Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Ginger Root Extract
2.2. Preparation of Elettaria cardamom Seed Extract
2.3. Preparation of Final Solution
2.4. Characterization
2.5. Photocatalytic Activity
3. Results and Discussion
3.1. Scanning Electron Microscopy
3.2. Energy-Dispersive X-ray Spectroscopy
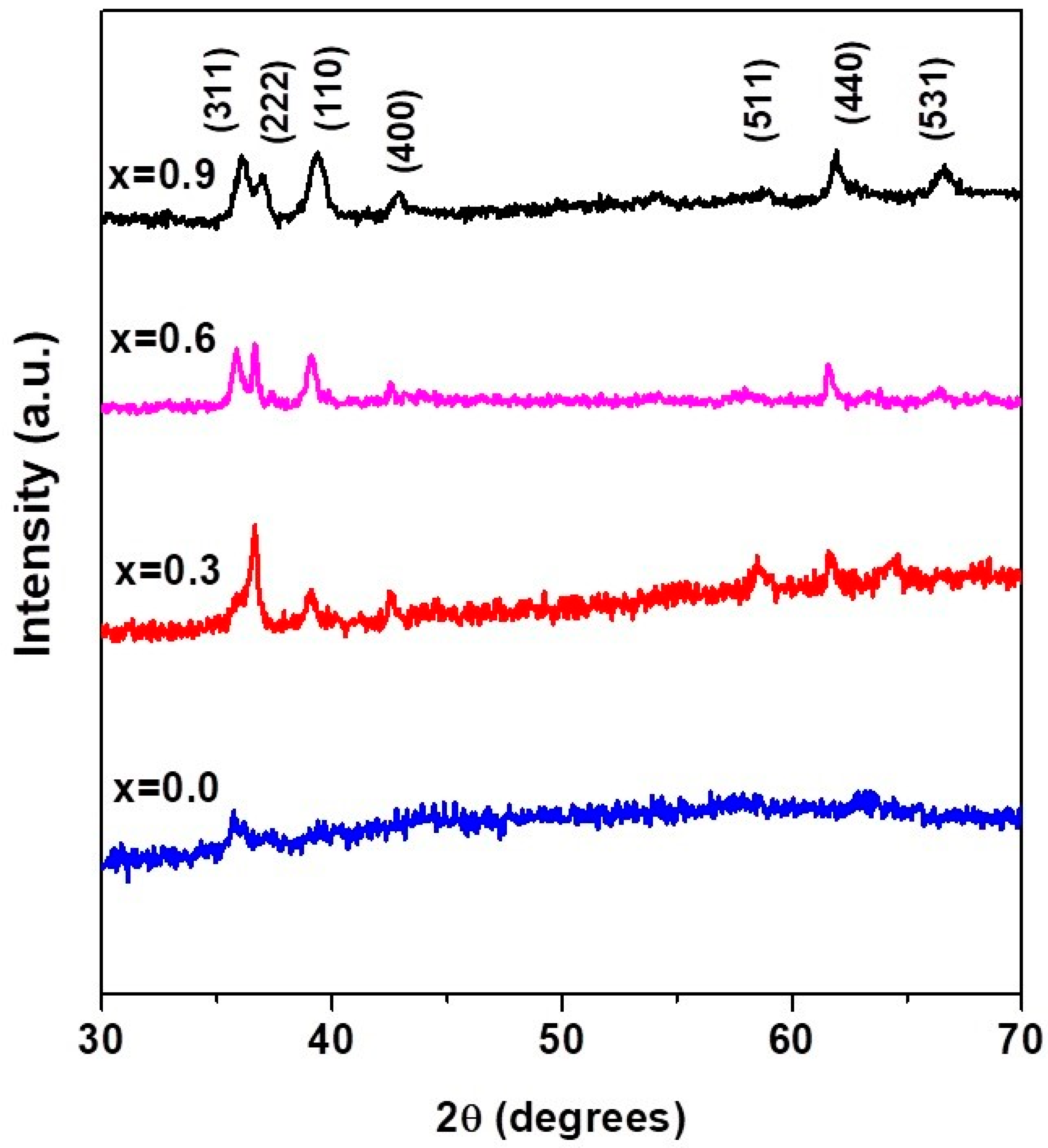
3.3. X-ray Diffraction Results
3.4. UV–Visible Spectroscopy Results
3.5. Photoluminescence Results
3.6. FTIR Spectroscopy
3.7. Photocatalytic Activity
4. Discussion
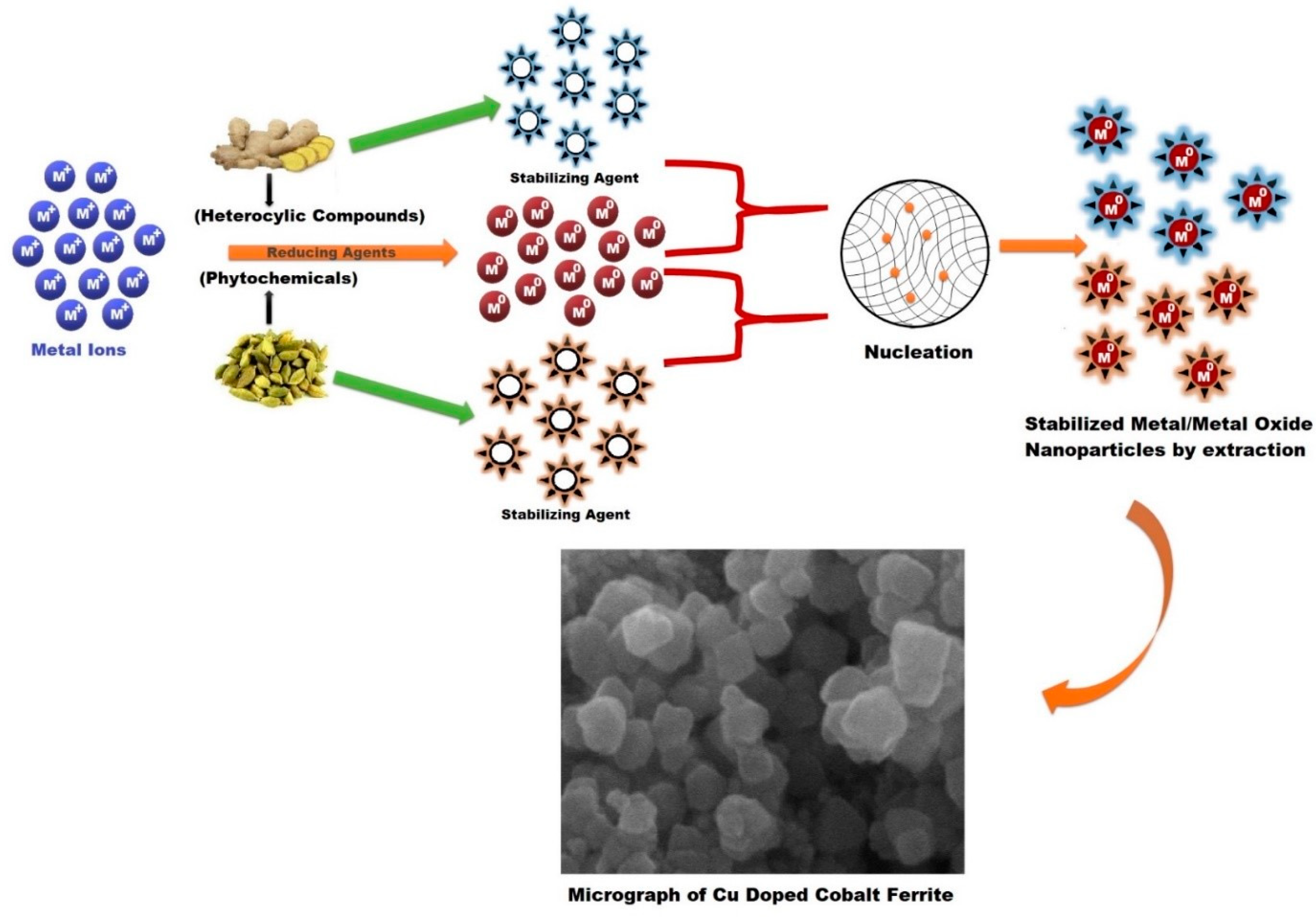
4.1. Mechanism for the Formation of Cobalt Ferrite Nanoparticles
4.2. Cu Concentration Effect on Lattice Parameter of Cobalt Ferrite Nanoparticles
4.3. Mechanism for Dyes’ Degradation
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Mehmood, T.; Shahzad Khan, B.; Mukhtar, A.; Chen, X.; Yi, P.; Tan, M. Mechanism for formation of fcc-cobalt nanowires in electrodeposition at ambient temperature. Mater. Lett. 2014, 130, 256–258. [Google Scholar] [CrossRef]
- Shahzad Khan, B.; Mehmood, T.; Mukhtar, A.; Tan, M. Effect of workfunction on the growth of electrodeposited Cu, Ni and Co nanowires. Mater. Lett. 2014, 137, 13–16. [Google Scholar] [CrossRef]
- Khan, B.S.; Mukhtar, A.; Mehmood, T.; Tan, M. Polarization curves of electrodepositing Ag and Cu nanowires. J. Nanosci. Nanotechnol. 2016, 16, 1–5. [Google Scholar] [CrossRef]
- Mukhtar, A.; Mehmood, T.; Khan, B.S.; Tan, M. Effect of Co2+ concentration on the crystal structure of electrodeposited Co nanowires. J. Cryst. Growth 2016, 441, 26–32. [Google Scholar] [CrossRef]
- Mehmood, T.; Khan, B.S.; Mukhtar, A.; Tan, M. Influence of bath temperature and pH on the structure of electrodeposited cobalt nanowires. Int. J. Mater. Res. 2015, 106, 957–961. [Google Scholar] [CrossRef]
- Mukhtar, A.; Khan, B.S.; Mehmood, T. Appropriate deposition parameters for formation of fcc Co–Ni alloy nanowires during electrochemical deposition process. Appl. Phys. A 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Köseoğlu, Y.; Alan, F.; Tan, M.; Yilgin, R.; Öztürk, M. Low temperature hydrothermal synthesis and characterization of Mn doped cobalt ferrite nanoparticles. Ceram. Int. 2012, 38, 3625–3634. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Paliychuk, N.; Yaremiy, I.; Moklyak, V. Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J. Alloys Compd. 2017, 694, 777–791. [Google Scholar] [CrossRef]
