Size-Dependent Persistent Luminescence of YAGG:Cr3+ Nanophosphors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Characterization Techniques
3. Results and Discussion
3.1. Microstructure
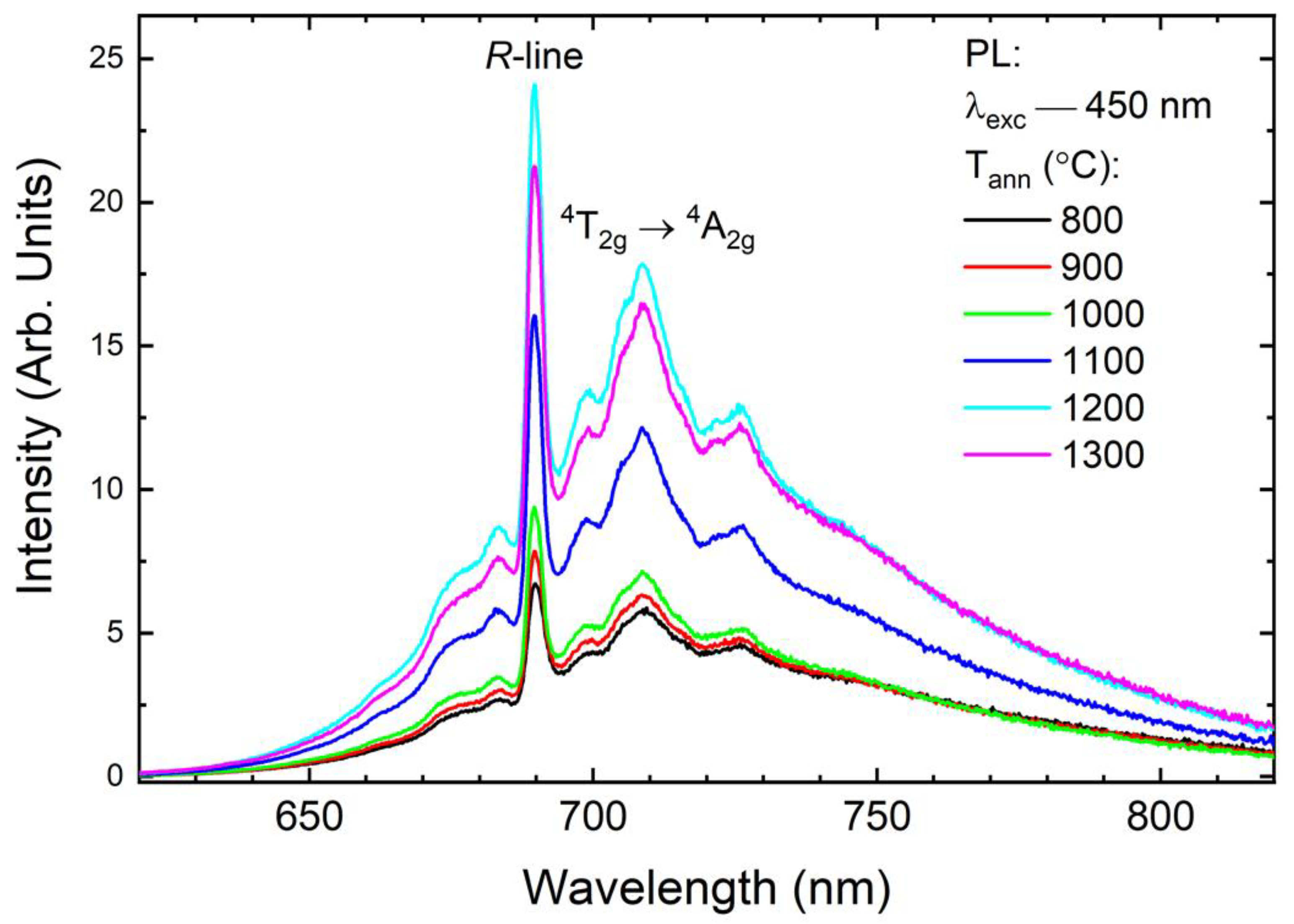
3.2. Photoluminescence
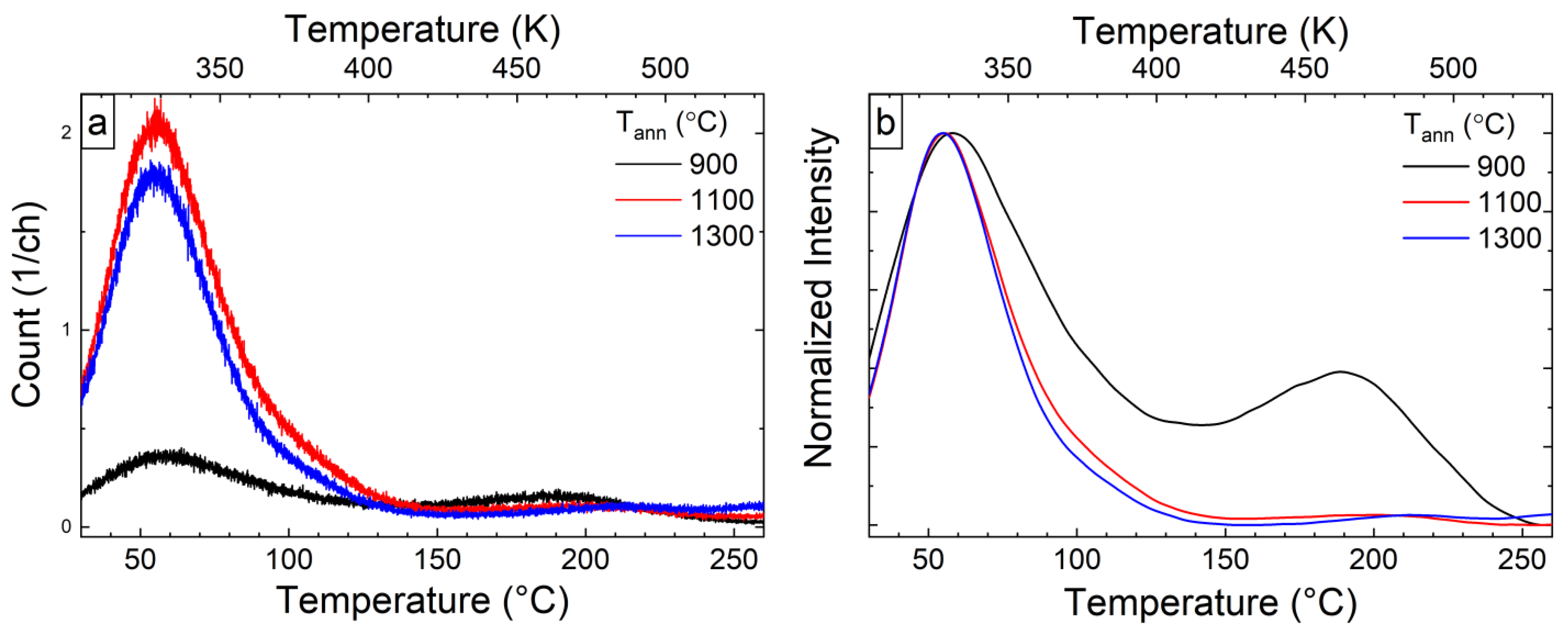
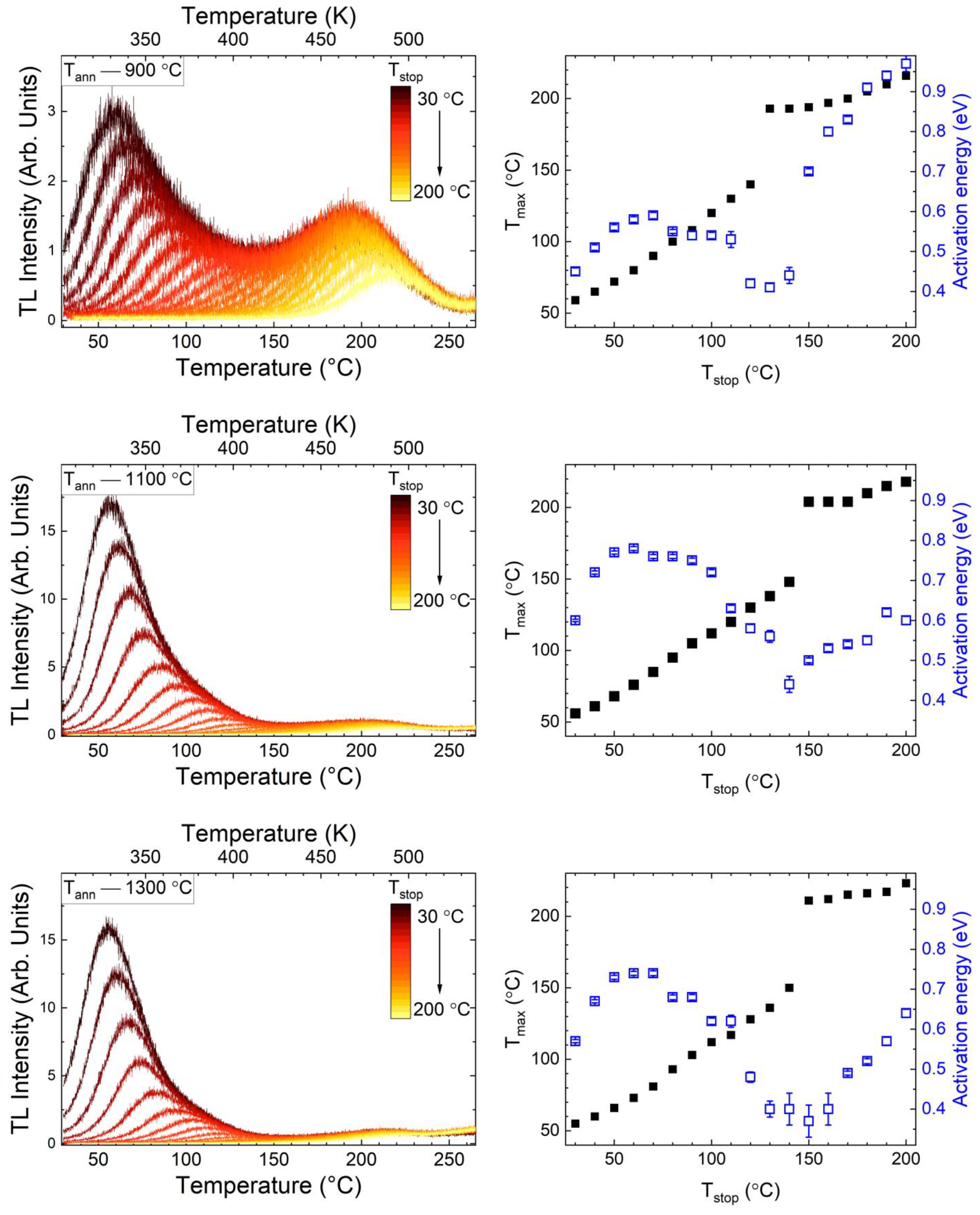
3.3. Thermoluminescence Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Tanabe, S. Persistent Luminescence Instead of Phosphorescence: History, Mechanism, and Perspective. J. Lumin. 2019, 205, 581–620. [Google Scholar] [CrossRef]
- Poelman, D.; Van der Heggen, D.; Du, J.; Cosaert, E.; Smet, P.F. Persistent Phosphors for the Future: Fit for the Right Application. J. Appl. Phys. 2020, 128, 240903. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D.; Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef] [Green Version]
- Bessière, A.; Sharma, S.K.; Basavaraju, N.; Priolkar, K.R.; Binet, L.; Viana, B.; Bos, A.J.J.; Maldiney, T.; Richard, C.; Scherman, D.; et al. Storage of Visible Light for Long-Lasting Phosphorescence in Chromium-Doped Zinc Gallate. Chem. Mater. 2014, 26, 1365–1373. [Google Scholar] [CrossRef]
- Hölsä, J. Persistent Luminescence Beats the Afterglow: 400 Years of Persistent Luminescence. Electrochem. Soc. Interface 2009, 18, 42–45. