Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Iron Ore Ultra-Fines
2.2. Correlations for the Evaluation of Using Iron Ore Ultra-Fines in a Fluidized Bed
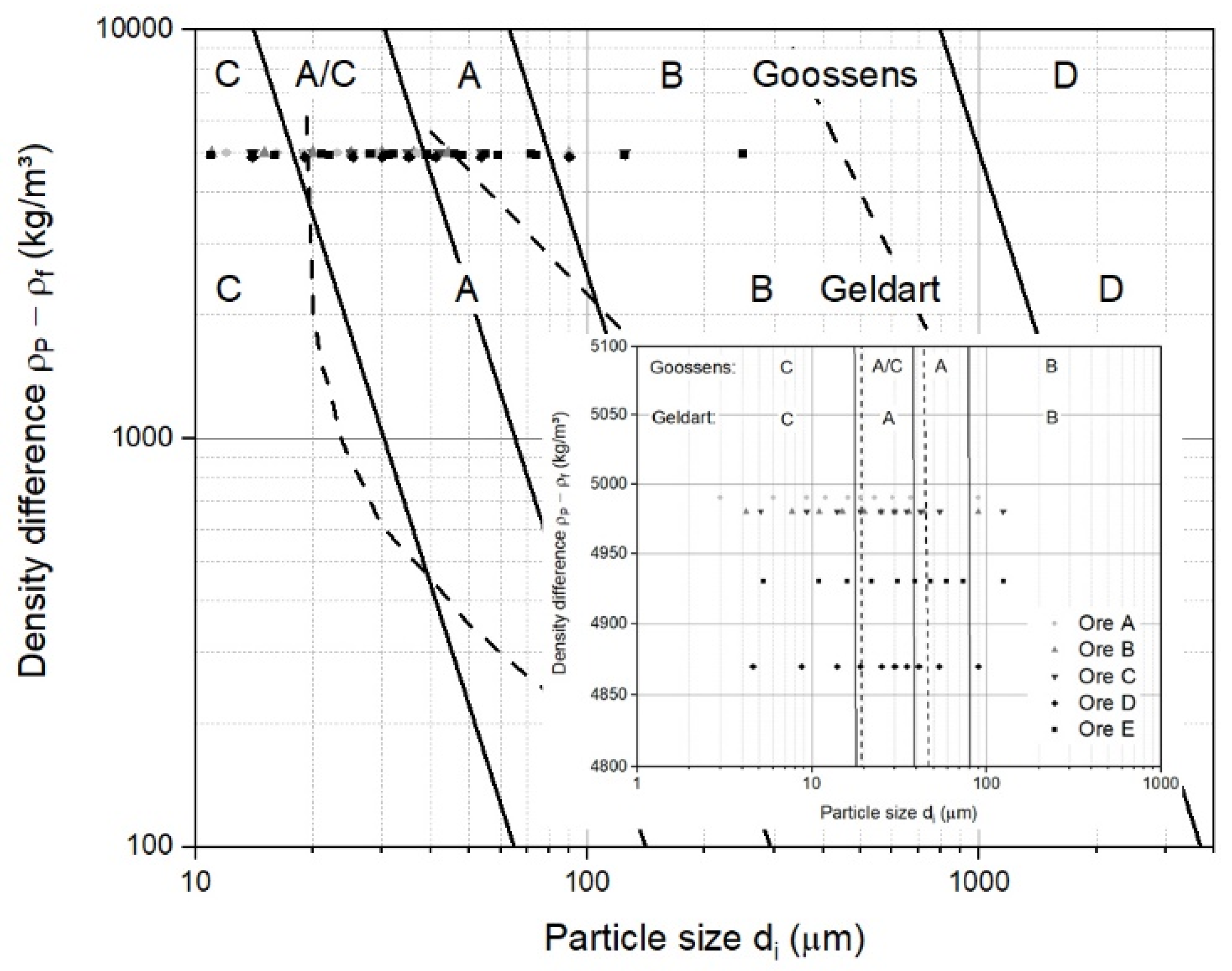
2.2.1. General Classification Diagram of Fluidized Particles
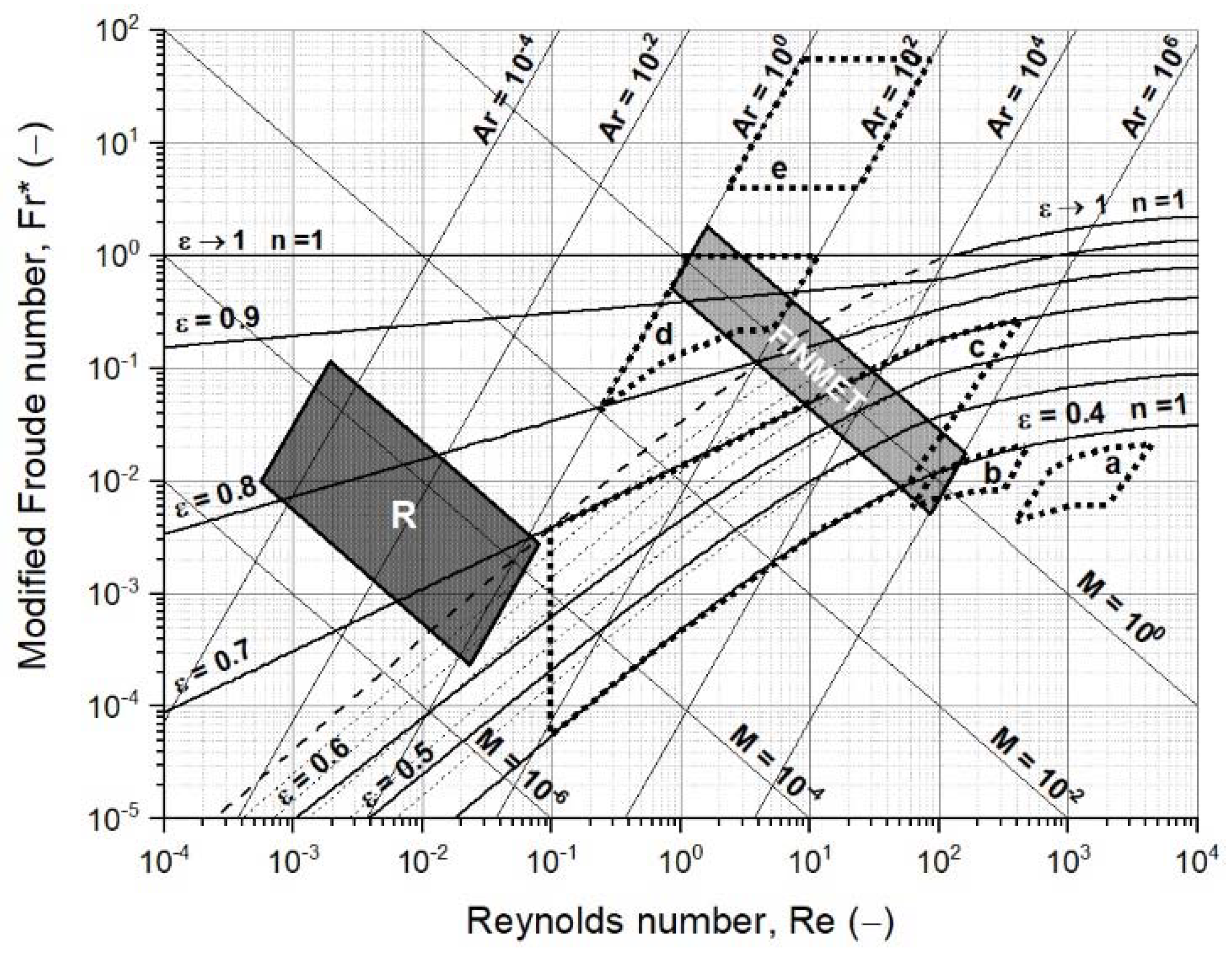
2.2.2. Fluidized State Diagram Following Reh’s Approach
2.2.3. Minimum Fluidization Velocity
3. Results and Discussion
3.1. Classification of Iron Ore Ultra-Fine Powders for a Fluidized Bed
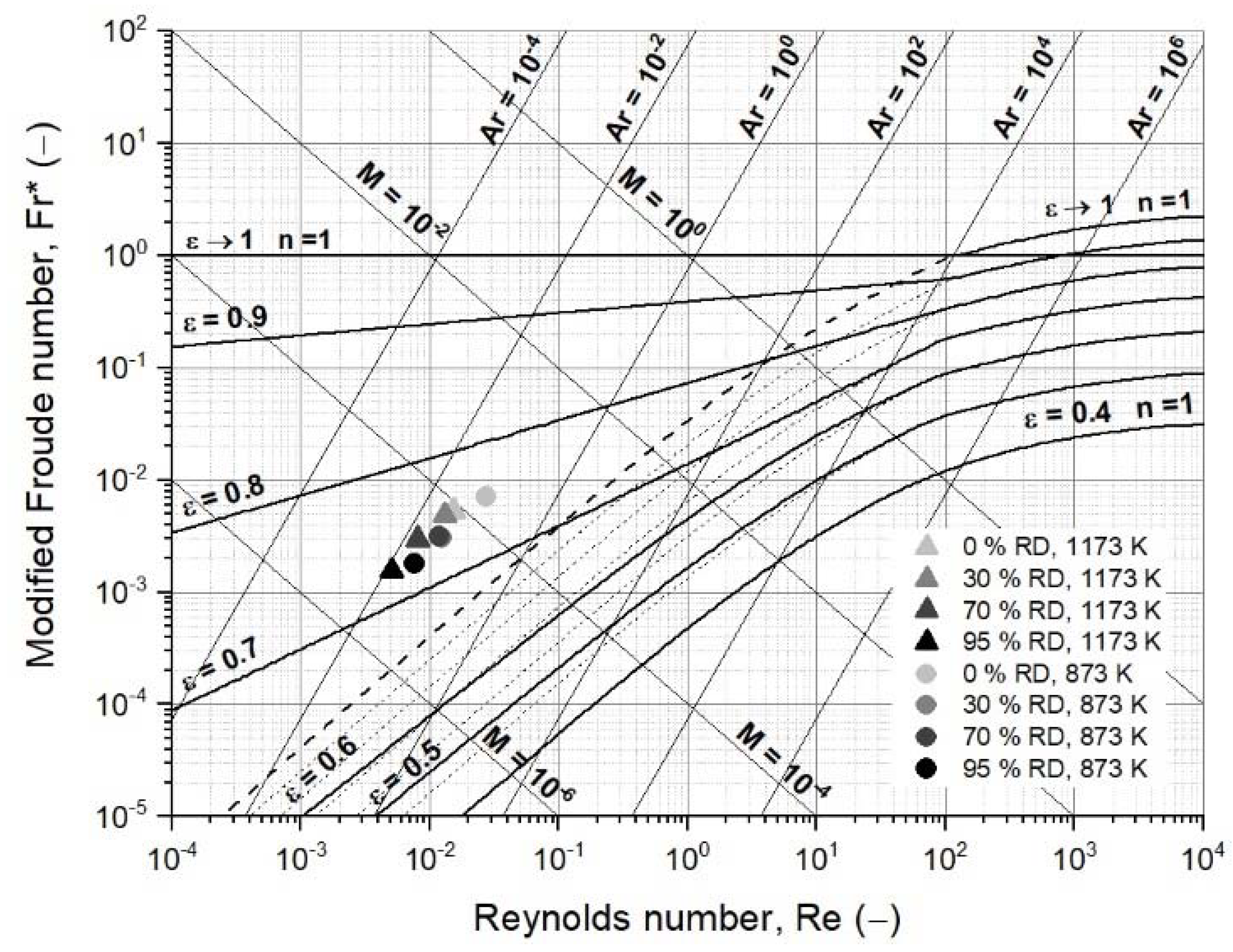
3.2. Operating Field of Iron Ore Ultra-Fines for a Fluidized Bed Reactor
- 1.
- Particle diameters, , in size range of 2 to 90 µm, to account for 95 wt.−%;
- 2.
- Densities of the ore and DRI, , between 5000 and 3500 kg/m3;
- 3.
- Gas densities, , and dynamic viscosities, , for the H2 and H2/H2O mixtures at temperatures of 873 to 1123 K and a pressure of 0.1 barg;
- 4.
- Superficial gas velocity, , between 0.15 and 0.30 m/s.
3.3. Mathematical Case Studies
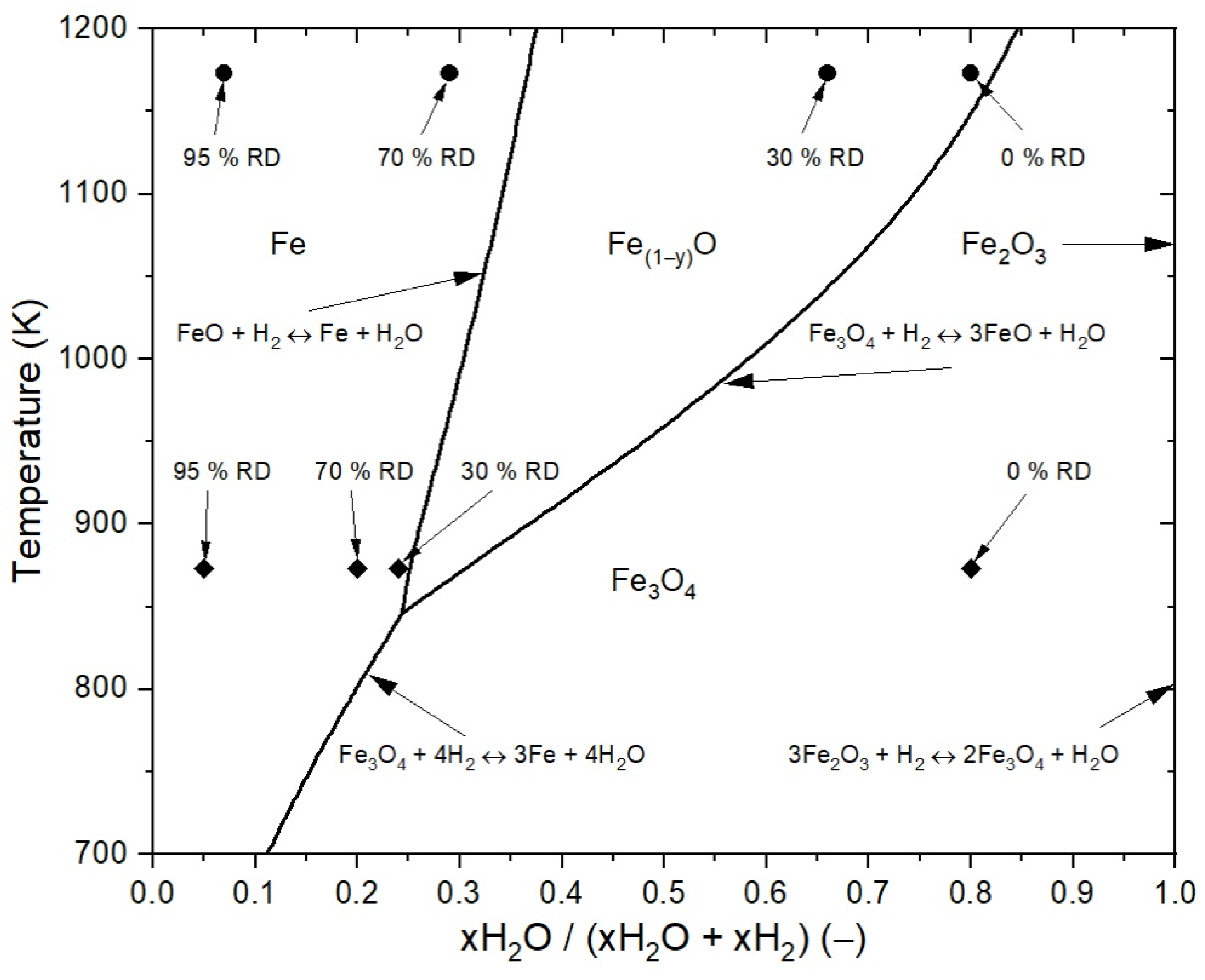
3.3.1. Assumptions of the Reduction Process for the Mathematical Case Studies
3.3.2. First Case Study: Fluidized Bed Reduction without Sticking
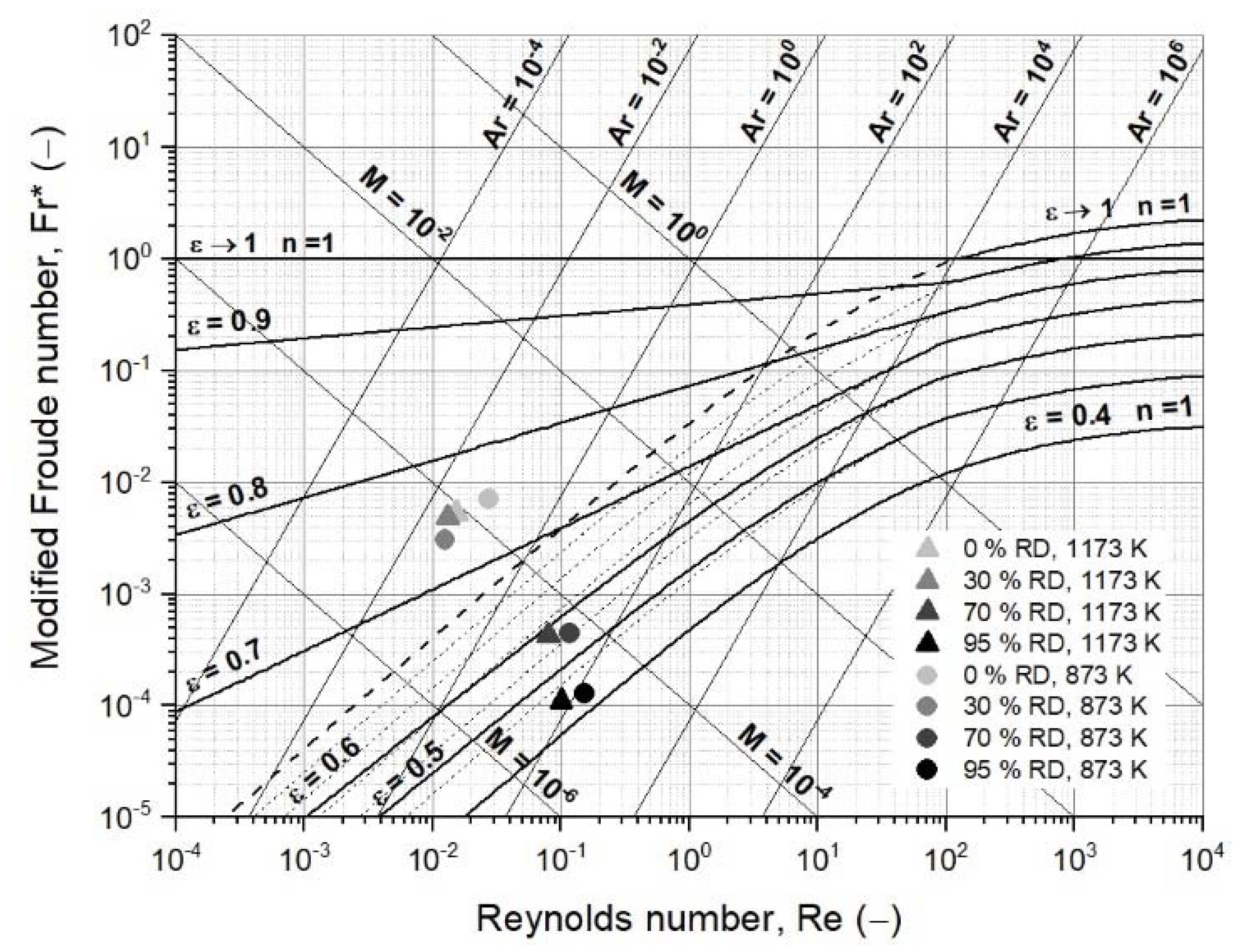
3.3.3. Second Case Study: Fluidized Bed Reduction with Sticking
4. Conclusions
- According to Geldart’s and Goossens’s classification, iron ore ultra-fines are mainly Group A, Group A/C, and Group C materials under ambient conditions and air. For the hydrogen-based reduction at higher temperatures, iron ore ultra-fines are mostly Group A/C and Group C, according to Goossens’s classification;
- The operating field for iron ore ultra-fines needs an extended version of the Reh diagram, and it is not positioned within the general fields of circulating or bubbling fluidized beds;