- Tahir, A.; Saeed, A.; Ramzan, I.; Hayat, S.S.; Ahmad, W.; Naeem, S.; Afzal, M.; Mukhtar, A.; Mehmood, T.; Khan, B.S. Mechanism for the formation of magnetite iron oxide nanostructures by Ficus carica dried fruit extract using green synthesis method. Appl. Nanosci. 2021, 11, 1857–1865. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.; Ismail, I.; Syazwan, M.; Zuikimi, M. Sol-gel auto-combustion synthesis of cobalt ferrite and it’s cytotoxicity properties. Dig. J. Nanomater. Biostruct. (DJNB) 2013, 8, 1601–1610. [Google Scholar]
- Akbari Moayyer, H.; Ataie, A. Investigation on Phase Evolution in the Processing of Nano-Crystalline Cobalt Ferrite by Solid-State Reaction Route. Adv. Mater. Res. 2014, 829, 767–771. [Google Scholar] [CrossRef]
- Saha, M.; Mukherjee, S.; Gayen, A. Microstructure, optical and magnetic properties of inverse spinel CoFe2O4 synthesized by microemulsion process assisted by CTAB and AOT. Aust. Ceram. Soc. 2016, 52, 150–162. [Google Scholar]
- Mehmood, T.; Mukhtar, A.; Wang, H.; Khan, B.S. Effect of deposition parameters on the crystal orientation and growth of Ag nanowires. Int. J. Mater. Res. 2016, 107, 283–286. [Google Scholar] [CrossRef]
- Mehmood, T.; Mukhtar, A.; Khan, B.S.; Wu, K. Growth mechanism of electrodeposited Fe, Co and Ni nanowires in the form of self-assembled arrays at fixed potential. Int. J. Electrochem. Sci 2016, 11, 6423–6431. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew. Chem. Int. Ed. 2009, 48, 4174–4179. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Zink, J.I. Spatial, temporal, and dose control of drug delivery using noninvasive magnetic stimulation. ACS Nano 2019, 13, 1292–1308. [Google Scholar] [CrossRef]
- Rajan, A.; Rajan, A.R.; Philip, D. Elettaria cardamomum seed mediated rapid synthesis of gold nanoparticles and its biological activities. OpenNano 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Vinotha, V.; Yazhiniprabha, M.; Raj, D.S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Govindarajan, M.; Vaseeharan, B. Biogenic synthesis of aromatic cardamom-wrapped zinc oxide nanoparticles and their potential antibacterial and mosquito larvicidal activity: An effective eco-friendly approach. J. Environ. Chem. Eng. 2020, 8, 104466. