma15134576





