Effect of TiC on Microstructure and Properties of Wear-Resistant Mo2FeB2 Claddings
Abstract
:1. Introduction
2. Materials and Methods
2.1. XRD Analysis and Microstructural Investigations
2.2. Hardness Measurement and Wear Tests
3. Results and Discussion
3.1. XRD Analysis
3.2. Microstructure and Composition
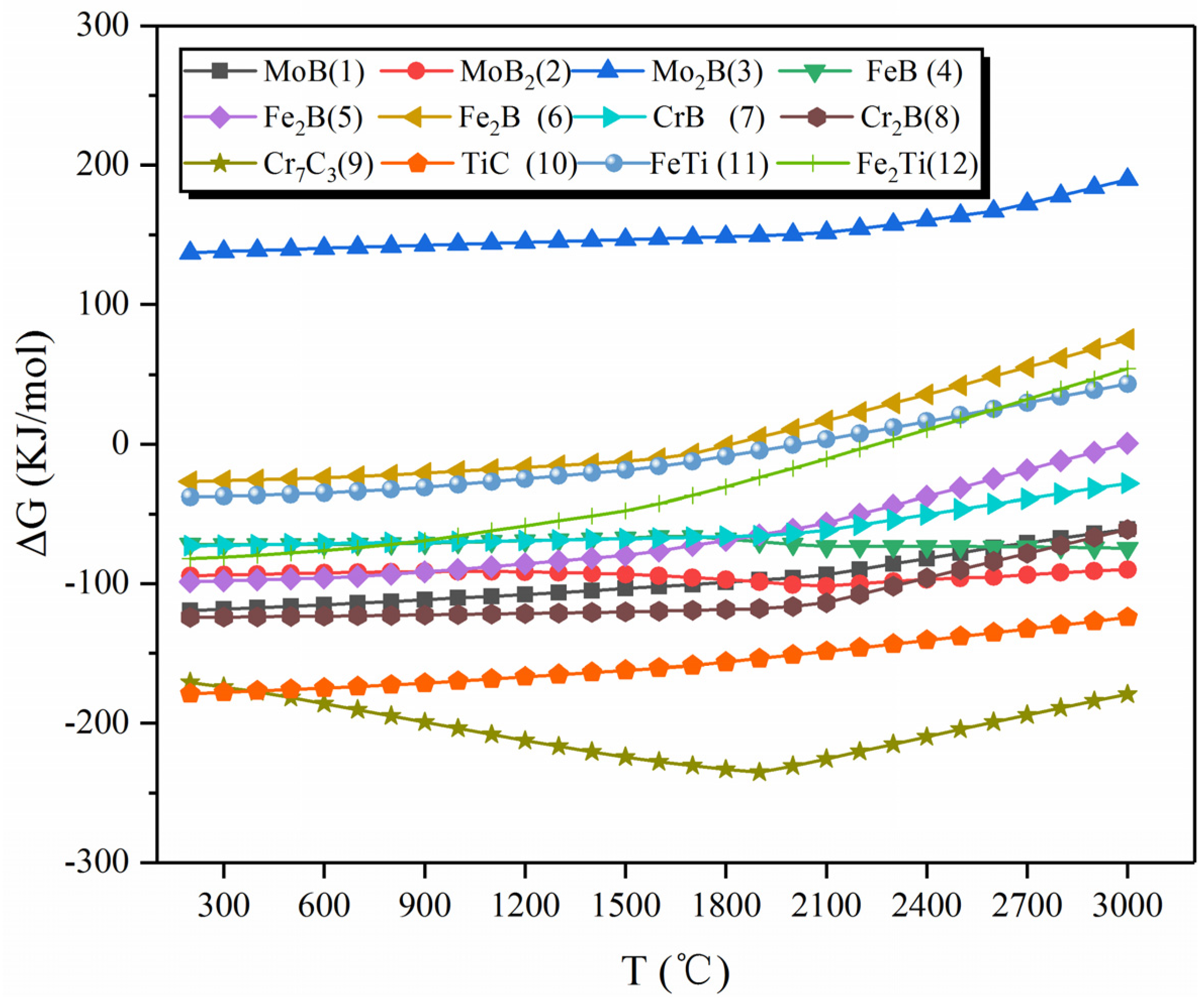
3.3. Thermodynamic Analysis
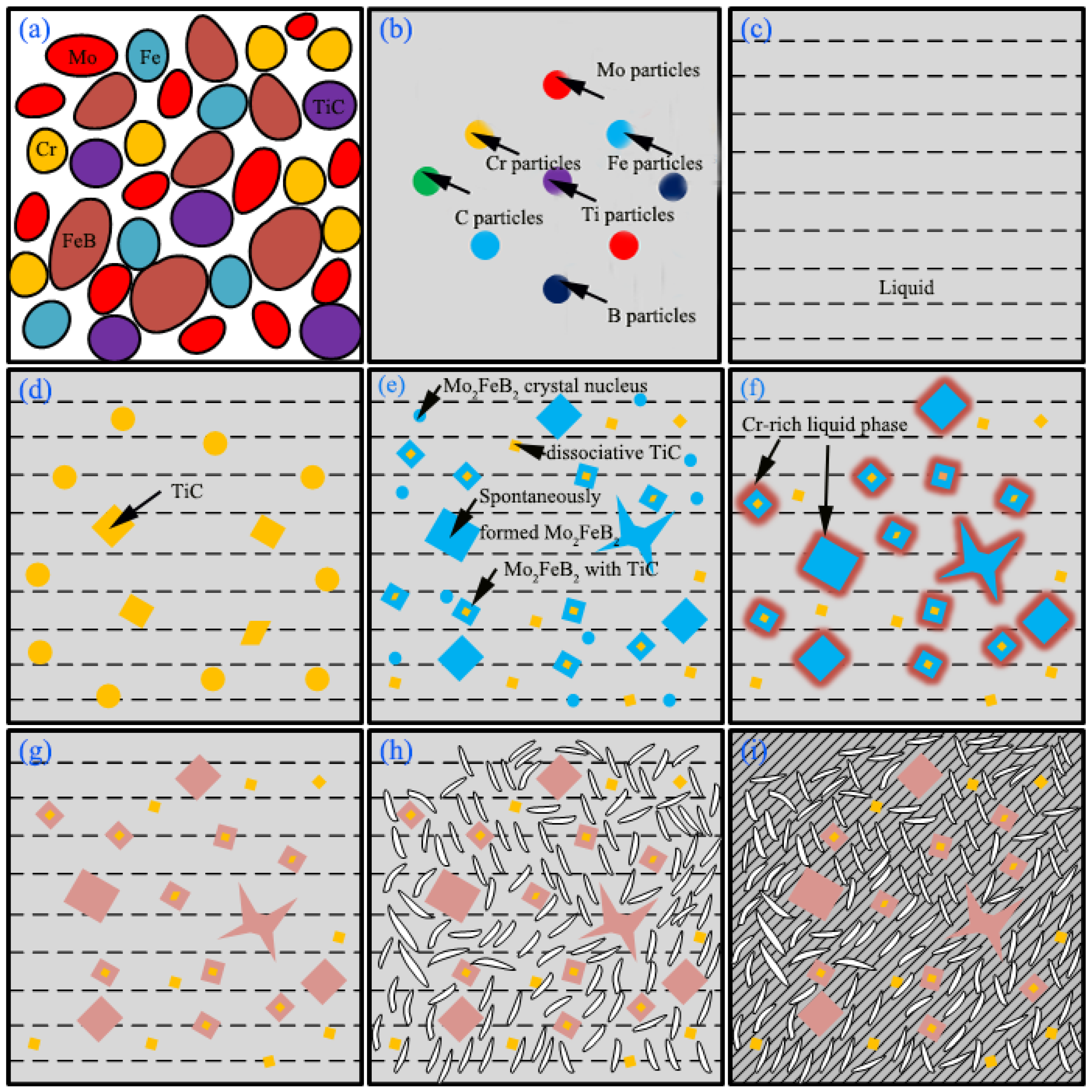
3.4. Microstructure Evolution
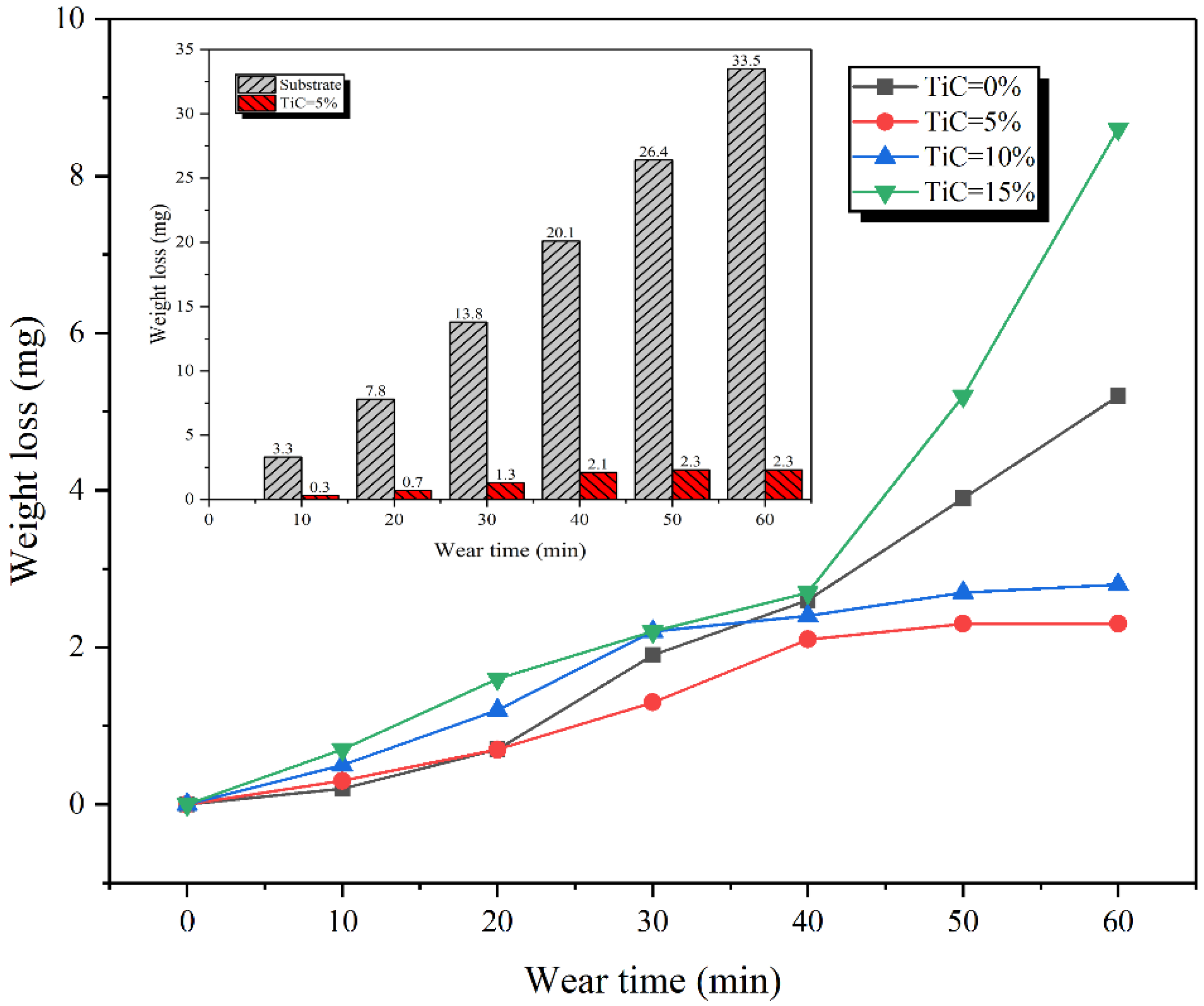
3.5. Wear Characteristics
4. Conclusions
- TiC content had important effects on the microstructure of cladding. The number of Mo2FeB2 increased with the increase of TiC content. Moreover, hard-phase Mo2FeB2 in cladding with TiC are finer compared with the cladding without TiC.
- TiC can serve as the non-spontaneous nucleation core of Mo2FeB2 in weld pool, which accelerated the nucleation and growth of Mo2FeB2 and made up for the problems of short weld pool time and insufficient metallurgical reaction.
- The addition of TiC to the alloy blocks significantly improves the wear resistance of the claddings. When the TiC content is 5%, the cladding has excellent wear resistance, which is 14.6 times that of the substrate.
