Effect of Oxygen Content on Microstructure and Tensile Properties of a 22Cr-5Al ODS Steel
Abstract
1. Introduction
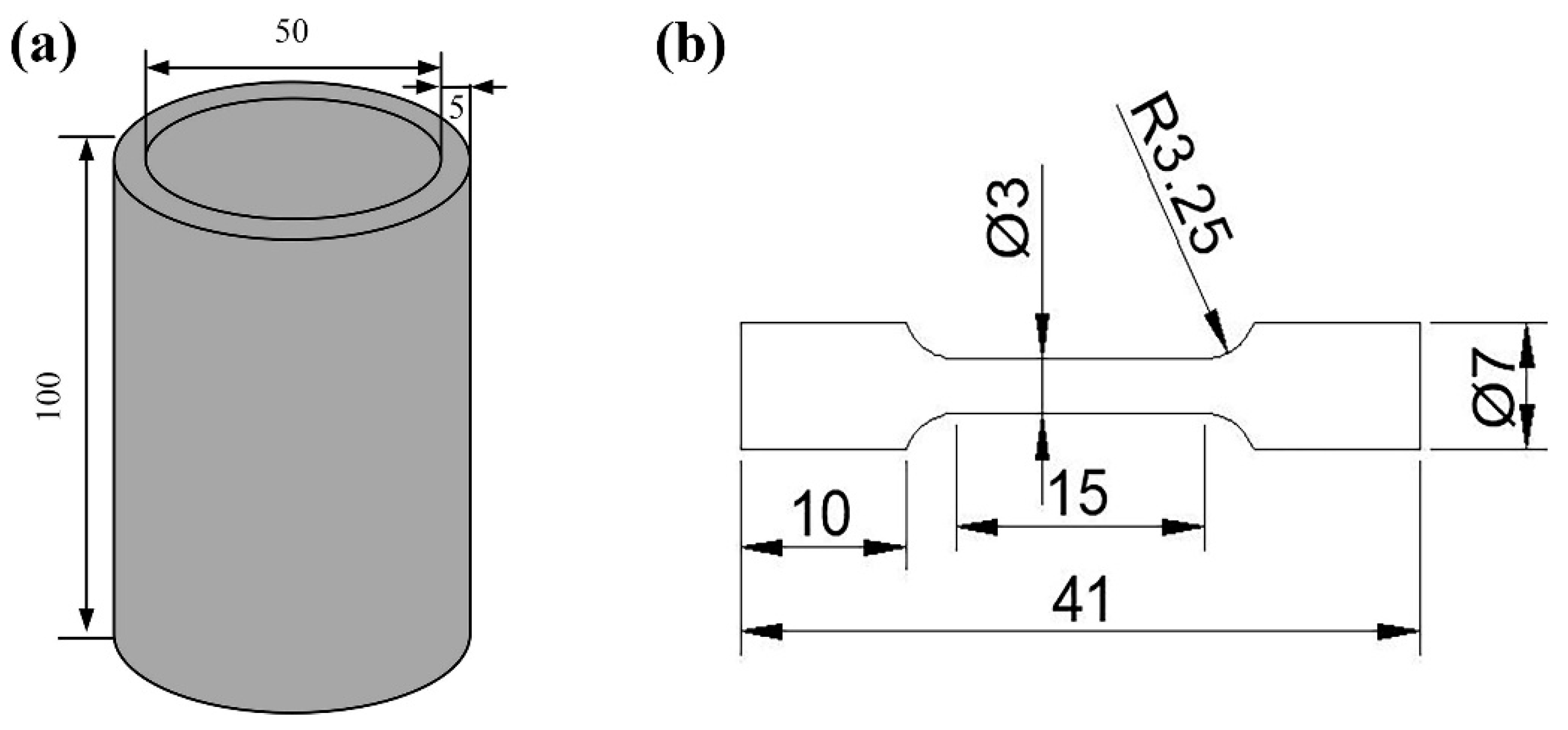