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels; crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Barth, T.F.; Posnjak, E. Spinel structures: With and without variate atom equipoints. Z. Für Krist-Cryst. Mater. 1932, 82, 325–341. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of spinel. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Nikam, D.S.; Jadhav, S.V.; Khot, V.M.; Bohara, R.; Hong, C.K.; Mali, S.S.; Pawar, S. Cation distribution, structural, morphological and magnetic properties of Co1−xZnxFe2O4 (x = 0–1) nanoparticles. RSC Adv. 2015, 5, 2338–2345. [Google Scholar] [CrossRef]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. J. Am. Chem. Soc. 2000, 122, 6263–6267. [Google Scholar] [CrossRef]
- Rajendran, M.; Pullar, R.; Bhattacharya, A.; Das, D.; Chintalapudi, S.; Majumdar, C. Magnetic properties of nanocrystalline CoFe2O4 powders prepared at room temperature: Variation with crystallite size. J. Magn. Magn. Mater. 2001, 232, 71–83. [Google Scholar] [CrossRef]
- Mooney, K.E.; Nelson, J.A.; Wagner, M.J. Superparamagnetic cobalt ferrite nanocrystals synthesized by alkalide reduction. Chem. Mater. 2004, 16, 3155–3161. [Google Scholar] [CrossRef]
- Zhen, L.; He, K.; Xu, C.; Shao, W. Synthesis and characterization of single-crystalline MnFe2O4 nanorods via a surfactant-free hydrothermal route. J. Magn. Magn. Mater. 2008, 320, 2672–2675. [Google Scholar] [CrossRef]
- Kim, D.-H.; Nikles, D.E.; Johnson, D.T.; Brazel, C.S. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 2008, 320, 2390–2396. [Google Scholar] [CrossRef]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Sugimoto, M. The past, present, and future of ferrites. J. Am. Ceram. Soc. 1999, 82, 269–280. [Google Scholar] [CrossRef]
- Gautam, S.; Muthurani, S.; Balaji, M.; Thakur, P.; Padiyan, D.P.; Chae, K.; Kim, S.; Asokan, K. Electronic Structure Studies of Nanoferrite CuxCo 1–xFe2O4 by X-ray Absorption Spectroscopy. J. Nanosci. Nanotechnol. 2011, 11, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Samavati, A.; Ismail, A. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method. Particuology 2017, 30, 158–163. [Google Scholar] [CrossRef]
- Melo, R.; Banerjee, P.; Franco, A. Hydrothermal synthesis of nickel doped cobalt ferrite nanoparticles: Optical and magnetic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 14657–14667. [Google Scholar] [CrossRef]