- The results of this paper provide an effective technical solution for the in situ synthesis of Mo2FeB2-based cermets by welding metallurgy. Based on studying the influence of Rare Earth and TiC on the size, quantity, distribution and properties of Mo2FeB2, the effect of compound addition of Rare Earth and TiC needs to be further studied.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jian, Y.X.; Huang, Z.F.; Liu, X.T.; Xing, J. Comparative investigation on the stability, electronic structures and mechanical properties of Mo2FeB2 and Mo2NiB2 ternary borides by first-principles calculations. Results Phys. 2019, 15, 102698. [Google Scholar] [CrossRef]
- Yupeng, S.; Zhifu, H.; Zhang, L.; Li, K.; Cao, Z.; Xiao, P.; Jian, Y. Sintering mechanism, microstructure evolution and nanomechanical properties of Cr-added Mo2FeB2 based cermets. Ceram. Int. 2020, 46, 15482–15491. [Google Scholar]
- Cheng, Z.; Li, X.Q.; Wang, B.; Qu, S.; Li, H. M3B2-type borides effect on the wide gap brazing of K417G alloy with mixed powder. J. Alloys Compd. 2020, 821, 153431. [Google Scholar] [CrossRef]
- Takagi, K. High tough boride base cermets produced by reaction sintering. Mater. Chem. Phys. 2001, 67, 214–219. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.G.; Zhong, J.; Xiang, Y. Feasibility of preparing Mo2FeB2-based cermet coating by electrospark deposition on high speed steel. Surf. Coat. Technol. 2016, 296, 58–64. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, J.S.; Li, C.N.; Geng, H.; Wang, L. Microstructure and mechanical properties of molybdenum-iron-boron-chromium cladding using argon arc welding. Mater. Sci. Technol. Ser. 2016, 32, 1694–1701. [Google Scholar] [CrossRef]
- Ivanov, M.B.; Vershinina, T.N.; Ivanisenko, V. The effect of composition and microstructure on hardness and toughness of Mo2FeB2 based cermets. Mater. Sci. Eng. A 2019, 763, 138117. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Zhang, J.J.; Zhang, J. Preparation of Mo2FeB2-based cermets with a core/rim structure by multi-step sintering approach. Ceram. Int. 2019, 45, 22371–22375. [Google Scholar] [CrossRef]