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, S.; Ahmed, M.H.; Khan, M.I.; Haque, N.; Raihan, G.A. A Review on Optical Applications, Prospects, and Challenges of Rare-Earth Oxides. ACS Appl. Electron. Mater. 2021, 3, 3715–3746. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ahmed, M.H.; Khan, M.I.; Miah, M.S.; Hossain, S. Recent Progress of Rare Earth Oxides for Sensor, Detector, and Electronic Device Applications: A Review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Castaing, V.; Arroyo, E.; Becerro, A.I.; Ocaña, M.; Lozano, G.; Míguez, H. Persistent Luminescent Nanoparticles: Challenges and Opportunities for a Shimmering Future. J. Appl. Phys. 2021, 130, 080902. [Google Scholar] [CrossRef]
- Xu, J.; Ueda, J.; Tanabe, S. Toward Tunable and Bright Deep-Red Persistent Luminescence of Cr3+ in Garnets. J. Am. Ceram. Soc. 2017, 100, 4033–4044. [Google Scholar] [CrossRef]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.Y.; Gupta, R.; et al. Photoluminescent Nanoparticles for Chemical and Biological Analysis and Imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef]
- Xu, J.; Ueda, J.; Zhuang, Y.; Viana, B.; Tanabe, S. Y3Al5−x GaxO12:Cr3+: A Novel Red Persistent Phosphor with High Brightness. Appl. Phys. Express 2015, 8, 042602. [Google Scholar] [CrossRef]
- Katayama, Y.; Viana, B.; Gourier, D.; Xu, J.; Tanabe, S. Photostimulation Induced Persistent Luminescence in Y3Al2Ga3O12:Cr3+. Opt. Mater. Express 2016, 6, 1405. [Google Scholar] [CrossRef]