- Sodaee, T.; Ghasemi, A.; Razavi, R.S. Microstructural characteristics and magnetic properties of gadolinium-substituted cobalt ferrite nanocrystals synthesized by hydrothermal processing. J. Cluster Sci. 2016, 27, 1239–1251. [Google Scholar] [CrossRef]
- Margabandhu, M.; Sendhilnathan, S.; Senthilkumar, S.; Gajalakshmi, D. Investigation of structural, morphological, magnetic properties and biomedical applications of Cu2+ substituted uncoated cobalt ferrite nanoparticles. Braz. Arch. Biol. Technol. 2017, 59, e16161046. [Google Scholar] [CrossRef] [Green Version]
- Sanpo, N.; Wang, J.; Berndt, C.C. Sol-gel synthesized copper-substituted cobalt ferrite nanoparticles for biomedical applications. J. Nano Res. 2013, 25, 110–121. [Google Scholar] [CrossRef]
- Naik, C.C.; Gaonkar, S.; Furtado, I.; Salker, A. Effect of Cu2+ substitution on structural, magnetic and dielectric properties of cobalt ferrite with its enhanced antimicrobial property. J. Mater. Sci. Mater. Electron. 2018, 29, 14746–14761. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Xing, Y.; Song, S.; Yu, S.; Shi, W.; Guo, X.; Yang, J.; Lei, Y.; Cao, F. Studies on the magnetism of cobalt ferrite nanocrystals synthesized by hydrothermal method. J. Solid State Chem. 2008, 181, 245–252. [Google Scholar] [CrossRef]
- Manikandan, A.; Sridhar, R.; Antony, S.A.; Ramakrishna, S. A simple aloe vera plant-extracted microwave and conventional combustion synthesis: Morphological, optical, magnetic and catalytic properties of CoFe2O4 nanostructures. J. Mol. Struct. 2014, 1076, 188–200. [Google Scholar] [CrossRef]
- GingaŞu, D.; Mindru, I.; Preda, S.; Calderon-Moreno, J.M.; Daniela, C.C.; Patron, L.; Diamandescu, L. Green synthesis of cobalt ferrite nanoparticles using plant extracts. Rev. Roum. Chim. 2017, 62, 645–653. [Google Scholar]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Ramalingam, R.J.; Al-Lohedan, H.A. Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Mater. Chem. Phys. 2018, 204, 410–419. [Google Scholar] [CrossRef]
- Gingasu, D.; Mindru, I.; Patron, L.; Calderon-Moreno, J.M.; Mocioiu, O.C.; Preda, S.; Stanica, N.; Nita, S.; Dobre, N.; Popa, M. Green synthesis methods of CoFe2O4 and Ag-CoFe2O4 nanoparticles using hibiscus extracts and their antimicrobial potential. J. Nanomater. 2016, 2016, 2106756. [Google Scholar] [CrossRef] [Green Version]
