Research Progress of Alumina-Forming Austenitic Stainless Steels: A Review
Abstract
:1. Introduction
2. The Types of AFA Steel and Design Method
2.1. The Types of AFA Steel
2.2. The Design Methods of AFA Steels
- (1)
- Phase Diagram
- (2)
- Thermodynamic Software
- (3)
- Genetic Algorithm
3. Precipitates of AFA Steel
3.1. Precipitates
3.1.1. Carbides
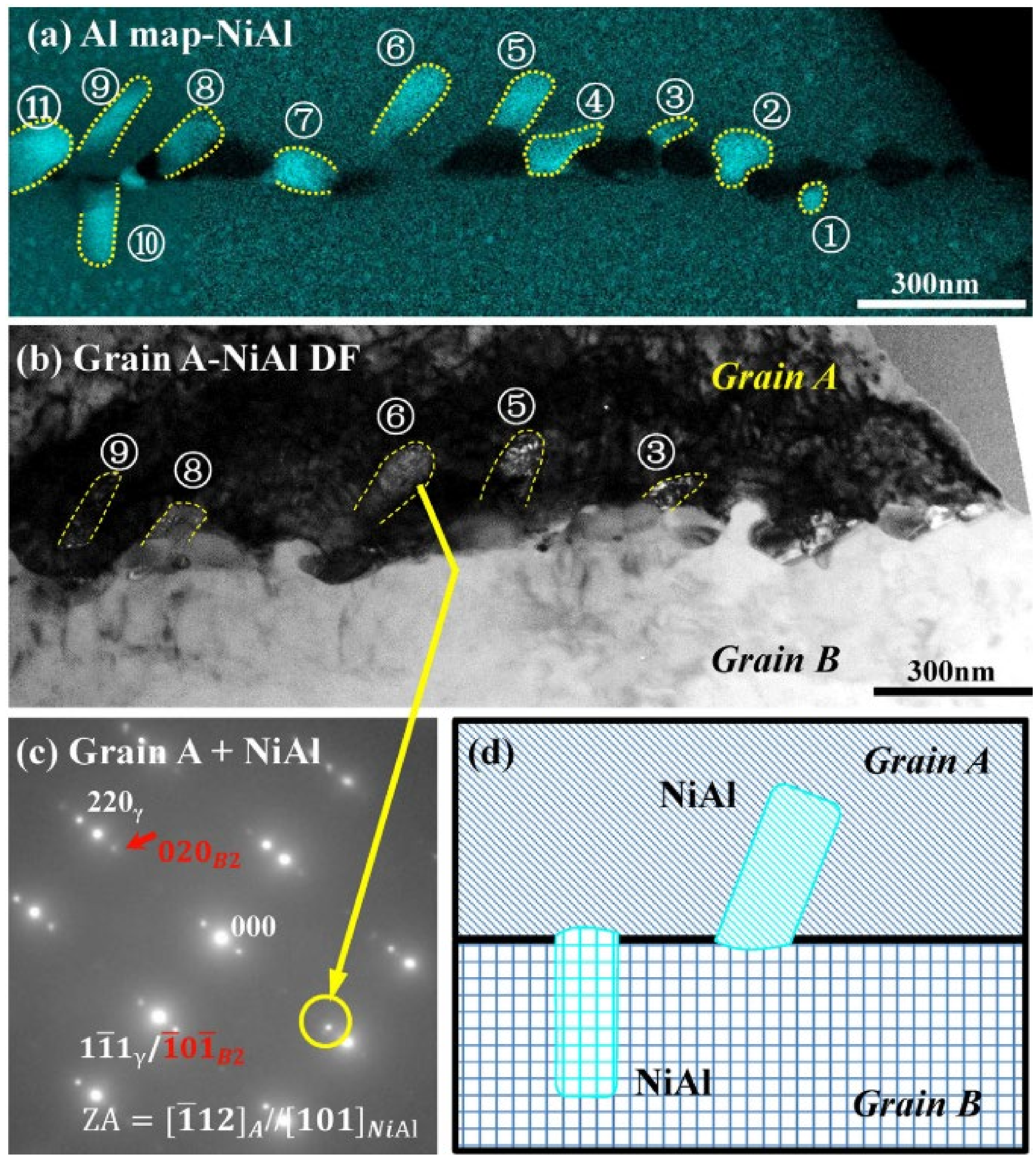
3.1.2. B2-NiAl
3.1.3. Fe2(Mo, Nb)-Laves
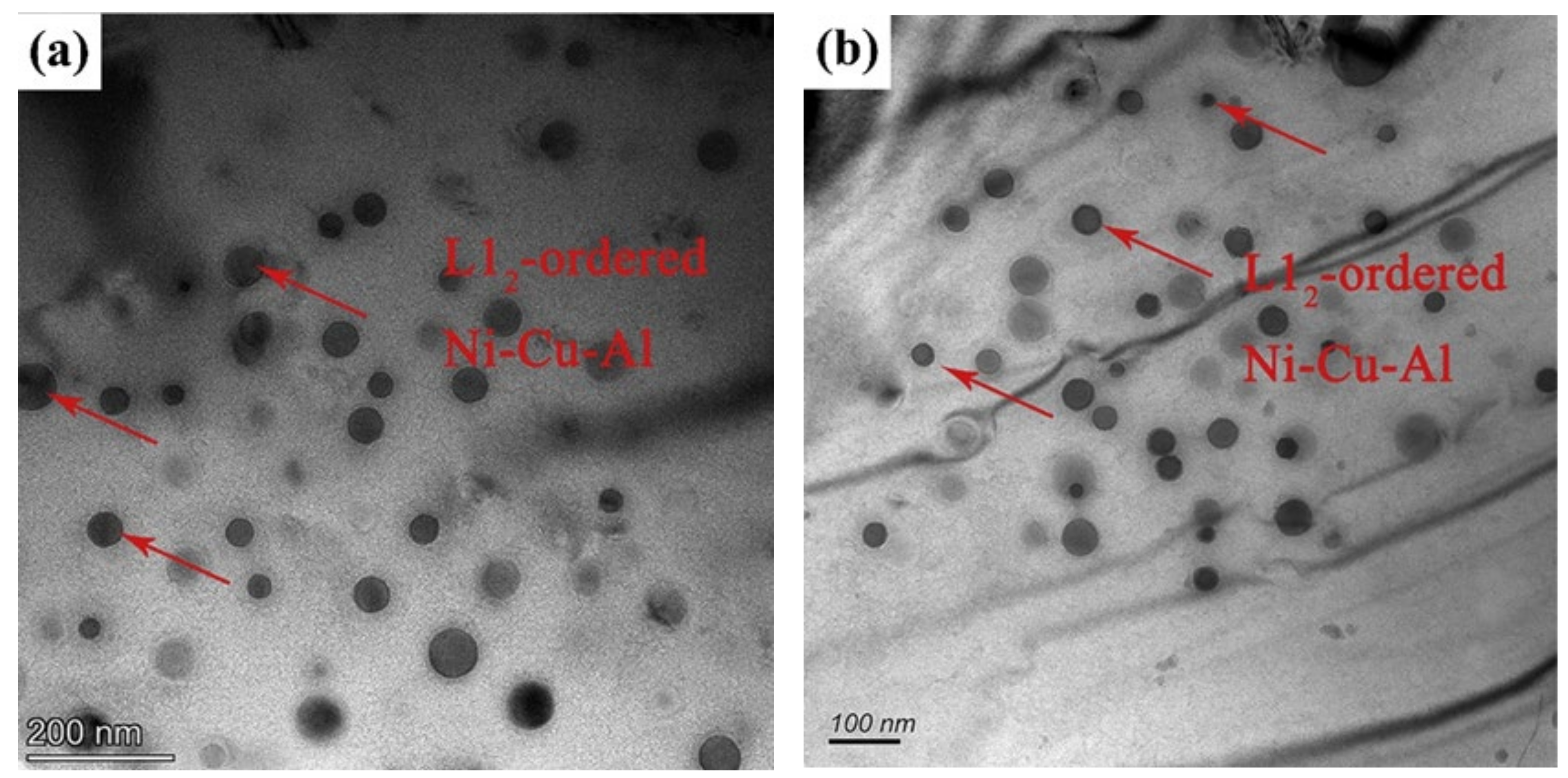
3.1.4. γ′ Phase
3.1.5. σ
3.2. Factors Affecting Precipitates in AFA Steels
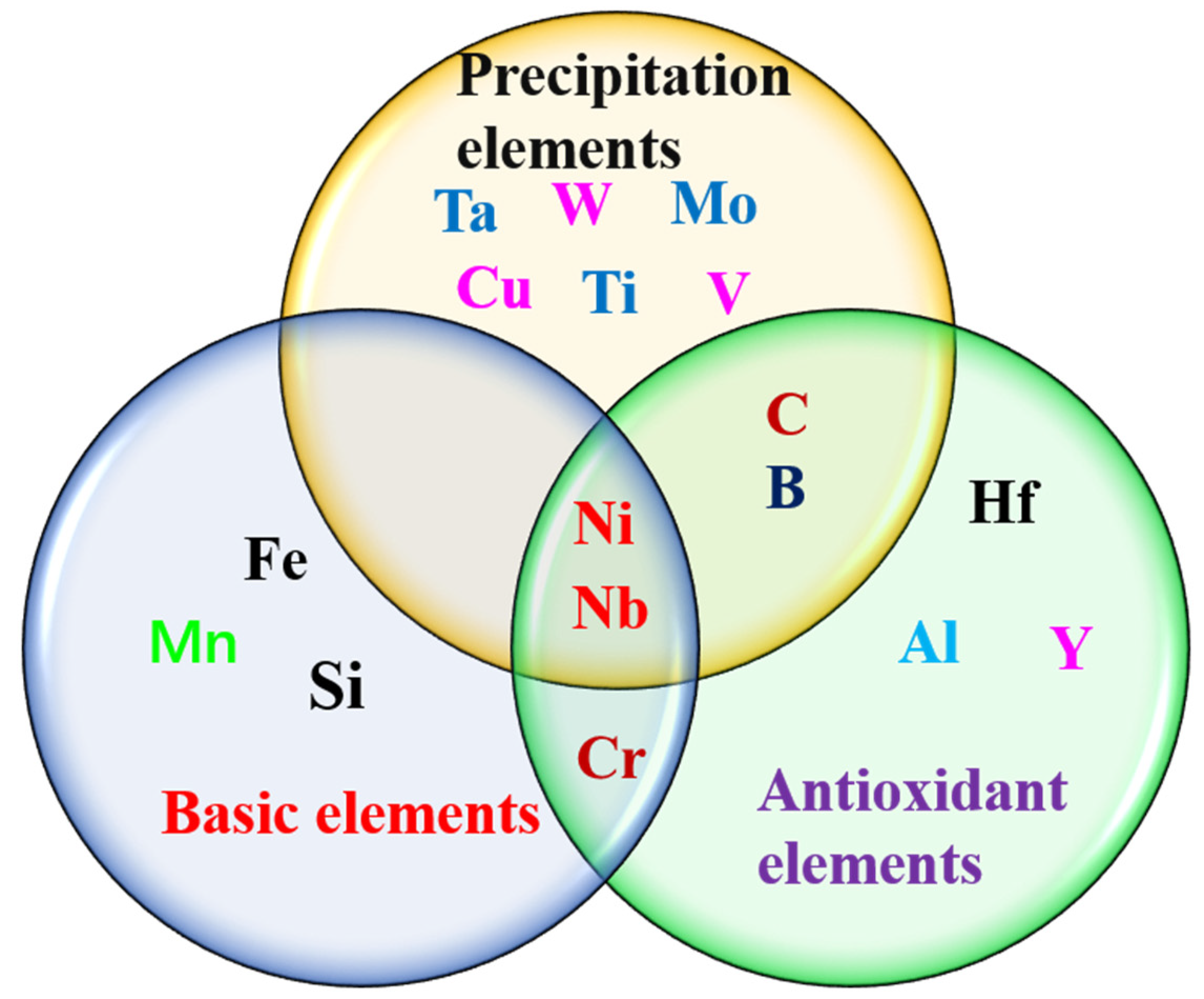
3.2.1. Alloy Elements
- (1)
- Elements affecting the carbides
- (2)
- Elements affecting B2-NiAl
- (3)
- Elements affecting the Laves phase
- (4)
- Elements affecting γ′
- (5)
- Elements Affecting σ
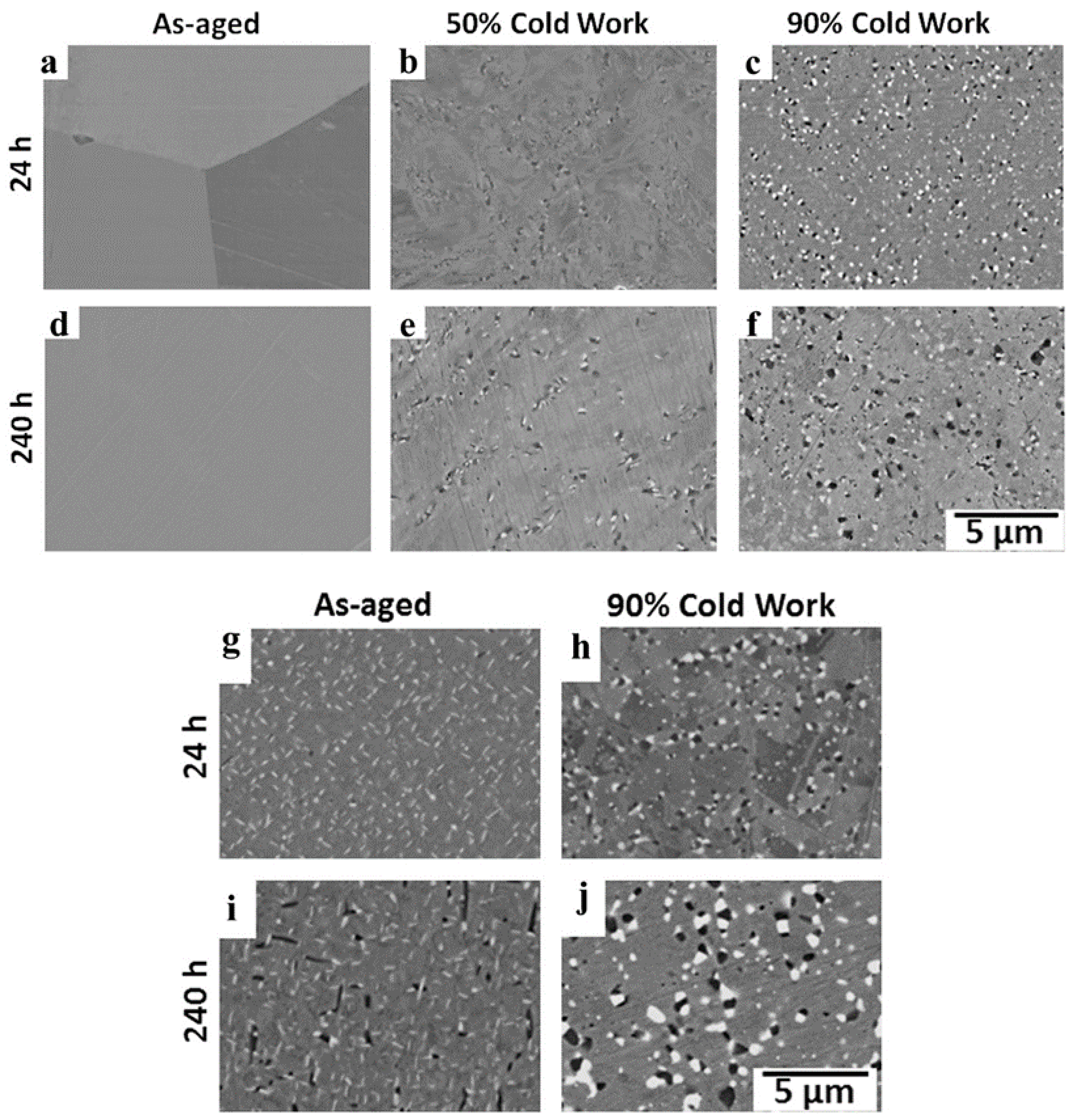
3.2.2. Pre-Strain
3.2.3. Heat TreatmentAging
- (1)
- Aging
- (2)
- Annealing
4. Corrosion Resistance
4.1. Oxidation Resistance in Different Environments
4.1.1. In Air
4.1.2. In Supercritical Water (SCW)
4.1.3. In Molten Sodium Sulfate
4.1.4. In Liquid Lead
4.1.5. In Metal Dusting
4.1.6. Other
4.2. The Influence of Alloy Elements on the Oxidation Resistance
4.2.1. The Elements to Stabilize Alumina-ScaleAluminum
- (1)
- Aluminum
- (2)
- Chromium
- (3)
- Niobium
4.2.2. The Elements to Improve Oxidation ResistanceNickel
- (1)
- Nickel
- (2)
- Carbon and boron
- (3)
- Hafnium and yttrium
4.2.3. The Elements to Degrade Oxidation Resistance
4.2.4. Other
5. Mechanical Properties
5.1. Hot Work-Ability
5.2. Short-Time Mechanical Properties
5.2.1. Reinforcing Phase
5.2.2. Short-Time Properties of AFA Steels Currently Tested
- (1)
- Tensile properties after pre-strain
- (2)
- Tensile properties after aging
- (3)
- Others
5.3. Creep Performance
5.3.1. Research Methods of Creep
5.3.2. Creep Properties of AFA Steels
6. Prospects
- (1)
- AFA steel composition is further optimized. The better the composition optimization effects of AFA steels, the stricter the composition control can be, and the cost will be reduced as much as possible to promote the industrialization of AFA steels.
- (2)
- Currently developed AFA steels easily form alumina protective scales in air. In contrast, the protective scales in other harsh environments are more complex, and the research results are relatively few, so systematic study is still needed.
- (3)
- Alloying elements have an important influence on the formation of precipitates and oxidation resistance, especially nickel, chromium, niobium, aluminum, etc., but the influence of aluminum on creep needs to study systematically. At present, our research group is carrying out research in this area. Due to the time-consuming creep test, there is no specific research result.