- Ueda, J.; Xu, J.; Takemura, S.; Nakanishi, T.; Miyano, S.; Segawa, H.; Tanabe, S. How Many Electron Traps Are Formed in Persistent Phosphors? ECS J. Solid State Sci. Technol. 2021, 10, 116003. [Google Scholar] [CrossRef]
- Fedyk, R.; Hreniak, D.; Łojkowski, W.; Strek, W.; Matysiak, H.; Grzanka, E.; Gierlotka, S.; Mazur, P. Method of Preparation and Structural Properties of Transparent YAG Nanoceramics. Opt. Mater. 2007, 29, 1252–1257. [Google Scholar] [CrossRef]
- Dai, Z.; Boiko, V.; Grzeszkiewicz, K.; Saladino, M.L.; Li, J.; Hreniak, D. Effect of Annealing Treatment on the Persistent Luminescence of Y3Al2Ga3O12:Ce3+,Cr3+,Pr3+ Ceramics. Opt. Mater. 2020, 105, 109888. [Google Scholar] [CrossRef]
- Xu, J.; Murata, D.; Ueda, J.; Viana, B.; Tanabe, S. Toward Rechargeable Persistent Luminescence for the First and Third Biological Windows via Persistent Energy Transfer and Electron Trap Redistribution. Inorg. Chem. 2018, 57, 5194–5203. [Google Scholar] [CrossRef] [PubMed]
- Boiko, V.; Dai, Z.; Markowska, M.; Leonelli, C.; Mortalò, C.; Armetta, F.; Ursi, F.; Nasillo, G.; Saladino, M.L.; Hreniak, D. Particle Size-Related Limitations of Persistent Phosphors Based on the Doped Y3Al2Ga3O12 System. Sci. Rep. 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Chen, Y.; Sun, C.; Fan, Y.; Yang, Y.; Liu, X.; Lu, L.; Zhao, M.; Zhang, H.; Zhao, D.; et al. X-ray-Activated Persistent Luminescence Nanomaterials for NIR-II Imaging. Nat. Nanotechnol. 2021, 16, 1011–1018. [Google Scholar] [CrossRef]