- Jolad, S.D.; Lantz, R.C.; Solyom, A.M.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Fresh organically grown ginger (Zingiber officinale): Composition and effects on LPS-induced PGE2 production. Phytochemistry 2004, 65, 1937–1954. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, J.J.; Jayaprakash, N.; Kombaiah, K.; Kaviyarasu, K.; Kennedy, L.J.; Ramalingam, R.J.; Al-Lohedan, H.A.; Mansoor-Ali, V.; Maaza, M. Bioreduction potentials of dried root of Zingiber officinale for a simple green synthesis of silver nanoparticles: Antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 177, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Manhar, A.K.; Bora, P.J.; Dolui, S.K.; Mandal, M. Evaluation of antioxidant and antibacterial activity of various aspect ratio gold (Au) nanorods. Adv. Mater. Lett. 2015, 6, 235–241. [Google Scholar] [CrossRef]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Vijayaraghavan, T.; Suriyaraj, S.; Selvakumar, R.; Venkateswaran, R.; Ashok, A. Rapid and efficient visible light photocatalytic dye degradation using AFe2O4 (A = Ba, Ca and Sr) complex oxides. Mater. Sci. Eng. B 2016, 210, 43–50. [Google Scholar] [CrossRef]
- Lisjak, D.; Ovtar, S.; Drofenik, M. The stability of BaFe 12 O 19 nanoparticles in polar solvents. J. Mater. Sci. 2011, 46, 2851–2859. [Google Scholar] [CrossRef]
- Vijaya, J.J.; Sekaran, G.; Bououdina, M. Effect of Cu2+ doping on structural, morphological, optical and magnetic properties of MnFe2O4 particles/sheets/flakes-like nanostructures. Ceram. Int. 2015, 41, 15–26. [Google Scholar] [CrossRef]
- Cullity, B.D. Answers to Problems: Elements of X-ray Diffraction; Addison-Wesley Publishing Company: Boston, MA, USA, 1978. [Google Scholar]
- Cullity, B.D.; Weymouth, J.W. Elements of X-ray Diffraction; Addison-Wesley Publishing Company Inc.: Reading, MA, USA, 1957. [Google Scholar]
- Gabal, M.; Ata-Allah, S. Effect of diamagnetic substitution on the structural, electrical and magnetic properties of CoFe2O4. Mater. Chem. Phys. 2004, 85, 104–112. [Google Scholar] [CrossRef]
- Abu Amsha, A. Synthesis and Characterization of Nanosized Metal Oxides. Master’s Thesis, Al Azhar University Gaza, Gaza, Palestinian, 2015. [Google Scholar]