- (4)
- At present, there is lagging research on the forming performance of AFA steels. The forming performance of the materials puts forward a better optimization plan for the design of the alloy composition and structure. Nowadays, the research on the forming performance of AFA steels is only limited to the hot compression simulation test, and the hot forming and cold forming of AFA steels are still not available. Corresponding processes such as the extrusion of heat transfer pipes and welding, etc., must be accelerated to improve the performance and applications of AFA steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viswanathan, R.; Bakker, W. Materials for ultrasupercritical coal power plants—Boiler materials: Part 1. J. Mater. Eng. Perform. 2001, 10, 81–95. [Google Scholar] [CrossRef]
- Viswanathan, R.; Sarver, J.; Tanzosh, J.M. Boiler materials for ultra-supercritical coal power plants—Steamside oxidation. J. Mater. Eng. Perform. 2006, 15, 255–274. [Google Scholar] [CrossRef]
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J.; Vitalis, B.; Purgert, R. U.S. Program on materials Technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming Austenitic Stainless Steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Brady, M.; Yamamoto, Y.; Lu, Z.; Maziasz, P.; Liu, C.; Pint, B.; Santella, M. Alumina-Forming Austenitics: A New Class of Heat-Resistant Stainless Steels; Stainless Steel World; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2008. [Google Scholar]
- Brady, M.P.; Gleeson, B.; Wright, I.G. Alloy Design Strategies for Promoting Protective Oxide-Scale Formation. J. Met. 2000, 52, 16–21. [Google Scholar] [CrossRef]
- Philip, J.; Maziasz John, P.; Shingledecker Neal, D.; Evans, N.D.; Pollard, M.J. Developing New Cast Austenitic Stainless Steels with Improved High-Temperature Creep Resistance. J. Press. Vessel. Technol. 2009, 131, 051404. [Google Scholar] [CrossRef]
- Pint, B.A.; Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Howe, J.Y.; Trejo, R.; Maziasz, P.J. Development of Alumina-Forming Austenitic Alloys for Advanced Recuperators. In ASME Turbo Expo 2009: Power for Land, Sea, and Air; International Gas Turbine Institute: Montreal, QC, Canada, 2009; pp. 271–280. [Google Scholar]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent Developments in Stainless Steels. Mater. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Muralidharan, G.; Rogers, H.; Pint, B.A. Deployment of Alumina Forming Austenitic, AFA Stainless Steel; ORNL/TM-2013/479; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2013. [Google Scholar]
- Pint, B.A.; Dryepondt, S.; Brady, M.P.; Yamamoto, Y. Field and Laboratory Evaluations of Commercial and Next-Generation Alumina-Forming Austenitic Foil for Advanced Recuperators. J. Eng. Gas Turbines Power 2016, 138, 122–136. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Muralidharan, G.; Pint, B.A.; Maziasz, P.J.; Shin, D.; Shassere, B.; Babu, S.S.; Kuo, C.H. Development of Creep-Resistant, Alumina-Forming Ferrous Alloys for High-Temperature Structural Use. In ASME 2018 Symposium on Elevated Temperature Application of Materials for Fossil, Nuclear, and Petrochemical Industries; American Society of Mechanical Engineers: New York, NY, USA, 2018. [Google Scholar]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Development of Alumina-Forming Austenitic Stainless Steels. In Proceedings of the 33rd International Technical Conference on Coal Utilization & Fuel Systems 2008, Clearwater, FL, USA, 1–5 June 2008; pp. 1–8. [Google Scholar]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L. The Development of Alumina-Forming Austenitic Stainless Steels for High-temperature Structural Use. J. Met. 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Pint, B.A.; Santella, M.L.; Maziasz, P.J.; Walker, L.R. On the Loss of Protective Scale Formation in Creep-Resistant, Alumina-Forming Austenitic Stainless Steels at 900 °C in Air. Mater. Sci. Forum 2008, 595–598, 725–732. [Google Scholar] [CrossRef]
- Brady, M.P.; Unocic, K.A.; Lance, M.J.; Santella, M.L.; Yamamoto, Y.; Walker, L.R. Increasing the Upper Temperature Oxidation Limit of Alumina Forming Austenitic Stainless Steels in Air with Water Vapor. Oxid. Met. 2011, 75, 337–357. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Pint, B.A. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scr. Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Jozaghi, T.; Wang, C.; Arroyave, R.; Karaman, I. Design of Alumina-forming Austenitic Stainless Steel Using Genetic Algorithms. Mater. Des. 2020, 186, 108198. [Google Scholar] [CrossRef]
- Wen, D.H.; Li, Z.; Jiang, B.B.; Wang, Q.; Chen, G.Q.; Tang, R.; Zhang, R.Q.; Dong, C.; Liaw, P.K. Effects of Nb/Ti/V/Ta on phase precipitation and oxidation resistance at 1073 K in alumina-forming austenitic stainless steels. Mater. Charact. 2018, 144, 86–98. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Walker, L.R. Composition, Microstructure, and Water Vapor Effects on Internal/External Oxidation of Alumina-Forming Austenitic Stainless Steels. Oxid. Met. 2009, 72, 311–333. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Santella, M.L.; Brady, M.P.; Bei, H.; Maziasz, P.J. Effect of Alloying Additions on Phase Equilibria and Creep Resistance of Alumina-Forming Austenitic Stainless Steels. Metall. Mater. Trans. A 2009, 40, 1868–1880. [Google Scholar] [CrossRef]
- Bei, H.; Yamamoto, Y.; Brady, M.P.; Santella, M.L. Aging effects on the mechanical properties of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2010, 527, 2079–2086. [Google Scholar] [CrossRef]
- Yanar, N.M.; Lutz, B.S.; Garcia-Fresnillo, L.; Brady, M.P.; Meier, G.H. The Effects of Water Vapor on the Oxidation Behavior of Alumina Forming Austenitic Stainless Steels. Oxid. Met. 2015, 84, 541–565. [Google Scholar] [CrossRef]
- Zhou, L.F.; Zeng, Z.P.; Brady, M.P. Chromium evaporation and oxidation characteristics of alumina-forming austenitic stainless steels for balance of plant applications in solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 21619–21633. [Google Scholar] [CrossRef]
- Zhao, B.; Fan, J.; Liu, Y.; Zhao, L.; Dong, X.; Sun, F.; Zhang, L. Formation of L12-ordered Precipitation in an Alumina-forming Austenitic Stainless Steel via Cu Addition and its Contribution to Creep/Rupture Resistance. Scr. Mater. 2015, 109, 64–67. [Google Scholar] [CrossRef]