- Boiko, V.; Zeler, J.; Markowska, M.; Dai, Z.; Gerus, A.; Bolek, P.; Zych, E.; Hreniak, D. Persistent Luminescence from Y3Al2Ga3O12 Doped with Ce3+ and Cr3+ after X-ray and Blue Light Irradiation. J. Rare Earths 2019, 37, 1200–1205. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; International Union of Crystallograhy; Oxford University Press: Oxford, UK, 1995; ISBN 9780198559122. [Google Scholar]
- McKeever, S.W.S. Thermoluminescence of Solids; Cambridge University Press: Cambridge, UK, 1985; ISBN 9780511564994. [Google Scholar]
- Chaika, M.; Paszkowicz, W.; Strek, W.; Hreniak, D.; Tomala, R.; Safronova, N.; Doroshenko, A.; Parkhomenko, S.; Dluzewski, P.; Kozłowski, M.; et al. Influence of Cr Doping on the Phase Composition of Cr,Ca:YAG Ceramics by Solid State Reaction Sintering. J. Am. Ceram. Soc. 2019, 102, 2104–2115. [Google Scholar] [CrossRef]
- Ayyub, P.; Palkar, V.R.; Chattopadhyay, S.; Multani, M. Effect of Crystal Size Reduction on Lattice Symmetry and Cooperative Properties. Phys. Rev. B 1995, 51, 6135. [Google Scholar] [CrossRef] [PubMed]
- Chaika, M.A.; Dulina, N.A.; Doroshenko, A.G.; Parkhomenko, S.V.; Gayduk, O.V.; Tomala, R.; Strek, W.; Hreniak, D.; Mancardi, G.; Vovk, O.M. Influence of Calcium Concentration on Formation of Tetravalent Chromium Doped Y3Al5O12 Ceramics. Ceram. Int. 2018, 44, 13513–13519. [Google Scholar] [CrossRef]
