Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions
Abstract
1. Introduction
2. Materials and Methods
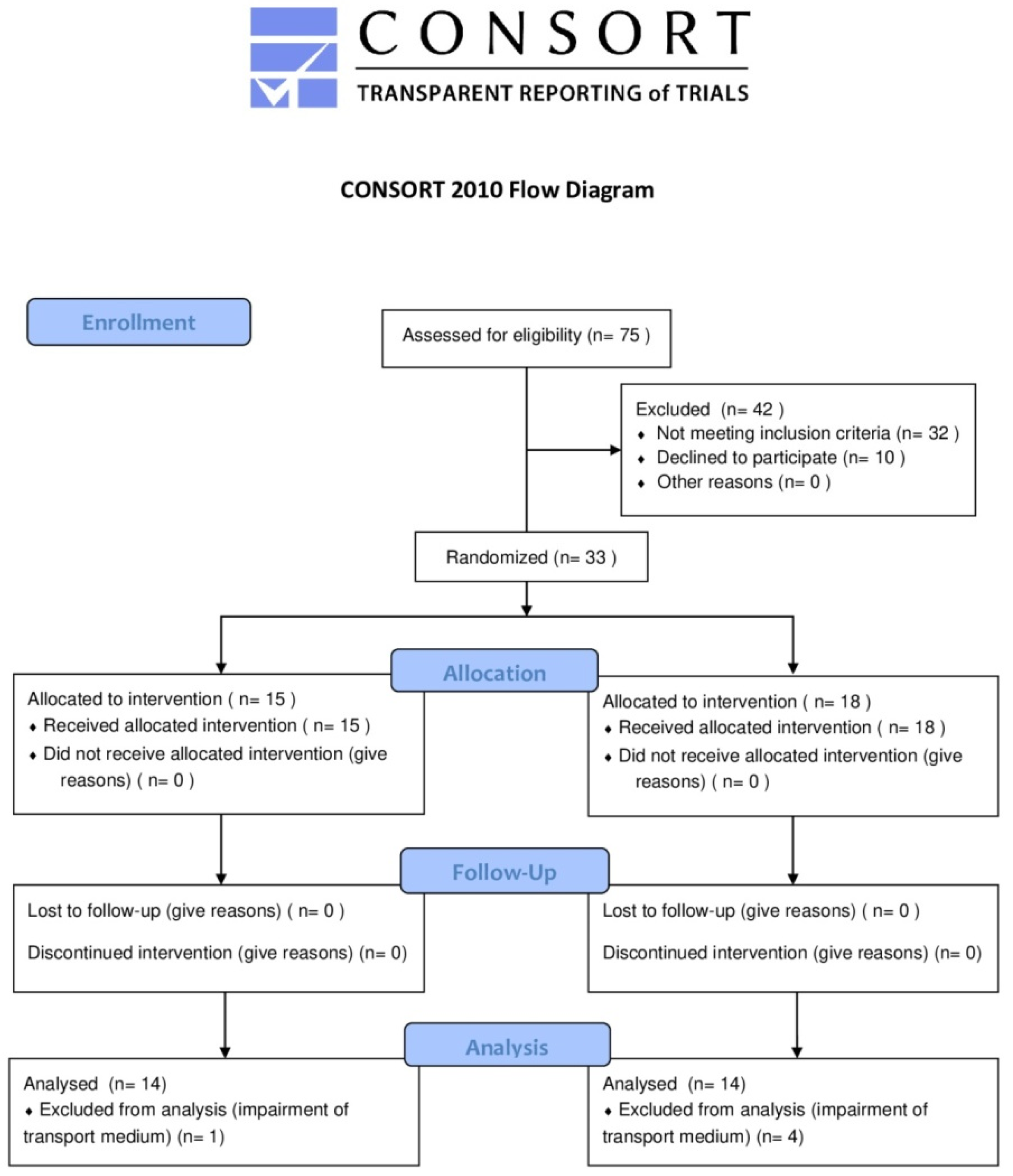
2.1. Trial Design, Inclusion, and Exclusion Criteria for Participants and Sample Size
2.2. Data Collection
