The Precipitation Behavior of a Cu-Ni-Si Alloy with Cr Addition Prepared by Heating-Cooling Combined Mold (HCCM) Continuous Casting
Abstract
:1. Introduction
2. Experimental Procedure
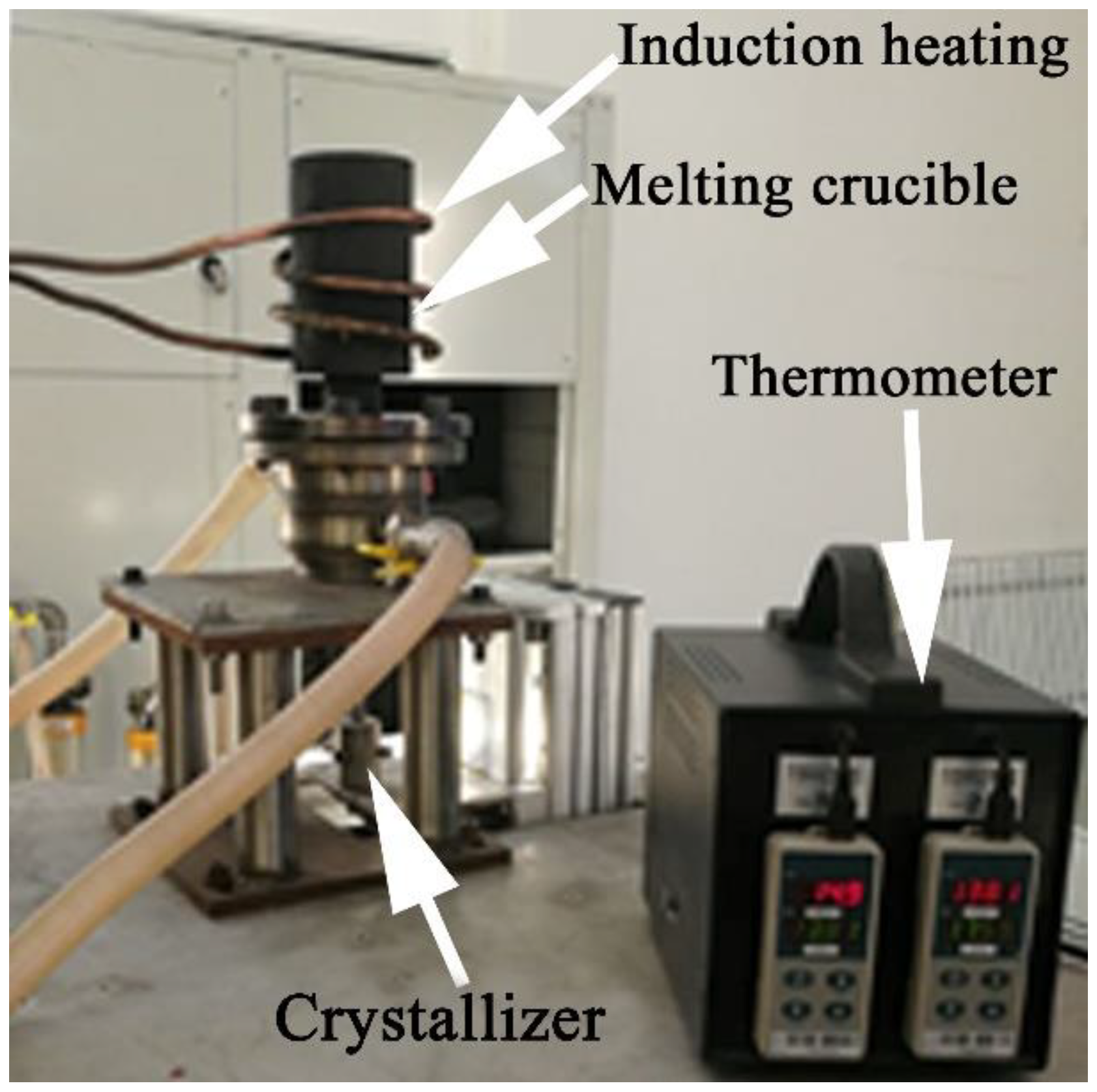
2.1. The Fabrication and Aging Treatment of Materials
2.2. Microstructure Observation
2.3. Mechanical Properties
3. Results
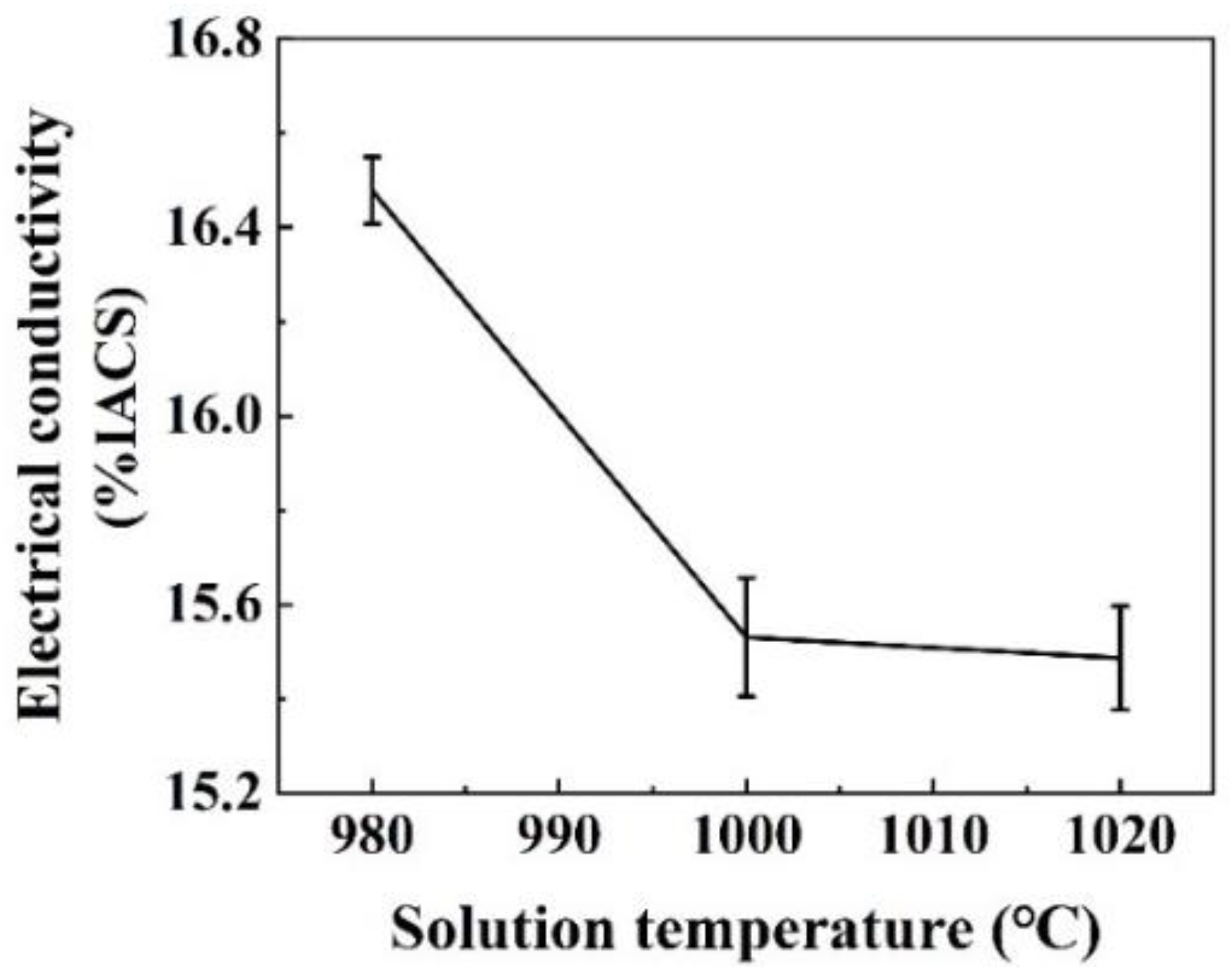
3.1. The Variation of the Mechanical Properties and Electrical Conductivities
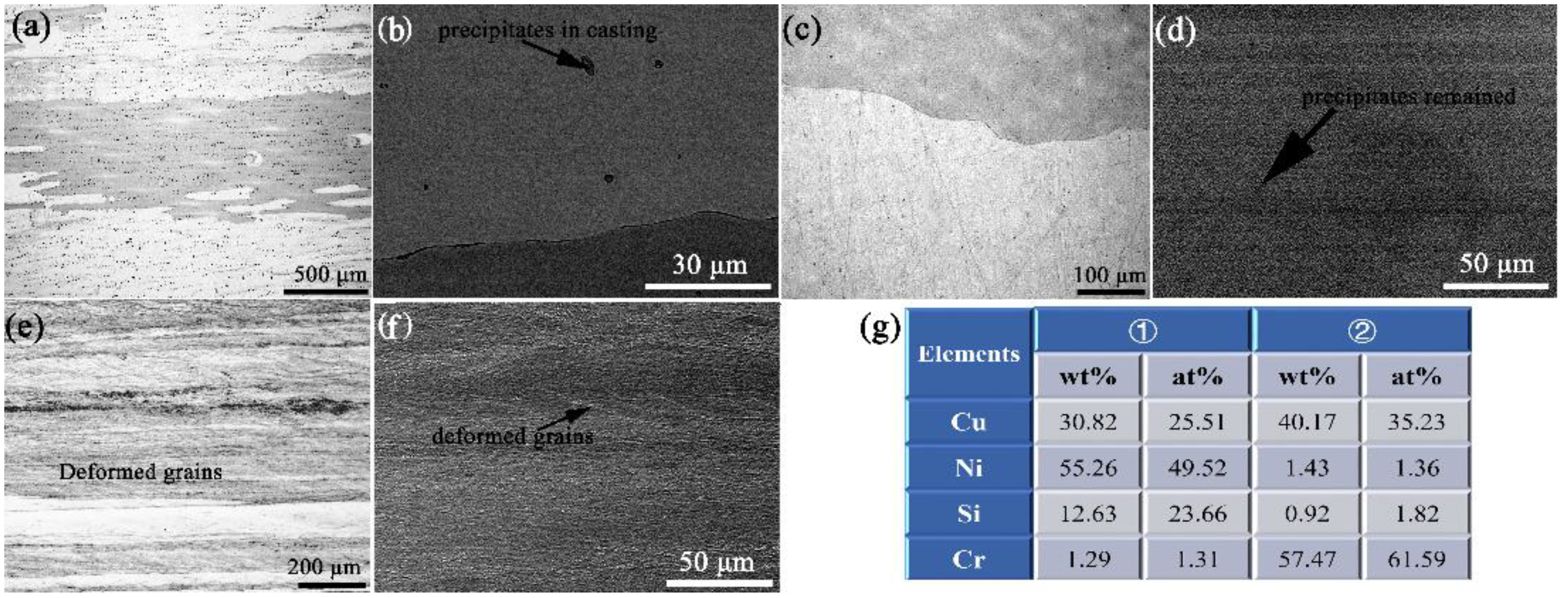
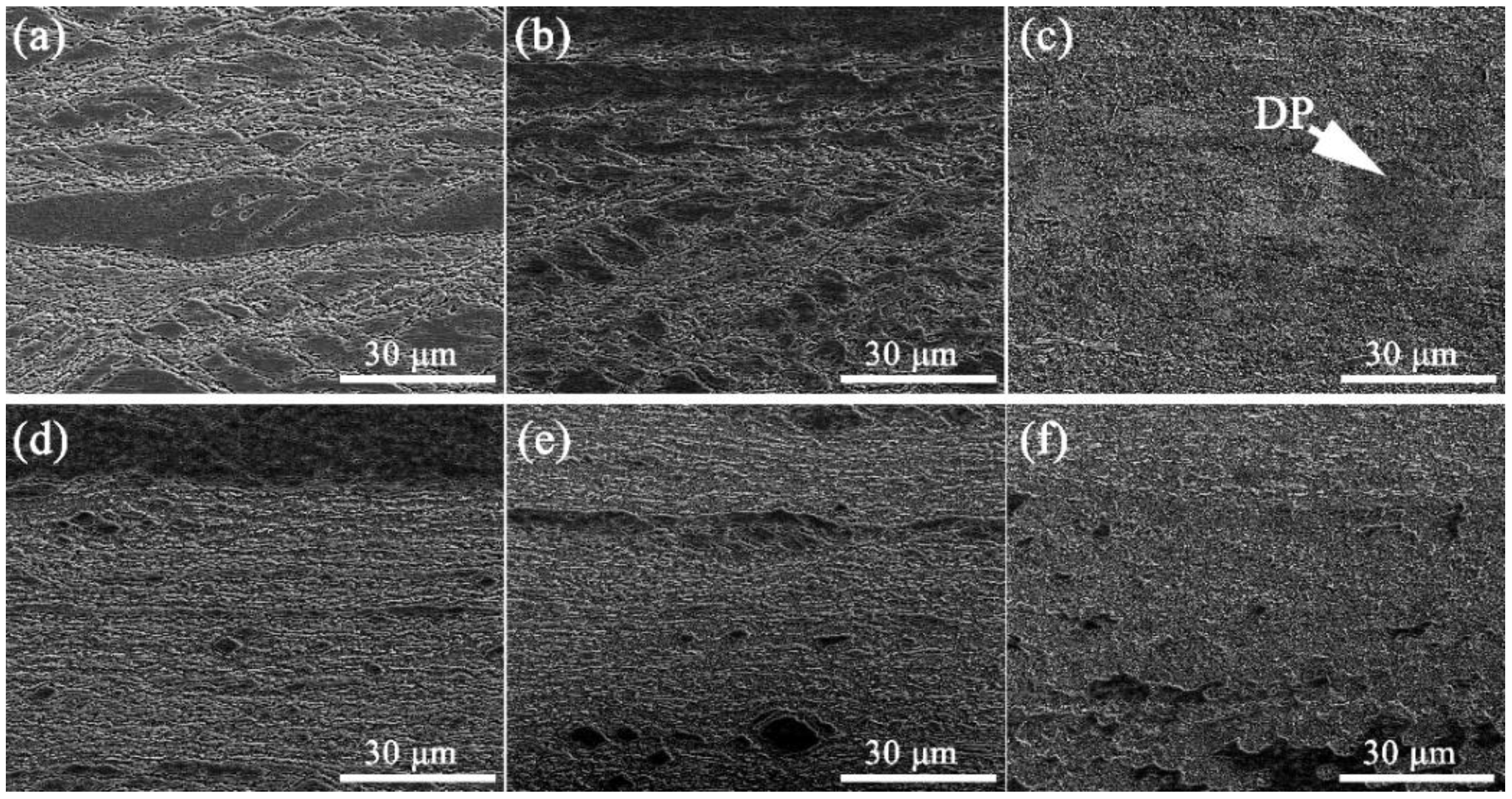
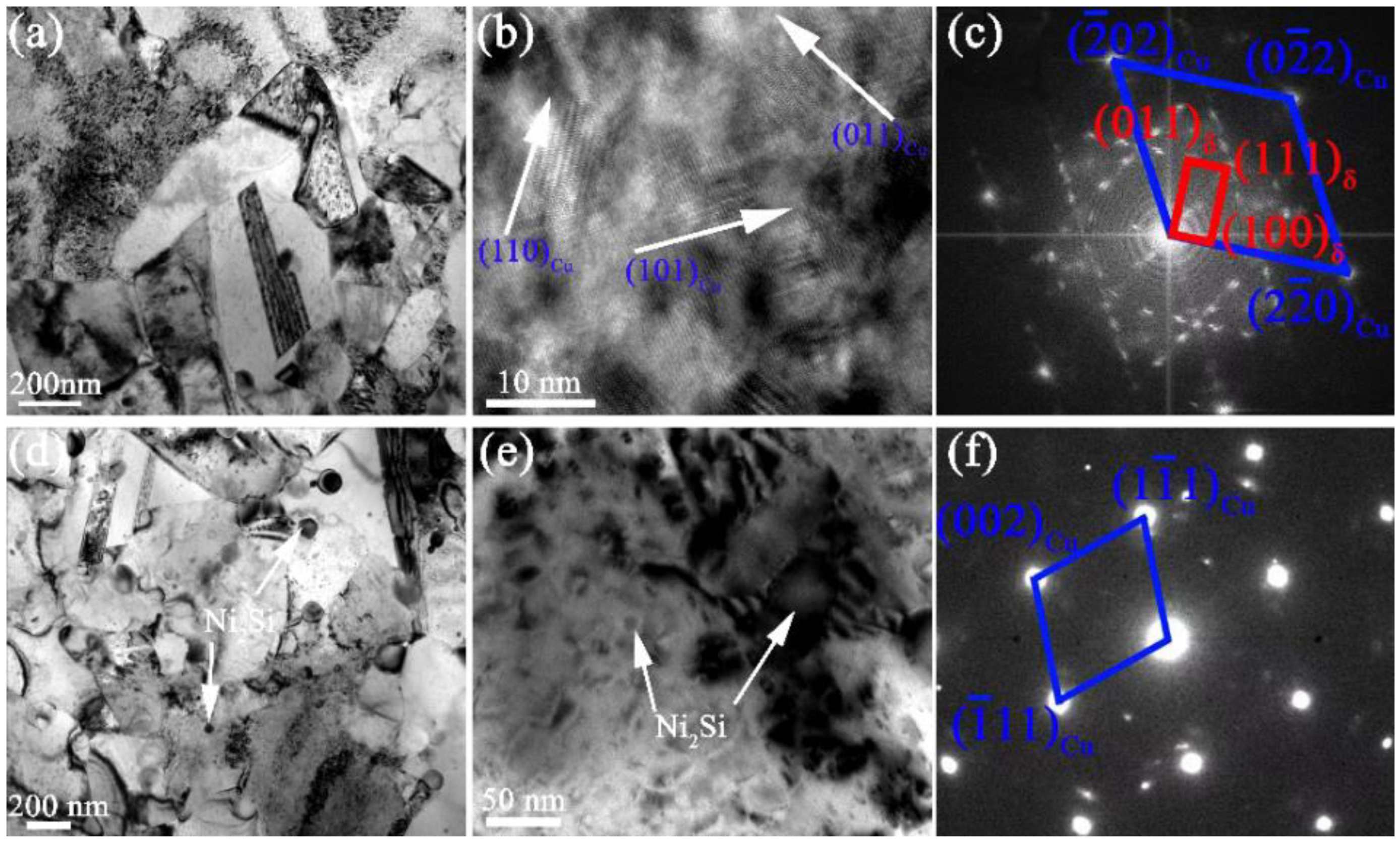
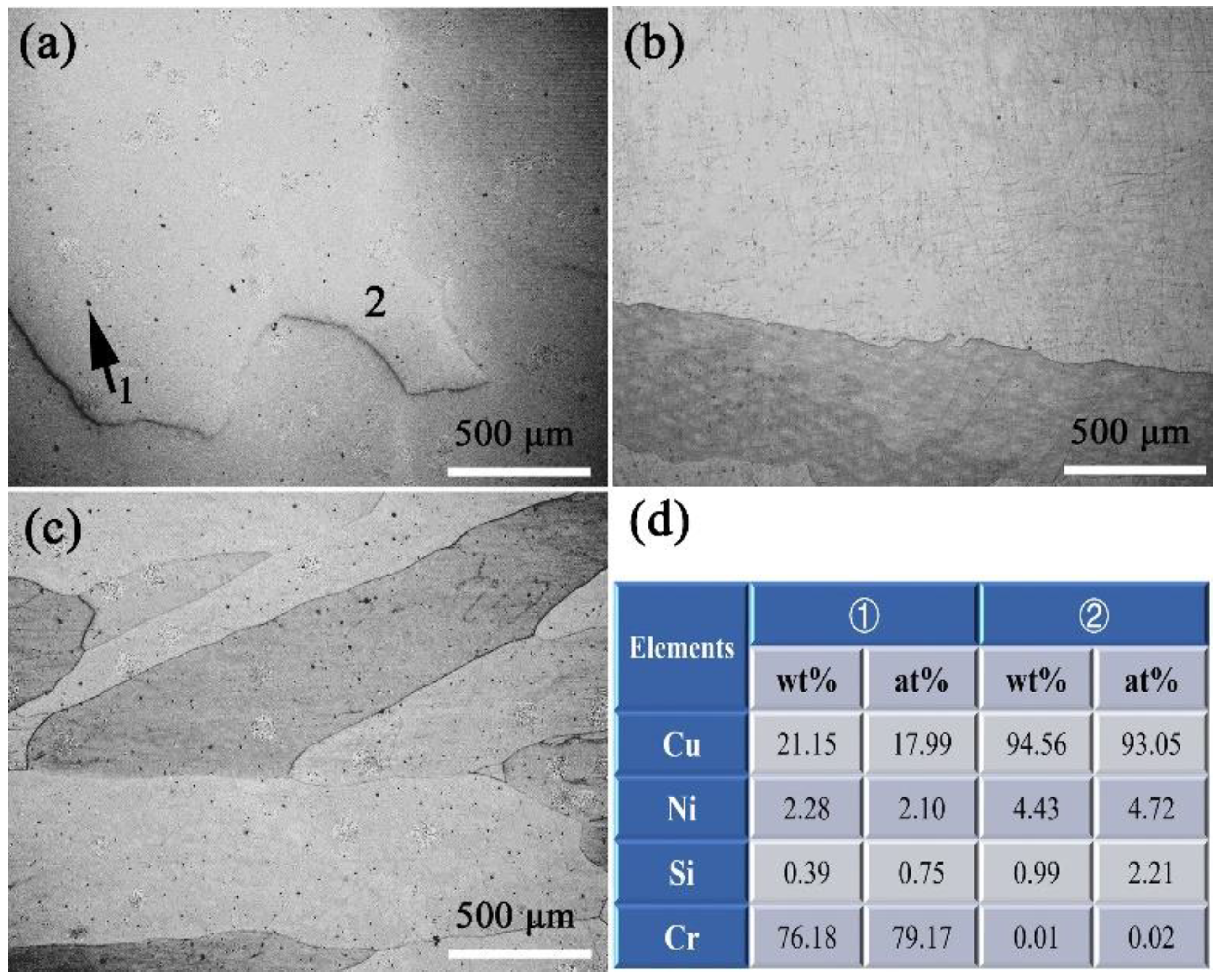
3.2. The Evolution of Microstructures
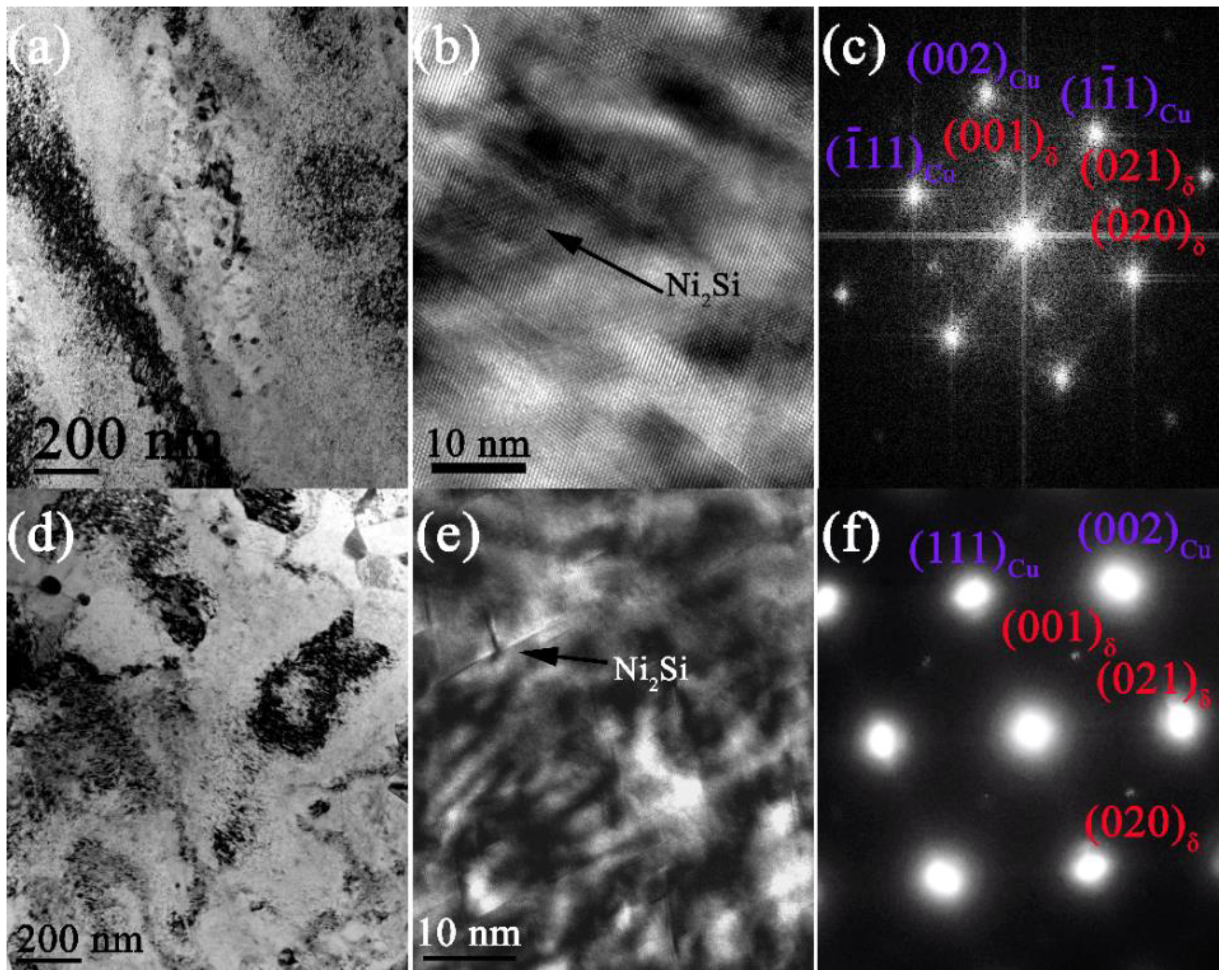
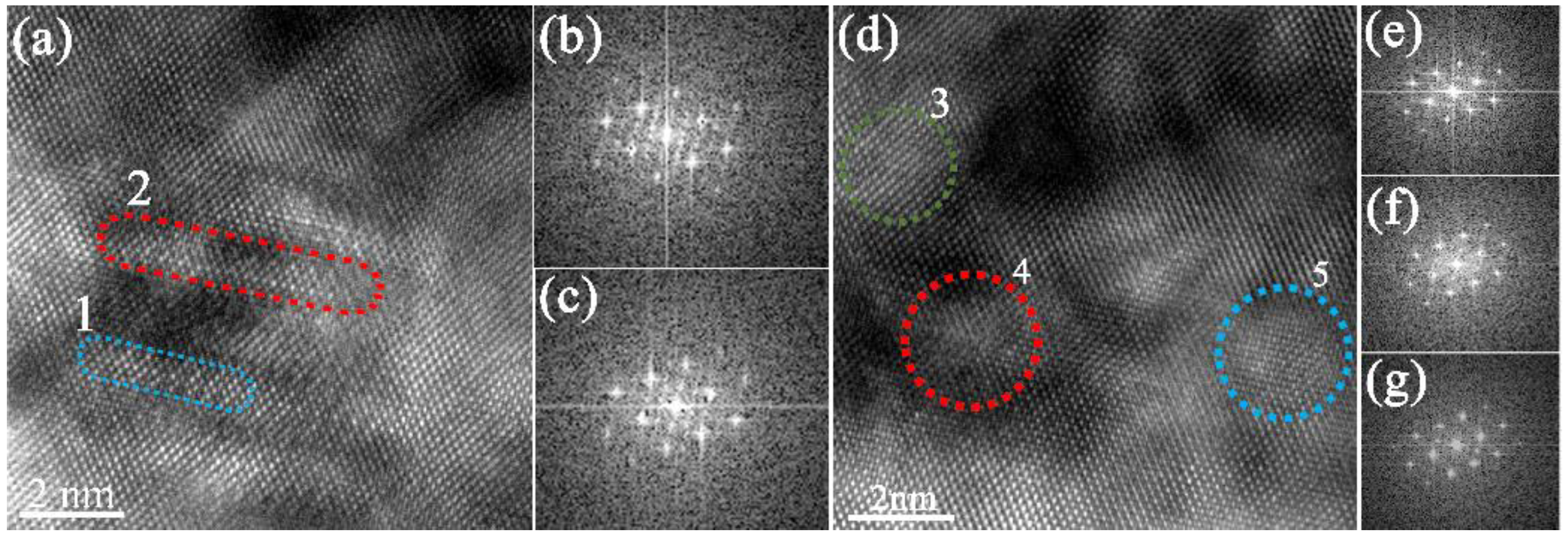
3.3. The Precipitation Process
4. Discussion
4.1. The Influence of HCCM on Deformation and Solution Process
4.2. The Influence of Addition of Cr on Precipitation Process
4.3. The Relationship between Precipitation and Strengthening
5. Conclusions
- (1)
- Both the size and amount of these δ-Ni2Si precipitates are smaller than those in the traditional casting ingots, due to the quite rapid cooling rate of the HCCM continuous casting method. The cold rolling and aging process are consequently conducted on the solution treated specimens without hot deformation, since the excellent homogeneity of matrix is obtained.
- (2)