- Dereń, P.J.; Malinowski, M.; Strȩk, W. Site Selection Spectroscopy of Cr3+ in MgAl2O4 Green Spinel. J. Lumin. 1996, 68, 91–103. [Google Scholar] [CrossRef]
- Chaika, M.A.; Tomala, R.; Strek, W.; Hreniak, D.; Dluzewski, P.; Morawiec, K.; Mateychenko, P.V.; Fedorov, A.G.; Doroshenko, A.G.; Parkhomenko, S.V.; et al. Kinetics of Cr3+ to Cr4+ Ion Valence Transformations and Intra-Lattice Cation Exchange of Cr4+ in Cr,Ca:YAG Ceramics Used as Laser Gain and Passive Q-Switching Media. J. Chem. Phys. 2019, 151, 134708. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Yoshiasa, A.; Yamanaka, T. Cation Distribution and Crystal Chemistry of Y3Al5−XGaxO12 (0 ≤ x ≤ 5) Garnet Solid Solutions. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 266–272. [Google Scholar] [CrossRef]
- Marezio, M.; Remeika, J.P.; Dernier, P.D. IUCr Cation Distribution in Y3Al5−cGacO12 Garnet. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1968, 24, 1670–1674. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Adachi, S. Review—Photoluminescence Properties of Cr3+-Activated Oxide Phosphors. ECS J. Solid State Sci. Technol. 2021, 10, 026001. [Google Scholar] [CrossRef]