- Zhao, B.; Chang, K.; Fan, J.; Chen, Z.; Dong, X.; Zhang, L. Annealing effects on precipitation and high-temperature properties of a Cu-containing alumina-forming austenitic steel. Mater. Lett. 2016, 176, 83–86. [Google Scholar] [CrossRef]
- Jang, M.H.; Moon, J.; Kang, J.Y.; Ha, H.Y.; Choi, B.G.; Lee, T.H.; Lee, C. Effect of tungsten addition on high-temperature properties and microstructure of alumina-forming austenitic heat-resistant steels. Mater. Sci. Eng. A 2015, 647, 163–169. [Google Scholar] [CrossRef]
- Zhao, B.; Fan, J.; Chen, Z.; Dong, X.; Sun, F.; Zhang, L. Evolution of precipitates in a Cu-containing alumina-forming austenitic steel after short-term mechanical tests. Mater. Charact. 2017, 125, 37–45. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Phaniraj, M.P.; Han, H.N.; Jang, J.; Zhou, Z. Evolution of microstructure and tensile properties of Fe-18Ni-12Cr based AFA steel during aging at 700 °C. Mater. Sci. Eng. A 2016, 672, 23–31. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.D.; Feng, J.K.; Zhang, R.Q.; Tang, R.; Zhou, Z.J. Microstructural evolution and mechanical properties of an Fe–18Ni–16Cr–4Al base alloy during aging at 950 °C. Int. J. Miner. Metall. Mater. 2016, 23, 314–322. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, Z.; Xu, S.; Wang, M. Hot deformation behavior and processing map of a Fe-25Ni-16Cr-3Al alumina-forming austenitic steel. Mater. Und Werkst. 2018, 49, 1135–1144. [Google Scholar] [CrossRef]
- Jiang, J.D.; Liu, Z.Y.; Gao, Q.Z.; Zhang, H.L.; Hao, A.M.; Qu, F.; Lin, X.P.; Li, H.J. The effect of isothermal aging on creep behavior of modified 2.5Al alumina-forming austenitic steel. Mater. Sci. Eng. A 2020, 797, 140219. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Zhang, Z.; Quan, X.; Liu, J.; Fang, X.; Han, P. Improved Oxidation Resistance of a New Aluminum-Containing Austenitic Stainless Steel at 800 °C in Air. Oxid. Met. 2017, 88, 301–314. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Q.; Zhang, H.; Luo, S.; Zhang, X.; Li, W.; Jiang, Y.; Li, H. EBSD analysis and mechanical properties of alumina-forming austenitic steel during hot deformation and annealing. Mater. Sci. Eng. A 2019, 755, 106–115. [Google Scholar] [CrossRef]
- Meng, H.; Wang, J.; Wang, L.; Fang, X.; Dong, N.; Zhang, C.; Han, P. The precipitation control in aged alumina-forming austenitic stainless steels Fe-15Cr-25Ni-3Al-NbWCu by W addition and its effect on the mechanical properties. Mater. Charact. 2020, 163, 110–233. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Xu, X.Q.; Mao, H.H.; Yan, Y.F.; Nieh, T.G.; Lu, Z.P. Plastic flow behaviour in an alumina-forming austenitic stainless steel at elevated temperatures. Mater Sci. Eng. A 2014, 594, 246–252. [Google Scholar] [CrossRef]
- Zhou, D.Q.; Zhao, W.X.; Mao, H.H.; Hu, Y.X.; Xu, X.Q.; Sun, X.Y.; Lu, Z.P. Precipitate characteristics and their effects on the high-temperature creep resistance of alumina-forming austenitic stainless steels. Mater. Sci. Eng. A 2015, 622, 91–100. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takeyama, M.; Lu, Z.P.; Liu, C.T.; Evans, N.D.; Maziasz, P.J.; Brady, M.P. Alloying Effects on Creep and Oxidation Resistance of Austenitic Stainless Steel Alloys Employing Intermetallic Precipitates. Intermetallics 2008, 16, 453–462. [Google Scholar] [CrossRef]
- Hu, B.; Trotter, G.; Baker, I.; Miller, M.K.; Yao, L.; Chen, S.; Cai, Z. The Effects of Cold Work on the Microstructure and Mechanical Properties of Intermetallic Strengthened Alumina-Forming Austenitic Stainless Steels. Metall. Mater. Trans. A 2015, 46, 3773–3785. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Muralidharan, G.; Brady, M.P. Development of L12-ordered Ni3, (Al, Ti)-strengthened alumina-forming austenitic stainless steel alloys. Scr. Mater. 2013, 69, 816–819. [Google Scholar] [CrossRef]
- Trotter, G.; Hu, B.; Sun, A.Y.; Harder, R.; Miller, M.K.; Yao, L.; Baker, I. Precipitation kinetics during aging of an alumina-forming austenitic stainless steel. Mater. Sci. Eng. A 2016, 667, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Santella, M.L.; Liu, C.T.; Evans, N.D.; Maziasz, P.J.; Brady, M.P. Evaluation of Mn Substitution for Ni in Alumina-forming Austenitic Stainless Steels. Mater. Sci. Eng. A 2009, 524, 176–185. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Wang, Q.; Gao, Y.; Dong, H.; Che, H.; Zhou, Z. Microstructure and mechanical property evolution of an AFA alloy with simple composition design during ageing at 700 °C. Mater. Sci. Eng. A 2020, 779, 139–157. [Google Scholar] [CrossRef]
- Wang, M.; Sun, H.; Zhou, Z. Creep behaviors of Fe-18Ni-12Cr based alumina forming austenitic steels with ultralow carbon. J. Mater. Sci. Met. Corros. 2021, 56, 9445–9457. [Google Scholar] [CrossRef]
- Brady, M.P.; Muralidharan, G.; Leonard, D.N. Long-Term Oxidation of Candidate Cast Iron and Stainless Steel Exhaust System Alloys from 650 to 800 °C in Air with Water Vapor. Oxid. Met. 2014, 82, 359–381. [Google Scholar] [CrossRef]
- Muralidharan, G.; Yamamoto, Y.; Brady, M.P.; Pint, B.A.; Voke, D.; Pankiw, R.I. Development of Cast Alumina-forming Austenitic Stainless Steel Alloys for use in High Temperature Process Environments. In Proceedings of the NACE Corrosion, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Brady, M.P.; Muralidharan, G.; Yamamoto, Y.; Pint, B.A. Development of 1100 °C Capable Alumina-Forming Austenitic Alloys. Oxid. Met. 2016, 87, 1–10. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Liu, C.T.; Takeyama, M.; Maziasz, P.J.; Pint, B.A. Alumina-Forming Austenitic Stainless Steels Strengthened by Laves Phase and MC Carbide Precipitates. Metall. Mater. Trans. A 2007, 38, 2737–2746. [Google Scholar] [CrossRef]
- Dong, N.; Jia, R.; Wang, J.; Fan, G.; Fang, X.; Han, P. Composition Optimum Design and Strengthening and Toughening Mechanisms of New Alumina-Forming Austenitic Heat-Resistant Steels. Metals 2019, 9, 921. [Google Scholar] [CrossRef] [Green Version]
- Elger, R.; Magnusson, H.; Frisk, K. Modelling Internal Nitridation in an Alumina-forming Austenitic Stainless Steel. Mater. Corros. 2017, 68, 143–150. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Kondo, S.; Okubo, N.; Kasada, R. Corrosion Behaviour of Al-added High Mn Austenitic steels in Molten Lead Bismuth Eutectic with Saturated and Low Oxygen Concentrations at 450 °C. Corros. Sci. 2020, 40, 108864. [Google Scholar] [CrossRef]