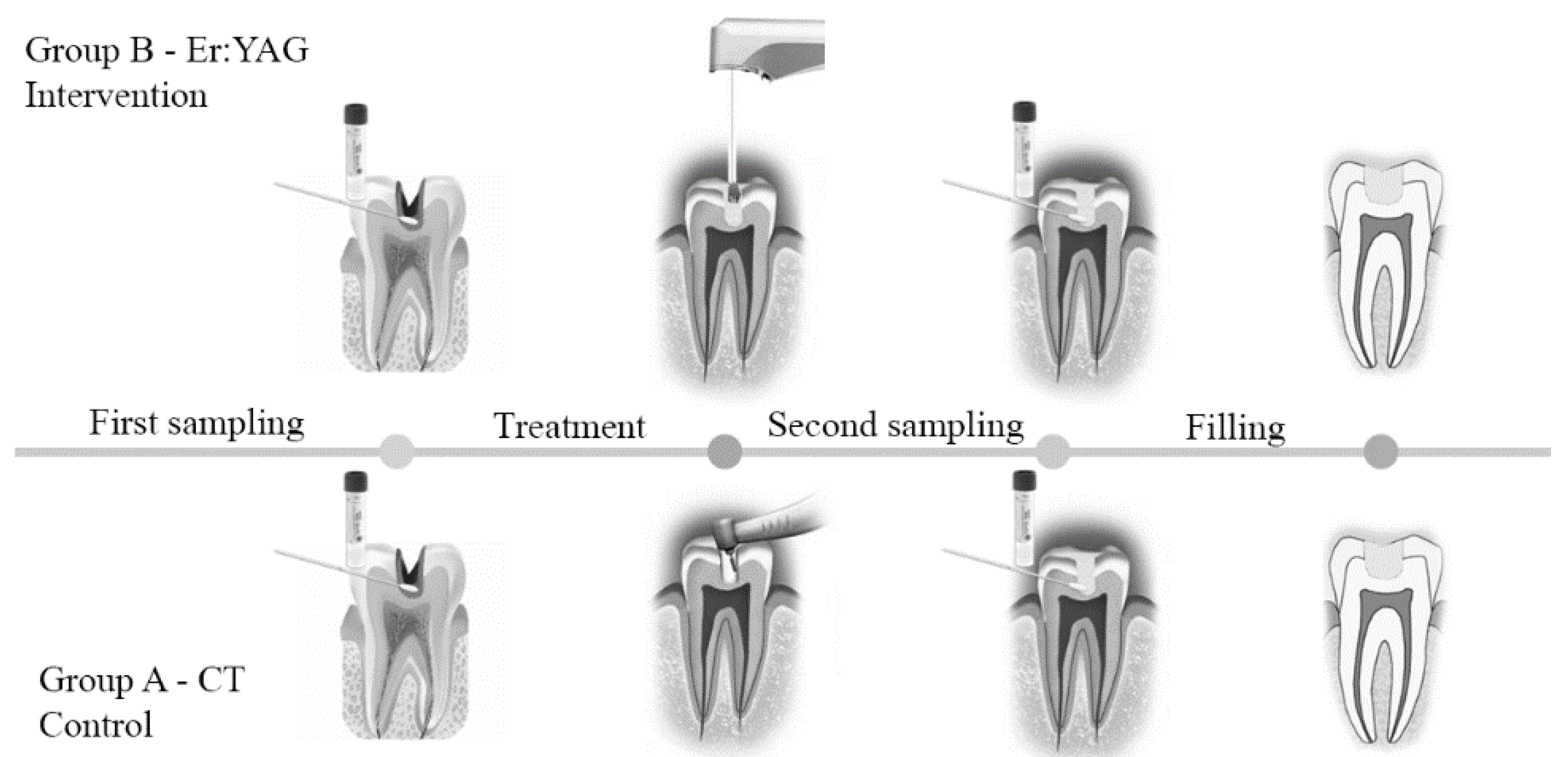
2.3. Clinical Procedures and Sample Collection
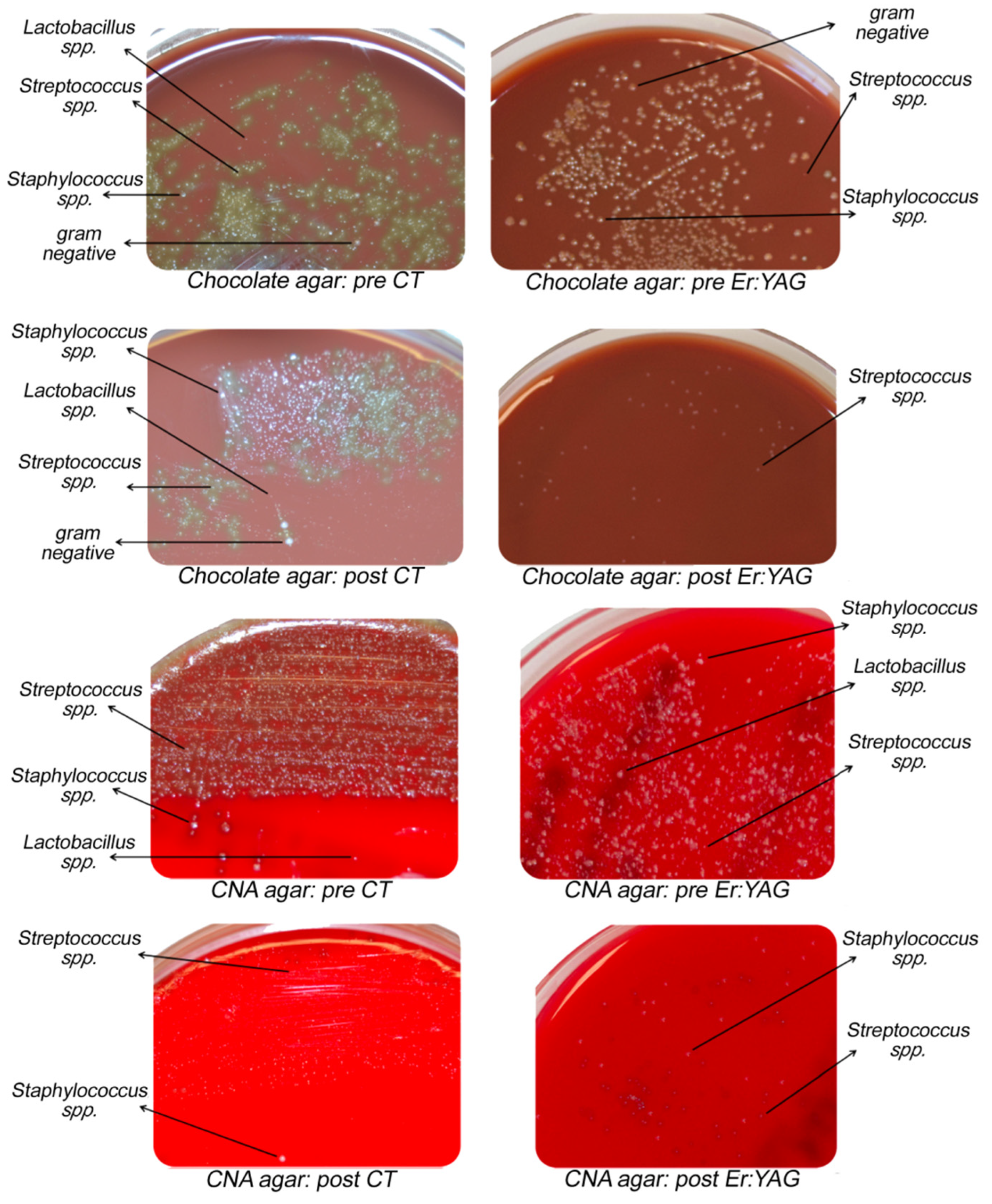
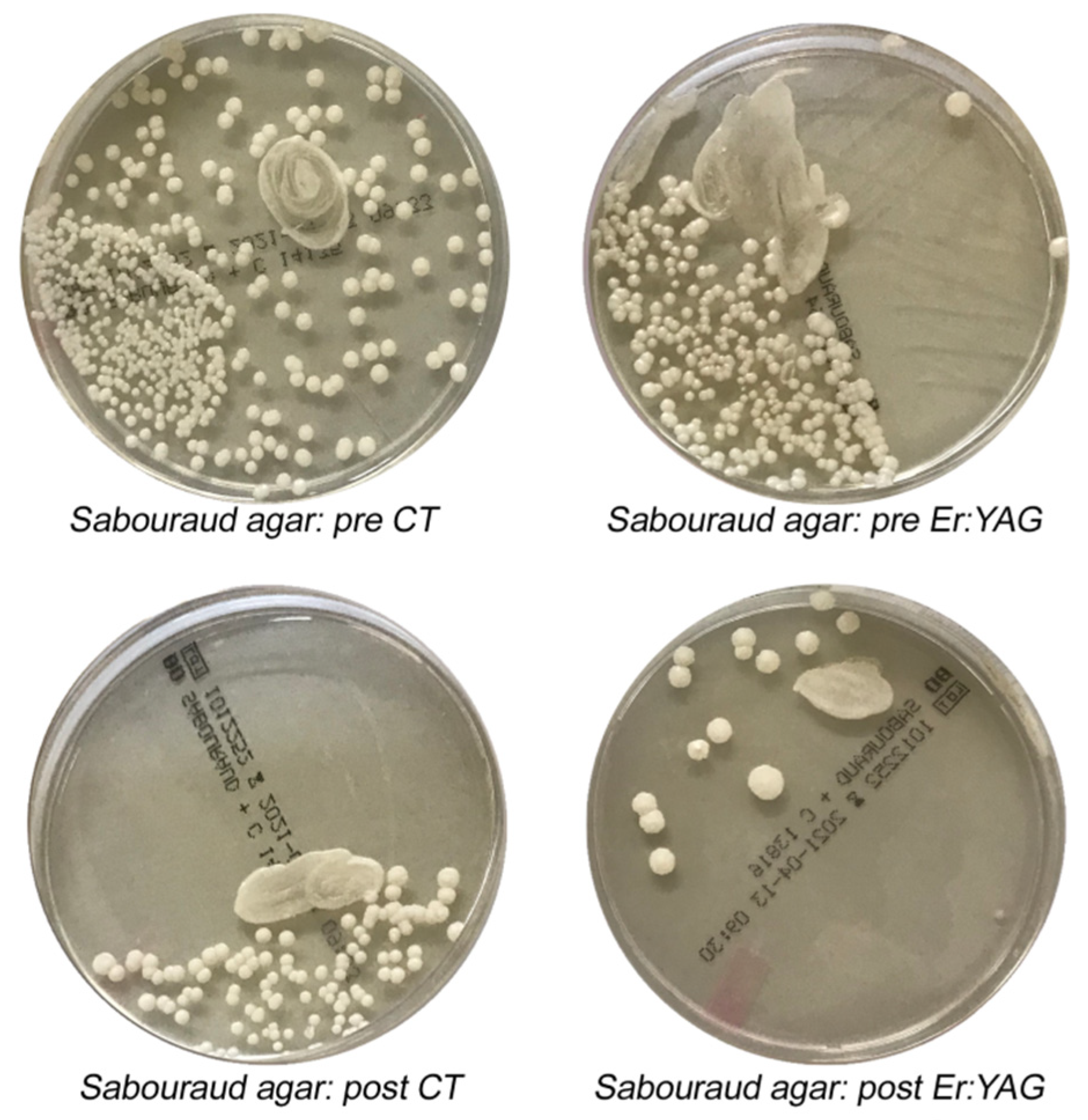
2.4. Microbiological Analysis and Procedures
2.5. Data Processing and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- Er:YAG laser irradiation allowed the complete minimally invasive removal of the carious process and the creation of a conservative therapeutic cavity preparation, increasing resistance to acid dissolution, modifying enamel surface ultrastructure, and creating a micro-retentive surface for adhesive restorations.
- (2)
- There were significant differences in terms of the reduction of the total microbial load following treatment between Er:YAG therapy and CT.