2. Materials and Experiment
3. Results
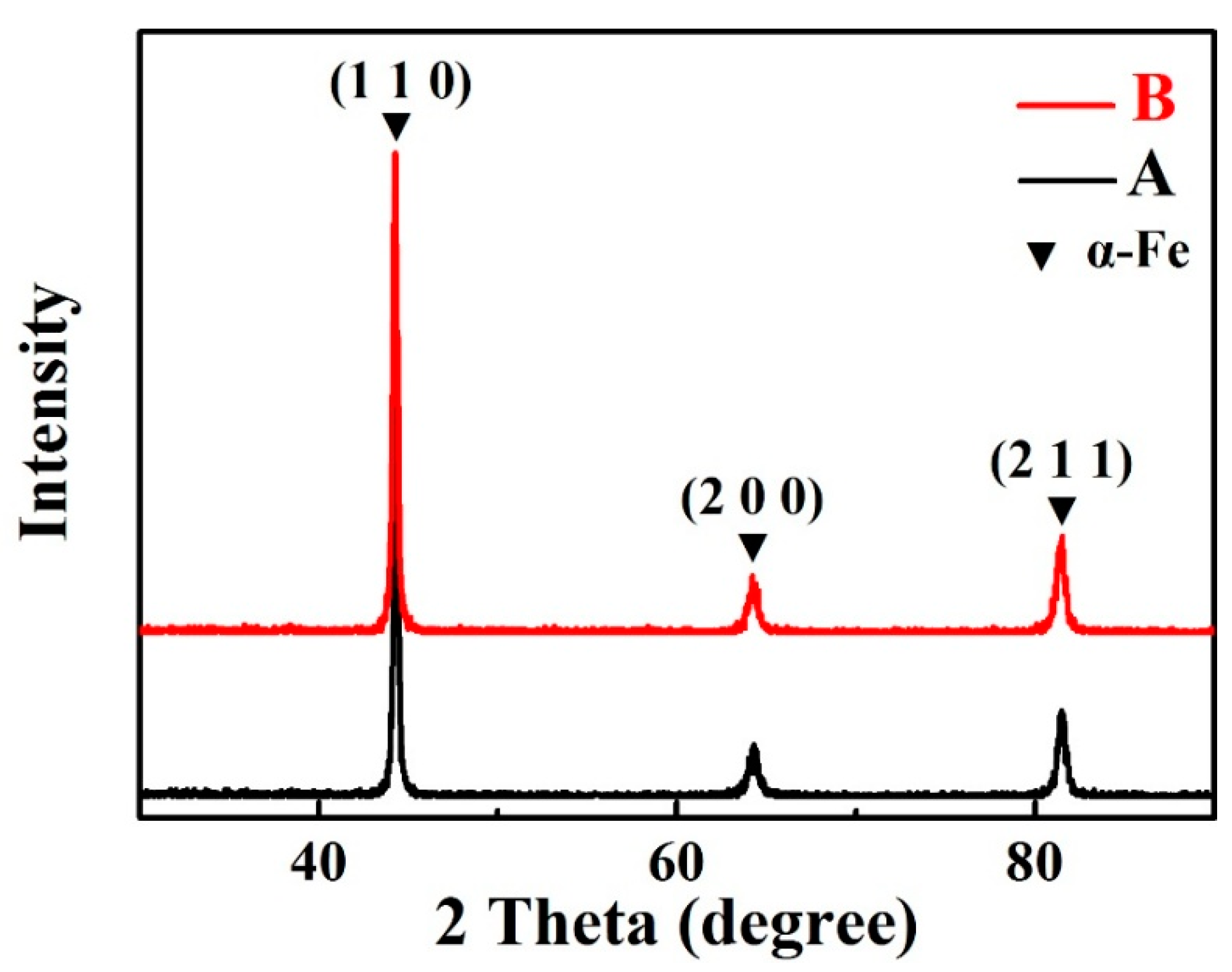
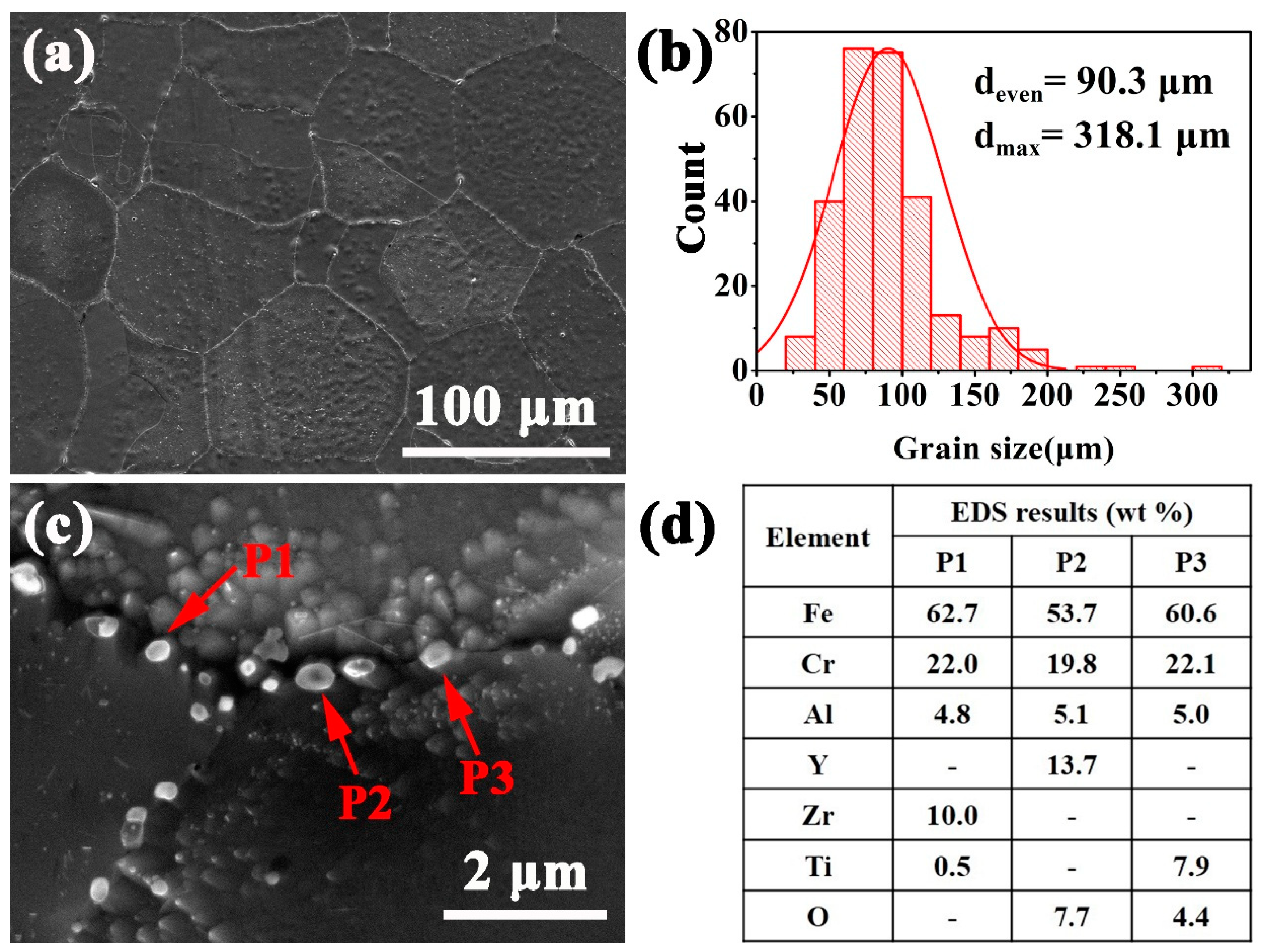
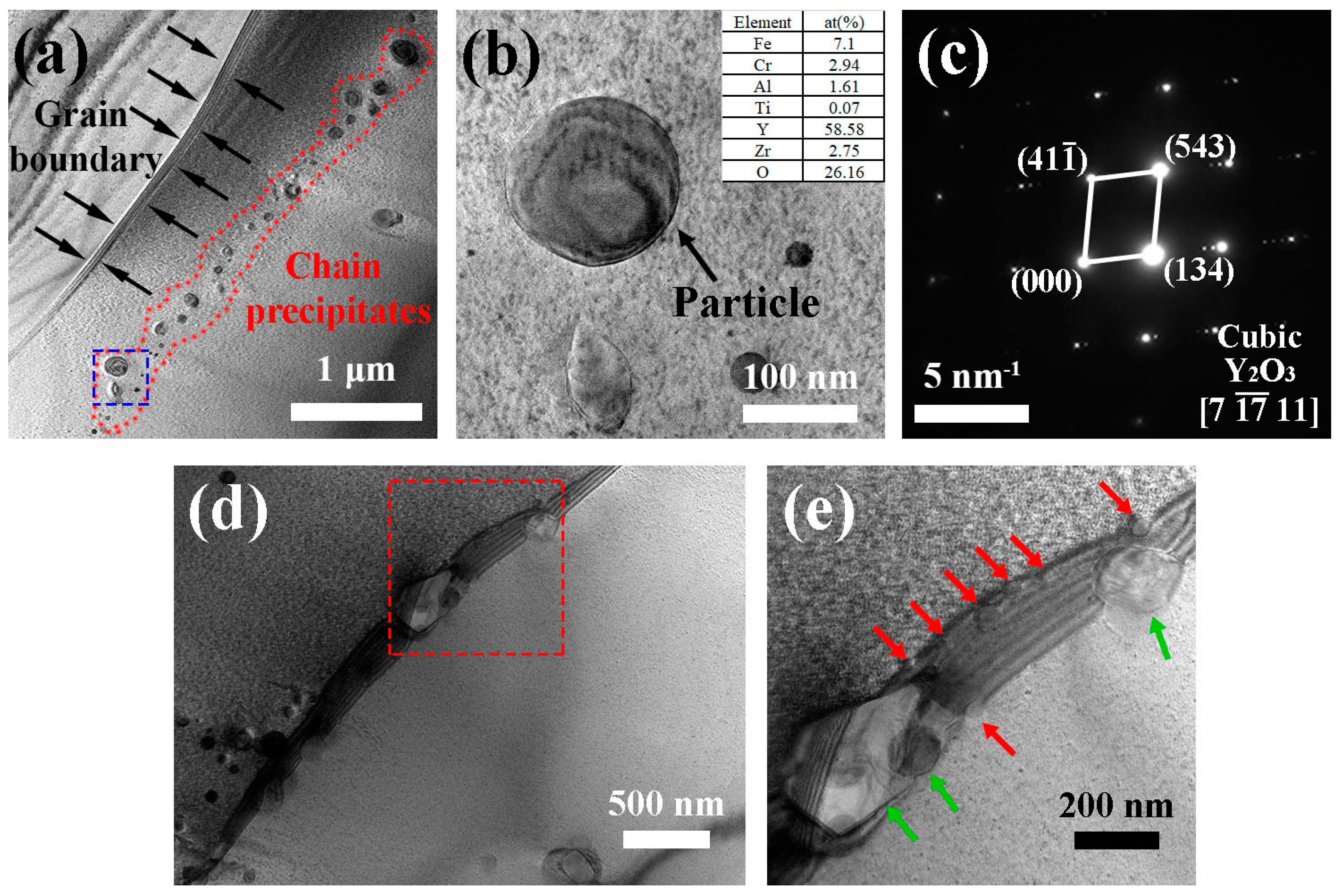
3.1. Microstructure