- Kumar, V.; Rana, A.; Kumar, N.; Pant, R.P. Investigations on Controlled-Size-Precipitated Cobalt Ferrite Nanoparticles. Int. J. Appl. Ceram. Technol. 2011, 8, 120–126. [Google Scholar] [CrossRef]
- Maksoud, M.; El-ghandour, A.; El-Sayyad, G.S.; Awed, A.; Fahim, R.A.; Atta, M.; Ashour, A.; El-Batal, A.I.; Gobara, M.; Abdel-Khalek, E. Tunable structures of copper substituted cobalt nanoferrites with prospective electrical and magnetic applications. J. Mater. Sci. Mater. Electron. 2019, 30, 4908–4919. [Google Scholar] [CrossRef]
- Maksoud, M.A.; El-Sayyad, G.S.; Ashour, A.; El-Batal, A.I.; Abd-Elmonem, M.S.; Hendawy, H.A.; Abdel-Khalek, E.; Labib, S.; Abdeltwab, E.; El-Okr, M. Synthesis and characterization of metals-substituted cobalt ferrite [Mx Co(1-x)Fe2O4; (M = Zn, Cu and Mn; x = 0 and 0.5)] nanoparticles as antimicrobial agents and sensors for Anagrelide determination in biological samples. Mater. Sci. Eng. C 2018, 92, 644–656. [Google Scholar] [CrossRef]
- Samavati, A.; Mustafa, M.; Ismail, A.; Othman, M.; Rahman, M. Copper-substituted cobalt ferrite nanoparticles: Structural, optical and antibacterial properties. Mater. Express 2016, 6, 473–482. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, P.; Kaur, G.; Dev, K.; Negi, P.; Sharma, R. Structural, morphological and optical properties of Eu-N co-doped zinc oxide nanoparticles synthesized using co-precipitation technique. Vacuum 2018, 155, 689–695. [Google Scholar] [CrossRef]
- Aslinjensipriya, A.; Narmadha, S.; Deepapriya, S.; John, D.R.; Grace, I.S.; Reena, R.S.; Chamundeeswari, A.; Jose, M.; Jerome, D.S. Synthesis and characterization of ZnO nanoparticles by novel sol gel technique. AIP Conf. Proc. 2020, 2244, 070013. [Google Scholar]
- Rahman, A.; Zulfiqar, S.; Musaddiq, S.; Shakir, I.; Warsi, M.F.; Shahid, M. Facile synthesis of Ce1-xFexO2/NiO/rGO ternary hybrid heterostructures with enhanced visible light mediated photocatalytic activity for waterborne pollutants. J. Photochem. Photobiol. A Chem. 2020, 397, 112583. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Si, S.; Mandal, T.K. Tryptophan-based peptides to synthesize gold and silver nanoparticles: A mechanistic and kinetic study. Chem.-A Eur. J. 2007, 13, 3160–3168. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.P.; Mukherjee, S. Disordered surface spins induced large exchange anisotropy in single-phase Sm3+ ions substituted nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2019, 489, 165320. [Google Scholar] [CrossRef]