- Excellent combination of mechanical property (hardness HV 250–270) and electrical conductivity (46–47% IACS) is obtained by the first step aging at 500 °C for 0.25 h and the second step aging at 450 °C for 1 h.
- (3)
- The nucleation and growth of δ-Ni2Si precipitates occurs around the boundaries of these Cr3Si cores, leading to an enhanced nucleation rate. The formation of DP is suppressed by the formation of Cr3Si cores before δ-Ni2Si. The precipitation process mainly containing CP is realized in the Cu-Ni-Si alloys containing (Ni + Si) ≥ 5 wt.% herein. A larger amount of nanometer precipitates can be obtained by introducing the two steps of aging process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, F.X.; Ning, H.L.; Ma, J.S. Precipitation in Cu-Ni-Si-Zn alloy for lead frame. Mater. Lett. 2003, 57, 2135–2139. [Google Scholar] [CrossRef]
- Monzen, R.; Watanabe, C. Microstructure and mechanical properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2008, 483, 117–119. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibutani, N.; Mimura, K.; Isshiki, M.; Waseda, Y. Improvement in strength and electrical conductivity of Cu-Ni-Si alloys by aging and cold rolling. J. Alloy. Compd. 2006, 417, 116–120. [Google Scholar] [CrossRef]
- Zhao, D.M.; Dong, Q.M.; Liu, P.; Kang, B.X.; Huang, J.L.; Jin, Z.H. Structure and strength of the age hardened Cu-Ni-Si alloy. Mater. Chem. Phys. 2003, 79, 81–86. [Google Scholar] [CrossRef]