- Shen, Y.; Bray, K. Effect of Pressure and Temperature on the Lifetime of in Yttrium Aluminum Garnet. Phys. Rev. B—Condens. Matter Mater. Phys. 1997, 56, 10882–10891. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the Absorption Spectra of Complex Ions II. J. Phys. Soc. Japan 1954, 9, 766–779. [Google Scholar] [CrossRef]
- Jørgensen, C.K. The Nephelauxetic Series. In Progress in Inorganic Chemistry; Cotton, F.A., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 1962; pp. 73–124. [Google Scholar]
- Głuchowski, P.; Małecka, M.; Stręk, W.; Ryba-Romanowski, W.; Solarz, P. Size Effect in Novel Red Efficient Garnet Nanophosphor. J. Phys. Chem. C 2017, 121, 25561–25567. [Google Scholar] [CrossRef]
- Dai, Z.; Boiko, V.; Grzeszkiewicz, K.; Markowska, M.; Ursi, F.; Hölsä, J.; Saladino, M.L.; Hreniak, D. Effect of Annealing Temperature on Persistent Luminescence of Y3Al2Ga3O12:Cr3+ Co-Doped with Ce3+ and Pr3+. Opt. Mater. 2020, 111, 110522. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Bos, A.J.J.; Poelman, D.; Smet, P.F. Revealing Trap Depth Distributions in Persistent Phosphors. Phys. Rev. B 2013, 87, 045126. [Google Scholar] [CrossRef] [Green Version]
- Bos, A.J.J. Theory of Thermoluminescence. Radiat. Meas. 2007, 41, S45–S56. [Google Scholar] [CrossRef]
- McKeever, S.W.S. On the Analysis of Complex Thermoluminescence. Glow-Curves: Resolution into Individual Peaks. Phys. Status Solidi 1980, 62, 331–340. [Google Scholar] [CrossRef]