- Yu, H.Z.; Liu, W.J.; Feng, P.; Zheng, Y. Synthesis and microstructure evolution during vacuum sintering of Mo2FeB2 based cermets. Int. J. Refract. Met. Hard Mater. 2014, 45, 48–52. [Google Scholar]
- Zhang, J.J.; Zheng, Y.; Zhou, W.; Zhang, G.; Ke, Z.; Dong, Z.; Lv, X. The influence of Mo/B atomic ratio on microstructural evolution and mechanical properties of Mo2FeB2-based cermets. Int. J. Mater. Res. 2019, 110, 664–671. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zheng, Y.; Zhou, W.; Zhang, G.; Ke, Z.; Dong, Z.; Feng, P. Effects of Cr content on the microstructure and mechanical properties of Mo2FeB2-based cermets prepared via vacuum sintering. Vacuum 2018, 155, 509–513. [Google Scholar] [CrossRef]
- Yu, H.Z.; Zheng, Y.; Liu, W.J.; Zheng, Z.; Xiong, W. Effect of V content on the microstructure and mechanical properties of Mo2FeB2 based cermets. Mater. Des. 2010, 31, 2680–2683. [Google Scholar] [CrossRef]
- Hao, W.; Yong, Z.; Zhang, J.; Zhang, G. Influence of multi-step sintering on microstructural evolution and interfacial characteristics of Mo2FeB2-based cermets. J. Am. Ceram. Soc. 2020, 103, 6040–6049. [Google Scholar]