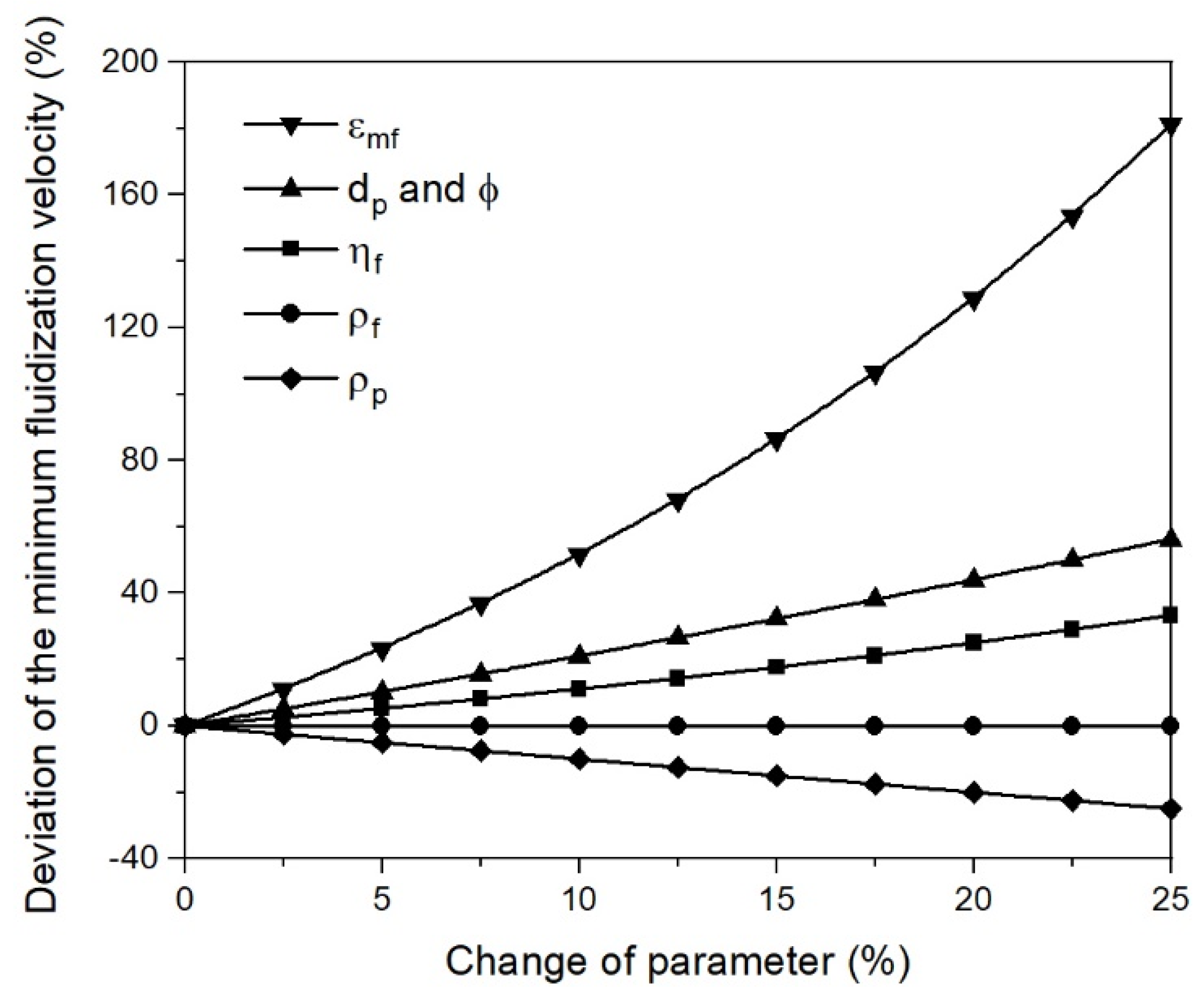
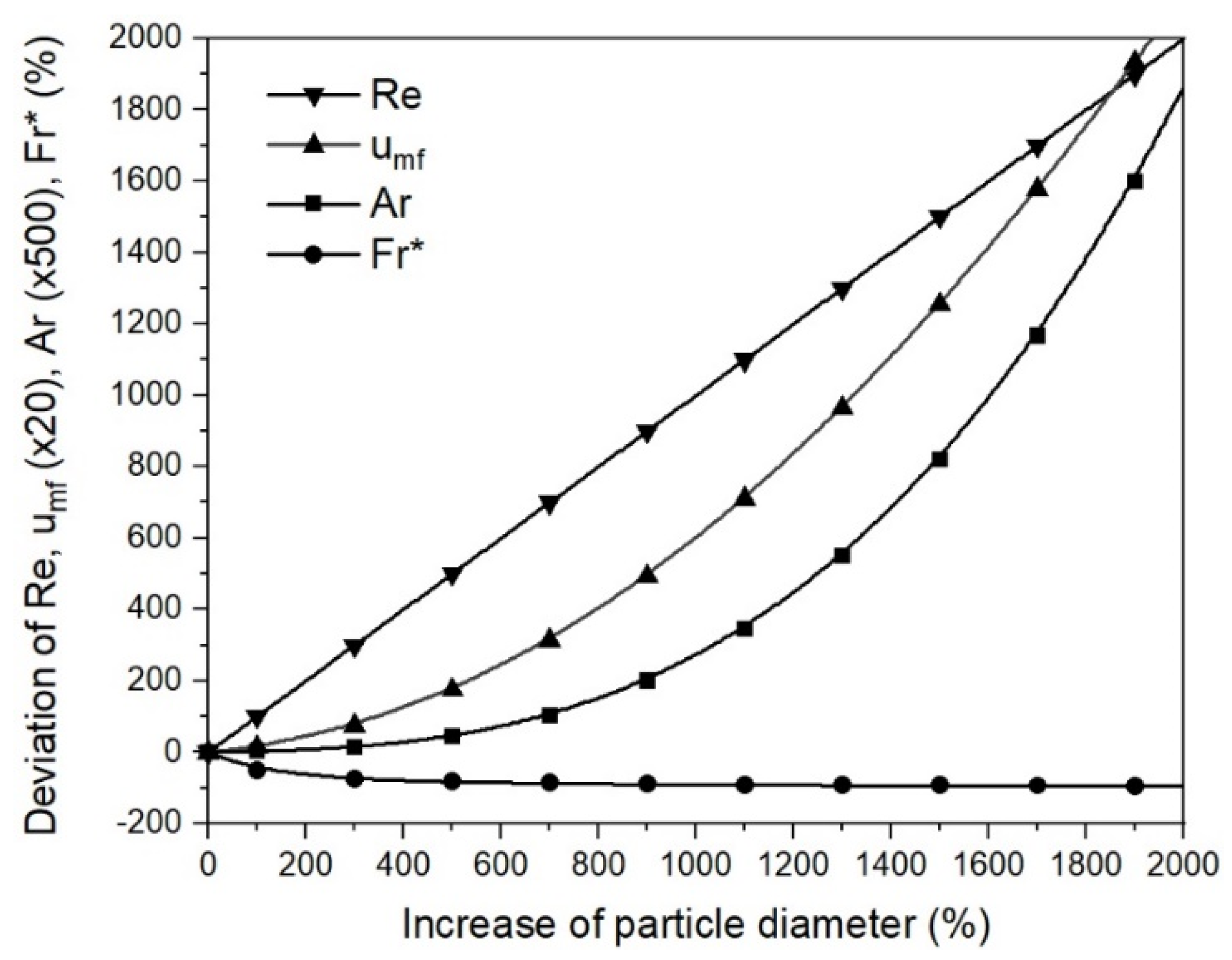
- Changing process conditions, such as the temperature and gas properties, hardly affects the fluidization conditions or the minimum fluidization velocity;
- Changing the characteristic diameter due to sticking significantly affects the fluidization conditions and the minimum fluidization velocity. Thus, the characteristic particle or agglomerate diameter is the most critical parameter for a stable fluidized bed direct reduction process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Description | Unit |
| Archimedes number | – | |
| Drag coefficient for a single particle | – | |
| Drag coefficient to account for mutual interactions between particles | – | |
| Characteristic diameter; i vol.−% underflow | µm | |
| Mean particle size | µm | |
| Amount of iron in the sample | mol | |
| Modified Froude number | – | |
| Gravity | m/s2 | |
| Gas oxidation degree | – | |
| Constant for laminar term | – | |
| Constant for turbulent term | – | |
| Liatschenko number | – | |
| Load factor | – | |
| Amount of oxygen in the sample | mol | |
| Pressure gauge | barg | |
| Reduction degree | % | |
| Reynolds number | – | |
| Temperature | K | |
| Superficial gas velocity in the reactor | m/s | |
| Minimum fluidization velocity | m/s | |
| Slip velocity | m/s | |
| Index to account for the mutual interactions between particles | – | |
| Pressure drop caused by gas flow through a packed bed | Pa | |
| Void fraction of the bulk material | – | |
| Void fraction of the bed material in the fluidized state | – | |