- (3)
- The reductions of the microbial load following the two treatments were different in various microbial populations. For the genus Streptococcus spp., the reduction rates with laser therapy were significant.
Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Moynihan, P.J.; Kelly, S.A. Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines. J. Dent. Res. 2014, 93, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hemadi, A.S.; Huang, R.; Zhou, Y.; Zou, J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int. J. Oral. Sci. 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Conrads, G.; de Soet, J.J.; Song, L.; Henne, K.; Sztajer, H.; Wagner-Döbler, I.; Zeng, A.P. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J. Oral. Microbiol. 2014, 3. [Google Scholar] [CrossRef]
- Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Flores-Estrada, J.; Uematsu, S.; Yamaguchi, R. Quantitative analysis of S. mutans and S. sobrinus cultivated independently and adhered to polished orthodontic composite resins. J. Appl.Oral. Sci. 2012, 20, 544–549. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001, 65, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Cardoso, M.; Coelho, A.; Lima, R.; Amaro, I.; Paula, A.; Marto, C.M.; Sousa, J.; Spagnuolo, G.; Marques Ferreira, M.; Carrilho, E. Efficacy and Patient’s Acceptance of Alternative Methods for Caries Removal-a Systematic Review. J. Clin. Med. 2020, 9, 3407. [Google Scholar] [CrossRef]
- Montedori, A.; Abraha, I.; Orso, M.; D’Errico, P.G.; Pagano, S.; Lombardo, G. Lasers for caries removal in deciduous and permanent teeth. Cochrane Database Syst. Rev. 2016, 9, CD010229. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Orso, M.; Abraha, I.; Capobianco, B.; Cianetti, S. Lasers to prevent dental caries: A systematic review. BMJ Open 2020, 10, e038638. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Pacheco, R.L.; Bussadori, S.K.; Santos, E.M.; Riera, R.; Latorraca, C.D.O.C.; Mota, P.; Bellotto, E.F.B.C.; Martimbianco, A.L.C. Effectiveness and Safety of Ozone Therapy in Dental Caries Treatment: Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2020, 20, 101472. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H. Dental caries-not just holes in teeth! A perspective. Mol. Oral. Microbiol. 2016, 31, 228–233. [Google Scholar] [CrossRef]
- Kallis, A.; Tolidis, K.; Gerasimou, P.; Dionysopoulos, D. Qualitative evaluation of hybrid layer formation using Er:YAG laser in QSP mode for tooth cavity preparations. Lasers Med. Sci. 2019, 34, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Nakhostin, A.; Namdar, F.; Chiniforush, N.; Hasani Tabatabaei, M. The Rate of Demineralization in the Teeth Prepared by Bur and Er:YAG Laser. J. Lasers Med. Sci. 2018, 9, 82–86. [Google Scholar] [CrossRef]
- Gurgan, S.; Kiremitci, A.; Cakir, F.Y.; Yazici, E.; Gorucu, J.; Gutknecht, N. Shear bond strength of composite bonded to erbium:yttrium-aluminum-garnet laser-prepared dentin. Lasers Med. Sci. 2009, 24, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Baraba, A.; Kqiku, L.; Gabrić, D.; Verzak, Ž.; Hanscho, K.; Miletić, I. Efficacy of removal of cariogenic bacteria and carious dentin by ablation using different modes of Er:YAG lasers. Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Medioni, E.; Rocca, J.P.; Fornaini, C.; Merigo, E. Histological evaluation of three techniques for caries removal. J. Oral. Sci. 2016, 58, 583–589. [Google Scholar] [CrossRef][Green Version]