- Jin, J.; Sun, J.S.; Wang, W.M.; Song, J.; Xu, H. Effect of rare earth on microstructure and wear resistance of In-Situ-Synthesized Mo2FeB2 ceramics-reinforced Fe-based cladding. Materials 2020, 13, 3633. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.H.; Peng, J.J.; Tao, Z.X.; Zhang, Z. Effect of TiC content on the microstructure and mechanical properties of Mo2FeB2 based cermets. Powder Metall. Technol. 2017, 27, 49–52. [Google Scholar]
- Li, W.H.; Ai, T.T.; Feng, X.M. Microstructure and properties of TiC-Mo2FeB2 composite cladding. Trans. Mater. Heat Treat. 2012, 33, 124–128. [Google Scholar]
- Wang, Y.G.; Li, Z.Q. Development of ternary-boride-based hard cladding material. Mater. Res. Bull. 2002, 37, 417–423. [Google Scholar]
- Takagi, K.; Koike, W.; Momozawa, A.; Fujima, T. Effects of Cr on the properties of Mo2NiB2 ternary boride. Solid. State Sci. 2012, 14, 1643–1647. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, F.T. Sintering thermodynamic analysis of Mo2FeB2 cermet prepared by in-situ reaction. Powder Metall. Technol. 2010, 28, 192–195. [Google Scholar]
- Lu, W.J.; Zhang, D.; Zhang, X.N.; Guo, S.L.; Wu, R.J. Growth mechanism of reinforcements in in-situ synthesized (TiB+TiC)/Ti composites. Trans. Nonferr. Metal. Soc. 2001, 11, 67–71. [Google Scholar]