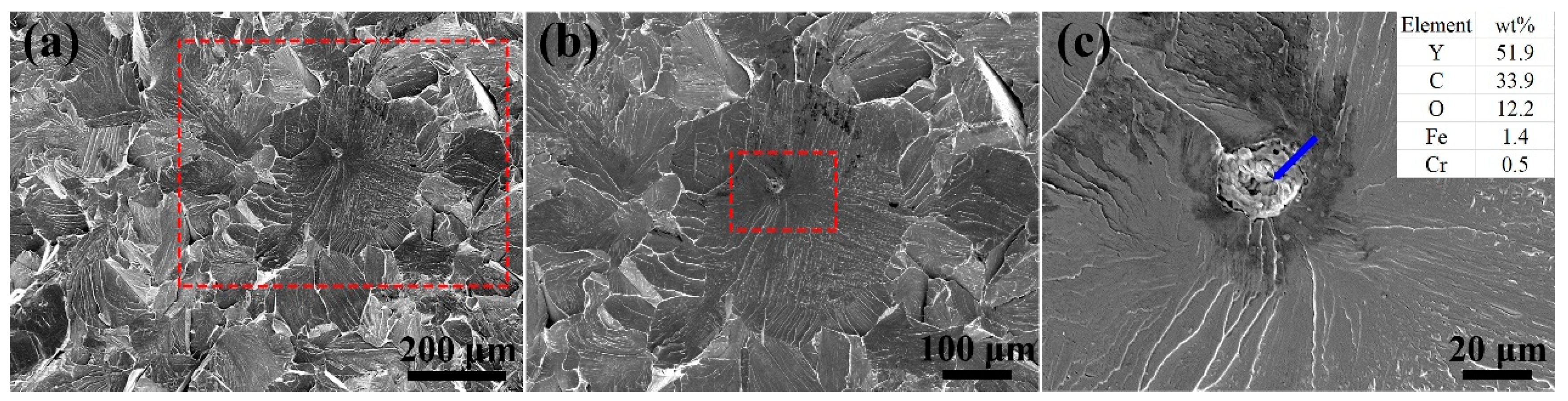
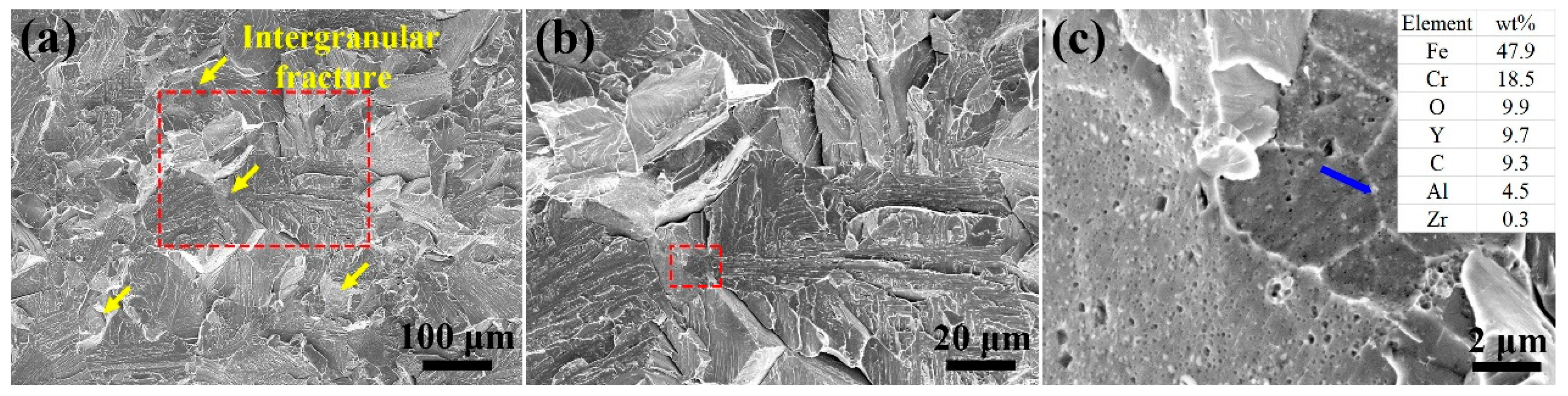
3.2. Tensile Test and Fracture Surface Analysis
4. Discussion
5. Conclusions
- The precipitates in the as-HIPed alloys mainly were Y-rich oxides. An increase in the oxygen content from 0.04 to 0.16 wt% led to an increase in the number and density of precipitates in the as-HIPed alloy. Meanwhile, the Y-rich precipitates segregated at and near grain boundaries and formed large-sized stripe/chain precipitates.
