Optimal Design of Ceramic Based Hip Implant Composites Using Hybrid AHP-MOORA Approach
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials and Hip Implant Composite Fabrication
2.2. Measurements
2.3. Wear Characterization
2.4. Determination and Implication of Criterion
- Criterion-1 (C-1): Density (g/cm3, Lower-is-better)
- Criterion-2 (C-2): Void content (Volume-%, Lower-is-better)
- Criterion-3 (C-3): Hardness (GPa, Higher-is-better)
- Criterion-4 (C-4): Indentation depth (nm, Lower-is-better)
- Criterion-5 (C-5): Elastic modulus (GPa, Higher-is-better)
- Criterion-6 (C-6): Fracture toughness (MPa.m1/2, Higher-is-better)
- Criterion-7 (C-7): Compressive strength (GPa, Higher-is-better)
- Criterion-8 (C-8): Wear (mm3/million cycles, Lower-is-better)
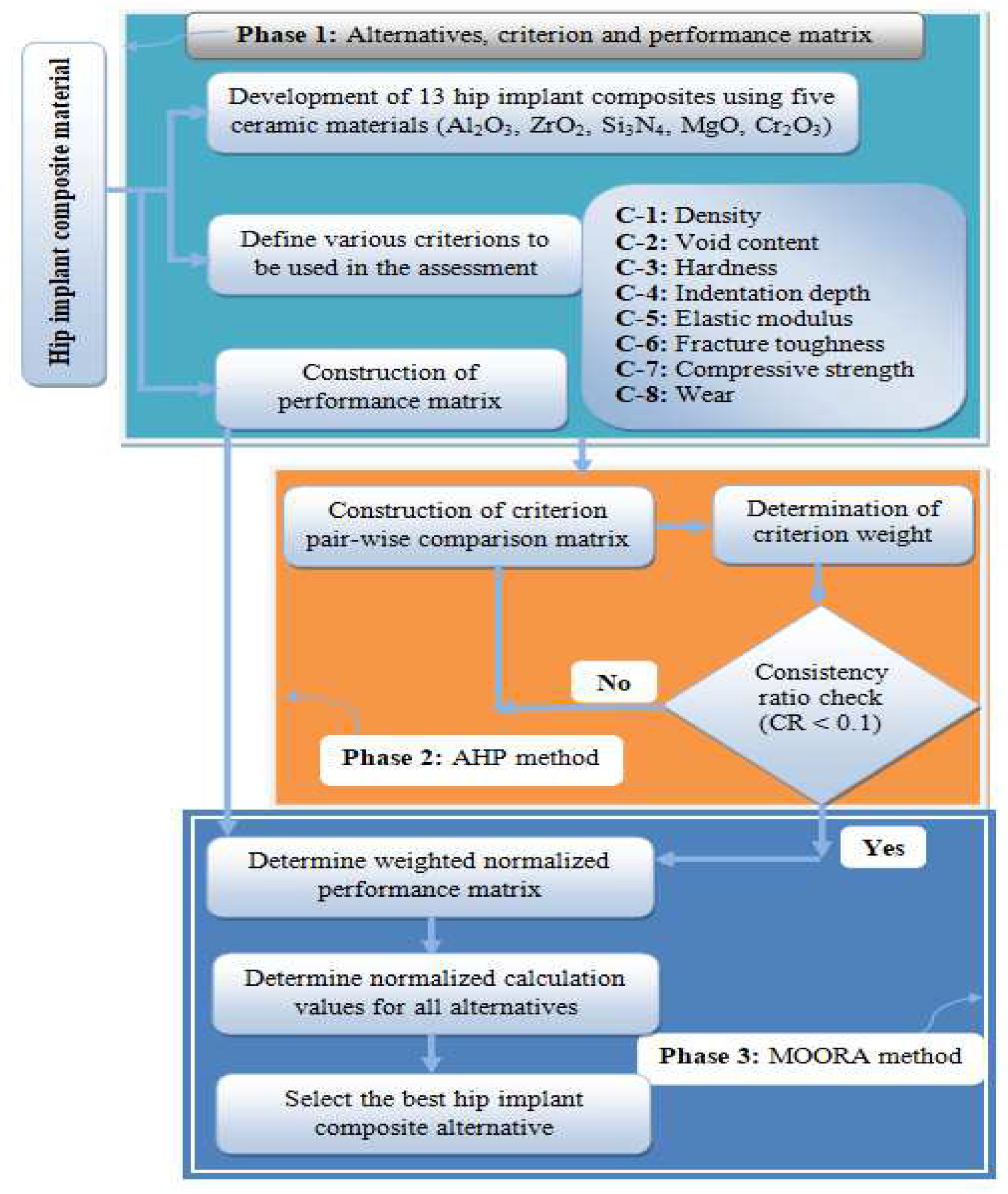
2.5. Evaluation Methodology
- Phase 1: Alternatives, criterion and creation of performance matrix
- Phase 2: AHP for criterion weight determination
- Phase 3: MOORA approach for alternatives ranking
2.5.1. Phase 1: Alternatives, Criterion and Creation of Performance Matrix
2.5.2. Phase 2: AHP for Criteria Weight Determination
2.5.3. Phase 3: MOORA Approach for Alternatives Ranking
3. Results and Discussion
3.1. Criteria Interpretation
3.2. Ranking of Alternative
3.2.1. Weight Calculation
3.2.2. Ranking Analysis
3.3. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Khanna, R.; Ong, J.L.; Oral, E.; Narayan, R.J. Progress in Wear Resistant Materials for Total Hip Arthroplasty. Coatings 2017, 7, 99. [Google Scholar] [CrossRef]
- Derar, H.; Shahinpoor, M. Recent patents and designs on hip replacement prostheses. Open Bio-Med. Eng. J. 2015, 9, 92. [Google Scholar] [CrossRef]
- Fernández-Fairén, M.; Torres-Perez, A.; Perez, R.; Punset, M.; Molmeneu, M.; Ortiz-Hernández, M.; Manero, J.M.; Gil, J. Early Short-Term Postoperative Mechanical Failures of Current Ceramic-on-Ceramic Bearing Total Hip Arthroplasties. Materials 2020, 13, 5318. [Google Scholar] [CrossRef]
- Bistolfi, A.; Ferracini, R.; Lee, G.C.; Mellano, D.; Guidotti, C.; Baino, F.; Verné, E. Ceramic-on-ceramic catastrophic liner failure in total hip arthroplasty: Morphological and compositional analysis of fractured ceramic components. Ceram. Int. 2021, 47, 11029–11036. [Google Scholar] [CrossRef]
- Tun, K.S.; Padnuru Sripathy, A.; Tekumalla, S.; Gupta, M. Development of Novel Lightweight Metastable Metal–(Metal + Ceramic) Composites Using a New Powder Metallurgy Approach. Materials 2020, 13, 3283. [Google Scholar] [CrossRef]
- Perrichon, A.; Reynard, B.; Gremillard, L.; Chevalier, J.; Farizon, F.; Geringer, J. A testing protocol combining shocks, hydrothermal ageing and friction, applied to Zirconia Toughened Alumina (ZTA) hip implants. J. Mech. Behav. Biomed. Mater. 2017, 65, 600–608. [Google Scholar] [CrossRef]
- Napier, R.J.; Shimmin, A.J. Ceramic-on-ceramic bearings in total hip arthroplasty: “The future is now”. Semin. Arthroplast. 2016, 27, 235–238. [Google Scholar] [CrossRef]
- Boutin, P. Experimental study of aluminium in surgery of hip. Presse Med. 1971, 79, 639–640. [Google Scholar]
- Piconi, C.; Sprio, S. Oxide Bioceramic Composites in Orthopedics and Dentistry. J. Compos. Sci. 2021, 5, 206. [Google Scholar] [CrossRef]
- Iwakiri, K.; Iwaki, H.; Minoda, Y.; Ohashi, H.; Takaoka, K. Alumina inlay failure in cemented polyethylene-backed total hip arthroplasty. Clin. Orthop. Relat. Res. 2008, 466, 1186–1192. [Google Scholar] [CrossRef]