- Ejenstam, J.; Szakálos, P. Long Term Corrosion Resistance of Alumina Forming Austenitic Stainless Steels in Liquid Lead. J. Nucl. Mater. 2015, 461, 164–170. [Google Scholar] [CrossRef]
- Jang, M.-H.; Kang, J.-Y.; Jang, J.H.; Lee, T.-H.; Lee, C. Hot Deformation Behavior and Microstructural Evolution of Alumina-forming Austenitic Heat-resistant Steels during Hot Compression. Mater. Charact. 2017, 123, 207–217. [Google Scholar] [CrossRef]
- Facco, A.; Couvrat, M.; Magné, D.; Roussel, M.; Guillet, A.; Pareige, C. Microstructure Influence on Creep Properties of Heat-resistant Austenitic Alloys with High Aluminum Content. Mater. Sci. Eng. A 2020, 783, 139276. [Google Scholar] [CrossRef]
- Wen, D.H.; Wang, Q.; Jiang, B.B.; Zhang, C.; Li, X.N.; Chen, G.Q.; Tang, R.; Zhang, R.Q.; Dong, C.; Liaw, P.K. Developing Fuel Cladding Fe-25Cr-22Ni Stainless Steels with High Microstructural Stabilities via Mo/Nb/Ti/Ta/W Alloying. Mater. Sci. Eng. A 2018, 719, 27–42. [Google Scholar] [CrossRef]
- Shin, D.; Yamamoto, Y.; Brady, M.P.; Lee, S.; Haynes, J.A. Modern Data Analytics Approach to Predict Creep of High-temperature Alloys. Acta Mater. 2019, 168, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Lee, T.H.; Heo, Y.U.; Han, Y.S.; Kang, J.Y.; Ha, H.Y.; Suh, D.W. Precipitation sequence and its effect on age hardening of alumina-forming austenitic stainless steel. Mater. Sci. Eng. A 2015, 645, 72–81. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, B.; Dong, X.; Sun, F.; Zhang, L. A Systematic Investigation of Precipitates in Matrix and at Grain Boundaries in an Alumina-Forming Austenitic Steel during Creep Testing at 700 °C. Metall. Mater. Trans. A 2020, 51, 4186–4194. [Google Scholar] [CrossRef]
- Wen, H.; Zhao, B.; Dong, X.; Zhou, J.; Wang, J.; Sun, F.; Zhang, L. How big is the difference between precipitation at twin boundary and normal grain boundary in an alumina-forming austenitic steel during creep at 700 °C. Mater. Lett. 2020, 274, 128019. [Google Scholar] [CrossRef]
- Brady, M.P.; Magee, J.; Yamamoto, Y.; Helmick, D.; Wang, L. Co-optimization of wrought alumina-forming austenitic stainless steel composition ranges for high-temperature creep and oxidation/corrosion resistance. Mater. Sci. Eng. A 2014, 590, 101–115. [Google Scholar] [CrossRef]
- Zhao, W.X.; Zhou, D.Q.; Jiang, S.H.; Wang, H.; Wu, Y.; Liu, X.J.; Wang, X.Z.; Lu, Z.P. Ultrahigh stability and strong precipitation strengthening of nanosized NbC in alumina-forming austenitic stainless steels subjecting to long-term high-temperature exposure. Mater. Sci. Eng. A 2018, 738, 295–307. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of strategies for high-temperature creep and oxidation resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A 2011, 42, 922–931. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Zhao, B.; Dong, X.; Sun, F.; Zhang, L. Preferential growth of coherent precipitates at grain boundary. Mater. Lett. 2020, 4, 126984. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.; Tsisar, V.; Schroer, C.; Zhou, Z. Investigation of microstructure and liquid lead corrosion behavior of a Fe-18Ni-16Cr-4Al base alumina-forming austenitic stainless steel. Mater. Res. Express 2020, 7, 026533. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Darolia, R.; Lahrman, D.; Field, R. The effect of iron, gallium and molybdenum on the room temperature tensile ductility of NiAl. Scr. Metall. Mater. 1992, 26, 1007–1012. [Google Scholar] [CrossRef]
- Baker, I. A review of the mechanical properties of B2 compounds. Mater. Sci. Eng. A 1995, 192, 1–13. [Google Scholar] [CrossRef]
- Trotter, G.; Baker, I. Orientation relationships of Laves phase and NiAl particles in an AFA stainless steel. Philos. Mag. 2015, 95, 4078–4094. [Google Scholar] [CrossRef]
- Baker, I.; Afonina, N.; Wang, Z.; Wu, M. Preliminary creep testing of the alumina-forming austenitic stainless steel Fe-20Cr-30Ni-2Nb-5Al. Mater. Sci. Eng. A 2018, 718, 492–498. [Google Scholar] [CrossRef]
- Ishikawa, H.; Zhang, C.; Chen, S.-W.; Yang, Z.-G. Precipitate Behavior in Fe–20Cr–30Ni–2Nb Austenitic Heat-Resistant Steel. Acta Metall. Sin. 2015, 28, 424–429. [Google Scholar] [CrossRef]
- Hu, B.; Baker, I. High temperature deformation of Laves phase precipitates in alumina-forming austenitic stainless steels. Mater. Lett. 2017, 195, 108–111. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Zhao, B.; Zhou, J.; Dong, X.; Chen, Q.; Sun, F.; Zhang, L. Early segregation and precipitation at triple junction in an alumina-forming austenitic steel. Mater. Lett. 2021, 283, 128802. [Google Scholar] [CrossRef]
- Andrew, P.; Baker, I. Analysis of the elevated temperature deformation mechanisms and grain boundary strengthening of the alumina-forming austenitic stainless steel Fe-20Cr-30Ni-2Nb-5Al. Mater. Sci. Eng. A 2021, 814, 141219. [Google Scholar] [CrossRef]
- Andrew, P.; Baker, I. Microstructural evolution of Fe–20Cr–30Ni–2Nb–5Al AFA steel during creep at 760 °C. Mater. Sci. Eng. A 2021, 806, 140602. [Google Scholar] [CrossRef]
- Trotter, G.; Baker, I. The effect of aging on the microstructure and mechanical behavior of the alumina-forming austenitic stainless steel Fe–20Cr–30Ni–2Nb–5Al. Mater. Sci. Eng. A 2015, 627, 270–276. [Google Scholar] [CrossRef]
- Trotter, G.; Rayner, G.; Baker, I.; Munroe, P.R. Accelerated precipitation in the AFA stainless steel Fe-20Cr-30Ni-2Nb-5Al via cold working. Intermetallics 2014, 53, 120–128. [Google Scholar] [CrossRef]
- Heo, Y.-U. A Strategy for Phase Identification of Precipitates in High Al-containing Austenitic and Ferritic Steels Using Electron Diffraction. Appl. Microsc. 2014, 44, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Padilha, A.F.; Escriba, D.M.; Materna-Morris, E.; Rieth, M.; Klimenkov, J.M. Precipitation in AISI 316L(N) during creep tests at 550 and 600 C up to 10 years. J. Nucl. Mater. 2007, 362, 132–138. [Google Scholar] [CrossRef]
- Andrew, P.; Baker, I. The formation mechanism, growth, and effect on the mechanical properties of precipitate free zones in the alumina-forming austenitic stainless steel Fe-20Cr-30Ni-2Nb-5Al during creep. Mater. Sci. Eng. A 2021, 820, 141561. [Google Scholar] [CrossRef]
- Hu, B.; Baker, I.; Kernion, S.J.; Yamamoto, Y.; Brady, M.P. Creep failure of a gamma prime-strengthened alumina-forming austenitic stainless steel. In Advances in Materials Technology for Fossil Power Plants, Proceeding of the Eighth International Conference, Albufeira, Algarve, Portugal, 11–14 October 2016; ASM International: Almere, The Netherlands, 2016. [Google Scholar]