- Hu, T.; Chen, J.H.; Liu, J.Z.; Liu, Z.R.; Wu, C.L. The crystallographic and morphological evolution of the strengthening precipitates in Cu-Ni-Si alloys. Acta Mater. 2013, 61, 1210–1219. [Google Scholar] [CrossRef]
- Yi, J.; Jia, Y.L.; Zhao, Y.Y.; Xiao, Z.; He, K.J.; Wang, Q.; Wang, M.P.; Li, Z. Precipitation behavior of Cu-3.0Ni-0.72Si alloy. Acta Mater. 2019, 166, 261–270. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Wang, M.P.; Zhang, L.; Xiao, Z.; Jia, Y.L. The evolution of microstructure in Cu-8.0Ni-1.8Si-0.15Mg alloy during aging. Mater. Sci. Eng. A 2010, 527, 6728–6733. [Google Scholar] [CrossRef]
- Mabuchi, M.; Higashi, K. Strengthening mechanisms of Mg-Si alloys. Acta Mater. 1996, 44, 4611–4618. [Google Scholar] [CrossRef]
- Huang, J.Z.; Xiao, Z.; Dai, J.; Li, Z.; Jiang, H.Y.; Wang, W.; Zhang, X.X. Microstructure and Properties of a Novel Cu-Ni-Co-Si-Mg Alloy with Super-high Strength and Conductivity. Mater. Sci. Eng. A 2019, 744, 754–763. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.M.; Yang, W.W.; Chen, D.L. Effect of aluminum on microstructure and property of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Watanabe, C.; Nishijima, F.; Monzen, R.; Tazaki, K. Mechanical Properties of Cu-4.0wt%Ni-0.95wt%Si Alloys with and without P and Cr Addition. Mater. Sci. Forum 2007, 90, 2321–2324. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.P.; Yi, Z.Y.; Chen, T.T.; Liu, R.Q.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu-Ni-Si alloy. J. Alloy. Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Xiao, Z.; Li, Z.; Qiu, W.T.; Jiang, H.Y.; Lei, Q.; Liu, Z.; Jiang, Y.B.; Zhang, S.J. Microstructure and properties of a Cu-Ni-Si-Co-Cr alloy with high strength and high conductivity. Mater. Sci. Eng. A 2019, 759, 396–403. [Google Scholar] [CrossRef]
- Horie, H.; Kammuri, K.; Imozuka, Y.; Tsuji, Y.; Watanabe, C. Effect of Co and P on the Discontinuous Precipitation Behavior in High Concentration Corson Alloy. Mater. Trans. 2020, 61, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.N.; Liu, X.F.; Yang, Y.H.; Du, M. Relationship and mechanism between microstructure and property of C70250 copper alloy strip prepared by temperature controlled mold continuous casting. Mater. Sci. Eng. A 2019, 767, 138428. [Google Scholar] [CrossRef]
- Watanabe, H.; Kunimine, T.; Watanabe, C.; Monzen, R.; Todaka, Y. Tensile deformation characteristics of a Cu-Ni-Si alloy containing trace elements processed by high-pressure torsion with subsequent aging. Mater. Sci. Eng. A 2018, 730, 10–15. [Google Scholar] [CrossRef]
- Kim, Y.G.; Seong, T.Y.; Han, J.H.; Ardell, A.J. Effect of heat treatment on precipitation behaviour in a Cu-Ni-Si-P alloy. J. Mater. Sci. 1986, 21, 1357–1362. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu-Ni-Si alloy. Intermetallics 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Lei, Q.; Qiu, W.T.; Luo, H.T. Hot deformation behavior of Cu-8.0Ni-1.8Si-0.15Mg alloy. Mater. Sci. Eng. A 2011, 528, 1641–1647. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Tian, B.H.; Jia, Y.L.; Liu, Y.; Song, K.X.; Volinsky, A.A. Co effects on Cu-Ni-Si alloys microstructure and physical properties. J. Alloy. Compd. 2019, 797, 1327–1337. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Y.L.; Li, Z.L.; Shan, Q.; Yu, W.; Tang, D. Microstructure evolution and dislocation strengthening mechanism of Cu-Ni-Co-Si alloy. Mater. Sci. Eng. A 2021, 826, 142023. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Tang, B.B.; Yu, F.X.; Shen, B. Evaluation of nanoscaled precipitates in a Cu-Ni-Si-Cr alloy during aging. J. Alloy. Compd. 2014, 614, 189–195. [Google Scholar] [CrossRef]
- Wang, W.; Kang, H.J.; Chen, Z.N.; Chen, Z.J.; Zou, C.L.; Li, R.G.; Yin, G.M.; Wang, T.M. Effects of Cr and Zr additions on microstructure and properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2016, 673, 378–390. [Google Scholar] [CrossRef]
- Wu, Y.K.; Li, Y.; Lu, J.Y.; Tan, S.; Jiang, F.; Sun, J. Correlations between microstructures and properties of Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2018, 731, 403–412. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, H.G.; Gu, J.W.; Hsu, C.E.; Wu, C.Y. Effects of heat treatment processes on the microstructures and properties of powder metallurgy produced Cu–Ni–Si–Cr alloy. Mater. Sci. Eng. A 2014, 619, 221–227. [Google Scholar] [CrossRef]
- Lei, J.G.; Huang, J.L.; Liu, P.; Jing, X.T.; Zhao, D.M.; Zhi, X. The effects of aging precipitation on the recrystallization of CuNiSiCr alloy. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2005, 20, 21–24. [Google Scholar]