- Al-Hajjar, M.; Carbone, S.; Jennings, L.M.; Begand, S.; Oberbach, T.; Delfosse, D.; Fisher, J. Wear of composite ceramics in mixed-material combinations in total hip replacement under adverse edge loading conditions. J. Biomed. Mater. Res. Part BAppl. Biomater. 2017, 105, 1361–1368. [Google Scholar] [CrossRef]
- Rahman, H.S.; Choudhury, D.; Osman, N.A.; Shasmin, H.N.; Abas, W.A. In vivo and in vitro outcomes of alumina, zirconia and their composited ceramic-on-ceramic hip joints. J. Ceram. Soc. Jpn. 2013, 121, 382–387. [Google Scholar] [CrossRef][Green Version]
- Jenabzadeh, A.R.; Pearce, S.J.; Walter, W.L. Total hip replacement: Ceramic-on-ceramic. Semin. Arthroplast. 2012, 23, 232–240. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Structure and thermomechanical properties of partially stabilized zirconia in the CaO-ZrO2 system. J. Am. Ceram. Soc. 1972, 55, 152–157. [Google Scholar] [CrossRef]
- Kaivosoja, E.; Tiainen, V.M.; Takakubo, Y.; Rajchel, B.; Sobiecki, J.; Konttinen, Y.T.; Takagi, M. Materials used for hip and knee implants. In Wear of Orthopaedic Implants and Artificial Joints; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2013; pp. 178–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, Y.; Li, W.; Jiang, S.; Cao, W.; Wu, Z.; Wang, K. Effect of MgO doping on properties of low zirconium content Ce-TZP/Al2O3 as a joint replacement material. Ceram. Int. 2017, 43, 2807–2814. [Google Scholar] [CrossRef]
- Shekhawat, D.; Singh, A.; Banerjee, M.K.; Singh, T.; Patnaik, A. Bioceramic composites for orthopaedic applications: A comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021, 147, 3013–3030. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kuwajima, H.; Masaki, T. Phase change and mechanical properties of ZrO2-Y2O3 solid electrolyte after ageing. SolidStateIon 1981, 3–4, 489–493. [Google Scholar] [CrossRef]
- Yoshimura, M.; Noma, T.; Kawabata, K.; Sōmiya, S. Role of H2O on the degradation process of Y-TZP. J. Mater. Sci. Lett. 1987, 6, 465–467. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Chevalier, J.; Cales, B.; Drouin, J. Low-temperature aging of Y-TZP ceramics. J. Am. Ceram. Soc. 1999, 82, 2150–2154. [Google Scholar] [CrossRef]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. SolidStateIon 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Kleiminger, L.; Li, T.; Li, K.; Kelsall, G.H. Syngas (CO-H2) production using high temperature micro-tubular solid oxide electrolysers. Electrochim. Acta 2015, 179, 565–577. [Google Scholar] [CrossRef]
- Arita, M.; Takahashi, Y.; Pezzotti, G.; Shishido, T.; Masoka, T.; Sano, K.; Yamamoto, K. Environmental stability and residual stresses in zirconia femoral head for total hip arthroplasty: In vitro aging versus retrieval studies. BioMedRes. Int. 2015, 2015, 638502. [Google Scholar] [CrossRef]
- Savin, A.; Craus, M.-L.; Turchenko, V.; Bruma, A.; Dubos, P.-A.; Malo, S.; Konstantinova, T.E.; Burkhovetsky, V.V. Monitoring Techniques of Cerium Stabilized Zirconia for Medical Prosthesis. Appl. Sci. 2015, 5, 1665–1682. [Google Scholar] [CrossRef]
- Solarino, G.; Spinarelli, A.; Virgilio, A.; Simone, F.; Baglioni, M.; Moretti, B. Outcomes of Ceramic Composite in Total Hip Replacement Bearings: A Single-Center Series. J. Compos. Sci. 2021, 5, 320. [Google Scholar] [CrossRef]
- Aherwar, A.; Patnaik, A.; Bahraminasab, M.; Singh, A. Preliminary evaluations on development of new materials for hip joint femoral head. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2019, 233, 885–899. [Google Scholar] [CrossRef]