| Void fraction of the bed material at the point of incipient fluidization | – | |
| Gas utilization | % | |
| Dynamic gas viscosity | Pa*s | |
| Bulk density | kg/m3 | |
| Density of the fluidizing medium | kg/m3 | |
| True particle density | kg/m3 | |
| Sphericity | – |
Appendix A
| Property | 0% RD | 30% RD | 70% RD | 95% RD |
|---|---|---|---|---|
| Characteristic particle diameter, (µm) | 25 | 25 | 25 | 25 |
| Sphericity, (−) | 0.70 | 0.70 | 0.70 | 0.70 |
| Gas density, (kg/m3) | 0.112 | 0.044 | 0.040 | 0.021 |
| Dynamic gas viscosity, (×10−6 Pas) | 33.0 | 28.3 | 27.4 | 22.0 |
| True particle density, (kg/m3) | 5000.0 | 4571.7 | 4000.6 | 3643.7 |
| Modified Froude number, Fr (−) | 7.04 × 10−3 | 3.02 × 10−3 | 3.14 × 10−3 | 1.81 × 10−3 |
| Reynolds number, Re (−) | 2.72 × 10−2 | 1.25 × 10−2 | 1.17 × 10−2 | 7.65 × 10−3 |
| Archimedes number, Ar (−) | 7.88 × 10−2 | 3.85 × 10−2 | 3.27 × 10−2 | 2.42 × 10−2 |
| Liatschenko number, M (−) | 2.55 × 10−4 | 5.02 × 10−5 | 4.90 × 10−5 | 1.85 × 10−5 |
| Void fraction of the bulk material at (−) | 0.65 | 0.65 | 0.65 | 0.65 |
| Minimum fluidization velocity, (m/s) | 0.0024 | 0.0025 | 0.0023 | 0.0026 |
| Property | 0% RD | 30% RD | 70% RD | 95% RD |
|---|---|---|---|---|
| Characteristic particle diameter, (µm) | 25 | 25 | 25 | 25 |
| Sphericity, (−) | 0.70 | 0.70 | 0.70 | 0.70 |
| Gas density, (kg/m3) | 0.084 | 0.071 | 0.038 | 0.018 |
| Dynamic gas viscosity, (×10−6 Pas) | 44.4 | 43.5 | 38.1 | 28.7 |
| True particle density, (kg/m3) | 5000.0 | 4571.7 | 4000.6 | 3643.7 |
| Modified Froude number, Fr (−) | 5.28 × 10−3 | 4.88 × 10−3 | 2.98 × 10−3 | 1.55 × 10−3 |
| Reynolds number, Re (−) | 1.52 × 10−2 | 1.31 × 10−2 | 7.99 × 10−3 | 5.03 × 10−3 |