- Hu, B.; Trotter, G.; Wang, Z.; Chen, S.; Cai, Z.; Baker, I. Effect of boron and carbon addition on microstructure and mechanical properties of the aged gamma-prime strengthened alumina-forming austenitic alloys. Intermetallics 2017, 90, 36–49. [Google Scholar] [CrossRef]
- Muralidharan, G.; Yamamoto, Y.; Brady, M.P.; Walker, L.R.; Meyer Iii, H.M.; Leonard, D.N. Development of Cast Alumina-Forming Austenitic Stainless Steels. JOM 2016, 68, 2803–2810. [Google Scholar] [CrossRef]
- Jang, M.H.; Kang, J.Y.; Jang, J.H.; Lee, T.H.; Lee, C. The role of phosphorus in precipitation behavior and its effect on the creep properties of alumina-forming austenitic heat-resistant steels. Mater. Sci. Eng. A 2017, 684, 14–21. [Google Scholar] [CrossRef]
- Qiuzhi, G.; Bingyi, L.; Qingshuang, M. Effect of Cu addition on microstructure and properties of Fe–20Ni–14Cr alumina-forming austenitic steel. Intermetallics 2021, 138, 107312. [Google Scholar] [CrossRef]
- Zhou, B.C.; Yang, T.; Zhou, G.; Wang, H.; Luan, J.H.; Jiao, Z.B. Mechanisms for suppressing discontinuous precipitation and improving mechanical properties of NiAl-strengthened steels through nanoscale Cu partitioning. Acta Mater. 2021, 205, 116561. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Sun, X.; Lu, Z.P. Effects of silicon additions on the oxide scale formation of an alumina-forming austenitic alloy. Corros. Sci. 2012, 65, 317–321. [Google Scholar] [CrossRef]
- Jang, M.H.; Kang, J.Y.; Jang, J.H.; Lee, T.H.; Lee, C. Improved creep strength of alumina-forming austenitic heat-resistant steels through W addition. Mater. Sci. Eng. A 2017, 696, 70–79. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, Q.; Zhang, H.; Zhang, X.; Li, H.; Liu, Z.; Liu, C. The effect of isothermal aging on microstructure and mechanical behavior of modified 2.5Al alumina-forming austenitic steel. Mater. Sci. Eng. A 2019, 748, 161–172. [Google Scholar] [CrossRef]
- Sun, Y.F.; Lv, Y.Z.; Zhang, Y.; Zhao, J.Y.; Wu, Y. Microstructural and properties evolution of austenitic heat resistant steel after addition of aluminum. Mater. Sci. Technol. 2013, 29, 511–516. [Google Scholar] [CrossRef]
- Moon, J.; Jang, M.-H.; Kang, J.-Y.; Lee, T.-H. The negative effect of Zr addition on the high temperature strength in alumina-forming austenitic stainless steels. Materials Characterization 2014, 87, 12–18. [Google Scholar] [CrossRef]
- Prince, A.; Petzow, G.; Effenberg, G. Ternary Alloys, a Comprehensive Compendium of Evaluated Constitutional Data and Phase Diagrams; VCH Publishing, Co.: Basel, Switzerland, 1991; Volume 4, pp. 597–629. [Google Scholar]
- Zhao, B.B.; Dong, X.P.; Sun, F.; Zhang, L.T. Impact of L12-Ordered Precipitation on the Strength of Alumina-Forming Austenitic Heat-Resistant Steels. Mater. Sci. Forum 2018, 941, 692–697. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, B.; Wang, Q.; Yu, F.; Li, X.; Tang, R.; Zhang, R.; Chen, G.; Dong, C. Influences of Mo/Zr minor-alloying on the phase precipitation behavior in modified 310S austenitic stainless steels at high temperatures. Mater. Des. 2017, 128, 34–46. [Google Scholar] [CrossRef]
- Jang, M.-H.; Kang, J.-Y.; Jang, J.H.; Lee, T.-H.; Lee, C. Microstructure control to improve creep strength of alumina-forming austenitic heat-resistant steel by pre-strain. Mater. Charact. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Gao, Q.; Qu, F.; Zhang, H.; Huo, Q. Austenite grain growth in alumina-forming austenitic steel. J. Mater. Res. 2016, 31, 1732–1740. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, Q.; Zhang, H.; Liu, Z.; Li, H. Microstructure and Mechanical Properties of 4Al Alumina-Forming Austenitic Steel after Cold-Rolling Deformation and Annealing. Materials 2020, 13, 2767. [Google Scholar] [CrossRef]
- Nie, S.H.; Chen, Y.; Ren, X.; Sridharan, K.; Allen, T.R. Corrosion of alumina-forming austenitic steel Fe–20Ni–14Cr–3Al–0.6Nb–0.1Ti in supercritical water. J. Nucl. Mater. 2010, 399, 231–235. [Google Scholar] [CrossRef]
- Sun, H.; Yang, H.; Wang, M.; Giron-Palomares, B.; Zhou, Z.; Zhang, L.; Zhang, G. The corrosion and stress corrosion cracking behavior of a novel alumina-forming austenitic stainless steel in supercritical water. J. Nucl. Mater. 2017, 484, 339–346. [Google Scholar] [CrossRef]
- Yan, Y.F.; Xu, X.Q.; Zhou, D.Q.; Wang, H.; Wu, Y.; Liu, X.J.; Lu, Z.P. Hot corrosion behaviour and its mechanism of a new alumina-forming austenitic stainless steel in molten sodium sulphate. Corros. Sci. 2013, 77, 202–209. [Google Scholar] [CrossRef]
- Lutz, B.S.; Yanar, N.M.; Holcomb, G.R.; Meier, G.H. Fireside Corrosion of Alumina-Forming Austenitic (AFA) Stainless Steels. Oxid. Met. 2016, 87, 575–602. [Google Scholar] [CrossRef]
- Zhang, J.; Speck, P.; Young, D.J. Metal Dusting of Alumina-Forming Creep-Resistant Austenitic Stainless Steels. Oxid. Met. 2011, 77, 167–187. [Google Scholar] [CrossRef]
- Put, A.R.V.; Unocic, K.A.; Brady, M.P.; Pint, B.A. Performance of chromina- and alumina-forming Fe- and Ni-base alloys exposed to metal dusting environments: The effect of water vapor and temperature. Corros. Sci. 2015, 92, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Elger, R.; Pettersson, R. Effect of Addition of 4% Al on the High Temperature Oxidation and Nitridation of a 20Cr–25Ni Austenitic Stainless Steel. Oxid. Met. 2014, 82, 469–490. [Google Scholar] [CrossRef]
- He, L.F.; Roman, P.; Leng, B.; Sridharan, K.; Anderson, M.; Allen, T.R. Corrosion behavior of an alumina forming austenitic steel exposed to supercritical carbon dioxide. Corros. Sci. 2014, 82, 67–76. [Google Scholar] [CrossRef]
- Rashidi, S.; Choi, J.P.; Stevenson, J.W.; Pandey, A.; Gupta, R.K. Effect of Aluminizing on the High-Temperature Oxidation Behavior of an Alumina-Forming Austenitic Stainless Steel. J. Met. 2018, 71, 109–115. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Shen, L.; Wu, B.; Zhao, K. Reason for negative effect of Nb addition on oxidation resistance of alumina-forming austenitic stainless steel at 1323 K. Corros. Sci. 2021, 191, 109754. [Google Scholar] [CrossRef]
- Shi, H.; Tang, C.; Jianu, A.; Fetzer, R.; Weisenburger, A. Oxidation behavior and microstructure evolution of alumina-forming austenitic & high entropy alloys in steam environment at 1200 °C. Corros. Sci. 2020, 26, 108654. [Google Scholar] [CrossRef]
- Pint, B.A. Optimization of Reactive-Element Additions to Improve Oxidation Performance of Alumina-Forming Alloys. J. ACERS 2003, 86, 686–695. [Google Scholar] [CrossRef]