- Wu, Y.K.; Li, Y.; Lu, J.Y.; Tan, S.; Jiang, F.; Sun, J. Effects of pre-deformation on precipitation behaviors and properties in Cu-Ni-Si-Cr alloy. Mater. Sci. Eng. A 2019, 742, 501–507. [Google Scholar] [CrossRef]
- Lei, Q.; Li, S.Y.; Zhu, J.L.; Xiao, Z.; Zhang, F.F.; Li, Z. Microstructural evolution, phase transition, and physics properties of a high strength Cu-Ni-Si-Al alloy. Mater. Charact. 2019, 147, 315–323. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Gao, Y.; Peng, X.; Derby, B. Microstructure and mechanical properties of a high strength Cu-Ni-Si alloy treated by combined aging processes. J. Alloy. Compd. 2017, 695, 2413–2423. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Wang, M.P.; Zhang, L.; Gong, S.; Xiao, Z.; Pan, Z.Y. Phase transformations behavior in a Cu-8.0Ni-1.8Si alloy. J. Alloy. Compd. 2011, 509, 3617–3622. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Zhu, A.; Qiu, W.; Liang, S. The transformation behavior of Cu-8.0Ni-1.8Si-0.6Sn-0.15Mg alloy during isothermal heat treatment. Mater. Charact. 2011, 62, 904–911. [Google Scholar] [CrossRef]
- Liao, W.N.; Liu, X.F.; Yang, Y.H.; Wang, S.Q.; Du, M. Effect of cold rolling reduction rate on mechanical properties and electrical conductivity of Cu-Ni-Si alloy prepared by temperature controlled mold continuous casting. Mater. Sci. Eng. A 2019, 763, 138068. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.X.; Zhao, F.; Liu, X.H.; Tian, Y.X. Effect of homogenization on second phases and mechanical properties of AA 5052 aluminum alloy tube billets fabricated by HCCM vertical continuous casting. J. Alloy. Compd. 2022, 901, 163645. [Google Scholar] [CrossRef]
- Fukamachi, K.; Kimura, M. Age-hardening structure and mechanism of Cu-3at%Ni-1.5at%Si Corson alloy. Mater. Sci. Eng. A 2022, 831, 142220. [Google Scholar] [CrossRef]
- Goto, M.; Yamamoto, T.; Han, S.Z.; Lim, S.H.; Kim, S.; Iwamura, T.; Kitamura, J.; Ahn, J.H.; Yakushiji, T.; Lee, J. Microstructure-dependent fatigue behavior of aged Cu-6Ni-1.5Si alloy with discontinuous/cellular precipitates. Mater. Sci. Eng. A 2019, 747, 63–72. [Google Scholar] [CrossRef]
- Han, S.Z.; Kang, J.; Kim, S.; Choi, S.; Kim, H.G.; Lee, J.; Kim, K.; Lim, S.H.; Han, B. Reliable and cost effective design of intermetallic Ni2Si nanowires and direct characterization of its mechanical properties. Sci. Rep. 2015, 5, 15050. [Google Scholar] [CrossRef] [Green Version]
- Han, S.Z.; Lim, S.H.; Kim, S.; Lee, J.; Goto, M.; Kim, H.G.; Han, B.; Kim, K.H. Increasing strength and conductivity of Cu alloy through abnormal plastic deformation of an intermetallic compound. Sci. Rep. 2016, 6, 30907. [Google Scholar] [CrossRef] [Green Version]
- Rdzawski, Z.; Stobrawa, J. Thermomechanical processing of Cu-Ni-Si-Cr-Mg alloy. Mater. Sci. Technol. 1993, 9, 142–149. [Google Scholar] [CrossRef]