- Bakhsh, A.A. Gamma-Ray Modified Polymer/Clay Composites: Synthesis, Characterization, and Formulation Optimization Using Multivariate Calculus and Graph Theory. Energies 2021, 14, 2724. [Google Scholar] [CrossRef]
- Shevtsov, S.; Zhilyaev, I.; Chang, S.-H.; Wu, J.-K.; Snezhina, N. Multi-Criteria Decision Approach to Design a Vacuum Infusion Process Layout Providing the Polymeric Composite Part Quality. Polymers 2022, 14, 313. [Google Scholar] [CrossRef]
- Singh, T. Utilization of cement bypass dust in the development of sustainable automotive brake friction composite materials. Arab. J. Chem. 2021, 14, 103324. [Google Scholar] [CrossRef]
- Paul, A.; Shukla, N.; Paul, S.K.; Trianni, A. Sustainable Supply Chain Management and Multi-Criteria Decision-Making Methods: A Systematic Review. Sustainability 2021, 13, 7104. [Google Scholar] [CrossRef]
- Bączkiewicz, A.; Kizielewicz, B.; Shekhovtsov, A.; Wątróbski, J.; Sałabun, W. Methodical Aspects of MCDM Based E-Commerce Recommender System. J. Theor. Appl. Electron. Commer. Res. 2021, 16, 2192–2229. [Google Scholar] [CrossRef]
- Singh, T. Optimum design based on fabricated natural fiber reinforced automotive brake friction composites using hybrid CRITIC-MEW approach. J. Mater. Res. Technol. 2021, 14, 81–92. [Google Scholar] [CrossRef]
- Simic, V.; Gokasar, I.; Deveci, M.; Karakurt, A. An integrated CRITIC and MABAC based type-2 neutrosophic model for public transportation pricing system selection. Socio-Econ. Plan. Sci. 2022, 80, 101157. [Google Scholar] [CrossRef]
- Singh, T. A hybrid multiple-criteria decision-making approach for selecting optimal automotive brake friction composite. Mater. Des. Processing Commun. 2021, 3, e266. [Google Scholar] [CrossRef]
- Akkaya, G.; Turanoğlu, B.; Öztaş, S. An integrated fuzzy AHP and fuzzy MOORA approach to the problem of industrial engineering sector choosing. Expert Syst. Appl. 2015, 42, 9565–9573. [Google Scholar] [CrossRef]
- Indrajayanthan, V.; Mohanty, N.K. Assessment of Clean Energy Transition Potential in Major Power-Producing States of India Using Multi-Criteria Decision Analysis. Sustainability 2022, 14, 1166. [Google Scholar] [CrossRef]
- Goswami, C.; Bhat, I.K.; Bathula, S.; Singh, T.; Patnaik, A. Physico-mechanical and surface wear assessment of magnesium oxide filled ceramic composites for hip implant application. Silicon 2019, 11, 39–49. [Google Scholar] [CrossRef]
- Aragón-Duartea, M.C.; Nevarez-Rascónb, A.; Esparza-Poncea, H.E.; Nevarez-Rascónb, M.M.; Talamantesa, R.P.; Ornelasa, C.; Mendez-Nonella, J.; González-Hernándezc, J.; Yacamánd, M.J.; Hurtado-Macías, A. Nanomechanical properties of zirconia- yttria and alumina zirconia- yttria biomedical ceramics, subjected to low temperature aging. Ceram. Int. 2017, 43, 3931–3939. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Anstis, G.R.; Chantikul, P.; Lawn, B.R.; Marshall, D.B. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Goswami, C.; Bhat, I.K.; Patnaik, A.; Singh, T.; Fekete, G. Fabrication of Ceramic Hip Implant Composites: Influence of silicon nitride on physical, mechanical and wear properties. Silicon 2020, 12, 1237–1245. [Google Scholar] [CrossRef]
- ISO-23317; Implants for Surgery-In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Organization for Standardization: Geneva, Switzerland, 2007.