- Takagi, K.; Ozaki, S.; Komai, M.; Matsuo, S.; Mizutani, N. Ceramic, powders, corrosion and advanced processing. Trans. Mater. Res. Soc. Jpn. 1994, 14, 475–478. [Google Scholar]
- Liang, Y.J.; Che, Y.C. Inorganic Thermodynamics Data Manual; Northeast University Press: Shenyang, China, 1993. [Google Scholar]
- Hao, S.M. Material Thermodynamic; Chemical Industry Press: Beijing, China, 2003. [Google Scholar]
- Wang, Y.K. Application of HSC chemistry software in university chemical scientific research. J. Henan Educ. Coll. 2013, 22, 28–30. [Google Scholar]
- Ide, T.; Ando, T. Reaction sintering of a Fe-6 wt% B-48wt%-Mo alloy in the presence of liquid phases. Metall. Mater. Trans. A 1989, 20, 17–24. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, F.T.; Feng, X.M. Effect of sintering temperature on the structure and properties of ternary boride hard alloys. Powder Metall. Technol. 2009, 27, 48–51. [Google Scholar]
- Sheng, N.C.; Hu, X.B.; Liu, J.D.; Jin, T. M3B2 and M5B3 formation in diffusion affected zone during transient liquid phase bonding single crystal superalloys. Metall. Mater. Trans. A 2015, 46, 1670–1677. [Google Scholar] [CrossRef]