- Cherepanov, A.N.; Cherepanova, V.K.; Fomin, V. Nucleation of a solid phase on spherical refractory nanoparticles. AIP Conf. Proc. 2019, 2125, 30063. [Google Scholar]
- Song, H.; Hoyt, J.J. A molecular dynamics study of heterogeneous nucleation at grain boundaries during solid-state phase transformations. Comp. Mater. Sci. 2016, 117, 151–163. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Xie, G.; Xue, W.; Fu, Y.; Wang, R.; Liu, X. The Precipitation Behavior of a Cu-Ni-Si Alloy with Cr Addition Prepared by Heating-Cooling Combined Mold (HCCM) Continuous Casting. Materials 2022, 15, 4521. https://doi.org/10.3390/ma15134521
Meng X, Xie G, Xue W, Fu Y, Wang R, Liu X. The Precipitation Behavior of a Cu-Ni-Si Alloy with Cr Addition Prepared by Heating-Cooling Combined Mold (HCCM) Continuous Casting. Materials. 2022; 15(13):4521. https://doi.org/10.3390/ma15134521
Chicago/Turabian StyleMeng, Xianghao, Guoliang Xie, Wenli Xue, Yilei Fu, Rui Wang, and Xinhua Liu. 2022. "The Precipitation Behavior of a Cu-Ni-Si Alloy with Cr Addition Prepared by Heating-Cooling Combined Mold (HCCM) Continuous Casting" Materials 15, no. 13: 4521. https://doi.org/10.3390/ma15134521
APA StyleMeng, X., Xie, G., Xue, W., Fu, Y., Wang, R., & Liu, X. (2022). The Precipitation Behavior of a Cu-Ni-Si Alloy with Cr Addition Prepared by Heating-Cooling Combined Mold (HCCM) Continuous Casting. Materials, 15(13), 4521. https://doi.org/10.3390/ma15134521