- Harnpornchai, N.; Wonggattaleekam, W. An Application of Neutrosophic Set to Relative Importance Assignment in AHP. Mathematics 2021, 9, 2636. [Google Scholar] [CrossRef]
- Singh, T.; Patnaik, A.; Fekete, G.; Chauhan, R.; Gangil, B. Application of hybrid analytical hierarchy process and complex proportional assessment approach for optimal design of brake friction materials. Polym. Compos. 2019, 40, 1602–1608. [Google Scholar] [CrossRef]
- Brauers, W.K.M. Multi-objective seaport planning by MOORA decision making. Ann. Oper. Res. 2013, 206, 39–58. [Google Scholar] [CrossRef]
- Brodny, J.; Tutak, M. Analyzing the Level of Digitalization among the Enterprises of the European Union Member States and Their Impact on Economic Growth. J. Open Innov. Technol. Mark. Complex. 2022, 8, 70. [Google Scholar] [CrossRef]
- Gupta, K.; Roy, S.; Poonia, R.C.; Kumar, R.; Nayak, S.R.; Altameem, A.; Saudagar, A.K.J. Multi-Criteria Usability Evaluation of mHealth Applications on Type 2 Diabetes Mellitus Using Two Hybrid MCDM Models: CODAS-FAHP and MOORA-FAHP. Appl. Sci. 2022, 12, 4156. [Google Scholar] [CrossRef]
- Li, W.; Gao, L. Rapid sintering of nanocrystalline ZrO2(3Y) by spark plasma sintering. J. Eur. Ceram. Soc. 2000, 20, 2441–2445. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Gardini, D.; Traverso, P.L.; Faga, M.G.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part II: Chemical stability and wear resistance in body environment. J. Mater. Sci. Mater. Med. 2008, 19, 2889. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 5, 6940–6946. [Google Scholar] [CrossRef]
- Bull, S.J.; Moharrami, N.; Langton, D. Mechanistic study of the wear of ceramic heads by metallic stems in modular implants. J. Bio- Tribo-Corros. 2017, 3, 7. [Google Scholar] [CrossRef]
- Sedlák, R.; Ivor, M.; Klimczyk, P.; Wyzga, P.; Podsiadlo, M.; Vojtko, M.; Dusza, J. Micro/Nano Indentation Testing of Spark Plasma Sintered Al2O3 + ZrO2 + cBN Ceramics. Ceramics 2021, 4, 40–53. [Google Scholar] [CrossRef]
- Benavente, R.; Salvador, M.D.; Penaranda-Foix, F.L.; Pallone, E.; Borrell, A. Mechanical properties and microstructural evolution of alumina–zirconia nanocomposites by microwave sintering. Ceram. Int. 2014, 40, 11291–11297. [Google Scholar] [CrossRef]
- Sarker, S.; Mumu, H.T.; Al-Amin, M.; Alam, M.Z.; Gafur, M.A. Impacts of inclusion of additives on physical, microstructural, and mechanical properties of Alumina and Zirconia toughened alumina (ZTA) ceramic composite: A review. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Arab, A.; Sktani, Z.D.I.; Zhou, Q.; Ahmad, Z.A.; Chen, P. Effect of MgO Addition on the Mechanical and Dynamic Properties of Zirconia Toughened Alumina (ZTA) Ceramics. Materials 2019, 12, 2440. [Google Scholar] [CrossRef]