- Kakade, S.; Kambale, R.; Ramanna, C.; Kolekar, Y. Crystal strain, chemical bonding, magnetic and magnetostrictive properties of erbium (Er3+) ion substituted cobalt-rich ferrite (Co1.1Fe1.9−xErxO4). RSC Adv. 2016, 6, 33308–33317. [Google Scholar] [CrossRef]
- Sharma, R.; Thakur, P.; Kumar, M.; Barman, P.; Sharma, P.; Sharma, V. Enhancement in AB super-exchange interaction with Mn2+ substitution in Mg-Zn ferrites as a heating source in hyperthermia applications. Ceram. Int. 2017, 43, 13661–13669. [Google Scholar] [CrossRef]
- Lakhani, V.; Pathak, T.; Vasoya, N.; Modi, K. Structural parameters and X-ray Debye temperature determination study on copper-ferrite-aluminates. Solid State Sci. 2011, 13, 539–547. [Google Scholar] [CrossRef]
- Vassiliou, J.K.; Mehrotra, V.; Russell, M.W.; Giannelis, E.P. Magnetic and optical properties of γ-Fe2O3 nanocrystals. J. Appl. Phys. 1993, 73, 5109. [Google Scholar] [CrossRef]
- Nair, S.S.; Mathews, M.; Anantharaman, M. Evidence for blueshift by weak exciton confinement and tuning of bandgap in superparamagnetic nanocomposites. Chem. Phys. Lett. 2005, 406, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Abdo, M.; El-Daly, A. Sm-substituted copper-cobalt ferrite nanoparticles: Preparation and assessment of structural, magnetic and photocatalytic properties for wastewater treatment applications. J. Alloys Compd. 2021, 883, 160796. [Google Scholar] [CrossRef]
- Muthukumaran, T.; Philip, J. Synthesis of water dispersible phosphate capped CoFe2O4 nanoparticles and its applications in efficient organic dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125755. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. Copper and cerium co-doped cobalt ferrite nanoparticles: Structural, morphological, optical, magnetic, and photocatalytic properties. Environ. Sci. Pollut. Res. 2019, 26, 19189–19206. [Google Scholar] [CrossRef]
- Ahmad, M.; Bhatti, I.; Qureshi, K.; Ahmad, N.; Nisar, J.; Zuber, M.; Ashar, A.; Khan, M.; Iqbal, M. Graphene oxide supported Fe2(MoO4)3 nano rods assembled round-ball fabrication via hydrothermal route and photocatalytic degradation of nonsteroidal anti-inflammatory drug. J. Mol. Liq. 2020, 301, 112343. [Google Scholar] [CrossRef]