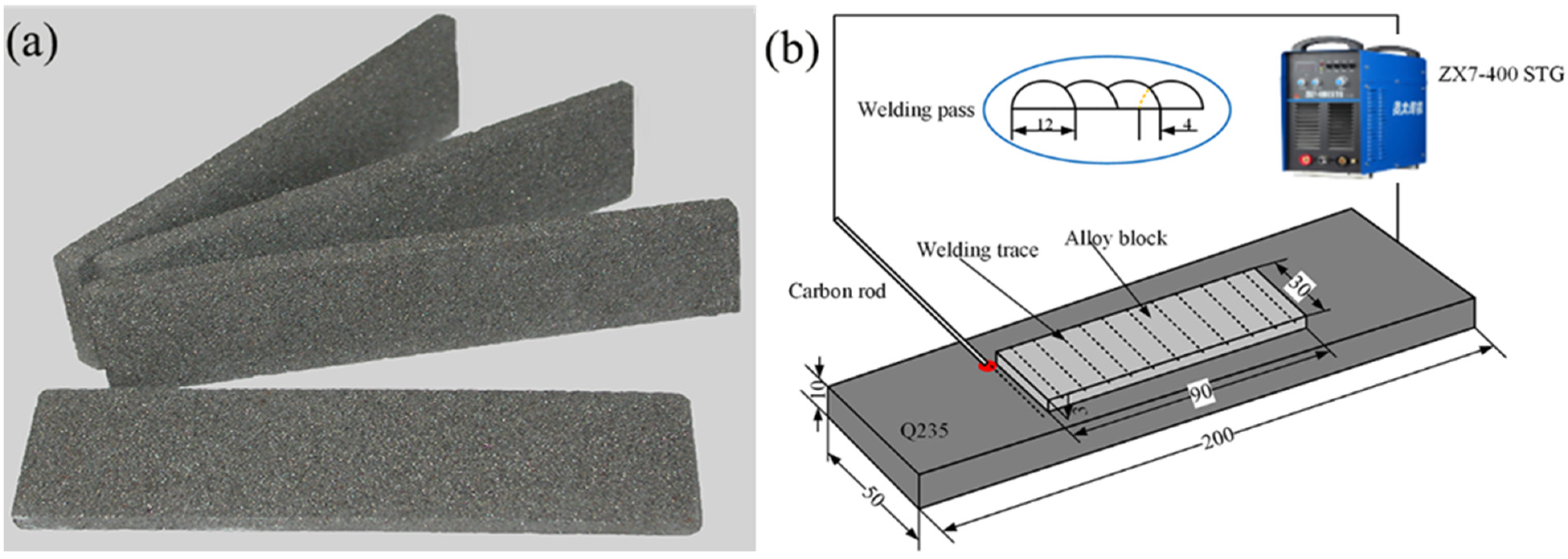
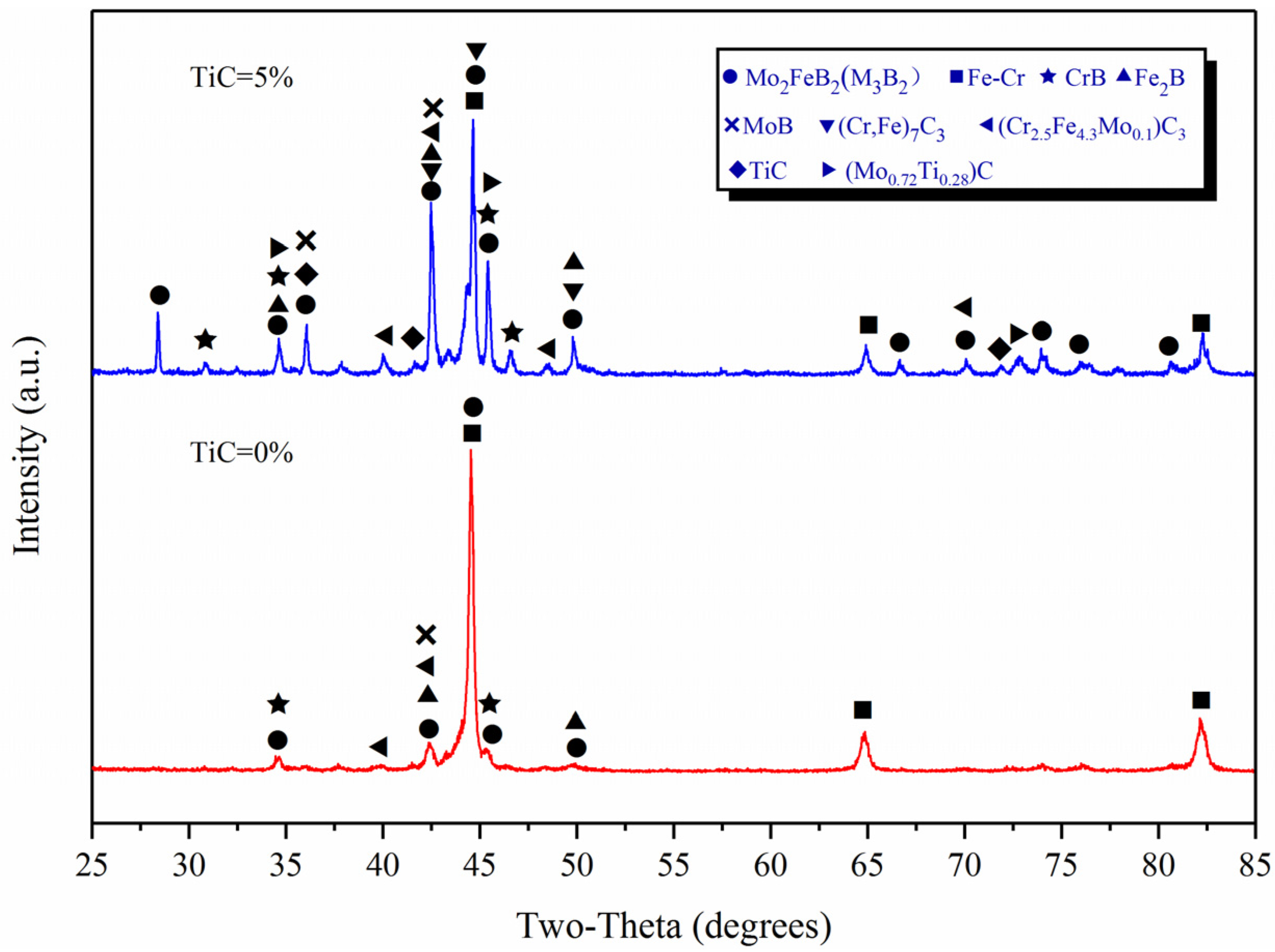

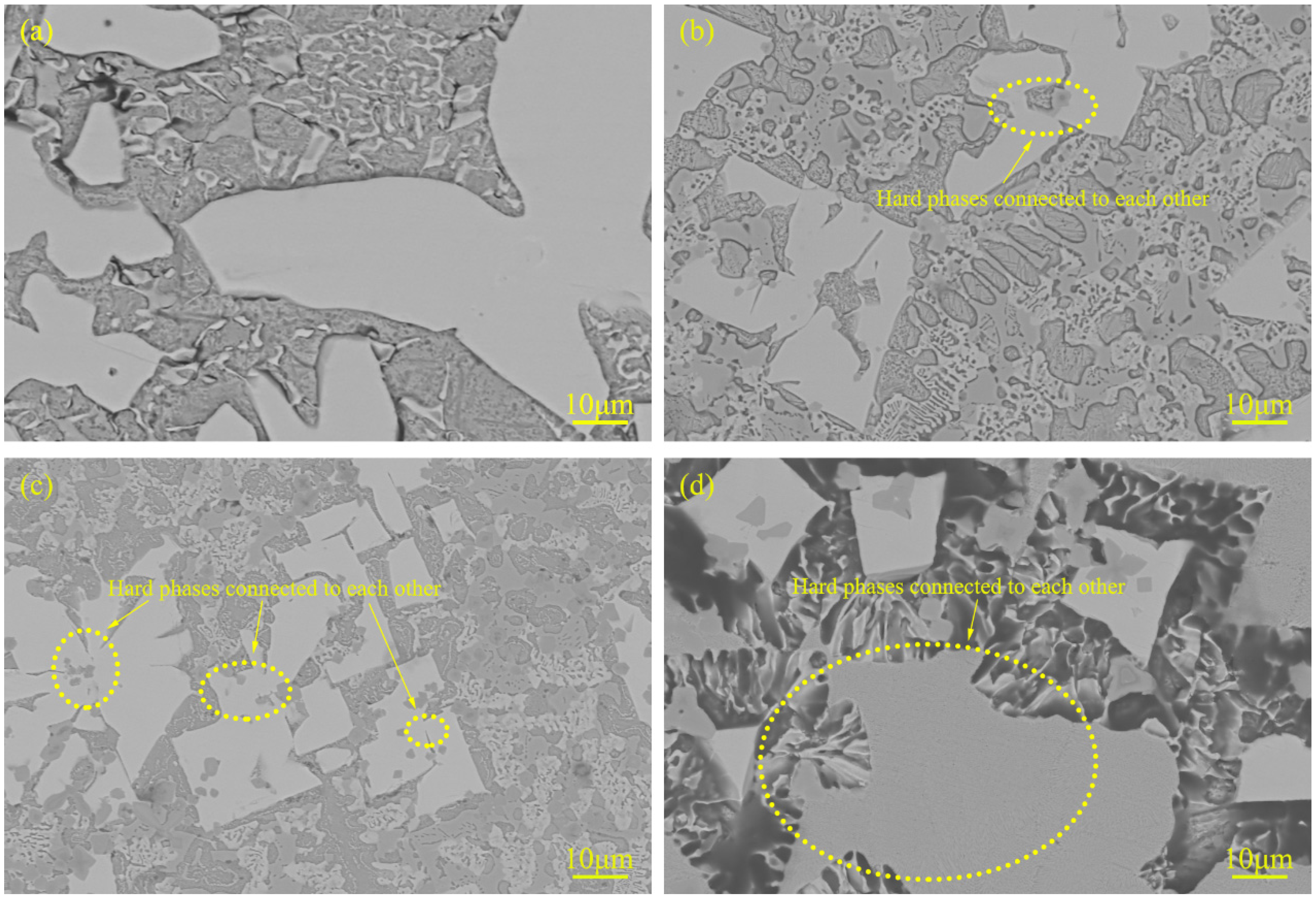
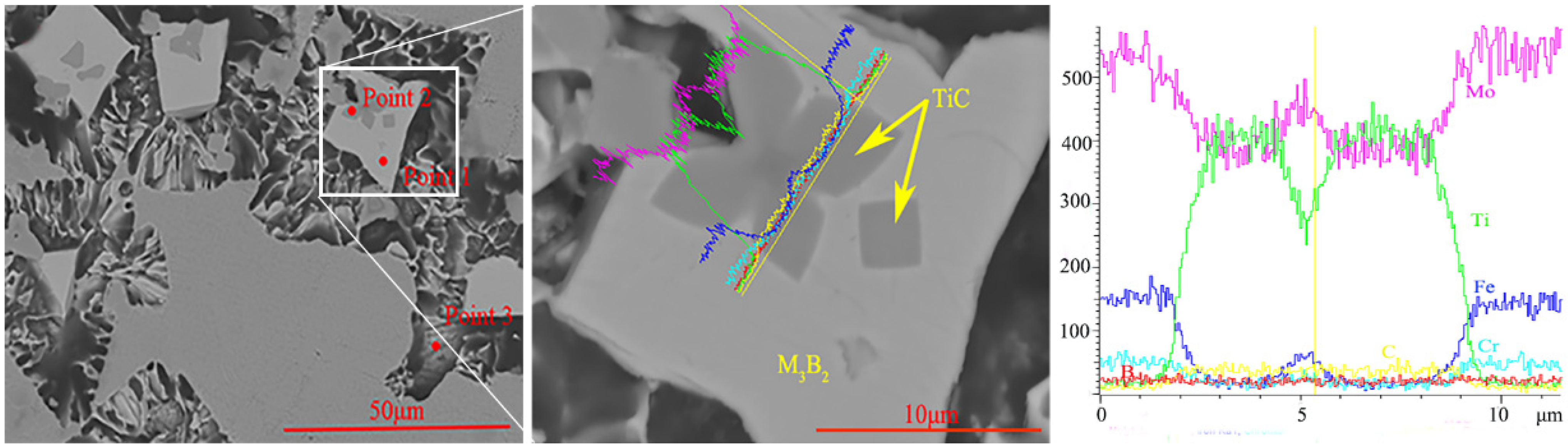
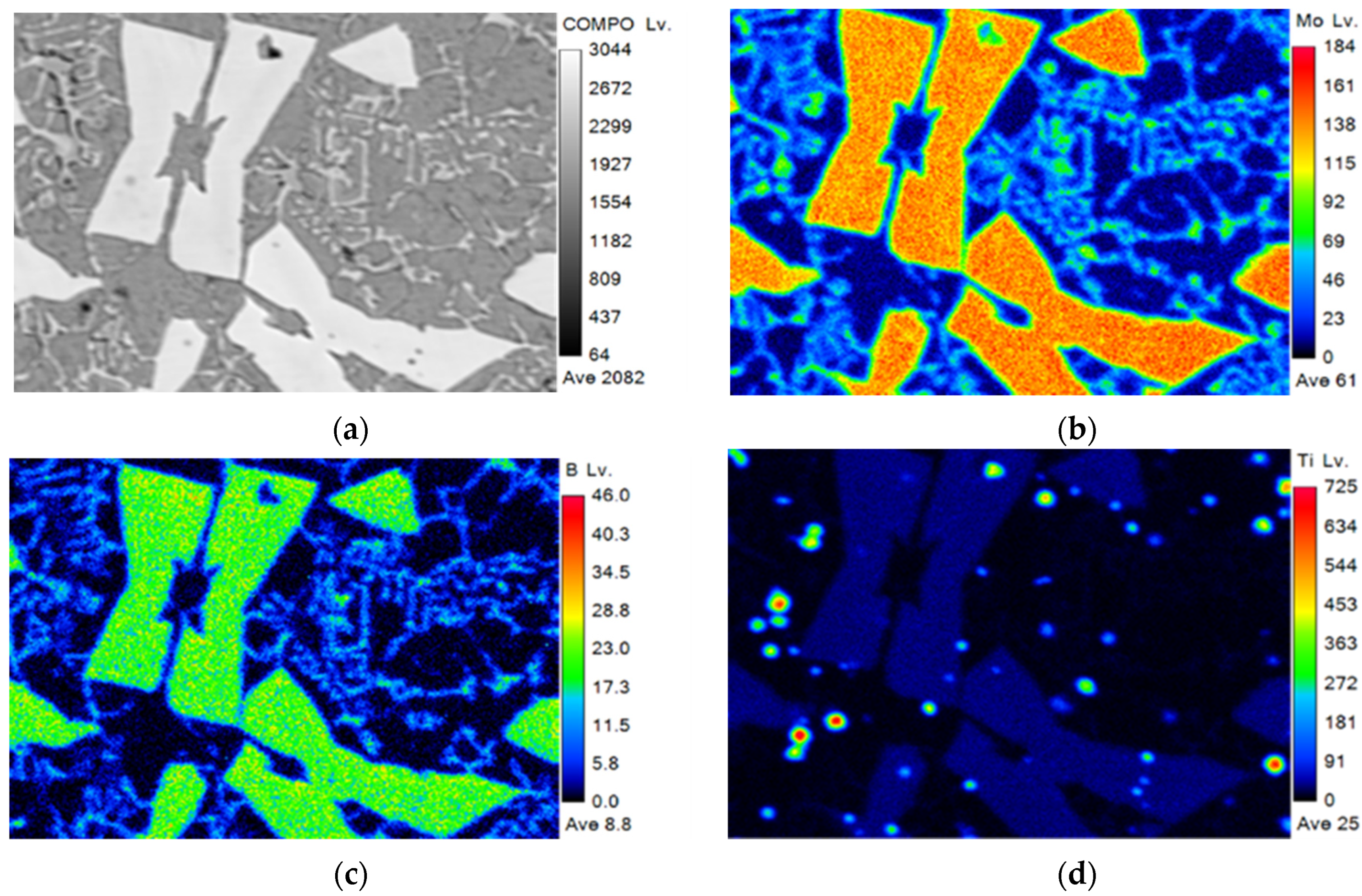


| Powder | Particle Size (μm) | Chemical Composition (wt.%) |
|---|---|---|
| Molybdenum (Mo) | 75–120 | Fe < 0.002,O < 0.1,Si < 0.001 |
| Iron (Fe) | 75–100 | C < 0.1, N < 0.1, O < 0.2 |
| Chromium (Cr) | 90–150 | O < 0.2, Fe < 0.18, N < 0.045 |
| Ferroboron (FeB) | 53–75 | C < 0.27, Si < 0.71 |
| Titanium carbide (TiC) | 2–4 | O < 0.06, S < 0.001 |
| Position | Element Mass Percentage (wt%) | |||||
|---|---|---|---|---|---|---|
| Mo | Fe | B | C | Cr | Ti | |
| Point 1 | 50.14 | 17.18 | 18.01 | 11.11 | 3.37 | 0.19 |
| Point 2 | 10.02 | 3.97 | 8.44 | 20.81 | 2.56 | 54.20 |
| Point 3 | 3.42 | 60.2 | 6.92 | 27.45 | 1.98 | 0.03 |
| Number | Chemical Reaction | Number | Chemical Reaction |
|---|---|---|---|
| 1 | [Mo] + [B] = MoB | 9 | 7[Cr] + 3[C] = Cr7C3 |
| 2 | [Mo] + 2[B] = MoB2 | 10 | [Ti] + [C] = TiC |