- Riu, D.-H.; Kong, Y.-M.; Kim, H.-E. Effect of Cr2O3 addition on microstructural evolution and me-chanical properties of Al2O3. Doh-Hyung. J. Eur. Ceram. Soc. 2000, 20, 1475–1481. [Google Scholar] [CrossRef]
- Heimann, R.B. Silicon Nitride, a Close to Ideal Ceramic Material for Medical Application. Ceramics 2021, 4, 208–223. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, B.S.; Lostak, J.; Janout, M. Advantages and disadvantages of ceramic on ceramic total hip arthroplasty: A review. Biomed. Pap. 2012, 156, 204–212. [Google Scholar] [CrossRef]
- Harding, D.; Loesener, G.; Ngyuen, B.; Blackburn, D.; Dixon, R.; Taylor, J. Comparison of Wear for Polycrystalline Diamond, Cobalt Chrome, and Polyethylene in a High Hertzian Stress Environment. Orthop. Proc. 2018, 94-B, SUPP_XL. [Google Scholar]
- Amaral, M.; Maru, M.M.; Rodrigues, S.P.; Gouvêa, C.P.; Trommer, R.M.; Oliveira, F.J.; Achete, C.A.; Silva, R.F. Extremely low wear rates in hip joint bearings coated with nanocrystalline diamond. Tribol. Int. 2015, 89, 72–77. [Google Scholar] [CrossRef]
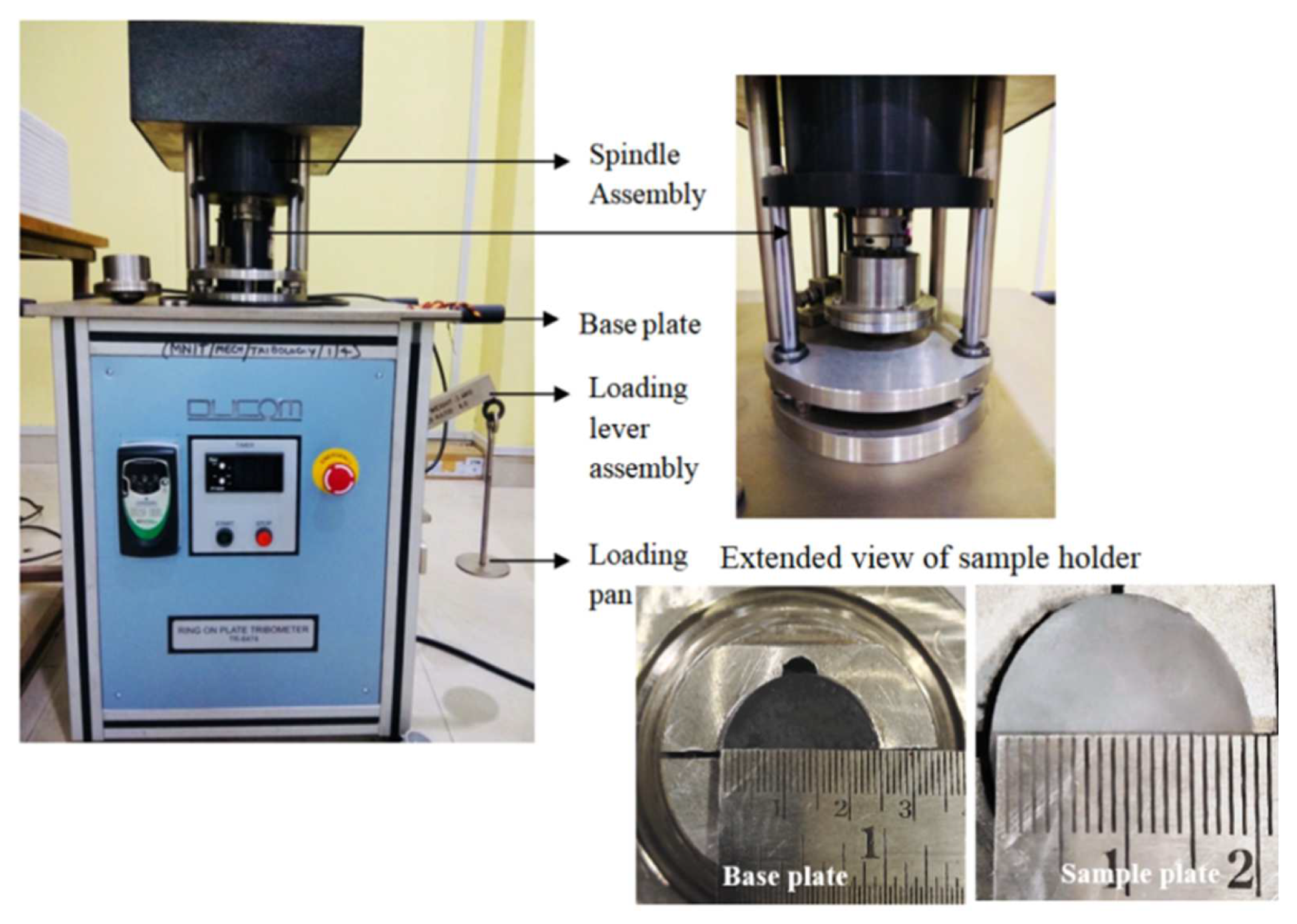
| Composition (wt.%) | Alternatives | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-1 | A-2 | A-3 | A-4 | A-5 | A-6 | A-7 | A-8 | A-9 | A-10 | A-11 | A-12 | A-13 | |
| Al2O3 | 72 | 71.25 | 70.5 | 69.75 | 73.5 | 72 | 69 | 75.5 | 73 | 68 | 93 | 83 | 63 |
| ZrO2 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 0 | 10 | 30 |
| Si3N4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 0 | 2.5 | 7.5 | 2.5 | 2.5 | 2.5 |
| MgO | 3 | 3 | 3 | 3 | 0 | 1.5 | 4.5 | 3 | 3 | 3 | 3 | 3 | 3 |
| Cr2O3 | 0 | 0.75 | 1.5 | 2.25 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Alternatives | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 |
|---|---|---|---|---|---|---|---|---|
| A-1 | 4.151 ± 0.001 | 0.0021 | 19.19 ± 0.68 | 79.03 ± 2.32 | 226.12 ± 6.85 | 04.19 ± 0.17 | 2.715 ± 0.07 | 0.0246 ± 0.0008 |
| A-2 | 4.154 ± 0.002 | 0.0033 | 21.34 ± 1.07 | 72.92 ± 1.82 | 239.10 ± 6.29 | 04.85 ± 0.24 | 2.772 ± 0.07 | 0.0152 ± 0.0005 |
| A-3 | 4.158 ± 0.002 | 0.0042 | 27.83 ± 1.16 | 69.61 ± 1.55 | 276.55 ± 9.88 | 11.41 ± 0.41 | 2.841 ± 0.07 | 0.0078 ± 0.0004 |
| A-4 | 4.161 ± 0.001 | 0.0054 | 20.93 ± 0.88 | 73.68 ± 1.67 | 232.05 ± 5.53 | 04.90 ± 0.15 | 2.796 ± 0.07 | 0.0127 ± 0.0006 |
| A-5 | 4.152 ± 0.001 | 0.0089 | 19.33 ± 0.76 | 86.03 ± 2.26 | 243.12 ± 6.57 | 05.75 ± 0.19 | 2.730 ± 0.08 | 0.0323 ± 0.0010 |
| A-6 | 4.156 ± 0.002 | 0.0063 | 20.99 ± 0.84 | 82.92 ± 1.68 | 244.10 ± 4.88 | 07.84 ± 0.27 | 2.793 ± 0.04 | 0.0276 ± 0.0008 |
| A-7 | 4.150 ± 0.002 | 0.0045 | 19.93 ± 0.72 | 87.68 ± 1.58 | 229.05 ± 4.16 | 05.87 ± 0.23 | 2.816 ± 0.03 | 0.0289 ± 0.0008 |