| Archimedes number, Ar (−) | 3.27 × 10−2 | 2.63 × 10−2 | 1.61 × 10−2 | 1.22 × 10−2 |
| Liatschenko number, M (−) | 1.07 × 10−4 | 8.51 × 10−5 | 3.18 × 10−5 | 1.04 × 10−5 |
| Void fraction of the bulk material at (−) | 0.65 | 0.65 | 0.65 | 0.65 |
| Minimum fluidization velocity, (m/s) | 0.0018 | 0.0017 | 0.0017 | 0.0020 |
| Property | 0% RD | 30% RD | 70% RD | 95% RD |
|---|---|---|---|---|
| Characteristic particle diameter, (µm) | 25 | 25 | 250 | 500 |
| Sphericity, (−) | 0.70 | 0.70 | 0.85 | 0.85 |
| Gas density, (kg/m3) | 0.112 | 0.044 | 0.040 | 0.021 |
| Dynamic gas viscosity, (×10−6 Pas) | 33.0 | 28.3 | 27.4 | 22.0 |
| True particle/agglomerate density, (kg/m3) | 5000.0 | 4571.7 | 4000.6 | 3643.7 |
| Modified Froude number, Fr (−) | 7.04 × 10−3 | 3.02 × 10−3 | 4.49 × 10−4 | 1.29 × 10−4 |
| Reynolds number, Re (−) | 2.72 × 10−2 | 1.25 × 10−2 | 1.17 × 10−1 | 1.53 × 10−1 |
| Archimedes number, Ar (−) | 7.88 × 10−2 | 3.85 × 10−2 | 3.27 × 10+1 | 1.94 × 10+2 |
| Liatschenko number, M (−) | 2.55 × 10−4 | 5.02 × 10−5 | 4.90 × 10−5 | 1.85 × 10−5 |
| Void fraction of the bulk material at (−) | 0.65 | 0.65 | 0.65 | 0.65 |
| Minimum fluidization velocity, (m/s) | 0.0024 | 0.0025 | 0.2363 | 1.0594 |
| Property | 0% RD | 30% RD | 70% RD | 95% RD |
|---|---|---|---|---|
| Characteristic particle diameter, (µm) | 25 | 25 | 250 | 500 |
| Sphericity, (−) | 0.70 | 0.70 | 0.85 | 0.85 |
| Gas density, (kg/m3) | 0.084 | 0.071 | 0.038 | 0.018 |
| Dynamic gas viscosity, (×10−6 Pas) | 44.4 | 43.5 | 38.1 | 28.7 |
| True particle/agglomerate density, (kg/m3) | 5000.0 | 4571.7 | 4000.6 | 3643.7 |
| Modified Froude number, Fr (−) | 5.28 × 10−3 | 4.88 × 10−3 | 4.26 × 10−4 | 1.11 × 10−4 |
| Reynolds number, Re (−) | 1.52 × 10−2 | 1.31 × 10−2 | 7.99 × 10−2 | 1.01 × 10−1 |