- Qureshi, K.; Ahmad, M.Z.; Bhatti, I.A.; Zahid, M.; Nisar, J.; Iqbal, M. Graphene oxide decorated ZnWO4 architecture synthesis, characterization and photocatalytic activity evaluation. J. Mol. Liq. 2019, 285, 778–789. [Google Scholar] [CrossRef]
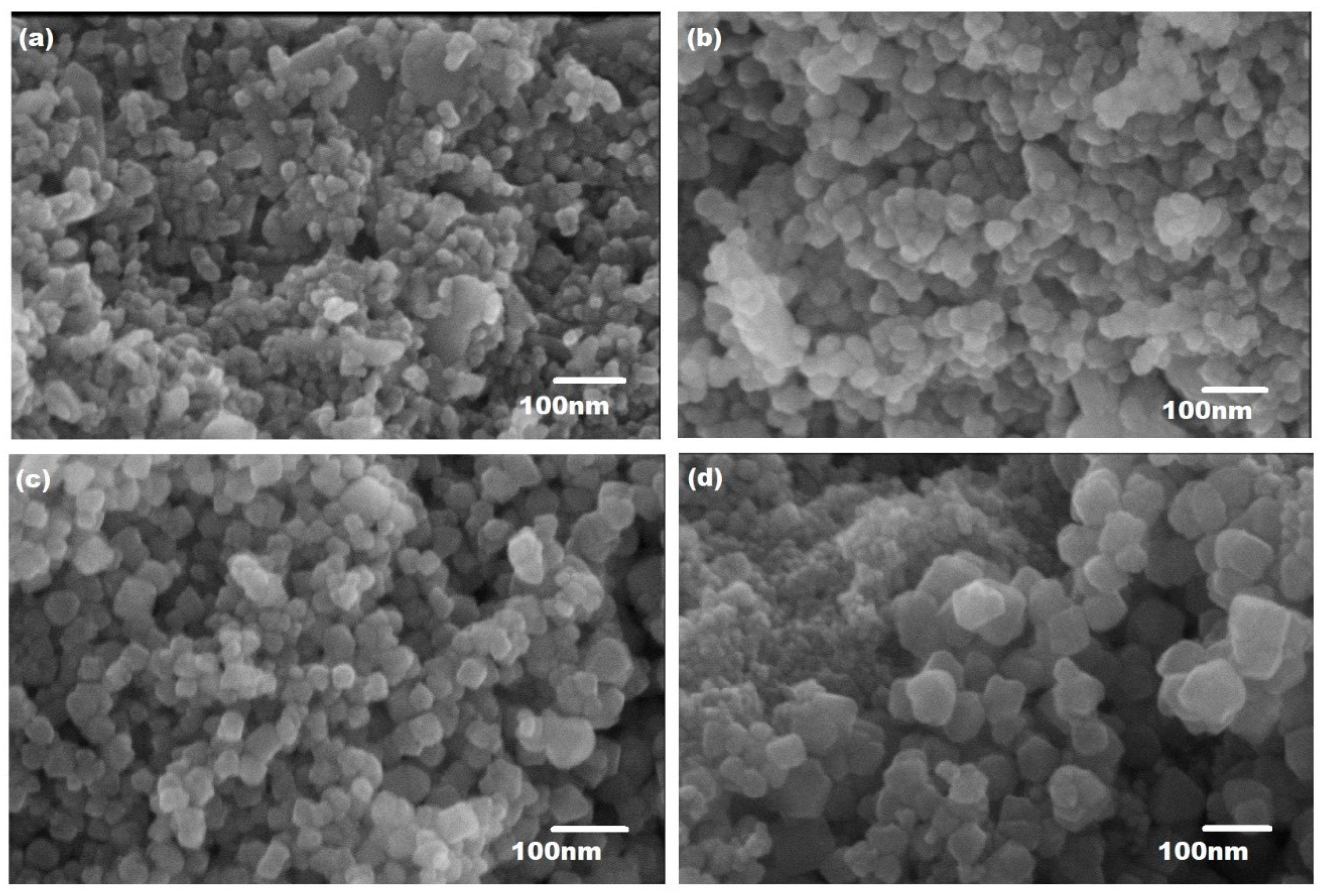
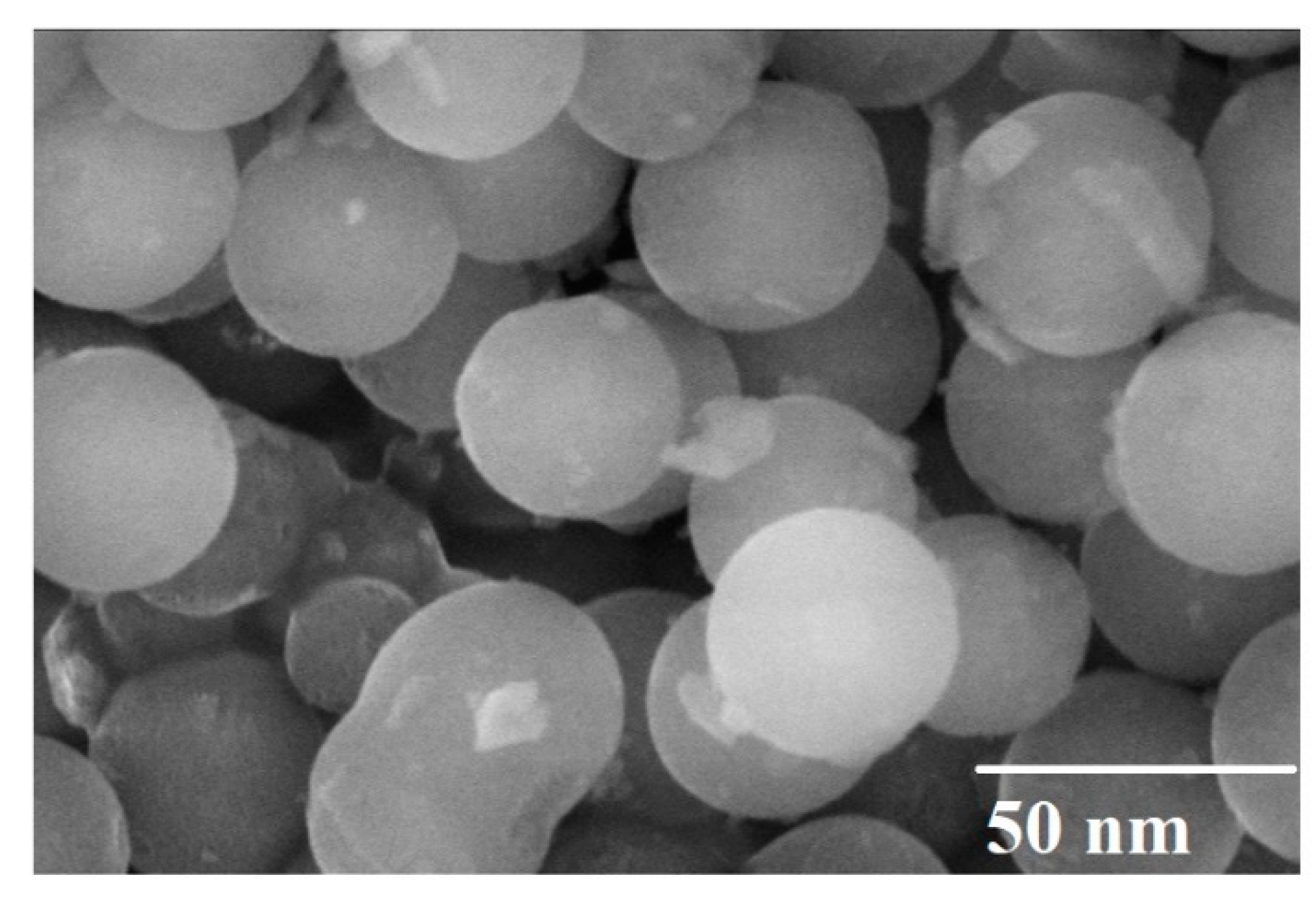

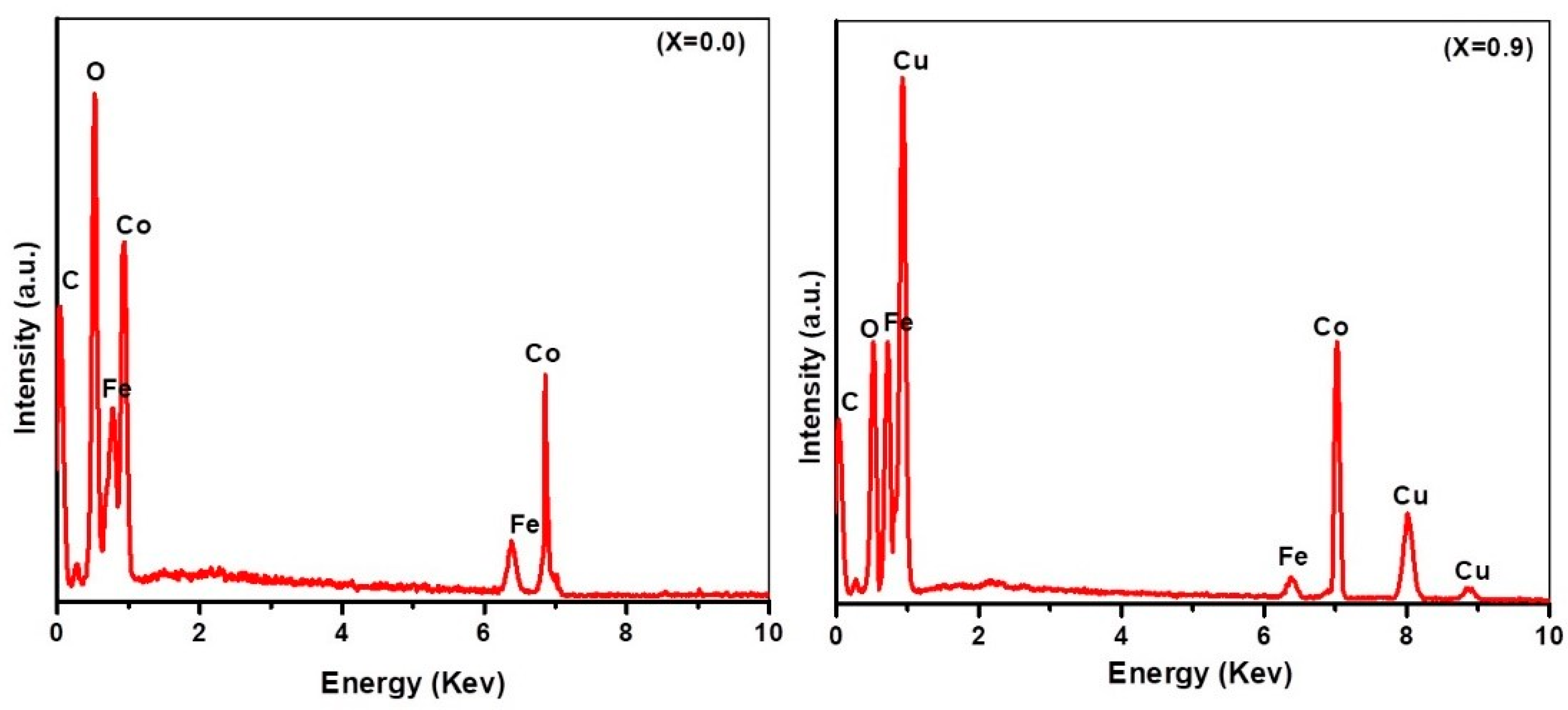
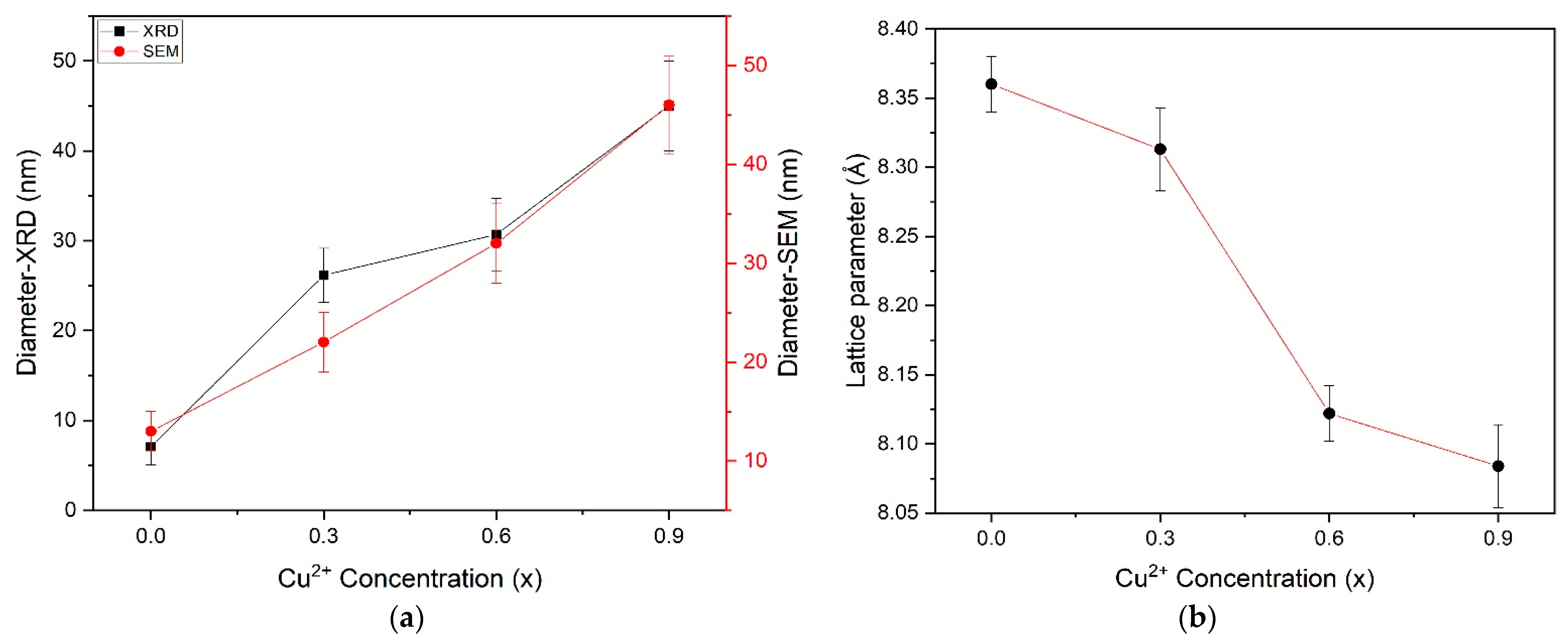


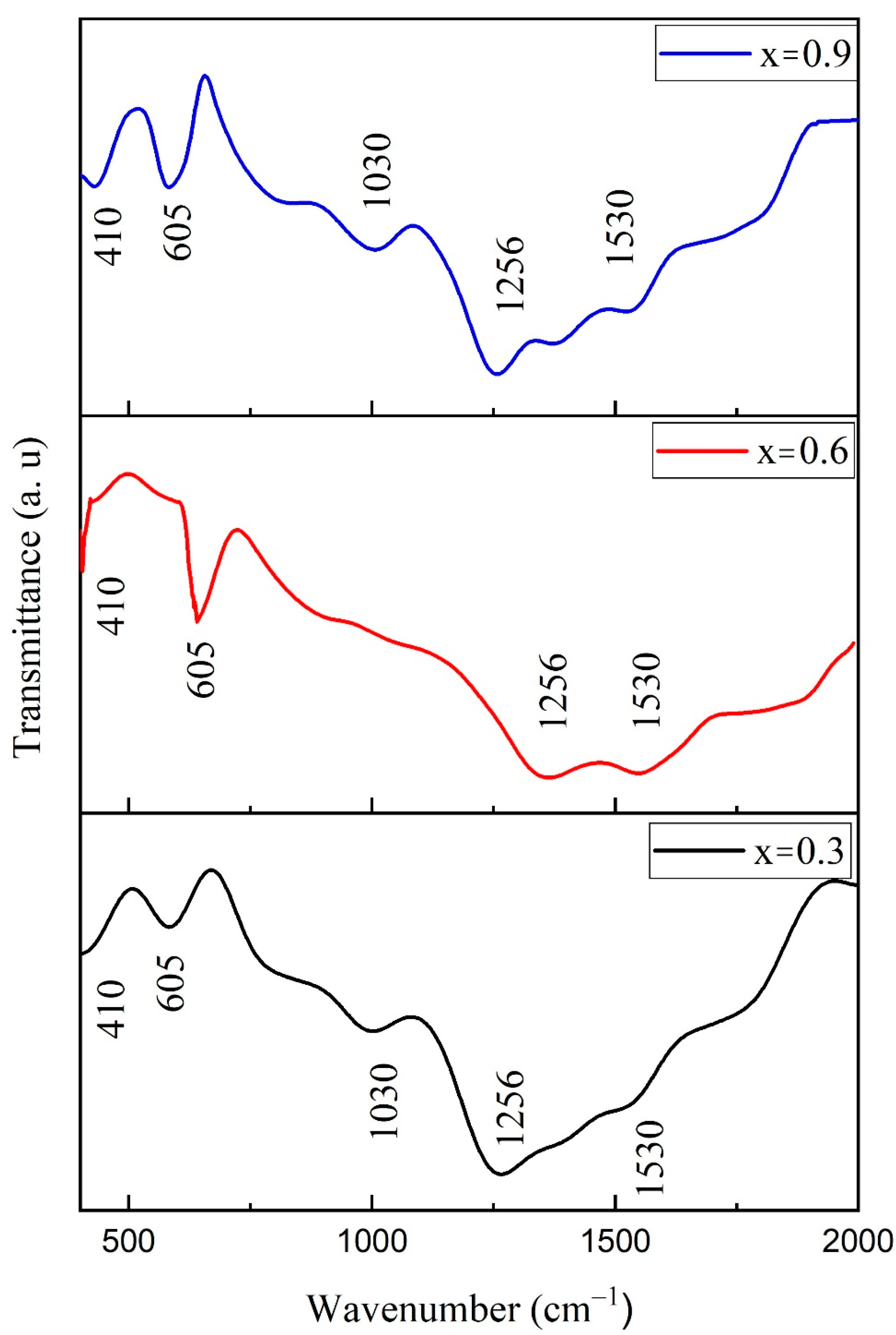
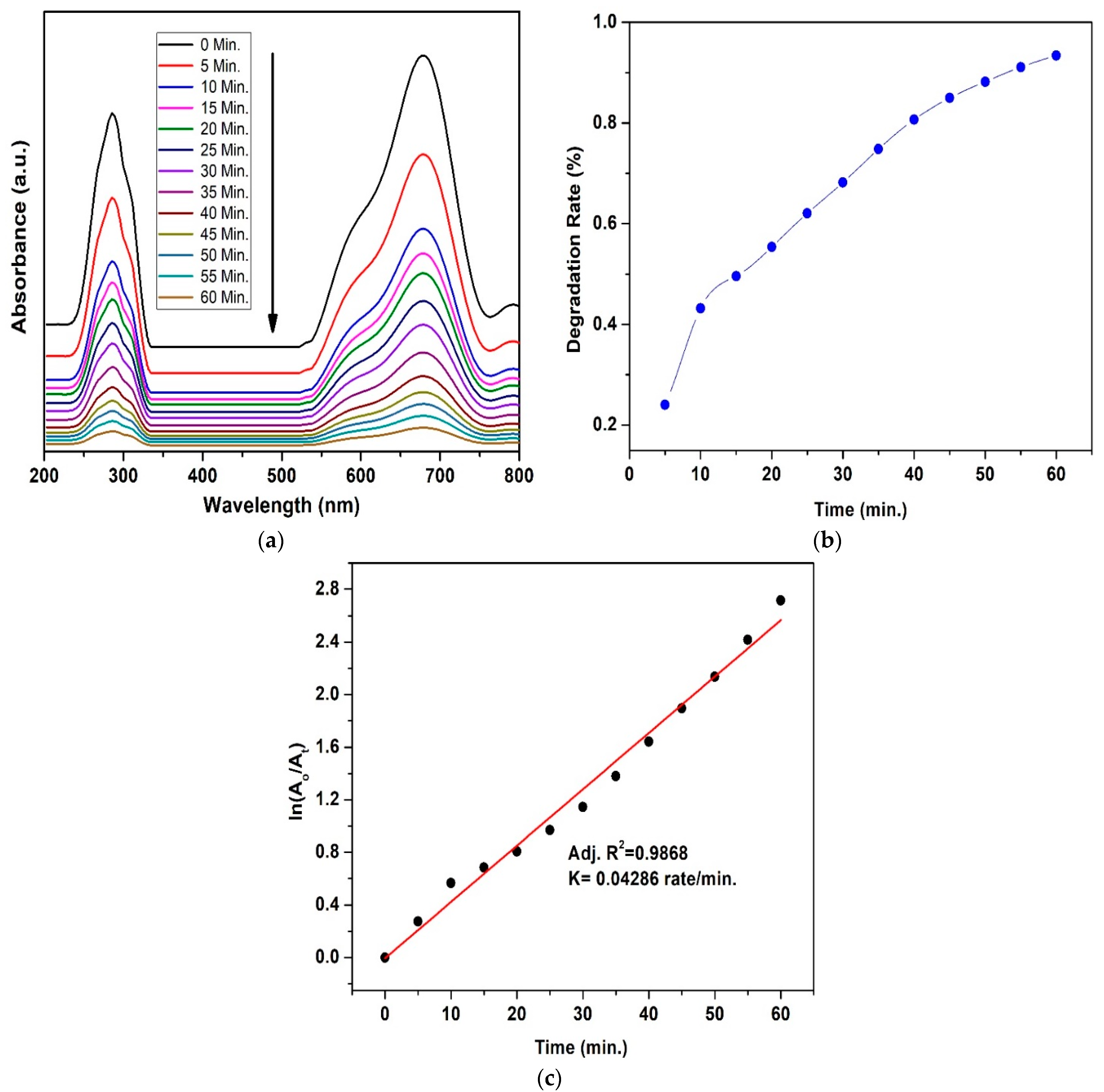
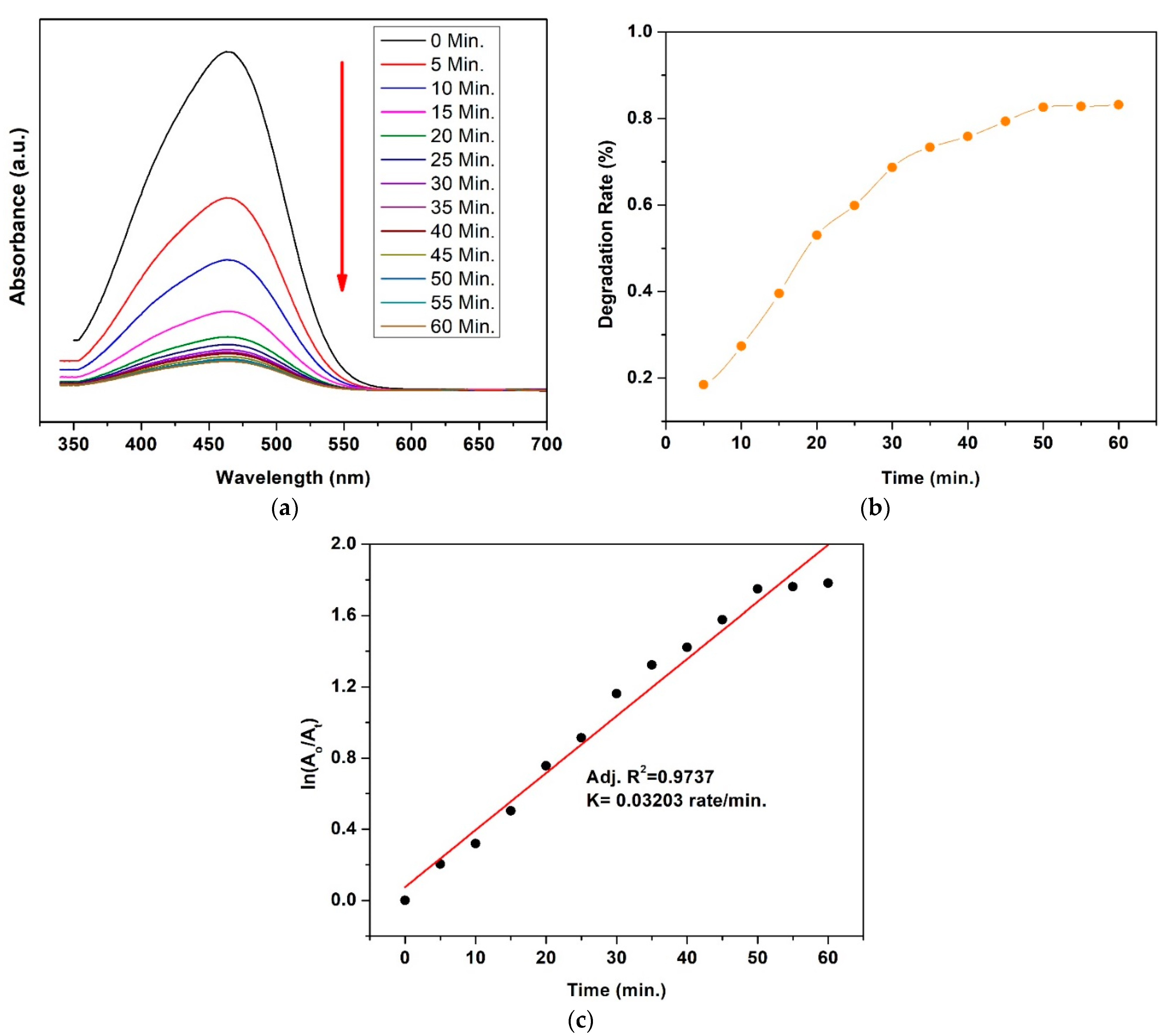
| Ferrite Composition (CuxCo1−xFe2O4) | Value of x | Element wt.% | ||||
|---|---|---|---|---|---|---|
| C | Co | Cu | Fe | O | ||
| CoFe2O4 | 0.0 | 2.37 | 63.06 | ----- | 11.06 | 23.51 |
| Co0.7Cu0.3 Fe2O4 | 0.3 | 3.66 | 21.25 | 41.29 | 8.63 | 25.17 |
| Co0.4Cu0.6 Fe2O4 | 0.6 | 3.65 | 11.06 | 54.14 | 8.04 | 23.11 |
| Co0.1Cu0.9 Fe2O4 | 0.9 | 3.55 | 3.08 | 65.14 | 5.63 | 22.60 |
| Ferrite Composition (CuxCo1−xFe2O4) | ~Diameter (nm) | |||||
|---|---|---|---|---|---|---|
| Scherer | SEM | |||||
| CoFe2O4 | 0.0 | 8.360 | 2.5095 | 7.06 | 13 | 584.2771 |
| Co0.7Cu0.3 Fe2O4 | 0.3 | 8.313 | 2.4532 | 26.15 | 22 | 574.4779 |
| Co0.4Cu0.6 Fe2O4 | 0.6 | 8.122 | 2.4461 | 30.69 | 32 | 535.783 |
| Co0.1Cu0.9 Fe2O4 | 0.9 | 8.084 | 2.4257 | 45 | 46 | 528.2979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkat, F.; Afzal, M.; Khan, B.S.; Saeed, A.; Bashir, M.; Mukhtar, A.; Mehmood, T.; Wu, K. Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route. Materials 2022, 15, 4374. https://doi.org/10.3390/ma15134374
Barkat F, Afzal M, Khan BS, Saeed A, Bashir M, Mukhtar A, Mehmood T, Wu K. Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route. Materials. 2022; 15(13):4374. https://doi.org/10.3390/ma15134374
Chicago/Turabian StyleBarkat, Faiqa, Marina Afzal, Babar Shahzad Khan, Adnan Saeed, Mahwish Bashir, Aiman Mukhtar, Tahir Mehmood, and Kaiming Wu. 2022. "Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route" Materials 15, no. 13: 4374. https://doi.org/10.3390/ma15134374
APA StyleBarkat, F., Afzal, M., Khan, B. S., Saeed, A., Bashir, M., Mukhtar, A., Mehmood, T., & Wu, K. (2022). Formation Mechanism and Lattice Parameter Investigation for Copper-Substituted Cobalt Ferrites from Zingiber officinale and Elettaria cardamom Seed Extracts Using Biogenic Route. Materials, 15(13), 4374. https://doi.org/10.3390/ma15134374