- Dong, N.; Qiao, Y.; Zhang, C.; Wang, J.; Fan, G.; Fang, X.; Han, P. Combined experiment and first-principles study of the formation of the Al2O3 layer in alumina-forming austenitic stainless steel. R. Soc. Chem. Adv. 2017, 7, 15727–15734. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Ha, H.-Y.; Kim, S.-D.; Park, J.Y.; Jang, M.-H.; Lee, T.-H. Effect of tungsten on the oxidation of alumina-forming austenitic stainless steel. Appl. Microsc. 2019, 49, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Liu, Z.; Li, H.; Zhang, H.; Jiang, C.; Hao, A.; Qu, F.; Lin, X. High-temperature oxidation behavior of modified 4Al alumina-forming austenitic steel: Effect of cold rolling. J. Mater. Sci. Technol. 2021, 68, 91–102. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, H.; Li, H.; Zhang, X.; Qu, F.; Jiang, Y.; Liu, Z.; Jiang, C. Hot deformation of alumina-forming austenitic steel: EBSD study and flow behavior. J. Mater. Sci. 2019, 54, 8760–8777. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Cheng, S.; Liu, C.; Wang, J. Effects of solution temperature on microstructure and mechanical properties of NF709 austenite heat resistant steel. Heat Treat. Met. 2013, 512, 241–245. [Google Scholar] [CrossRef]
- Hu, B.; Baker, I. The effect of thermo-mechanical treatment on the high temperature tensile behavior of an alumina-forming austenitic steel. Mater. Sci. Eng. A 2016, 651, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Wen-Tai, H.; Honeycombe, R.W.K. Structure of centrifugally cast austenitic stainless steels: Part 1, HK 40 as cast and after creep between 750 and 1000 °C. Mater. Sci. Technol. 1985, 1, 385–389. [Google Scholar] [CrossRef]
- Clark, C.L. Report on Influence of Recrystallization Temperature and Grain Size on the Creep Characteristics of Non-Ferrous Alloys; The Detroit Edison Company: Detroit, MI, USA, 2006. [Google Scholar] [CrossRef]
- Larson, F.R.; Miller, J. A Time-Temperature Relationship for Rupture and Creep Stresses. Trans. ASME 1952, 74, 765–775. [Google Scholar]
- Wang, M.; Sun, H.; Zheng, W.; Zhou, Z. Creep behavior of an alumina-forming austenitic steel with simple alloy design. Mater. Today Commun. 2020, 25, 101303. [Google Scholar] [CrossRef]



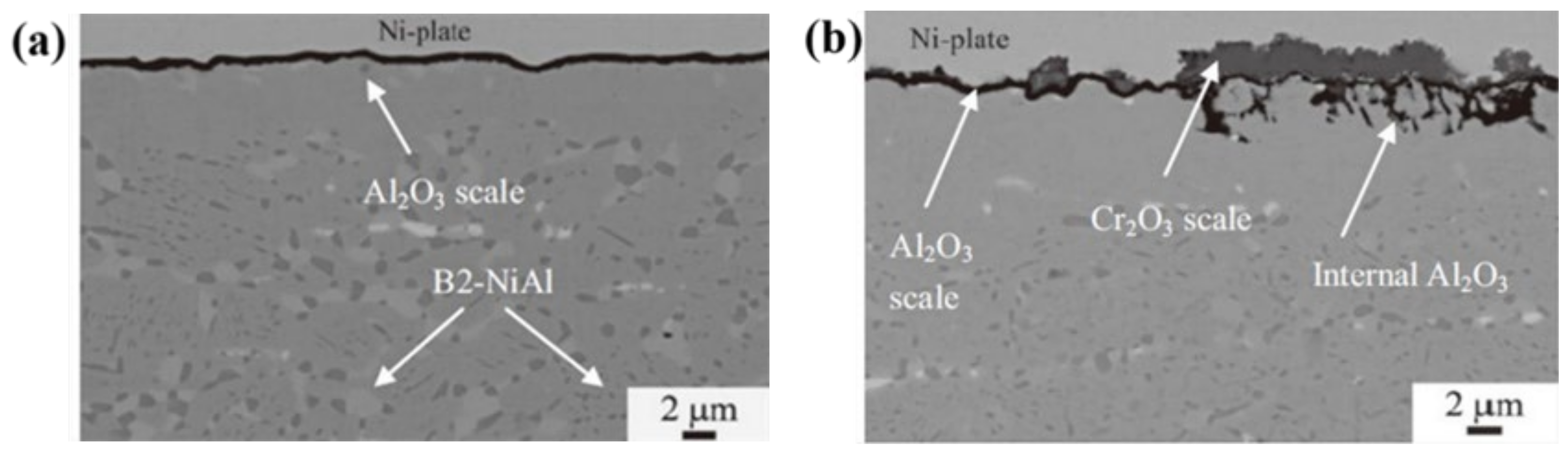


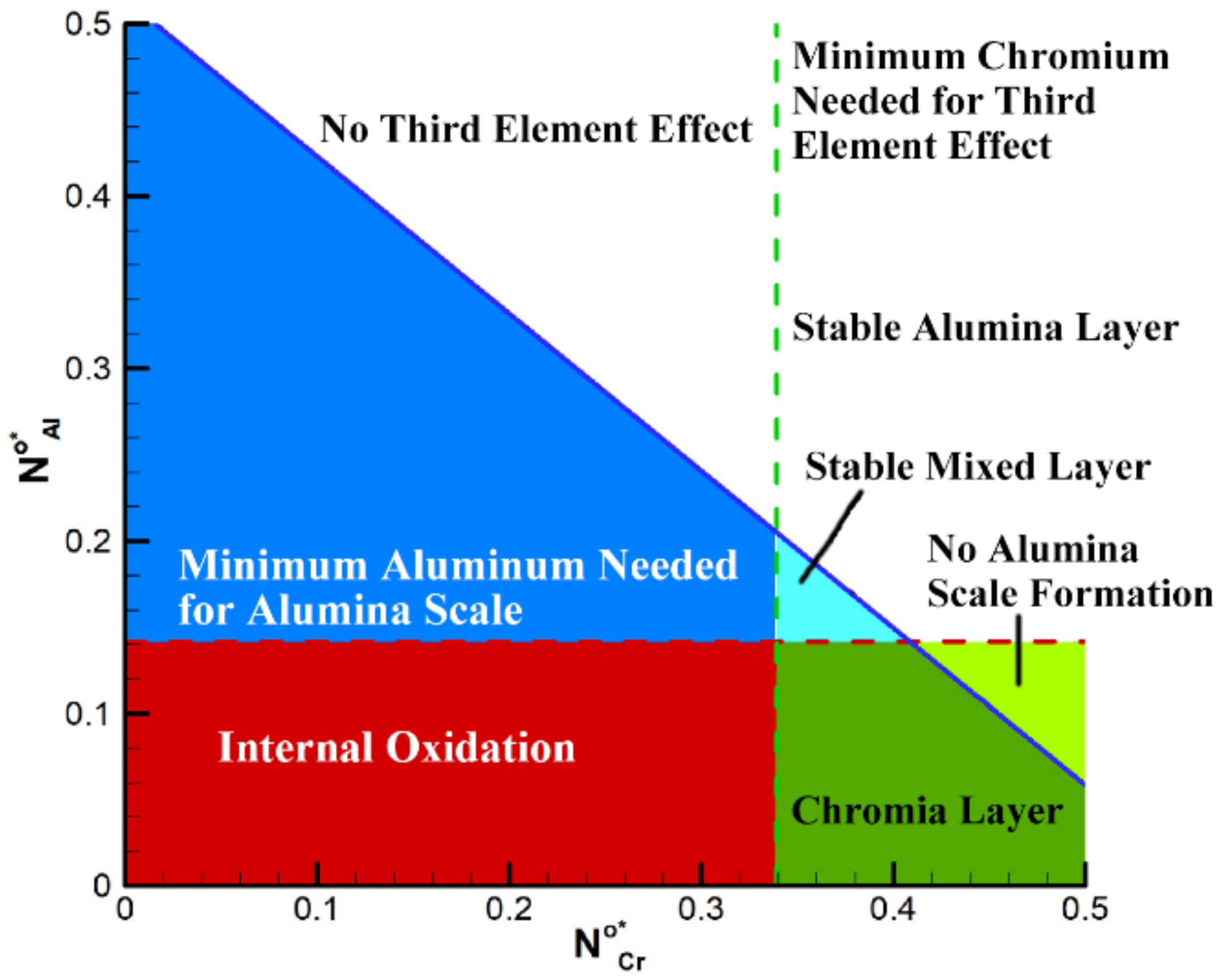
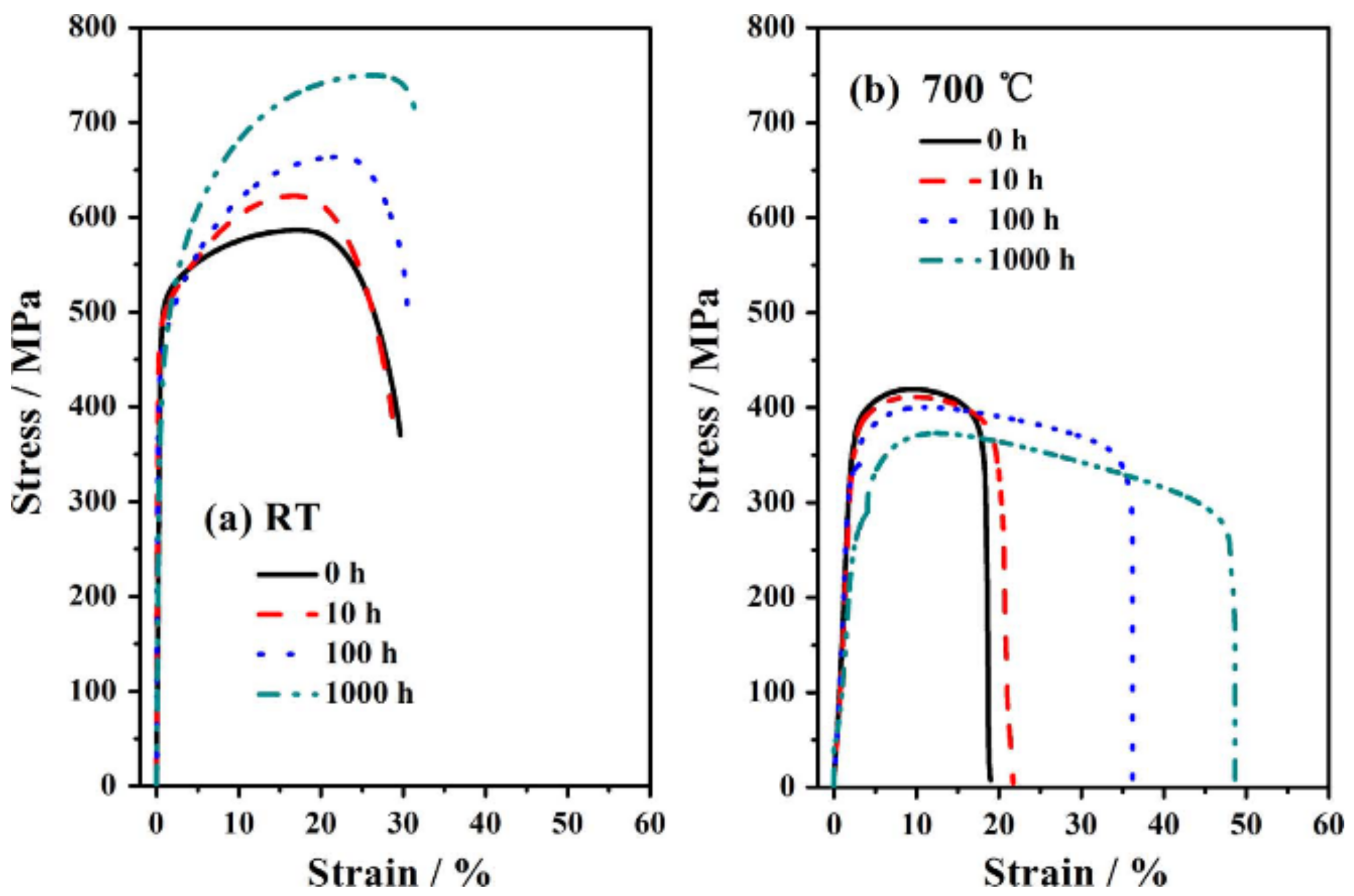

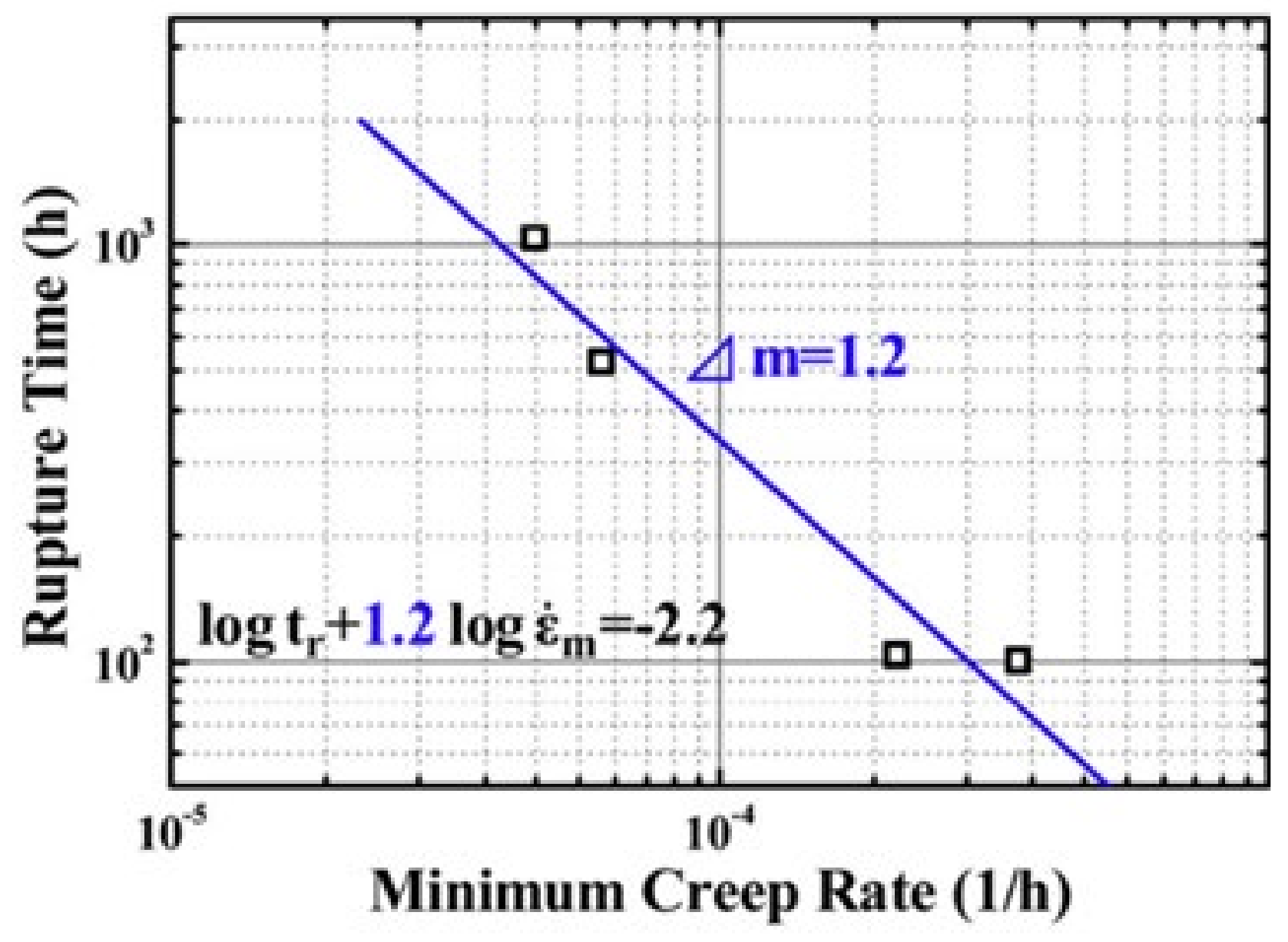
| Name | Composition (wt.%) | References |
|---|---|---|
| Baseline AFA: Fe-(20–25) Ni-(14–15) Cr-(2.5–3.5) Al-(1–3) Nb-2Mn-(0–4) Mo/W-0.5Cu + B, C, P wt.% Corrosion limit: Up to ~700–850 °C | ||
| AFA 2-1 (HTUPS 4) | Fe-20Ni-14.3Cr-2.5Al-0.9Nb-2.5Mo-2Mn-0.15Si-0.08C-0.01B-0.04P | [4] |
| A | Fe-26Ni-14Cr-2.8Al-0.6Nb-1.3Mo-0.15W-0.2Mn-0.2Si-0.04C | [15] |
| AFA + Al/C | Fe-25Ni-15Cr-4Al-2.5Nb-0.1C-0.01B | [16] |
| 13 | Fe-21Ni-14Cr-2.3Al-3Nb-0.19V-0.02C-0.01B | [17] |
| AFA-SS-M | Fe-17Ni-15Cr-3Al-2Mo-9Mn-0.2Si | [18] |
| AFA-NbTa | Fe-20Ni-18Cr-2.5Al-2.3Mo-0.45Nb-0.89Ta | [19] |
| 3–0.6 | Fe-20Ni-14Cr-3Al-0.6Nb-2Mn-2Mo-W-0.1Ti-0.5Cu-0.13Si-0.1C-0.04 P-0.001S | [20] |
| 3–0.4 | Fe-20Ni-14Cr-3Al-0.4Nb-2Mn-2Mo-W-0.1Ti-0.5Cu-0.13Si-0.1C-0.04 P-0.007S | |
| 3–2.5 | Fe-20Ni-14Cr-3Al-2.5Nb-2Mn-2Mo-0.9W-0.1Ti-0.5Cu-0.15Si-0.08C-0.04 P | |
| A-0.9 | Fe-14Cr-2.5Al-20Ni-0.9Nb-0.01Cu-2.5Mo-2Mn-0.15Si-0.02V-0.01Ti-0.075C-0.01B-0.043P | [21] |
| B-1.0 | Fe-12Cr-3Al-20Ni-Nb-0.5Cu-2Mo-2Mn-0.15Si-W-0.1C-0.007B-0.002P | |
| C-1.0 | Fe-14Cr-4Al-20Ni-Nb-0.5Cu-2Mo-2Mn-0.15Si-W-0.1C-0.007B-0.002P | |
| AFA1 | Fe-14Cr-3Al-20Ni-Nb-0.5Cu-2Mo-2Mn-0.15Si-W-0.1C-0.007B-0.02P | [22] |
| AFA5 | Fe-12Cr-4Al-20Ni-Nb-0.5Cu-2Mo-2Mn-0.15Si-W-0.1C-0.007B-0.02P | |
| AFA6 | Fe-12Cr-4Al-25Ni-Nb-0.5Cu-2Mo-2Mn-0.15Si-W-0.1C-0.007B-0.02P | |
| OC 4 | Fe-25Ni-14Cr-3.5Al-2.5Nb-2Mn-0.5Cu-2Mo-0.16W-0.16Si-0.1C-0.05Ti-0.05V-0.02P-0.009B-W | [23,24] |
| AFA-Cu | Fe-20Ni-14Cr-2.5Mo-2.8Cu-2.25Al-2Mn-0.5Nb-0.2V-0.15Si-0.04C-0.01B | [25,26] |
| AFAW | Fe-20Ni-14Cr-2.5Al-Nb-0.16Si-0.1C-0.02V-2W | [27] |
| A-0 | Fe-20Ni-14Cr-1.9Al-0.5Nb-0.18Si-0.046C-2.36Mo-1.26Mn-0.22V-0.012B | [25] |
| AFA-Cu | Fe-20Ni-14Cr-1.9Al-0.5Nb-0.18Si-0.046C-2.36Mo-1.26Mn-0.22V-0.012B-2.8Cu | [25,28] |
| Fe-18Ni-12Cr based | Fe-20Ni-12Cr-2Mo-0.4Si-0.03Mn-2.3Al-0.8Nb-0.02C | [29] |
| 316 based AFA | Fe-18Ni-16Cr-2Mo-0.3Si-0.03Mn-4Al-0.4Nb-0.01C | [30] |
| AFA | Fe-25Ni-16Cr-3Al-2W-0.3Si-0.4Nb-0.04Y | [31] |
| 2.5Al AFA | Fe-14Cr-20Ni-2.5Al-1.5Nb-0.13Si-0.01Ti-2.2Mn-2.2Mo-0.06C-0.03W | [32] |
| 22Cr-25Ni AFA | Fe-25Ni-22Cr-0.45Nb-1.5/2.5/3.5Al-0.8Mn-2.75Cu-0.2Si-0.06C | [33] |
| 310S AFA | Fe-(18-23)Cr-20Ni-2.5Al-2.3Mo-0.08C-0.45Nb-Ti/V/Ta | [19] |
| AFA | Fe-14Cr-20Ni-2.5Al-1.5Nb-0.13Si-0.01Ti-2Mn-0.06C-2Mo-0.03W | [34] |
| Fe-15Cr-25Ni-3Al-NbWCu | Fe-15Cr-25Ni-3Al-0.5Nb-0/2.5W-2.8Cu-0.01P-0.8Mn-0.3Si-0.08C | [35] |
| High performance AFA: Fe-(25–30) Ni-(12–15) Cr-(3.5–4.5) Al-(1–3) Nb-0.1Hf/Zr-0.02Y-2Mn-(0–4) Mo/W-0.5Cu + B, C, P Corrosion limit: up to 800–950 °C | ||
| 4–1(Hf, Y) | Fe-25Ni-12Cr-4Al-Nb-2Mn-2Mo-W-0.5Cu-0.15Si-0.1C-0.025P/(−0.14Hf-0.024Y) | [20] |
| AFA | Fe-25Ni-18Cr-3Al-1.5Nb-1.5Mo-0.15Si-0.08C-0.01B-0.04P-0.15Hf-0.01Y | [36] |
| AFA + Al/C | Fe-25Ni-15Cr-3Al-2.5Nb-0.1C-0.01B-0.009Y-0.13Hf +Cr, Si, Al, C, B | [16] |
| NF709 base AFA | Fe-25Ni-18Cr-3Al-(0.5-1.5)Nb-1.5Mo-0.15Si-0.08C-0.01B-0.04P-0.15Hf-(0–0. 1)Y/(−0.1Ti) | [37] |
| OC11 | Fe-25Ni-15Cr-4Al-2.5Nb-2Mn-0.15Si-2Mo-0.11C-0.01B-0.5Cu-0.18Hf-0.03Y | [24] |
| AFA-super alloy: Fe-(30–35) Ni-(14–19) Cr-(2.5–3.5) Al-3Nb + B, C, Ti, Zr Corrosion limit: up to 750–850 °C | ||
| OC 8 | Fe-32Ni-18Cr-3Al-3.3Nb-2Mn-0.15Cu-0.15Mo-0.16W-0.13Si-0.1C-0.05Ti-0.05V-0.14W | [23] |
| 2–3.3 | Fe-32Ni-19Cr-2.4Al-3.3Nb-2Mn-0.01C-0.005 P | [20] |
| DAFA 29 (32ZCB) | Fe-20Cr-30Ni-2Nb -5Al (at. %) Fe-32Ni-14Cr-(3–4)Al-3Nb-(0–0.3)Zr-0.1C-0.01B-0.15Si-(1–3)Ti | [38,39,40] |
| DAFA 26(32Z) | Fe-14Cr-32Ni-3Nb-0.15Si-3Al-2Ti-0.3Zr-0.1C-0.01B-0.1Mo | [40,41] |
| low nickel (wtNi. % < 20%), Corrosion limit: Up to ~650 °C, Lower-cost, low-Ni AFA alloy | ||
| HC-2 | Fe-14Cr-5Mn-12Ni-3Cu-2.5Al-0.6Nb-0.1C-0.007B-0.002N | [42] |
| Simple AFA 18-12-Al 18-12-AlNb | Fe-18Ni-12Cr-2Mo-0.3Si-3Al Fe-18Ni-12Cr-2Mo-0.3Si-2.5Al-0.6Nb-0.02C | [43,44] |
| Cast AFA: Fe-25Ni-14Cr-(0–4) Mo/W-(0.5–1) Si-3.5Al-2Mn-1Nb-0.05V-W-0.05Ti-(0.2–0.5) C-0.5Cu + B Corrosion limit: up to ~750–850 °C | ||
| CAFA 4 | Fe-25Ni-14Cr-2Mo-0.5Si-3.5Al-2Mn-1Nb-0.05V-W-0.05Ti-0.3C-0.5Cu-0.02P | [45] |
