Medium- and Long-Term Re-Treatment of Root Canals Filled with a Calcium Silicate-Based Sealer: An Experimental Ex Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Size Calculation
2.3. Sample Selection
2.4. Study Procedure
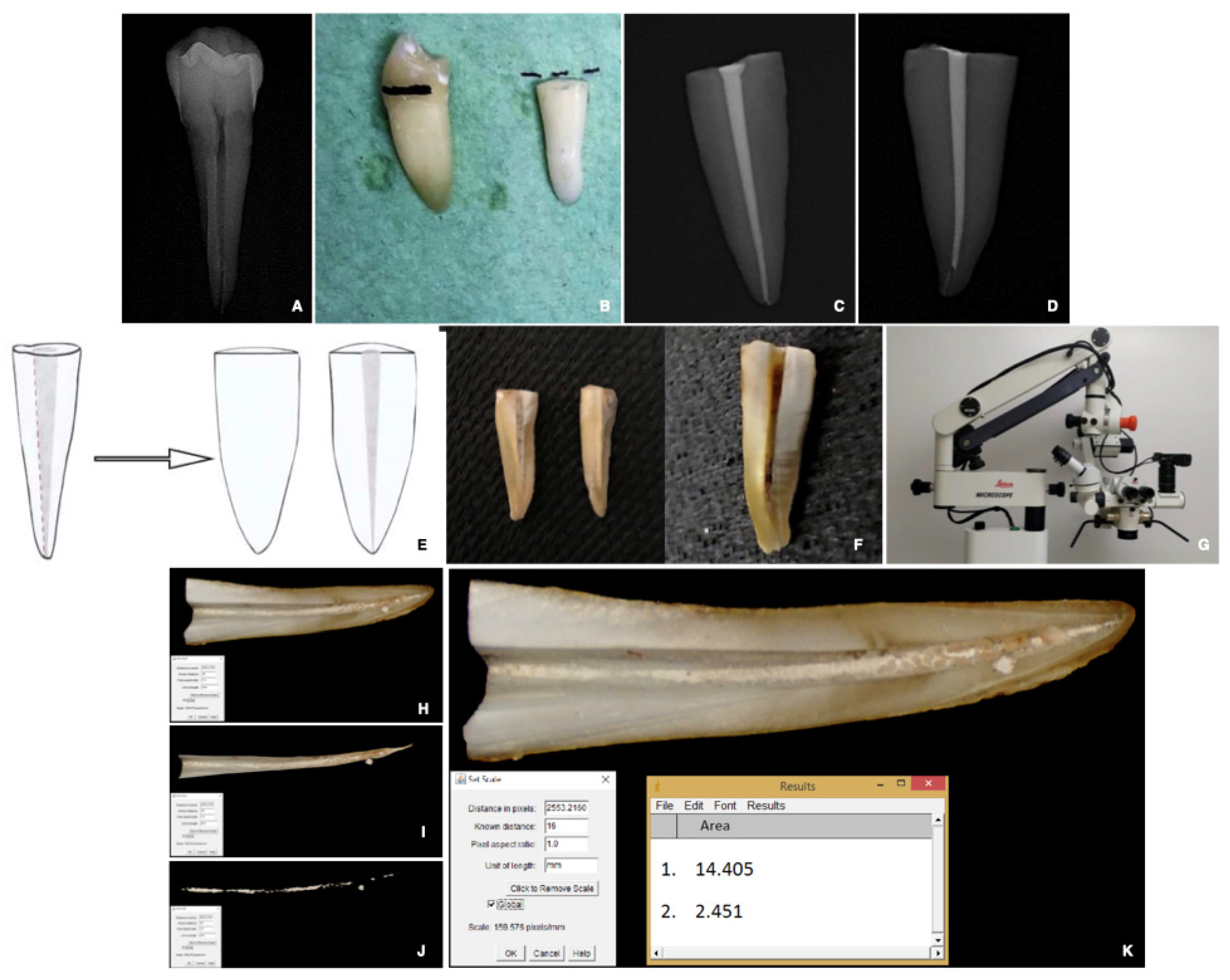
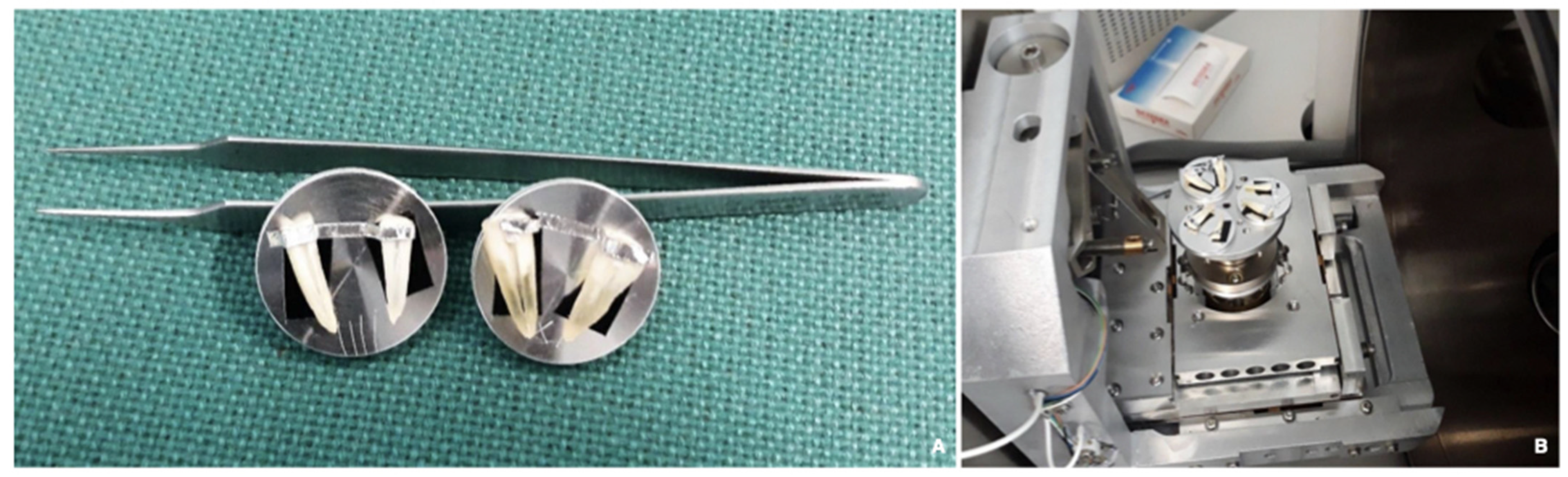
2.4.1. Specimen and Root Canal Preparation
2.4.2. Root Canal Obturation
- BR: single-cone GP and BioRootTM RCS, re-treated after 1 month;
- AH: warm vertical condensation of GP and AH Plus re-treated after one month;
- BR*: single-cone GP and BioRootTM RCS, re-treated after 12 months.