- Sójka, M.; Zeler, J.; Zych, E. Effect of Ge:Si Ratio and Charging Energy on Carriers Trapping in Y2(Ge,Si)O5:Pr Powders Observed with Thermoluminescence Methods. J. Alloys Compd. 2021, 858, 157676. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, H.; Hai, O.; Dong, Y.; Liu, S.; Gao, S. Effect of Oxygen Vacancies on the Persistent Luminescence of Y3Al2Ga3O12:Ce3+,Yb3+Phosphors. Inorg. Chem. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Magdalene Mashangva, M.; Nara Singh, T.B.S. Estimation of Optimal Trapping Parameters Relevant to Persistent Luminescence. Indian J. Pure Appl. Phys. 2011, 49, 583–589. [Google Scholar]
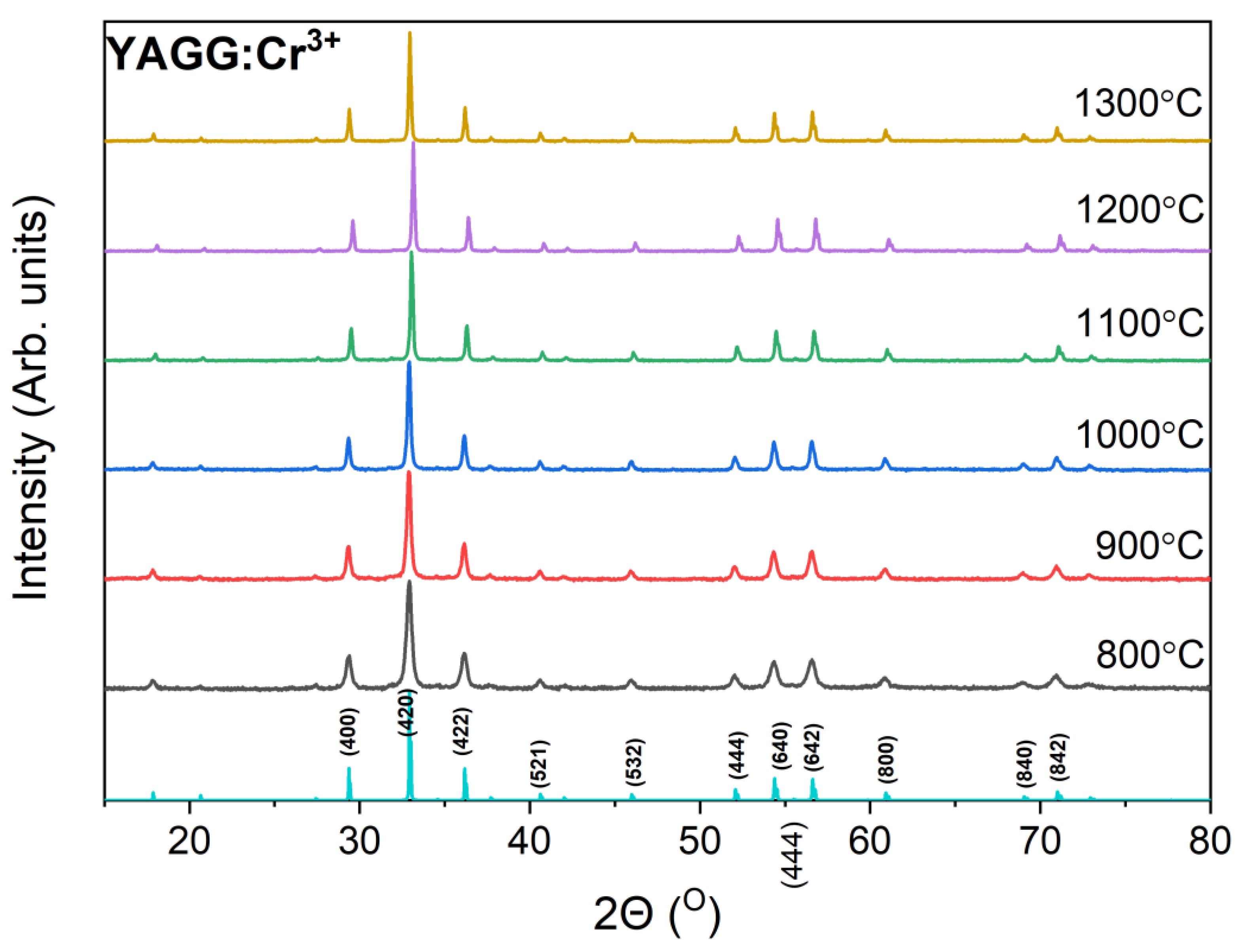
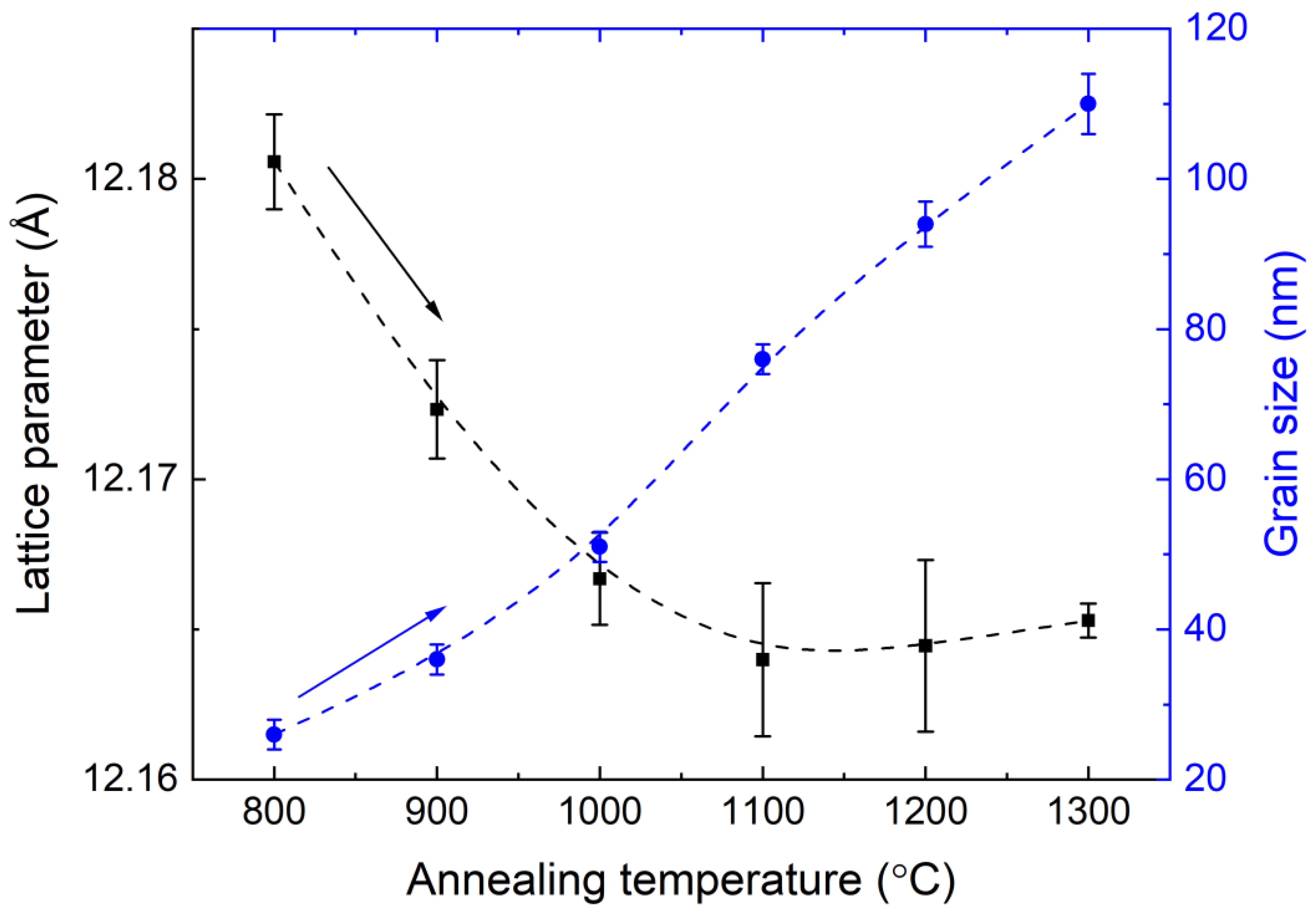
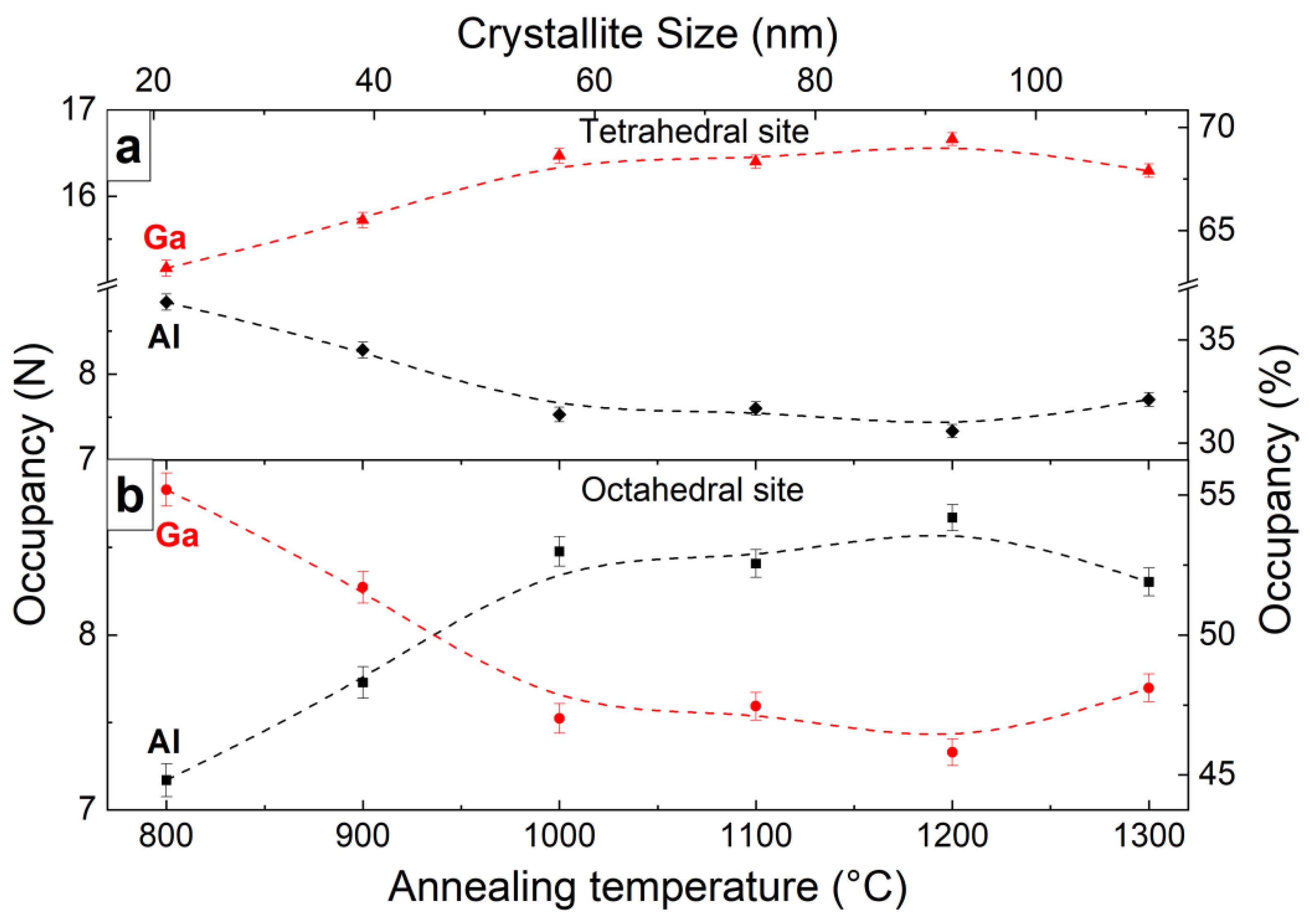
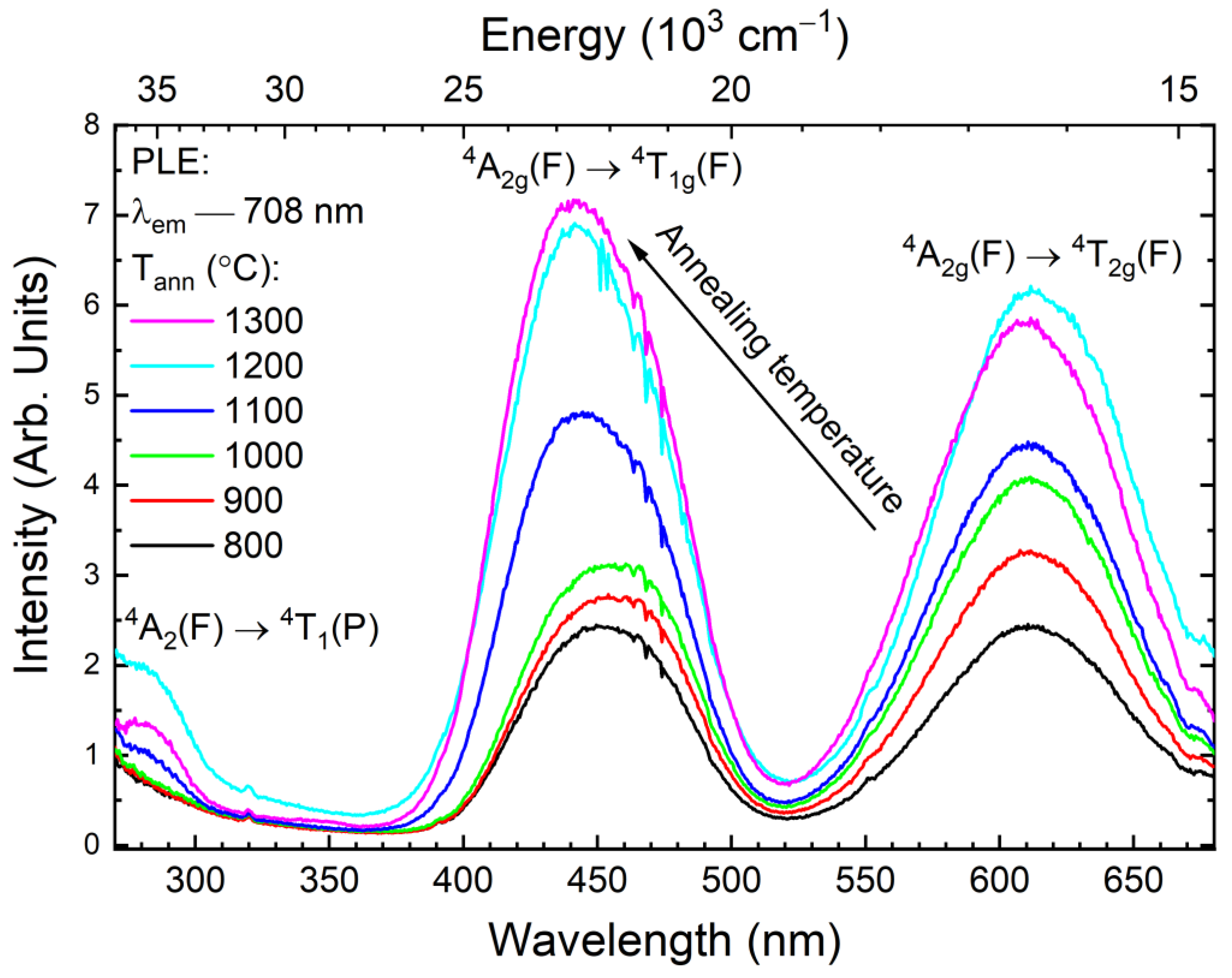
| Annealing Temperature, °C | a, Å | DXRPD, nm | ε | N(A) | N(D) | fGa |
|---|---|---|---|---|---|---|
| 800 | 12.1806(16) | 26(2) | 0.00223(10) | 8.83(9) | 15.16(9) | 0.632(7) |
| 900 | 12.1723(16) | 36(2) | 0.00159(9) | 8.27(9) | 15.72(9) | 0.655(7) |
| 1000 | 12.1667(15) | 51(2) | 0.00113(8) | 7.52(9) | 16.47(9) | 0.687(8) |
| 1100 | 12.1640(26) | 76(2) | 0.00077(6) | 7.59(8) | 16.40(8) | 0.684(7) |
| 1200 | 12.1645(29) | 94(2) | 0.00061(7) | 7.33(8) | 16.66(8) | 0.695(7) |
| 1300 | 12.1653(6) | 110(2) | 0.00053(4) | 7.70(8) | 16.30(8) | 0.679(7) |
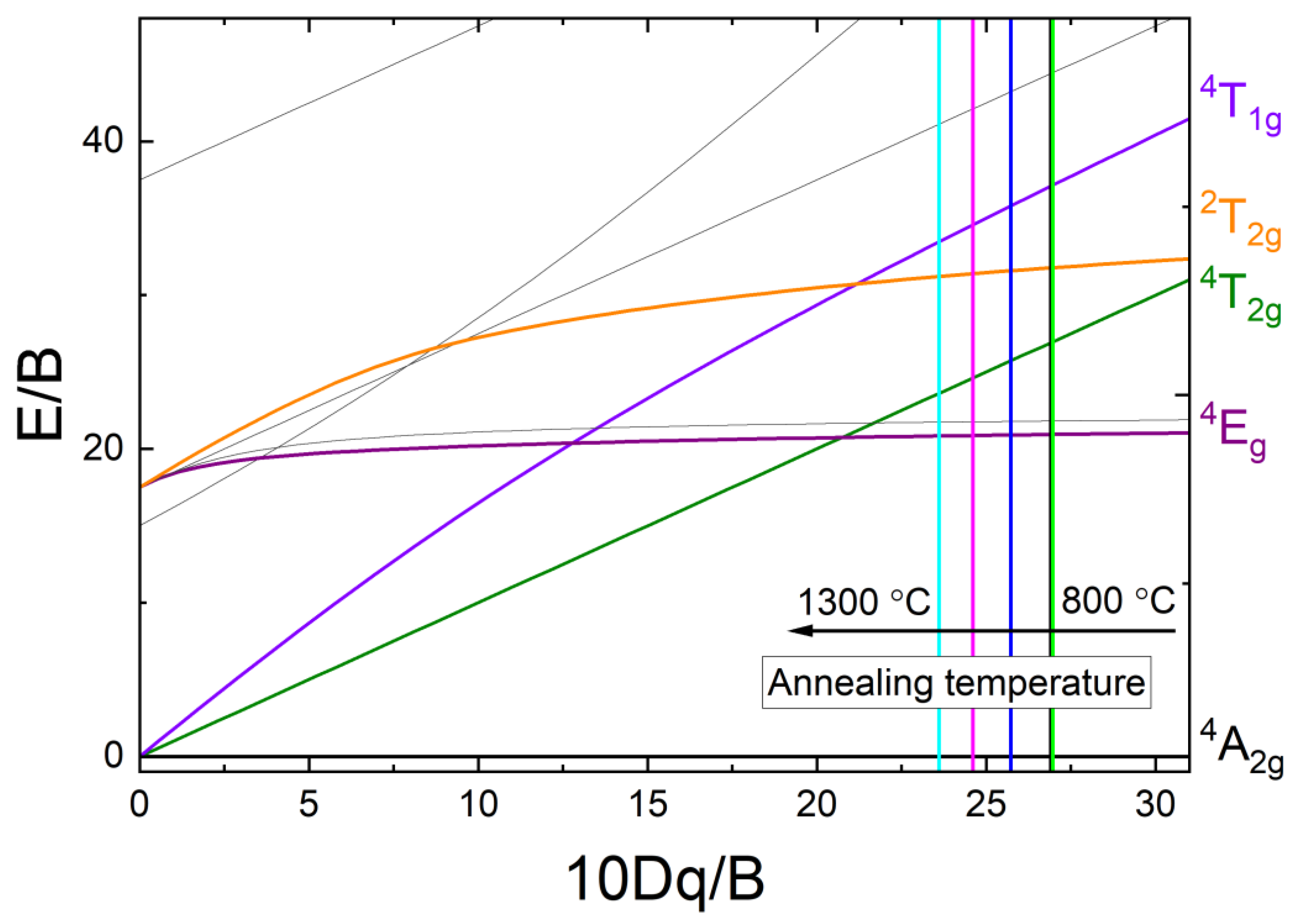