- Melo, M.A.; Lima, J.P.; Passos, V.F.; Rodrigues, L.K. The influence of dentin demineralization on morphological features of cavities using Er:YAG laser. Photomed Laser Surg. 2015, 33, 22–28. [Google Scholar] [CrossRef]
- Chen, M.L.; Ding, J.F.; He, Y.J.; Chen, Y.; Jiang, Q.Z. Effect of pretreatment on Er:YAG laser-irradiated dentin. Lasers Med. Sci. 2015, 30, 753–759. [Google Scholar] [CrossRef]
- Cebe, F.; Bulbul, M.; Simsek, I.; Cebe, M.A.; Ozturk, B. Effect of Erbium:Yttrium aluminum garnet laser on bond strength of a total-etch adhesive system to caries-affected dentin on gingival wall. Niger. J. Clin. Pract. 2017, 20, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Sharon-Buller, A.; Block, C.; Savion, I.; Sela, M. Reduced bacteria levels in cavities prepared by Er: YAG laser. J. Oral Laser Appl. 2003, 3, 153–155. [Google Scholar]
- Nelson, D.G.; Wefel, J.S.; Jongebloed, W.L.; Featherstone, J.D. Morphology, histology and crystallography of human dental enamel treated with pulsed low-energy infrared laser radiation. Caries Res. 1987, 21, 411426. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Valério, R.A.; Borsatto, M.C.; Campos Serra, M.; Fernandes Polizeli, S.A.; Alencar Nemezio, M.; Galo, R.; Aires, C.P.; dos Santos, A.C.; Milori Corona, S.A. Caries removal in deciduous teeth using an Er:YAG laser: A randomized split-mouth clinical trial. Clin. Oral. Investig. 2016, 20, 65–73. [Google Scholar] [CrossRef]
- Ornellas, P.O.; Antunes, L.S.; Motta, P.C.; Mendonça, C.; Póvoa, H.; Fontes, K.; Iorio, N.; Antunes, L.A.A. Antimicrobial Photodynamic Therapy as an Adjunct for Clinical Partial Removal of Deciduous Carious Tissue: A Minimally Invasive Approach. Photochem. Photobiol. 2018, 94, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Joyston-Bechal, S.; Beighton, D. Microbiological validation of assessments of caries activity during cavity preparation. Caries Res. 1993, 27, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Pitts, N.B.; Tellez, M.; Banerjee, A.; Deery, C.; Douglas, G.; Eggertsson, H.; Ekstrand, K.; Ellwood, R.; Gomez, J.; et al. The International Caries Classification and Management System (ICCMS™) An Example of a Caries Management Pathway. BMC Oral. Healthe 2015, 15 (Suppl. 1), S9. [Google Scholar] [CrossRef]
- Jew, J.; Chan, K.H.; Darling, C.L.; Fried, D. Selective removal of natural caries lesions from dentin and tooth occlusal surfaces using a diode-pumped Er:YAG laser. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10044, 100440I. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Shi, H.; Ma, Z.; Lv, B.; Xie, M. Er:YAG laser application in caries removal and cavity preparation in children: A meta-analysis. Lasers Med. Sci. 2019, 34, 273–280. [Google Scholar] [CrossRef]
- Belcheva, A.; Shindova, M. Subjective Acceptance of Pediatric Patients during Cavity Preparation with Er:YAG Laser and Conventional Rotary Instruments. J. IMAB 2014, 20, 631–633. [Google Scholar] [CrossRef]
- Raj, S.; Agarwai, M.; Aradya, K.; Konde, S.; Nagakishore, V. Evaluation of Dental Fear in Children During DentalVisit using Children’s Fear SurveySchedule-Dental Subscale. Int. J. Clin.Pediatr. Dent. 2013, 6, 12–15. [Google Scholar] [CrossRef]