2.4.3. Re-Treatment Procedure
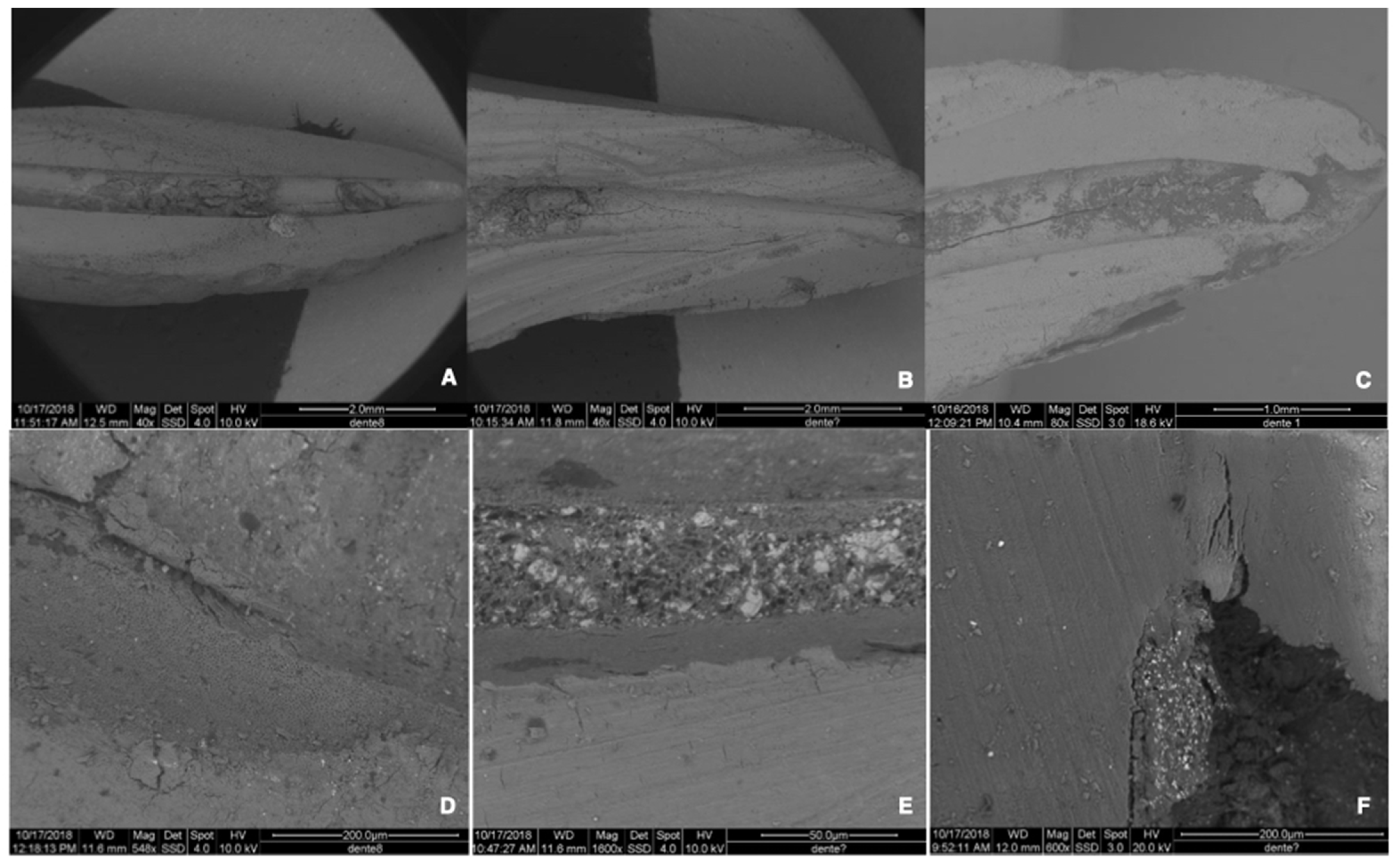
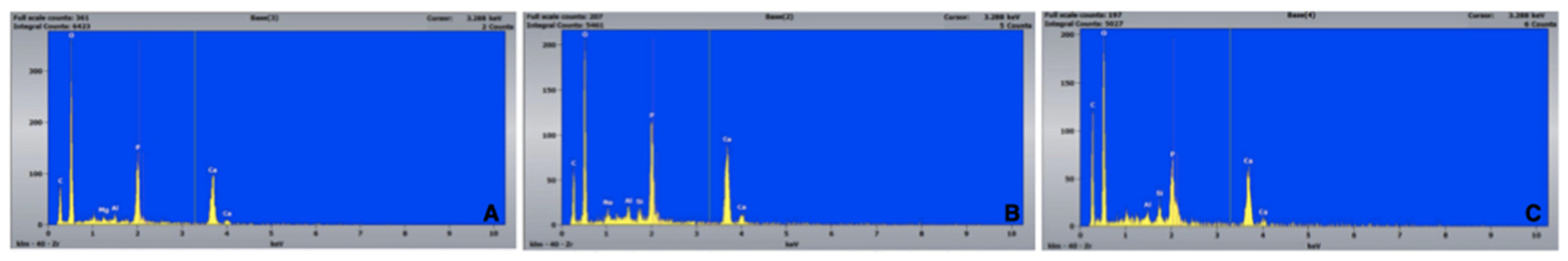
2.4.4. Preparation and Evaluation of the Re-Treated Samples
2.4.5. Data Presentation and Statistical Analysis
3. Results
3.1. Percentage of Residual Filling Materials in the Whole Surface of the Canal [RFM (%)]
3.2. Percentage of Residual Filling Materials in the Apical 3rd of the Canal (Apical 3rd RFM (%))
3.3. Time Required for Re-Treatment Procedure
3.4. Re-Establishing Working Length and Patency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, S. Prognosis of initial endodontic therapy. Endod. Top. 2002, 2, 59–88. [Google Scholar] [CrossRef] [Green Version]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, J.F.; Antunes, H.S.; Pérez, A.R.; Alves, F.R.F.; Mdala, I.; Silva, E.J.N.L.; Belladonna, F.G.; Rôças, I.N. The Apical Root Canal System of Teeth with Posttreatment Apical Periodontitis: Correlating Microbiologic, Tomographic, and Histopathologic Findings. J. Endod. 2020, 46, 1195–1203. [Google Scholar] [CrossRef]
- Löst, C. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Tyagi, S.; Tyagi, P.; Mishra, P. Evolution of root canal sealers: An insight story. Eur. J. Gen. Dent. 2013, 2, 199. [Google Scholar] [CrossRef]
- Leonardo, M.R.; Da Silva, L.; Tanomaru-Filho, M.; da Silva, R.S. Release of formaldehyde by 4 endodontic sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 221–225. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; Mcdonald, F.; Ford, T.R.P. Physical and Chemical Properties of a New Root-End Filling Material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Bardini, G.; Musu, D.; Mezzena, S.; Dettori, C.; Cotti, E. Combined management of apical root fracture and avulsion of two maxillary permanent central incisors: A case report. Dent. J. 2021, 9, 39. [Google Scholar] [CrossRef]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef]
- Wang, Z. Bioceramic materials in endodontics. Endod. Top. 2015, 32, 30. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review-Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pongprueksa, P.; Van Landuyt, K.; Chen, Z.; Pedano, M.; Van Meerbeek, B.; De Munck, J. Correlative micro-Raman/EPMA analysis of the hydraulic calcium silicate cement interface with dentin. Clin. Oral Investig. 2016, 20, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Hadis, M.; Wang, J.; Zhang, Z.J.; Di Maio, A.; Camilleri, J. Interaction of hydraulic calcium silicate and glass ionomer cements with dentine. Materialia 2020, 9, 100515. [Google Scholar] [CrossRef]