| A-8 | 4.190 ± 0.001 | 0.0044 | 19.45 ± 0.61 | 76.14 ± 2.82 | 235.85 ± 5.24 | 08.63 ± 0.26 | 2.718 ± 0.05 | 0.0213 ± 0.0009 |
| A-9 | 4.176 ± 0.002 | 0.0038 | 28.64 ± 1.10 | 67.93 ± 1.66 | 280.18 ± 6.83 | 11.84 ± 0.48 | 2.861 ± 0.05 | 0.0076 ± 0.0003 |
| A-10 | 4.142 ± 0.003 | 0.0043 | 22.69 ± 0.71 | 72.18 ± 2.01 | 245.12 ± 4.71 | 09.93 ± 0.34 | 2.810 ± 0.06 | 0.0139 ± 0.0007 |
| A-11 | 3.920 ± 0.002 | 0.0045 | 19.93 ± 0.22 | 79.74 ± 2.28 | 263.12 ± 7.97 | 08.47 ± 0.22 | 2.814 ± 0.06 | 0.0196 ± 0.0006 |
| A-12 | 4.048 ± 0.002 | 0.0032 | 28.81 ± 0.96 | 63.25 ± 1.64 | 291.00 ± 9.72 | 11.97 ± 0.28 | 2.894 ± 0.06 | 0.0071 ± 0.0002 |
| A-13 | 4.307 ± 0.002 | 0.0058 | 20.79 ± 0.72 | 78.83 ± 1.43 | 253.05 ± 7.23 | 07.45 ± 0.21 | 2.818 ± 0.05 | 0.0127 ± 0.0003 |
| C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 | |
|---|---|---|---|---|---|---|---|---|
| C-1 | 1 | 1.5 | 0.5 | 0.67 | 0.33 | 0.25 | 0.67 | 0.17 |
| C-2 | 0.67 | 1 | 0.25 | 0.5 | 0.2 | 0.17 | 0.33 | 0.13 |
| C-3 | 2 | 4 | 1 | 1.5 | 2 | 0.33 | 1.50 | 0.25 |
| C-4 | 1.5 | 2 | 0.67 | 1 | 0.67 | 0.25 | 0.5 | 0.2 |
| C-5 | 3 | 5 | 0.5 | 1.5 | 1 | 0.5 | 2 | 0.33 |
| C-6 | 4 | 6 | 3 | 4 | 2 | 1 | 4 | 0.5 |
| C-7 | 1.50 | 3 | 0.67 | 2 | 0.5 | 0.25 | 1 | 0.2 |
| C-8 | 6 | 8 | 4 | 5 | 3 | 2 | 5 | 1 |
| Criterion | Consistency Parameters | ||
|---|---|---|---|
| C-1 | 0.048 | ||
| C-2 | 0.030 | ||
| C-3 | 0.108 | λmax = 8.23 | |
| C-4 | 0.062 | CI = 0.033 | CR = 0.023 |
| C-5 | 0.111 | RI = 1.41 | |
| C-6 | 0.227 | ||
| C-7 | 0.074 | ||
| C-8 | 0.340 | ||
| Alternatives | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 |
|---|---|---|---|---|---|---|---|---|
| A-1 | 0.2778 | 0.1167 | 0.2358 | 0.2867 | 0.2494 | 0.1390 | 0.2691 | 0.3465 |
| A-2 | 0.2780 | 0.1833 | 0.2623 | 0.2645 | 0.2637 | 0.1609 | 0.2747 | 0.2141 |
| A-3 | 0.2783 | 0.2333 | 0.3420 | 0.2525 | 0.3050 | 0.3786 | 0.2815 | 0.1099 |
| A-4 | 0.2785 | 0.3000 | 0.2572 | 0.2673 | 0.2560 | 0.1626 | 0.2771 | 0.1789 |
| A-5 | 0.2779 | 0.4944 | 0.2376 | 0.3121 | 0.2682 | 0.1908 | 0.2705 | 0.4549 |
| A-6 | 0.2781 | 0.3500 | 0.2580 | 0.3008 | 0.2692 | 0.2602 | 0.2768 | 0.3887 |
| A-7 | 0.2777 | 0.2500 | 0.2449 | 0.3181 | 0.2526 | 0.1948 | 0.2791 | 0.4070 |
| A-8 | 0.2804 | 0.2444 | 0.2390 | 0.2762 | 0.2601 | 0.2864 | 0.2693 | 0.3000 |
| A-9 | 0.2795 | 0.2111 | 0.3520 | 0.2464 | 0.3090 | 0.3929 | 0.2835 | 0.1070 |
| A-10 | 0.2772 | 0.2389 | 0.2789 | 0.2618 | 0.2704 | 0.3295 | 0.2785 | 0.1958 |
| A-11 | 0.2623 | 0.2500 | 0.2449 | 0.2893 | 0.2902 | 0.2811 | 0.2789 | 0.2761 |
| A-12 | 0.2709 | 0.1778 | 0.3541 | 0.2294 | 0.3210 | 0.3972 | 0.2868 | 0.1000 |
| A-13 | 0.2882 | 0.3222 | 0.2555 | 0.2860 | 0.2791 | 0.2472 | 0.2793 | 0.1789 |