| Archimedes number, Ar (−) | 3.27 × 10−2 | 2.63 × 10−2 | 1.12 × 10+1 | 6.83 × 10+1 |
| Liatschenko number, M (−) | 1.07 × 10−4 | 8.51 × 10−5 | 4.54 × 10−5 | 1.49 × 10−5 |
| Void fraction of the bulk material at (−) | 0.65 | 0.65 | 0.65 | 0.65 |
| Minimum fluidization velocity, (m/s) | 0.0018 | 0.0017 | 0.1701 | 0.8178 |
| Deviation and Trend | (µm) Increase | (kg/m3) Decrease | (kg/m3) Decrease | (kg/ms) Decrease | (−) Increase | (−) Increase |
|---|---|---|---|---|---|---|
| 0.0 | 25.0 | 5000 | 0.122 | 3.30 × 10−5 | 0.55 | 0.65 |
| 2.5 | 25.6 | 4875 | 0.119 | 3.22 × 10−5 | 0.56 | 0.67 |
| 5.0 | 26.3 | 4750 | 0.116 | 3.14 × 10−5 | 0.58 | 0.68 |
| 7.5 | 26.9 | 4625 | 0.113 | 3.05 × 10−5 | 0.59 | 0.70 |
| 10.0 | 27.5 | 4500 | 0.110 | 2.97 × 10−5 | 0.61 | 0.72 |
| 12.5 | 28.1 | 4375 | 0.107 | 2.89 × 10−5 | 0.62 | 0.73 |
| 15.0 | 28.8 | 4250 | 0.104 | 2.81 × 10−5 | 0.63 | 0.75 |
| 17.5 | 29.4 | 4125 | 0.101 | 2.72 × 10−5 | 0.65 | 0.76 |
| 20.0 | 30.0 | 4000 | 0.098 | 2.64 × 10−5 | 0.66 | 0.78 |
| 22.5 | 30.6 | 3875 | 0.095 | 2.56 × 10−5 | 0.67 | 0.80 |
| 25.0 | 31.3 | 3750 | 0.092 | 2.48 × 10−5 | 0.69 | 0.81 |
References
- Eder, W. Environment–Climate–Energy: Quo vadis, industry? Berg Huettenmaenn. Monatsh. 2017, 162, 494–497. [Google Scholar] [CrossRef]
- Fischedick, M.; Marzinkowski, J.; Winzer, P.; Weigel, M. Techno-economic evaluation of innovative steel production technologies. J. Clean. Prod. 2014, 84, 563–580. [Google Scholar] [CrossRef] [Green Version]
- European Environment Agency. Greenhouse Gas Emission Trend Projections and Target. Available online: https://www.eea.europa.eu/data-and-maps/figures/greenhouse-gas-emission-trend-projections (accessed on 14 March 2022).