- Buchanan, L.S. The continuous wave of condensation technique: A convergence of conceptual and procedural advances in obturation. Dent. Today 1994, 13, 80, 82, 84–85. [Google Scholar]
- Camps, J.; Jeanneau, C.; El Ayachi, I.; Laurent, P.; About, I. Bioactivity of a Calcium Silicate-based Endodontic Cement (BioRoot RCS): Interactions with Human Periodontal Ligament Cells in Vitro. J. Endod. 2015, 41, 1469–1473. [Google Scholar] [CrossRef]
- Camilleri, J. Sealers and warm gutta-percha obturation techniques. J. Endod. 2015, 41, 72–78. [Google Scholar] [CrossRef]
- Oltra, E.; Cox, T.C.; LaCourse, M.R.; Johnson, J.D.; Paranjpe, A. Retreatability of two endodontic sealers, EndoSequence BC Sealer and AH Plus: A micro-computed tomographic comparison. Restor. Dent. Endod. 2017, 42, 19. [Google Scholar] [CrossRef]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of root canal treatments using a new calcium silicate root canal sealer: A non-randomized clinical trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Bardini, G.; Casula, L.; Ambu, E.; Musu, D.; Mercadè, M.; Cotti, E. A 12-month follow-up of primary and secondary root canal treatment in teeth obturated with a hydraulic sealer. Clin. Oral Investig. 2021, 25, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Ersahan, S.; Aydin, C. Solubility and apical sealing characteristics of a new calcium silicate-based root canal sealer in comparison to calcium hydroxide-, methacrylate resin- and epoxy resin-based sealers. Acta Odontol. Scand. 2013, 71, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Pushpa, S.; Maheshwari, C.; Maheshwari, G.; Sridevi, N.; Duggal, P.; Ahuja, P. Effect of pH on solubility of white Mineral Trioxide Aggregate and Biodentine: An in vitro study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2018, 12, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Prüllage, R.K.; Urban, K.; Schäfer, E.; Dammaschke, T. Material Properties of a Tricalcium Silicate–containing, a Mineral Trioxide Aggregate–containing, and an Epoxy Resin–based Root Canal Sealer. J. Endod. 2016, 42, 1784–1788. [Google Scholar] [CrossRef]
- Kebudi Benezra, M.; Schembri Wismayer, P.; Camilleri, J. Interfacial Characteristics and Cytocompatibility of Hydraulic Sealer Cements. J. Endod. 2018, 44, 1007–1017. [Google Scholar] [CrossRef]
- Weller, R.N.; Tay, K.C.Y.; Garrett, L.V.; Mai, S.; Primus, C.M.; Gutmann, J.L.; Pashley, D.H.; Tay, F.R. Microscopic appearance and apical seal of root canals filled with gutta-percha and ProRoot Endo Sealer after immersion in a phosphate-containing fluid. Int. Endod. J. 2008, 41, 977–986. [Google Scholar] [CrossRef]