| 3 | 2[Mo] + [B] = Mo2B | 11 | [Ti] + [Fe] = FeTi |
| 4 | [Fe] + [B] = FeB | 12 | [Ti] + 2[Fe] = Fe2Ti |
| 5 | 2[Fe] + [B] = Fe2B | 13 | [Fe] + 2MoB = Mo2FeB2 |
| 6 | [Fe] + FeB = Fe2B | 14 | 2[Mo] + 2FeB = Mo2FeB2 + [Fe] |
| 7 | [Cr] + [B] = CrB | 15 | 2[Mo] + 2Fe2B = Mo2FeB2 + 3[Fe] |
| 8 | [Cr] + 2[B] = CrB2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Sun, J.; Jin, J.; Xu, H. Effect of TiC on Microstructure and Properties of Wear-Resistant Mo2FeB2 Claddings. Materials 2022, 15, 4441. https://doi.org/10.3390/ma15134441
Sun Y, Sun J, Jin J, Xu H. Effect of TiC on Microstructure and Properties of Wear-Resistant Mo2FeB2 Claddings. Materials. 2022; 15(13):4441. https://doi.org/10.3390/ma15134441
Chicago/Turabian StyleSun, Yiqun, Junsheng Sun, Jun Jin, and Hu Xu. 2022. "Effect of TiC on Microstructure and Properties of Wear-Resistant Mo2FeB2 Claddings" Materials 15, no. 13: 4441. https://doi.org/10.3390/ma15134441
APA StyleSun, Y., Sun, J., Jin, J., & Xu, H. (2022). Effect of TiC on Microstructure and Properties of Wear-Resistant Mo2FeB2 Claddings. Materials, 15(13), 4441. https://doi.org/10.3390/ma15134441