- Krause, F.; Braun, A.; Lotz, G.; Kneist, S.; Jepsen, S.; Eberhard, J. Evaluation of selective caries removal in deciduous teeth by a fluorescence feedback-controlled Er: YAG laser in vivo. Clin. Oral. Investig. 2008, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Matthews, S.; Pitts, N.B.; Longbottom, C.; Nugent, Z.J. A clinical evaluation of an Erbium:YAG laser for dental cavity preparation. Br. Dent. J. 2000, 188, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Polizeli, S.; Curylofo-Zotti, F.A.; Valério, R.A.; Nemezio, M.A.; Souza-Gabriel, A.E.; Borsatto, M.C.; Corona, S. Selective Removal of Necrotic Dentin in Primary Teeth Using Laser Irradiation: One-Year Clinical Evaluation of Composite Restorations. J. Lasers Med. Sci. 2019, 10, 108–116. [Google Scholar] [CrossRef]
- Schwass, D.R.; Leichter, J.W.; Purton, D.G.; Swain, M.V. Evaluating the efficiency of caries removal using an Er:YAG laser driven by fluorescence feedback control. Arch. Oral. Biol. 2013, 58, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Denbesten, P.K.; White, J.M.; Pelino, J.E.P.; Furnish, G.; Silveira, A.; Parkins, F.M. The Safety and Effectiveness of an Er:YAG Laser for Caries Removal and Cavity Preparation in Children. Med. Laser Appl. 2001, 16, 215–222. [Google Scholar] [CrossRef]
- Mosskull Hjertton, P.; Bågesund, M. Er:YAG laser or high-speed bur for cavity preparation in adolescents. Acta Odontol. Scand. 2013, 71, 610–615. [Google Scholar] [CrossRef]
- Cvar, J.F.; Ryge, G. Reprint of criteria for the clinical evaluation of dental restorative materials. Clin. Oral Investig. 2005, 9, 215–232. [Google Scholar] [CrossRef]
- Apel, C.; Birker, L.; Meister, J.; Weiss, C.; Gutknecht, N. The caries-preventive potential of subablative Er:YAG and Er:YSGG laser radiation in an intraoral model: A pilot study. Photomed. Laser Surg. 2004, 22, 312–317. [Google Scholar] [CrossRef]
- Ramalho, K.M.; Hsu, C.Y.; de Freitas, P.M. Erbium Lasers for the Prevention of Enamel and Dentin Demineralization: A Literature Review. Photomed. Laser Surg. 2015, 33, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Al-Maliky, M.A.; Frentzen, M.; Meister, J. Laser-assisted prevention of enamel caries: A 10-year review of the literature. Lasers Med. Sci. 2020, 35, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, R.; Andersson, E.V.; Lingström, P.; Gabre, P. A Randomized Controlled Trial Comparing Er:YAG Laser and Rotary Bur in the Excavation of Caries—Patients’ Experiences and the Quality of Composite Restoration. Open Dent. J. 2018, 31, 443–454. [Google Scholar] [CrossRef]
- Koyuturk, A.E.; Ozmen, B.; Cortcu, M.; Tokay, U.; Tosun, G.; Erhan, S.M. Effects of Er:YAG laser on bond strength of self-etching adhesives to caries-affected dentin. Microsc.Res. Tech. 2014, 77, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Paddick, J.S.; Brailsford, S.R.; Kidd, E.A.; Beighton, D. Phenotypic and genotypic selection of microbiota surviving under dental restorations. Appl. Environ. Microbiol. 2005, 71, 2467–2472. [Google Scholar] [CrossRef]
- Cecchini, R.C.; Zezell, D.M.; de Oliveira, E.; de Freitas, P.M.; Eduardo, C.d.P. Effect of Er:YAG laser on enamel acid resistance: Morphological and atomic spectrometry analysis. Lasers Surg. Med. 2005, 37, 366–372. [Google Scholar] [CrossRef]
- Guven, Y.; Aktoren, O. Shear bond strength and ultrastructural interface analysis of different adhesive systems to Er:YAG laser-prepared dentin. Lasers Med. Sci. 2015, 30, 769–778. [Google Scholar] [CrossRef]
- Bohari, M.R.; Chunawalla, Y.K.; Ahmed, B.M. Clinical evaluation of caries removal in primary teeth using conventional, chemomechanical and laser technique: An in vivo study. J. Contemp. Dent. Pract. 2012, 13, 40–47. [Google Scholar] [CrossRef]
- Pelagalli, J.; Gimbel, C.B.; Hansen, R.T.; Swett, A.; Winn, D.W. Investigational study of the use of Er:YAG laser versus dental drill for caries removal and cavity preparation-phase I. J. Clin. Laser Med. Surg. 1997, 15, 109–115. [Google Scholar] [CrossRef]