- Urban, K.; Neuhaus, J.; Donnermeyer, D.; Schäfer, E.; Dammaschke, T. Solubility and pH Value of 3 Different Root Canal Sealers: A Long-term Investigation. J. Endod. 2018, 44, 1736–1740. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Parrilli, A.P.; Fini, M.; Prati, C.; Dummer, P.M.H. 3D micro-CT analysis of the interface voids associated with Thermafil root fillings used with AH Plus or a flowable MTA sealer. Int. Endod. J. 2013, 46, 253–263. [Google Scholar] [CrossRef]
- Milanovic, I.; Milovanovic, P.; Antonijevic, D.; Dzeletovic, B.; Djuric, M.; Miletic, V. Immediate and Long-Term Porosity of Calcium Silicate–Based Sealers. J. Endod. 2020, 46, 515–523. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Prati, C. MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term study of sealing ability. Int. Endod. J. 2010, 43, 889–901. [Google Scholar] [CrossRef]
- Pedullà, E.; Abiad, R.S.; Conte, G.; Khan, K.; Lazaridis, K.; Rapisarda, E.; Neelakantan, P. Retreatability of two hydraulic calcium silicate-based root canal sealers using rotary instrumentation with supplementary irrigant agitation protocols: A laboratory-based micro-computed tomographic analysis. Int. Endod. J. 2019, 52, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Kakoura, F.; Pantelidou, O. Retreatability of root canals filled with Gutta percha and a novel bioceramic sealer: A scanning electron microscopy study. J. Conserv. Dent. 2018, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- Pedullà, E.; Plotino, G.; Grande, N.M.; Avarotti, G.; Gambarini, G.; Rapisarda, E.; Mannocci, F. Shaping ability of two nickel–titanium instruments activated by continuous rotation or adaptive motion: A micro-computed tomography study. Clin. Oral Investig. 2016, 20, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Roggendorf, M.J.; Legner, M.; Ebert, J.; Fillery, E.; Frankenberger, R.; Friedman, S. Micro-CT evaluation of residual material in canals filled with Activ GP or GuttaFlow following removal with NiTi instruments. Int. Endod. J. 2010, 43, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Grotra, D.; Sharma, S. Retreatability of 2 mineral trioxide aggregate-based root canal sealers: A cone-beam computed tomography analysis. J. Endod. 2013, 39, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Bunne, C.; Schäfer, E.; Dammaschke, T. Retreatability of three calcium silicate-containing sealers and one epoxy resin-based root canal sealer with four different root canal instruments. Clin. Oral Investig. 2018, 22, 811–817. [Google Scholar] [CrossRef]