| Weighted Matrix | Ranking of Alternatives | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternatives | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | C-7 | C-8 | Ranking | |||
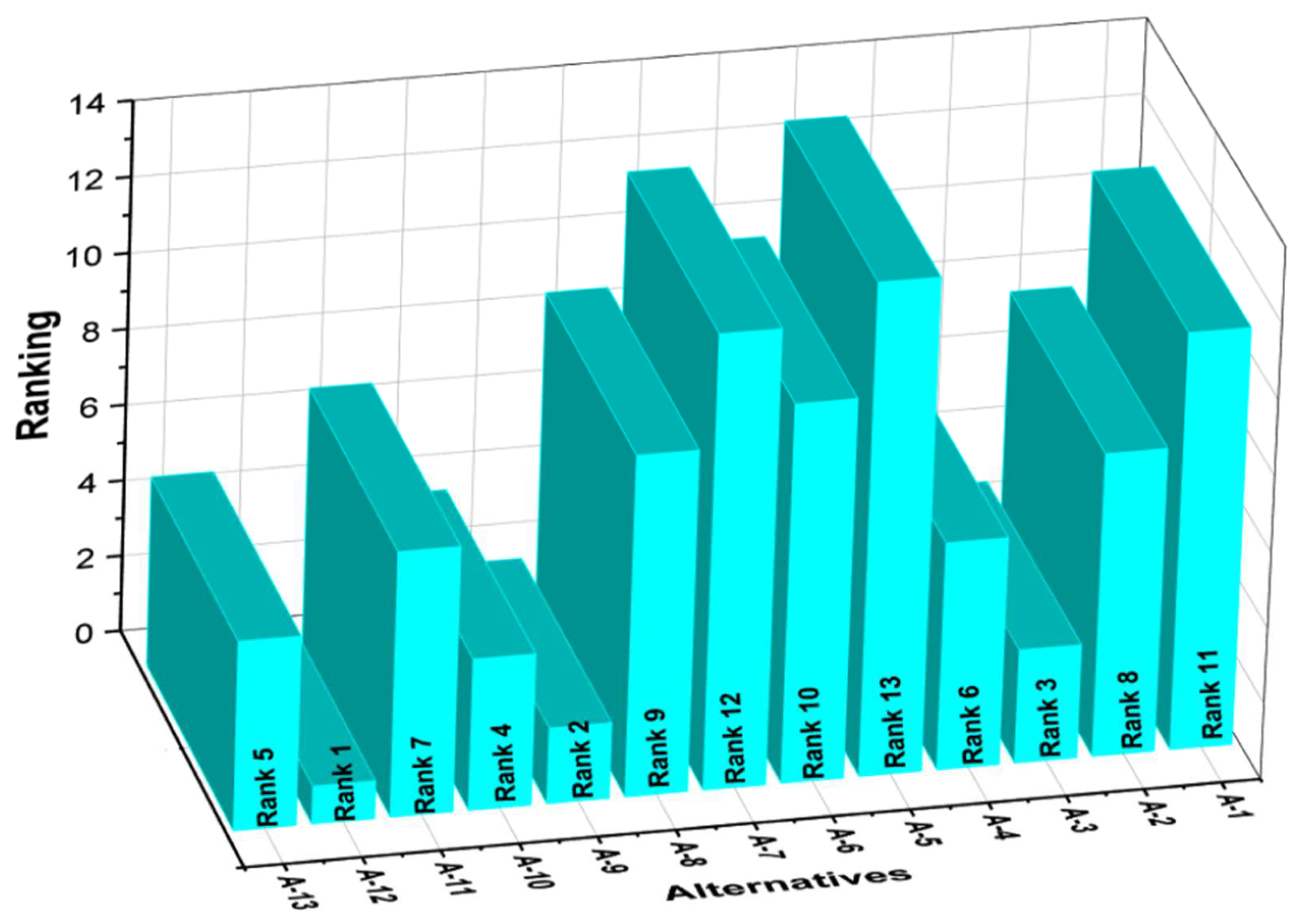
| A-1 | 0.0133 | 0.0035 | 0.0255 | 0.0178 | 0.0277 | 0.0316 | 0.0199 | 0.1178 | 0.1046 | 0.1524 | −0.0478 | 11 |
| A-2 | 0.0133 | 0.0055 | 0.0283 | 0.0164 | 0.0293 | 0.0365 | 0.0203 | 0.0728 | 0.1145 | 0.1080 | 0.0064 | 8 |
| A-3 | 0.0134 | 0.0070 | 0.0369 | 0.0157 | 0.0339 | 0.0860 | 0.0208 | 0.0374 | 0.1776 | 0.0734 | 0.1042 | 3 |
| A-4 | 0.0134 | 0.0090 | 0.0278 | 0.0166 | 0.0284 | 0.0369 | 0.0205 | 0.0608 | 0.1136 | 0.0998 | 0.0139 | 6 |
| A-5 | 0.0133 | 0.0148 | 0.0257 | 0.0193 | 0.0298 | 0.0433 | 0.0200 | 0.1547 | 0.1188 | 0.2022 | −0.0834 | 13 |
| A-6 | 0.0134 | 0.0105 | 0.0279 | 0.0186 | 0.0299 | 0.0591 | 0.0205 | 0.1322 | 0.1373 | 0.1747 | −0.0374 | 10 |
| A-7 | 0.0133 | 0.0075 | 0.0265 | 0.0197 | 0.0280 | 0.0442 | 0.0207 | 0.1384 | 0.1194 | 0.1789 | −0.0596 | 12 |
| A-8 | 0.0135 | 0.0073 | 0.0258 | 0.0171 | 0.0289 | 0.0650 | 0.0199 | 0.1020 | 0.1396 | 0.1399 | −0.0003 | 9 |
| A-9 | 0.0134 | 0.0063 | 0.0380 | 0.0153 | 0.0343 | 0.0892 | 0.0210 | 0.0364 | 0.1825 | 0.0714 | 0.1111 | 2 |
| A-10 | 0.0133 | 0.0072 | 0.0301 | 0.0162 | 0.0300 | 0.0748 | 0.0206 | 0.0666 | 0.1555 | 0.1033 | 0.0523 | 4 |
| A-11 | 0.0126 | 0.0075 | 0.0265 | 0.0179 | 0.0322 | 0.0638 | 0.0206 | 0.0939 | 0.1431 | 0.1319 | 0.0112 | 7 |
| A-12 | 0.0130 | 0.0053 | 0.0382 | 0.0142 | 0.0356 | 0.0902 | 0.0212 | 0.0340 | 0.1853 | 0.0666 | 0.1187 | 1 |
| A-13 | 0.0138 | 0.0097 | 0.0276 | 0.0177 | 0.0310 | 0.0561 | 0.0207 | 0.0608 | 0.1354 | 0.1020 | 0.0333 | 5 |
| Alternatives | Weight Level | ||||||
|---|---|---|---|---|---|---|---|
| −15% | −10% | −5% | Original | +5% | +10% | +15% | |
| A-1 | −0.0538 (11) | −0.0517 (11) | −0.0497 (11) | −0.0477 (11) | −0.0457 (11) | −0.0437 (11) | −0.0416 (11) |
| A-2 | 0.0003 (8) | 0.0024 (8) | 0.0045 (8) | 0.0065 (8) | 0.0086 (8) | 0.0106 (8) | 0.0127 (8) |
| A-3 | 0.0917 (3) | 0.0959 (3) | 0.1001 (3) | 0.1043 (3) | 0.1086 (3) | 0.1128 (3) | 0.1170 (3) |
| A-4 | 0.0072 (6) | 0.0095 (6) | 0.0117 (6) | 0.0139 (6) | 0.0162 (6) | 0.0184 (7) | 0.0206 (7) |
| A-5 | −0.0936 (13) | −0.0901 (13) | −0.0867 (13) | −0.0833 (13) | −0.0800 (13) | −0.0766 (13) | −0.0731 (13) |
| A-6 | −0.0487 (10) | −0.0448 (10) | −0.0411 (10) | −0.0373 (10) | −0.0335 (10) | −0.0297 (10) | −0.0259 (10) |
| A-7 | −0.0685 (12) | −0.0654 (12) | −0.0625 (12) | −0.0595 (12) | −0.0565 (12) | −0.0535 (12) | −0.0505 (12) |
| A-8 | −0.0116 (9) | −0.0078 (9) | −0.0040 (9) | −0.0002 (9) | 0.0036 (9) | 0.0074 (9) | 0.0112 (9) |
| A-9 | 0.0983 (2) | 0.1026 (2) | 0.1069 (2) | 0.1112 (2) | 0.1155 (2) | 0.1198 (2) | 0.1241 (2) |
| A-10 | 0.0404 (4) | 0.0444 (4) | 0.0484 (4) | 0.0524 (4) | 0.0564 (4) | 0.0603 (4) | 0.0643 (4) |