| Microrganism | Phylum | Gram-Positive | Gram-Negative |
|---|---|---|---|
| Staphylococcus aureus | Firmicutes | x | |
| Staphylococcus epidermidis | Firmicutes | x | |
| Streptococcus oralis | Firmicutes | x | |
| Streptococcus mutans | Firmicutes | x | |
| Streptococcus salivarius | Firmicutes | x | |
| Haemophilus parainfluenzae | Proteobacteria | x | |
| Neisseria flava | Proteobacteria | x | |
| Neisseria sicca | Proteobacteria | x | |
| Candida albicans | |||
| Lactobacillus casei | Firmicutes | x | |
| Lactobacillus collinoides | Firmicutes | x | |
| Lactobacillus salivarius | Firmicutes | x | |
| Prevotella oralis | Bacteroidetes | x | |
| Prevotella denticola | Bacteroidetes | x | |
| Streptococcus anginosus | Firmicutes | x | |
| Streptococcus cristatus | Firmicutes | x | |
| Streptococcus gordonii | Firmicutes | x | |
| Streptococcus mitis | Firmicutes | x | |
| Streptococcus milleri | Firmicutes | x | |
| Streptococcus pyogenes | Firmicutes | x | |
| Streptococcus sanguinis | Firmicutes | x | |
| Streptococcus parasanguinis | Firmicutes | x | |
| Actinobacillus hominis | Proteobacteria | x | |
| Actinomyces odontolyticus | Actinobacteria | x | |
| Clostridium perfringens | Firmicutes | x | |
| Corynebacterium propinquum | Actinobacteria | x | |
| Escherichia coli | Proteobacteria | x | |
| Fusobacterium nucleatum | Fusobacteria | x | |
| Neisseria mucosa | Proteobacteria | x | |
| Neisseria perflava | Proteobacteria | x | |
| Staphylococcus hominis | Firmicutes | x | |
| Staphylococcus warneri | Firmicutes | x |
| Sample Collection | Percentage of CFUs Reduction after Treatments (%) | Mean CFUs (SD) | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | CT | Er:YAG | |||
| Pre | Post | Pre | Post | |||
| Total microrganisms | 80.6% | 91% * | 3.69 × 104 (4.56 × 104) | 7.78 × 103 (2.25 × 104) | 5.15 × 104 (4.77 × 104) | 5.52 × 103 (1.44 × 104) |
| Streptococcus spp. | 72.4% | 90.2% * | 6.04 × 104 (4.64 × 104) | 1.84 × 104 (3.54 × 104) | 8.01 × 104 (3.96 × 104) | 9.40 × 103 (2.18 × 104) |
| Lactobacillus spp. | 100% | 100% | 5.50 × 103 (4.50 × 103) | 0 (0) | 1 × 103 (0) | 0 (0) |
| Candida spp. | 89.5% | 94.4% | 3.17 × 103 (3.39 × 103) | 5.00 × 102 (5.00 × 102) | 3.06 × 104 (4.40 × 104) | 2.00 × 103 (3.61 × 103) |
| p value | 0.05 | 0.05 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, C.; Pagano, S.; Bozza, S.; Ciurnella, E.; Lomurno, G.; Capobianco, B.; Coniglio, M.; Cianetti, S.; Marinucci, L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials 2021, 14, 2387. https://doi.org/10.3390/ma14092387
Valenti C, Pagano S, Bozza S, Ciurnella E, Lomurno G, Capobianco B, Coniglio M, Cianetti S, Marinucci L. Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials. 2021; 14(9):2387. https://doi.org/10.3390/ma14092387
Chicago/Turabian StyleValenti, Chiara, Stefano Pagano, Silvia Bozza, Enrico Ciurnella, Giuseppe Lomurno, Benito Capobianco, Maddalena Coniglio, Stefano Cianetti, and Lorella Marinucci. 2021. "Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions" Materials 14, no. 9: 2387. https://doi.org/10.3390/ma14092387
APA StyleValenti, C., Pagano, S., Bozza, S., Ciurnella, E., Lomurno, G., Capobianco, B., Coniglio, M., Cianetti, S., & Marinucci, L. (2021). Use of the Er:YAG Laser in Conservative Dentistry: Evaluation of the Microbial Population in Carious Lesions. Materials, 14(9), 2387. https://doi.org/10.3390/ma14092387