- De Souza, P.F.; Goncalves, L.C.O.; Marques, A.A.F.; Junior, E.C.S.; da Garcia, L.D.F.R.; de Carvalho, F.M.A. Root canal retreatment using reciprocating and continuous rotary nickel-titanium instruments. Eur. J. Dent. 2015, 9, 234–239. [Google Scholar] [CrossRef]
- De Azevêdo Rios, M.; Villela, A.M.; Cunha, R.S.; Velasco, R.C.; De Martin, A.S.; Kato, A.S.; Da Silveira Bueno, C.E. Efficacy of 2 reciprocating systems compared with a rotary retreatment system for gutta-percha removal. J. Endod. 2014, 40, 543–546. [Google Scholar] [CrossRef]
- Versiani, M.A.; Ordinola-Zapata, R.; Keleş, A.; Alcin, H.; Bramante, C.M.; Pécora, J.D.; Sousa-Neto, M.D. Middle mesial canals in mandibular first molars: A micro-CT study in different populations. Arch. Oral Biol. 2016, 61, 130–137. [Google Scholar] [CrossRef]
- Crozeta, B.M.; Silva-Sousa, Y.T.C.; Leoni, G.B.; Mazzi-Chaves, J.F.; Fantinato, T.; Baratto-Filho, F.; Sousa-Neto, M.D. Micro-Computed Tomography Study of Filling Material Removal from Oval-shaped Canals by Using Rotary, Reciprocating, and Adaptive Motion Systems. J. Endod. 2016, 42, 793–797. [Google Scholar] [CrossRef]
- Agrafioti, A.; Koursoumis, A.D.; Kontakiotis, E.G. Re-establishing apical patency after obturation with Gutta-percha and two novel calcium silicate-based sealers. Eur. J. Dent. 2015, 9, 457–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, D.; Solomon, E.; Spears, R.; He, J. Retreatability of a bioceramic root canal sealing material. J. Endod. 2011, 37, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Alsubait, S.; Alhathlol, N.; Alqedairi, A.; Alfawaz, H. A micro-computed tomographic evaluation of retreatability of BioRoot RCS in comparison with AH Plus. Aust. Endod. J. 2021, 47, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okiji, T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int. Endod. J. 2011, 44, 1081–1087. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int. Endod. J. 2013, 46, 808–814. [Google Scholar] [CrossRef]
- Neelakantan, P.; Nandagopal, M.; Shemesh, H.; Wesselink, P. The effect of root dentin conditioning protocols on the push-out bond strength of three calcium silicate sealers. Int. J. Adhes. Adhes. 2015, 60, 104–108. [Google Scholar] [CrossRef]
| Variables | Mean | Median | Standard Deviation | Min | Max | Range | IQR |
|---|---|---|---|---|---|---|---|
| RFM (%) * | 29,104 | 28,900 | 9192 | 13,460 | 48,920 | 35,460 | 14,060 |
| Apical 3rd RFM (%) ** | 18,025 | 14,355 | 13,759 | 0.540 | 68,230 | 67,690 | 20,730 |