| A-11 | 0.0004 (7) | 0.0041 (7) | 0.0077 (7) | 0.0113 (7) | 0.0149 (7) | 0.0185 (6) | 0.0222 (6) |
| A-12 | 0.1051 (1) | 0.1093 (1) | 0.1135 (1) | 0.1178 (1) | 0.1220 (1) | 0.1262 (1) | 0.1305 (1) |
| A-13 | 0.0237 (5) | 0.0270 (5) | 0.0302 (5) | 0.0334 (5) | 0.0366 (5) | 0.0399 (5) | 0.0431 (5) |
| Alternatives | Weight Level | ||||||
|---|---|---|---|---|---|---|---|
| −15% | −10% | −5% | Original | +5% | +10% | +15% | |
| A-1 | −0.0285 (11) | −0.0348 (11) | −0.0414 (11) | −0.0477 (11) | −0.0540 (11) | −0.0614 (11) | −0.0669 (11) |
| A-2 | 0.0191 (8) | 0.0150 (8) | 0.0106 (8) | 0.0065 (8) | 0.0024 (8) | −0.0028 (8) | −0.0060 (8) |
| A-3 | 0.1138 (3) | 0.1108 (3) | 0.1073 (3) | 0.1043 (3) | 0.1014 (3) | 0.0971 (3) | 0.0949 (3) |
| A-4 | 0.0238 (7) | 0.0206 (7) | 0.0172 (6) | 0.0139 (6) | 0.0107 (6) | 0.0065 (6) | 0.0041 (6) |
| A-5 | −0.0610 (13) | −0.0685 (13) | −0.0758 (13) | −0.0833 (13) | −0.0908 (13) | −0.0991 (13) | −0.1057 (13) |
| A-6 | −0.0165 (10) | −0.0234 (10) | −0.0304 (10) | −0.0373 (10) | −0.0442 (10) | −0.0520 (10) | −0.0581 (10) |
| A-7 | −0.0378 (12) | −0.0450 (12) | −0.0523 (12) | −0.0595 (12) | −0.0667 (12) | −0.0748 (12) | −0.0811 (12) |
| A-8 | 0.0169 (9) | 0.0113 (9) | 0.0054 (9) | −0.0002 (9) | −0.0058 (9) | −0.0125 (9) | −0.0173 (9) |
| A-9 | 0.1209 (2) | 0.1178 (2) | 0.1142 (2) | 0.1112 (2) | 0.1082 (2) | 0.1037 (2) | 0.1015 (2) |
| A-10 | 0.0650 (4) | 0.0609 (4) | 0.0565 (4) | 0.0524 (4) | 0.0483 (4) | 0.0430 (4) | 0.0397 (4) |
| A-11 | 0.0275 (6) | 0.0222 (6) | 0.0166 (7) | 0.0113 (7) | 0.0060 (7) | −0.0003 (7) | −0.0048 (7) |
| A-12 | 0.1276 (1) | 0.1245 (1) | 0.1208 (1) | 0.1178 (1) | 0.1147 (1) | 0.1102 (1) | 0.1080 (1) |
| A-13 | 0.0437 (5) | 0.0403 (5) | 0.0368 (5) | 0.0334 (5) | 0.0300 (5) | 0.0256 (5) | 0.0231 (5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, T.; Goswami, C.; Patnaik, A.; Lendvai, L. Optimal Design of Ceramic Based Hip Implant Composites Using Hybrid AHP-MOORA Approach. Materials 2022, 15, 3800. https://doi.org/10.3390/ma15113800
Singh T, Goswami C, Patnaik A, Lendvai L. Optimal Design of Ceramic Based Hip Implant Composites Using Hybrid AHP-MOORA Approach. Materials. 2022; 15(11):3800. https://doi.org/10.3390/ma15113800
Chicago/Turabian StyleSingh, Tej, Chandramani Goswami, Amar Patnaik, and László Lendvai. 2022. "Optimal Design of Ceramic Based Hip Implant Composites Using Hybrid AHP-MOORA Approach" Materials 15, no. 11: 3800. https://doi.org/10.3390/ma15113800
APA StyleSingh, T., Goswami, C., Patnaik, A., & Lendvai, L. (2022). Optimal Design of Ceramic Based Hip Implant Composites Using Hybrid AHP-MOORA Approach. Materials, 15(11), 3800. https://doi.org/10.3390/ma15113800