- Primetals Technologies Austria GmbH. HYFOR Pilot Plant under Operation—The Next Step for Carbon Free, Hydrogen-Based Direct Reduction Is Done. Available online: https://www.primetals.com/press-media/news/hyfor-pilot-plant-under-operation-the-next-step-for-carbon-free-hydrogen-based-direct-reduction-is-done (accessed on 14 March 2022).
- Klima-und Energiefonds. CO2-Freie Roheisenproduktion in Pilotanlage Erfolgreich Gestartet. Available online: https://energieforschung.at/projekt/fluidred-hot-bench-scale-plant/ (accessed on 18 March 2022).
- Hasanbeigi, A.; Arens, M.; Price, L. Alternative emerging ironmaking technologies for energy-efficiency and carbon dioxide emissions reduction: A technical review. Renew. Sustain. Energy Rev. 2014, 33, 645–658. [Google Scholar] [CrossRef]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of hydrogen direct reduction for fossil-free steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2 Part II. Low temperature reduction of magnetite. Thermochim. Acta 2007, 447, 75–88. [Google Scholar] [CrossRef]
- Rechberger, K.; Spanlang, A.; Sasiain Conde, A.; Wolfmeir, H.; Harris, C. Green hydrogen-based direct reduction for low-carbon steelmaking. Steel Res. Int. 2020, 91, 2000110. [Google Scholar] [CrossRef]
- Kirschen, M.; Badr, K.; Pfeifer, H. Influence of direct reduced iron on the energy balance of the electric arc furnace in steel industry. Energy 2011, 36, 6146–6155. [Google Scholar] [CrossRef]
- Lang, S.; Haimi, T.; Köpf, M. Circored Fine Ore Direct Reduction Plus DRI Smelting: Proven Technologies for the Transition Towards Green Steel. In REWAS 2022: Energy Technologies and CO2 Management (Volume II), 1st ed.; Tesfaye, F., Zhang, L., Guillen, D.P., Sun, Z., Baba, A.A., Neelameggham, N.R., Zhang, M., Verhulst, D.E., Alam, S., Eds.; Springer International Publishing, Imprint Springer: Cham, Switzerland, 2022; pp. 61–71. ISBN 978-3-030-92558-1. [Google Scholar]
- World Steel Association. World Steel in Figures 2021. Available online: https://worldsteel.org/publications/bookshop/world-steel-in-figures-2021/ (accessed on 14 March 2022).
- Xu, C.C.; Cang, D.-Q. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar] [CrossRef]
- Lucena, R.; Whipp, R.; Abarran, W. Finmet plant operation at Orinoco Iron. Stahl Eisen 2007, 127, 67–80. [Google Scholar]
- Schenk, J.L. Recent status of fluidized bed technologies for producing iron input materials for steelmaking. Particuology 2011, 9, 14–23. [Google Scholar] [CrossRef]
- Plaul, F.J.; Böhm, C.; Schenk, J.L. Fluidized-bed technology for the production of iron products for steelmaking. J. S. Afr. Inst. Min. Metall. 2009, 109, 121–128. [Google Scholar]
- Nuber, D.; Eichberger, H.; Rollinger, B. Circored fine ore direct reduction. Stahl Eisen 2006, 126, 47–51. [Google Scholar]
- Elmquist, S.A.; Weber, P.; Eichberger, H. Operational results of the Circored fine ore direct reduction plant in Trinidad. Stahl Eisen 2002, 122, 59–64. [Google Scholar]
- Fruehan, R.J.; Li, Y.; Brabie, L.; Kim, E.-J. Final stage of reduction of iron ores by hydrogen. Scand. J. Metall. 2005, 34, 205–212. [Google Scholar] [CrossRef]
- Kim, W.-H.; Lee, S.; Kim, S.-M.; Min, D.-J. The retardation kinetics of magnetite reduction using H2 and H2–H2O mixtures. Int. J. Hydrogen Energy 2013, 38, 4194–4200. [Google Scholar] [CrossRef]
- Hayashi, S.; Iguchi, Y. Factors affecting the sticking of fine iron ores during fluidized bed reduction. ISIJ Int. 1992, 32, 962–971. [Google Scholar] [CrossRef]
- Gudenau, H.W.; Fang, J.; Hirata, T.; Gebel, U. Fluidized bed reduction as the prestep of smelting reduction. Steel Res. Int. 1989, 60, 138–144. [Google Scholar] [CrossRef]
- He, S.; Sun, H.; Hu, C.; Li, J.; Zhu, Q.; Li, H. Direct reduction of fine iron ore concentrate in a conical fluidized bed. Powder Technol. 2017, 313, 161–168. [Google Scholar] [CrossRef]
- Ezz, S.Y. Gaseous reduction of fine iron ore in the fluidized state. Trans. Am. Inst. Min. Metall. Eng. 1960, 218, 709–715. [Google Scholar]
- Langston, B.G.; Stephens, F.M. Self-agglomerating fluidized-bed reduction. J. Met. 1960, 12, 312–316. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Guo, Z.; Tang, Q. Agglomeration/defluidization in a fluidized bed reduction of Fe2O3 particles by CO: Influences of iron precipitation on particle cohesiveness. Powder Technol. 2014, 256, 13–19. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, B.; Wang, Z.; Guo, Z. Insight of iron whisker sticking mechanism from iron atom diffusion and calculation of solid bridge radius. Metall. Mater. Trans. B 2014, 45, 2050–2056. [Google Scholar] [CrossRef]