| Times required for re-treatment procedure (min) | 2124 | 2075 | 0.865 | 1000 | 4520 | 3520 | 1000 |
| Variables | Mean | Standard Deviation |
|---|---|---|
| RFM (%) * | 29,104 | 9192 |
| Apical 3rd RFM (%) ** | 18,025 | 13,759 |
| Times required for re-treatment procedure (min) | 2124 | 0.865 |
| Dependent Variables | (I) Groups | (J) Groups | Mean Difference (I–J) | Sig. | Confidence Interval 95% | |
|---|---|---|---|---|---|---|
| Inferior | Superior | |||||
| RFM (%) * | AH, AH-1 | BR, BR-1 | 7126 | 0.149 | −1701 | 15,952 |
| BR*, BR*-1 | −1775 | 1000 | −10,601 | 7051 | ||
| BR, BR-1 | AH, AH-1 | −7126 | 0.149 | −15,952 | 1701 | |
| BR*, BR*-1 | −8901 | 0.048 | −17,727 | −0.074 | ||
| BR*, BR*-1 | AH, AH-1 | 1775 | 1000 | −7051 | 10,601 | |
| BR, BR-1 | 8901 | 0.048 | 0.074 | 17,727 | ||
| Times required for re-treatment procedure (min) | AH, AH-1 | BR, BR-1 | −0.981 | 0.0001 | −1397 | −0.565 |
| BR*, BR*-1 | −0.988 | 0.0078 | −1687 | −0.288 | ||
| BR, BR-1 | AH, AH-1 | 0.981 | 0.0001 | 0,565 | 1397 | |
| BR*, BR*-1 | −0.007 | 0.9852 | −0.741 | 0.728 | ||
| BR*, BR*-1 | AH, AH-1 | 0.988 | 0.0078 | 0.288 | 1687 | |
| BR, BR-1 | 0.007 | 0.9852 | −0.728 | 0.741 | ||
| Variables | Overall | Subgroups (WL) | Subgroups (WL-1) |
|---|---|---|---|
| BR | 23.76 (9.41) | 22.37 (5.46) | 33.09 (7.14) |
| BR* | 32.66 (8.05) | 32.24 (9.55) | 25.15 (12.66) |
| AH | 30.89 (8.19) | 27.98 (10.04) | 33.79 (5.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardini, G.; Cotti, E.; Congiu, T.; Caria, C.; Aru, D.; Mercadè, M. Medium- and Long-Term Re-Treatment of Root Canals Filled with a Calcium Silicate-Based Sealer: An Experimental Ex Vivo Study. Materials 2022, 15, 3501. https://doi.org/10.3390/ma15103501
Bardini G, Cotti E, Congiu T, Caria C, Aru D, Mercadè M. Medium- and Long-Term Re-Treatment of Root Canals Filled with a Calcium Silicate-Based Sealer: An Experimental Ex Vivo Study. Materials. 2022; 15(10):3501. https://doi.org/10.3390/ma15103501
Chicago/Turabian StyleBardini, Giulia, Elisabetta Cotti, Terenzio Congiu, Claudia Caria, Davide Aru, and Montse Mercadè. 2022. "Medium- and Long-Term Re-Treatment of Root Canals Filled with a Calcium Silicate-Based Sealer: An Experimental Ex Vivo Study" Materials 15, no. 10: 3501. https://doi.org/10.3390/ma15103501
APA StyleBardini, G., Cotti, E., Congiu, T., Caria, C., Aru, D., & Mercadè, M. (2022). Medium- and Long-Term Re-Treatment of Root Canals Filled with a Calcium Silicate-Based Sealer: An Experimental Ex Vivo Study. Materials, 15(10), 3501. https://doi.org/10.3390/ma15103501