| Annealing Temperature, °C | υ2/υ1 Ratio | Dq/B | B2, cm−1 | β = B2/B0 | Crystallite Size DXRPD, nm |
|---|---|---|---|---|---|
| 800 | 1.378 | 27.203 | 828.889 | 0.805 | 26(2) |
| 900 | 1.378 | 27.203 | 828.889 | 0.805 | 36(2) |
| 1000 | 1.378 | 27.203 | 828.889 | 0.805 | 51(2) |
| 1100 | 1.391 | 26.045 | 874.601 | 0.849 | 76(2) |
| 1200 | 1.417 | 23.730 | 980.021 | 0.951 | 94(2) |
| 1300 | 1.404 | 24.887 | 925.828 | 0.899 | 110(2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiko, V.; Dai, Z.; Chaika, M.; Grzeszkiewicz, K.; Li, J.; Strek, W.; Hreniak, D. Size-Dependent Persistent Luminescence of YAGG:Cr3+ Nanophosphors. Materials 2022, 15, 4407. https://doi.org/10.3390/ma15134407
Boiko V, Dai Z, Chaika M, Grzeszkiewicz K, Li J, Strek W, Hreniak D. Size-Dependent Persistent Luminescence of YAGG:Cr3+ Nanophosphors. Materials. 2022; 15(13):4407. https://doi.org/10.3390/ma15134407
Chicago/Turabian StyleBoiko, Vitalii, Zhengfa Dai, Mykhailo Chaika, Karina Grzeszkiewicz, Jiang Li, Wieslaw Strek, and Dariusz Hreniak. 2022. "Size-Dependent Persistent Luminescence of YAGG:Cr3+ Nanophosphors" Materials 15, no. 13: 4407. https://doi.org/10.3390/ma15134407
APA StyleBoiko, V., Dai, Z., Chaika, M., Grzeszkiewicz, K., Li, J., Strek, W., & Hreniak, D. (2022). Size-Dependent Persistent Luminescence of YAGG:Cr3+ Nanophosphors. Materials, 15(13), 4407. https://doi.org/10.3390/ma15134407