- Guo, L.; Bao, Q.; Gao, J.; Zhu, Q.; Guo, Z. A review on prevention of sticking during fluidized bed reduction of fine iron ore. ISIJ Int. 2020, 60, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Spreitzer, D.; Schenk, J.L. Iron ore reduction by hydrogen using a laboratory scale fluidized bed reactor: Kinetic investigation—Experimental setup and method for determination. Metall. Mater. Trans. B 2019, 50, 2471–2484. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Liu, R.; Fang, J. Experimental study on sticking behavior of fluidized bed in reduction process. Adv. Mater. Res. 2012, 482, 1354–1357. [Google Scholar] [CrossRef]
- Zheng, H.; Spreitzer, D.; Wolfinger, T.; Schenk, J.; Xu, R. Effect of prior oxidation on the reduction behavior of magnetite-based iron ore during hydrogen-induced fluidized bed reduction. Metall. Mater. Trans. B 2021, 52, 1955–1971. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Guo, Z.; Tang, Q. Defluidization behavior of iron powders at elevated temperature: Influence of fluidizing gas and particle adhesion. Powder Technol. 2012, 230, 225–231. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Z.; Guo, Z.; Tang, Q. Prediction of defluidization behavior of iron powder in a fluidized bed at elevated temperatures: Theoretical model and experimental verification. Powder Technol. 2013, 249, 175–180. [Google Scholar] [CrossRef]
- Zhong, Y.; Gao, J.; Wang, Z.; Guo, Z. Influence of particle size distribution on agglomeration/defluidization of iron powders at elevated temperature. ISIJ Int. 2017, 57, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lei, C.; Zhu, Q. Reduction of fine iron ore via a two-step fluidized bed direct reduction process. Powder Technol. 2014, 254, 1–11. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, Q.; Li, H. Experimental and theoretical study on the fluidization behaviors of iron powder at high temperature. Chem. Eng. Sci. 2014, 118, 50–59. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, Q.; Fan, C.; Pan, F.; Li, H.; Xie, Z. Influence of reduction condition on the morphology of newly formed metallic iron during the fluidized bed reduction of fine iron ores and its corresponding agglomeration behavior. Steel Res. Int. 2016, 87, 789–797. [Google Scholar] [CrossRef]
- Komatina, M.; Gudenau, H.W. The sticking problem during direct reduction of fine iron ore in the fluidized bed. Metall. Mater. Eng. 2004, 10, 309–328. [Google Scholar] [CrossRef]
- Krumbein, W.C.; Sloss, L.L. Stratigraphy and Sedimentation; W. H. Freeman and Company: San Francisco, CA, USA, 1951. [Google Scholar]
- Geldart, D. Types of gas fluidization. Powder Technol. 1973, 7, 285–292. [Google Scholar] [CrossRef]
- Goossens, W.R.A. Classification of fluidized particles by Archimedes number. Powder Technol. 1998, 98, 48–53. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Richardson, J.F.; Zaki, W.N. The sedimentation of a suspension of uniform spheres under conditions of viscous flow. Chem. Eng. Sci. 1954, 3, 65–73. [Google Scholar] [CrossRef]
- Grace, J.R. Contacting modes and behaviour classification of gas-solid and other two-phase suspensions. Can. J. Chem. Eng. 1986, 64, 353–363. [Google Scholar] [CrossRef]
- Shaul, S.; Rabinovich, E.; Kalman, H. Typical fluidization characteristics for Geldart’s classification groups. Part. Sci. Technol. 2014, 32, 197–205. [Google Scholar] [CrossRef]
- Molerus, O. Interpretation of Geldart’s type A, B, C and D powders by taking into account interparticle cohesion forces. Powder Technol. 1982, 33, 81–87. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Fluidization behavior and reducibility of iron ore fines during hydrogen-induced fluidized bed reduction. Particuology 2020, 52, 36–46. [Google Scholar] [CrossRef]
- Reh, L. Das Wirbeln von Körnigem Gut im Schlanken Diffusor als Grenzzustand Zwischen Wirbelschicht und Pneumatischer Förderung. Ph.D. Thesis, Technische Hochschule Karlsruhe, Karlsruhe, Germany, 1961. [Google Scholar]
- Reh, L. Auswahlkriterien für nichtkatalytische Gas/Feststoff-Hochtemperaturreaktoren. Chem. Ing. Tech. 1977, 49, 786–795. [Google Scholar] [CrossRef]