| CAFA 7 | Fe-25Ni-14Cr-2Mo-0.9Si-3.5Al-2Mn-1Nb-0.01V-W-0.1Ti-0.3C-0.5Cu-0.01P-0.01B | [46] |
| HTCAFA 4 | Fe-35Ni-25Cr-Si-4Al-1Nb-0.3C-0.02P-0.01B+0.15Hf-0.03Y | [46,47] |
| Precipitates | Influencing Elements |
|---|---|
| Carbides | Nb, Ti, V, Ta, Mn, P, W, C |
| B2-NiAl | Nb, Al, Cu, Si |
| Laves | Nb, W, Si and C |
| γ’ phase | Ni, Al, Zr, W, Cu |
| σ | Cr, Mo, Ti, Zr and V |
| Alloy | Condition | Weight Change (mg/cm2) | ||
|---|---|---|---|---|
| Temperature (°C) | Water Vapor | Time (h) | ||
| 3-0.6 (Fe-20Ni-14Cr-3Al-0.6Nb) [20] | 650 | 10% | 10000 | 0.02 |
| HC-2 (Fe-14Cr-12Ni-4.7Mn-2.5Al) [42] | 650 | 10% | 5000 | 0.2 |
| HTUPS 4 (Fe-20Ni-14Cr-2.5Al-0.86Nb) [4] | 800 | 10% | 1000 | 0.09 |
| 13 (Fe-21Ni-14Cr-2.3Al-3Nb) [17] | 800 (900) | dry 10% | 500 | 0.05 (0.15) 0.045 |
| AFA-SS-M (Fe-17Ni-15.3Cr-3.1Al-2.3Mo-9Mn) [18] | 800 | dry | 1000 | 5 |
| 3-2.5 (Fe-20Ni-14Cr-3Al-2.5Nb) [20] | 800 | 10% | 5000 | 0.2 |
| NF709-4 (Fe-25Ni-18Cr-3Al-0.8Nb) [65] | 800 | dry 10% | 2000 800 | 0.088 0.075 |
| OC4 (Fe-14Cr-25Ni-3.5Al-2.5Nb) [82] | 800 | 10% | 5000 | 0.3 |
| CAFA4 (Fe-25Ni-14Cr-3.5Al-1Nb-2Mn-0.5Si-2Mo) [82] | 800 700 | 10% | 5000 | 0.25 0.1 |
| 22Cr-25Ni (Fe-25Ni-22Cr-2.5/3.5Al-0.45Nb) [33] | 800 | dry | 120 | 0.13 |
| AFA-NbTa (Fe-20Ni-18Cr-2.5Al-2.3Mo-0.45Nb-0.89Ta) [19] | 800 | dry | 500 | 0.15 |
| AFA + Al/C (Fe-25Ni-15Cr-4Al-2.5Nb-0.1C-0.01B) [16] | 800 | 10% | 8000 | 0.2 |
| 4 (Fe-35Ni-25Cr-4Al+Nb, C) [47] | 1100 | 10% | 1000 | 1 |
| A (Fe-26Ni-14Cr-2.8Al-0.6Nb) [15] | 900 | dry | 4000 | 2.5 |
| AFA + B/C (Fe-25Ni-15Cr-3Al-2.5Nb-0.1C-0.107B) [16] | 900 | 10% | 1000 | 0.2 |
| Name | YS (MPa)/UTS (MPa)/Elongation (pct) | Heat Treatment Condition | |
|---|---|---|---|
| Room Temperature | 750 °C | ||
| A-0.9 | 523/650/22 | 349/407/26 | SA + 10% CW |
| DAFA 29(32ZCB) | 560//22 | As received | |
| 1280//5.1 | CR + 2.4 h Anneal | ||
| 1070//5.1 | CR + 24 h | ||
| 800//5.1 | CR + 240 h | ||
| 1150//6.2 | SA + CR + 2.4 h | ||
| 1020//6.2 | SA + CR + 24 h | ||
| 750//6.2 | SA + CR + 240 h | ||
| AFA-Cu | 700 °C | ||
| (A1230) | 394/450/20 | Annealing (1230 °C) + 10% CW | |
| B-1.0 | 261/613/51 | 201/357/32 | SA |
| C-1.0 | 268/644/49 | 232/373/39 | SA |
| AFA1 | 240/570/40 | 210/380/44 | SA |
| 420/890/28 | 220/300/37 | Aged (750 °C, 500 h) | |
| AFA5 | 250/620/60 | 215/385/38 | SA |
| 430/930/22 | 240/290/34 | Aged (750 °C, 500 h) | |
| AFA6 | 270/670/58 | 220/390/32 | SA |
| 450/930/33 | 280/310/34 | Aged (750 °C, 500 h) | |
| NF709-4 | 330/490 | SA | |
| Fe-18Ni-12Cr based | 700 °C | ||
| 487/586/28 | 355/420/15 | As received | |
| 471/622/26.9 | 350/410/17.5 | Aged (700 °C, 10 h) | |
| 432/663/28.9 | 325/400/31.5 | Aged (700 °C, 100 h) | |
| 386/749/29.7 | 265/375/45.5 | Aged (700 °C, 1000 h) | |
| 316 based AFA Dual phase steel | 663/962/24.6 | As received | |
| 431/831/32 | Aged (950 °C, 10 h) | ||
| 383/790/30 | Aged (950 °C, 50 h) | ||
| 391/785/32 | Aged (950 °C, 100 h) | ||
| AFA Fe-16Cr-3Al-2W-0.3Si-0.4Nb-0.04Y | 592/779/27 | 700 °C 474/483/48 | SA |
| Al-modified | 205/338 | SA, | |
| 322/502 | SA + Aged (800 °C, 2.4 h) | ||
| 362/707 | SA + Aged (800 °C, 24 h) | ||
| 351/715 | SA + Aged (800 °C, 240 h) | ||
| 383/736 | SA + Aged (800 °C, 1325 h) | ||
| 2.5Al AFA | 573/703/27 | As received | |
| 595/781/22 | Aged (700 °C, 500 h) | ||
| 581/741/19 | Aged (700 °C, 3000 h) | ||
| Fe-14Cr-20Ni-2.5Al-1.5Nb-0.13Si-0.01Ti-2Mn-0.06C-2Mo-0.03W | 638.9/774/41 | HR | |
| 526/698/53 | HR (20%) | ||
| 715/843/35 | HR (40%) | ||
| 757/849/25 | HR (70%) | ||
| 834/924/7.3 | HR (90%) | ||
| 494/757/37 | Annealing | ||
| 455/680/49 | Annealing +HR (20%) | ||
| 590/773/43 | Annealing + HR (40%) | ||
| 684/828/29 | Annealing + HR (70%) | ||
| 829/976/8.4 | Annealing + HR (90%) | ||
| Fe-15Cr-25Ni-3Al-NbWCu | 372/689/31.8 | SA + Aged (700 °C, 72 h) | |
| Fe-15Cr-25Ni-3Al-NbCu | 454/824/31.8 | Aged (700 °C, 72 h) | |
| 593/841/41 | As received | ||
| 731/916/28 | Aged (700 °C, 2 h) | ||
| 1223/1310/26 | Aged (700 °C, 20 h) | ||
| 4Al-AFA | 951/1140/23 | Aged (700 °C, 100 h) | |
| 1000/1184/25 | Aged (700 °C, 500 h) | ||
| 978/1110/19 | Aged (700 °C, 1000 h) | ||
| 636/1005/27 | As received | ||
| 1153/1464/23 | Aged (700 °C, 2 h) | ||
| 4Al-2Cu-AFA | 1137/1402/28 | Aged (700 °C, 20 h) | |
| 1075/1309/20 | Aged (700 °C, 100 h) | ||
| 996/1225/17 | Aged (700 °C, 500 h) | ||
| 914/1181/27 | Aged (700 °C, 1000h) | ||
| Composition | Creep Condition | Fracture Life (h) | Strengthening Phase |
|---|---|---|---|
| HTUPS 4 | 750 °C/170 MPa | 2200 h | NbC |
| A-0.9 | 750 °C/170 MPa | 357.6 | NbC, Laves, B2 |
| B-1.0 | 750 °C/170 MPa | 337.1 | NbC, Laves, B2, M23C6 |
| C-1.0 | 750 °C/170 MPa | 408.1 | NbC, Laves, B2, M23C6 |
| AFAW (Fe-20Ni-14Cr-2.5Al-Nb-0.16Si-0.1C-0.02V-2W) | 700 °C/160 MPa | 1598 | Finer Laves |
| AFA-0 | 750 °C/150 MPa | 1537 | B2, Laves |
| AFA-Cu A1230 | 750 °C/150 MPa 700 °C/240 MPa 700 °C/150 MPa | 2047 648 2821 | Nano L12 containing Cu |
| NF709 base /0.5Nb0.08C0.1Ti | 750 °C/100 MPa 750 °C/100 MPa | 410 4500 | Laves-Fe2Nb, sigma 10% cold work |
| NF709 base 1.5Nb0.15C | 750 °C/100 MPa | 230 | Primary NbC, sigma |
| NF709 base 1.0Nb0.1C | 750 °C/100 MPa | 460 | Primary NbC, Laves, sigma |
| CAFA 4 | 750 °C/100 MPa | 3500 | Fine L12 |
| CAFA 6 | 750 °C/100 MPa | 10326 | Fine M23C6 |
| WAFA | 700 °C/160 MPa 700 °C/200 MPa | 4100 1044 3119 | Laves Pre-strain |
| HC-2 | 750 °C/100 MPa | 484 | (Ni, Fe)Al type B2, M23C6, α-Cu and NbC |
| 32ZCB (DAFA 29) | 750 °C/100 MPa | 3008 | L12, laves, B2 |
| DAFA 26 | 760 °C/75 MPa | 4921 | Annealing at 800 °C 2.4 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Fan, C.; Sun, H.; Chen, F.; Guo, J.; Huang, T. Research Progress of Alumina-Forming Austenitic Stainless Steels: A Review. Materials 2022, 15, 3515. https://doi.org/10.3390/ma15103515
Liu L, Fan C, Sun H, Chen F, Guo J, Huang T. Research Progress of Alumina-Forming Austenitic Stainless Steels: A Review. Materials. 2022; 15(10):3515. https://doi.org/10.3390/ma15103515
Chicago/Turabian StyleLiu, Ling, Cuilin Fan, Hongying Sun, Fuxiao Chen, Junqing Guo, and Tao Huang. 2022. "Research Progress of Alumina-Forming Austenitic Stainless Steels: A Review" Materials 15, no. 10: 3515. https://doi.org/10.3390/ma15103515
APA StyleLiu, L., Fan, C., Sun, H., Chen, F., Guo, J., & Huang, T. (2022). Research Progress of Alumina-Forming Austenitic Stainless Steels: A Review. Materials, 15(10), 3515. https://doi.org/10.3390/ma15103515