- Bruni, G.; Lettieri, P.; Newton, D.; Barletta, D. An investigation of the effect of the interparticle forces on the fluidization behaviour of fine powders linked with rheological studies. Chem. Eng. Sci. 2007, 62, 387–396. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H. Estimation of agglomerate size for cohesive particles during fluidization. Powder Technol. 1999, 101, 57–62. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H. Force balance modelling for agglomerating fluidization of cohesive particles. Powder Technol. 2000, 111, 60–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J. Prediction of dense phase voidage for group C+ fluidized bed reactor. Chem. Eng. J. 2020, 402, 126217. [Google Scholar] [CrossRef]
- Iwadate, Y.; Horio, M. Prediction of agglomerate sizes in bubbling fluidized beds of group C powders. Powder Technol. 1998, 100, 223–236. [Google Scholar] [CrossRef]
- Zhou, T.; Li, H. Effects of adding different size particles on fluidization of cohesive particles. Powder Technol. 1999, 102, 215–220. [Google Scholar] [CrossRef]
- Seville, J.P.K.; Willett, C.D.; Knight, P.C. Interparticle forces in fluidisation: A review. Powder Technol. 2000, 113, 261–268. [Google Scholar] [CrossRef]
- Massimilla, L.; Donsì, G. Cohesive forces between particles of fluid-bed catalysts. Powder Technol. 1976, 15, 253–260. [Google Scholar] [CrossRef]
- Xie, H.-Y. The role of interparticle forces in the fluidization of fine particles. Powder Technol. 1997, 94, 99–108. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.; Jin, Y.; Bi, H.T. On impacts of solid properties and operating conditions on the performance of gas-solid fluidization systems. Powder Technol. 2007, 172, 167–176. [Google Scholar] [CrossRef]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Moujahid, S.E.; Rist, A. The nucleation of iron on dense wustite: A morphological study. Metall. Trans. B 1988, 19, 787–802. [Google Scholar] [CrossRef]
- Nicolle, R.; Rist, A. The mechanism of whisker growth in the reduction of wüstite. Metall. Trans. B 1979, 10, 429–438. [Google Scholar] [CrossRef]
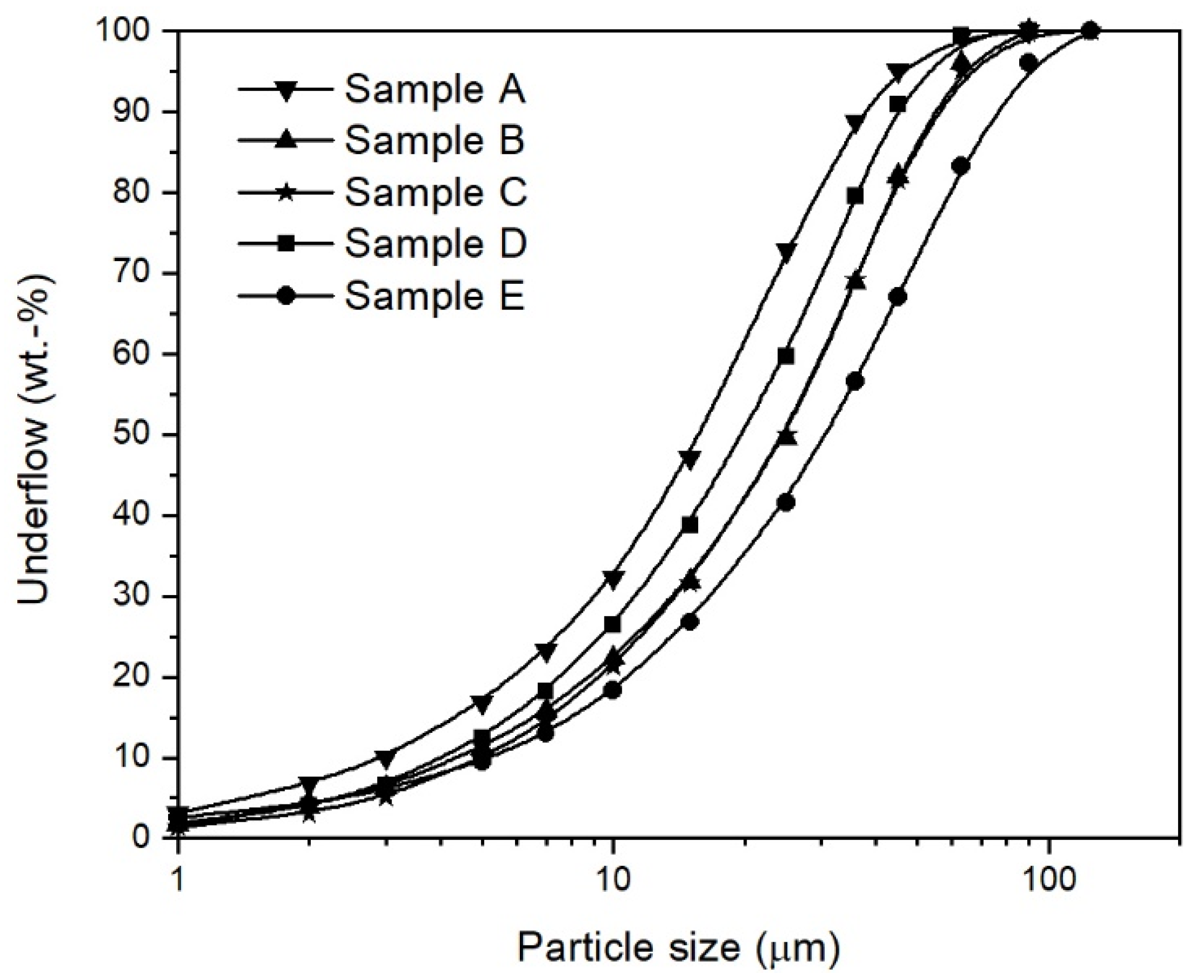
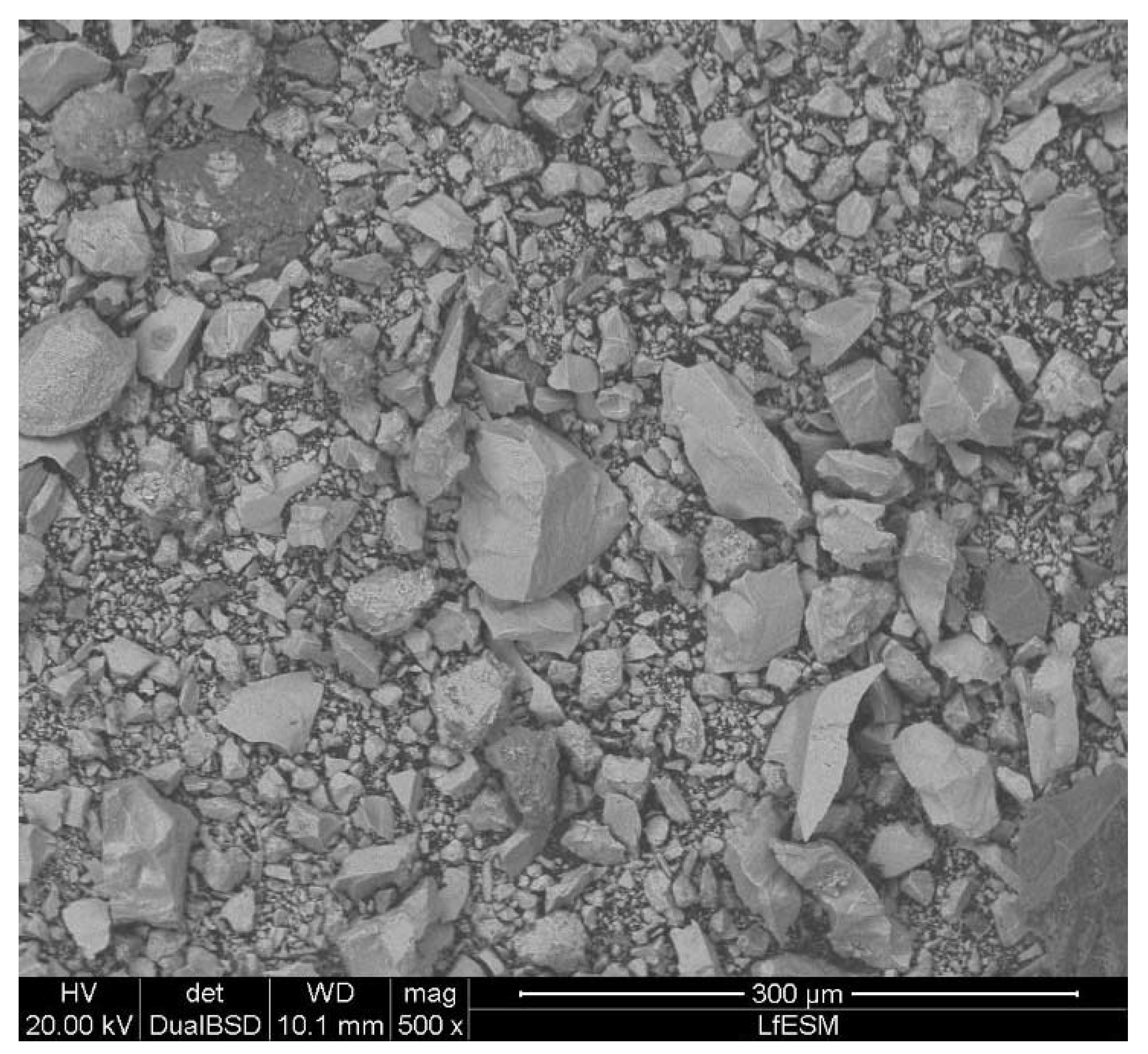
| Property | Sample A | Sample B | Sample C | Sample D | Sample E |
|---|---|---|---|---|---|
| d10 (µm) | 3.0 | 4.2 | 5.1 | 4.6 | 5.3 |
| d50 (µm) | 16.1 | 20.0 | 25.0 | 25.2 | 31.1 |
| d90 (µm) | 36.9 | 44.0 | 54.2 | 53.5 | 73.8 |
| Maximum particle size (µm) | 90.0 | 90.0 | 125.0 | 90.0 | 125.0 |
| True particle density, (kg/m3) | 4990 | 4980 | 4980 | 4870 | 4930 |
| Bulk density, (kg/m3) | 1925 | 1988 | 2117 | 1996 | 2093 |
| Void fraction of the bulk material, (−) | 0.61 | 0.60 | 0.57 | 0.59 | 0.58 |
| Sphericity, (−) | 0.75 | 0.65 | 0.60 | 0.70 | 0.60 |
| Fetot (wt.−%) | 68.06 | 69.76 | 69.09 | 67.31 | 66.96 |
| FeO (wt.−%) | 23.25 | 30.03 | 27.30 | 27.60 | 25.70 |
| SiO2 (wt.−%) | 2.84 | 2.32 | 3.78 | 6.65 | 2.33 |
| CaO (wt.−%) | 0.13 | 0.15 | 0.01 | 0.01 | 0.93 |
| Al2O3 (wt.−%) | 1.68 | 0.04 | 0.08 | 0.01 | 0.69 |
| MgO (wt.−%) | 0.27 | 0.19 | 0.49 | 0.42 | 0.56 |
| Property | 0% RD | 30% RD | 70% RD | 95% RD |
|---|---|---|---|---|
| Temperature, T (K) | 1173/873 | 1173/873 | 1173/873 | 1173/873 |
| Pressure gauge, p (barg) | 0.1 | 0.1 | 0.1 | 0.1 |
| Superficial gas velocity, (m/s) | 0.25 | 0.25 | 0.25 | 0.25 |
| Void fraction of the bulk material, (−) | 0.78 | 0.78 | 0.78 | 0.78 |
| Slip velocity, (m/s) | 0.321 | 0.321 | 0.321 | 0.321 |
| Gas utilization, (%) | 80 | 80 | 80 | 20 |
| Gas composition for 1173 K, xH2O (−) | 0.8 (1 × 0.8) | 0.66 (0.83 × 0.8) | 0.29 (0.37 × 0.8) | 0.07 (0.37 × 0.2) |
| Gas composition for 873 K, xH2O (−) | 0.8 (1 × 0.8) | 0.24 (0.30 × 0.8) | 0.20 (0.25 × 0.8) | 0.05 (0.25 × 0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfinger, T.; Spreitzer, D.; Schenk, J. Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation. Materials 2022, 15, 3943. https://doi.org/10.3390/ma15113943
Wolfinger T, Spreitzer D, Schenk J. Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation. Materials. 2022; 15(11):3943. https://doi.org/10.3390/ma15113943
Chicago/Turabian StyleWolfinger, Thomas, Daniel Spreitzer, and Johannes Schenk. 2022. "Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation" Materials 15, no. 11: 3943. https://doi.org/10.3390/ma15113943
APA StyleWolfinger, T., Spreitzer, D., & Schenk, J. (2022). Using Iron Ore Ultra-Fines for Hydrogen-Based Fluidized Bed Direct Reduction—A Mathematical Evaluation. Materials, 15(11), 3943. https://doi.org/10.3390/ma15113943