- With the increase in oxygen content from 0.04 to 0.16 wt%, the ultimate tensile strength of the as-HIPed alloy increased from 604 to 669 MPa, and the yield strength increased from 468 to 506 MPa. However, a brittle fracture occurred in both as-HIPed alloys, which could be attributed to the segregation of large-sized oxides and the stripe/chain Y-rich precipitates at and near grain boundaries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent Developments in Irradiation-Resistant Steels. Annu. Rev. Mater. Sci. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Eiselt, C.C.; Schendzielorz, H.; Seubert, A.; Hary, B.; de Carlan, Y.; Diano, P.; Perrin, B.; Cedat, D. ODS-materials for high temperature applications in advanced nuclear systems. Nucl. Mater. Energy 2016, 9, 22–28. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Ijiri, Y.; Oono, N.; Ukai, S.; Ohtsuka, S.; Kaito, T.; Matsukawa, Y. Oxide particle–dislocation interaction in 9Cr-ODS steel. Nucl. Mater. Energy 2016, 9, 378–382. [Google Scholar] [CrossRef]
- Oono, N.; Tang, Q.X.; Ukai, S. Oxide particle refinement in Ni-based ODS alloy. Mater. Sci. Eng. A 2016, 649, 250–253. [Google Scholar] [CrossRef]
- Song, M.; Sun, C.; Jang, J.; Han, C.H.; Kim, T.K.; Hartwig, K.T.; Zhang, X. Microstructure refinement and strengthening mechanisms of a 12Cr ODS steel processed by equal channel angular extrusion. J. Alloys Compd. 2013, 577, 247–256. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Effect of zirconium addition on the microstructure and mechanical properties of ODS ferritic steels containing aluminum. J. Nucl. Mater. 2014, 444, 462–468. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Wang, D.; Liu, C. Effect of zirconium addition on the microstructure and mechanical properties of 15Cr-ODS ferritic Steels consolidated by hot isostatic pressing. Fusion Eng. Des. 2017, 114, 33–39. [Google Scholar] [CrossRef]
- Darling, K.A.; Rajagopalan, M.; Komarasamy, M.; Bhatia, M.A.; Hornbuckle, B.C.; Mishra, R.S.; Solanki, K.N. Extreme creep resistance in a microstructurally stable nanocrystalline alloy. Nature 2016, 537, 378–381. [Google Scholar] [CrossRef]
- Yano, Y.; Sekio, Y.; Tanno, T.; Kato, S.; Inoue, T.; Oka, H.; Ohtsuka, S.; Furukawa, T.; Uwaba, T.; Kaito, T.; et al. Ultra-high temperature creep rupture and transient burst strength of ODS steel claddings. J. Nucl. Mater. 2019, 516, 347–353. [Google Scholar] [CrossRef]
- Hayashi, T.; Sarosi, P.M.; Schneibel, J.H.; Mills, M.J. Creep response and deformation processes in nanocluster-strengthened ferritic steels. Acta Mater. 2008, 56, 1407–1416. [Google Scholar] [CrossRef]
- Jaumier, T.; Vincent, S.; Vincent, L.; Desmorat, R. Creep and damage anisotropies of 9%Cr and 14%Cr ODS steel cladding. J. Nucl. Mater. 2019, 518, 274–286. [Google Scholar] [CrossRef]
- Hilger, I.; Boulnat, X.; Hoffmann, J.; Testani, C.; Bergner, F.; De Carlan, Y.; Ferraro, F.; Ulbricht, A. Fabrication and characterization of oxide dispersion strengthened (ODS) 14Cr steels consolidated by means of hot isostatic pressing, hot extrusion and spark plasma sintering. J. Nucl. Mater. 2016, 472, 206–214. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Lewandowska, M.; Kurzydlowski, K.; Baluc, N. Reduced activation ODS ferritic steel - recent development in high speed hot extrusion processing. Phys. Status Solidi (A) 2010, 207, 1128–1131. [Google Scholar] [CrossRef]
- Karak, S.K.; Majumdar, J.D.; Witczak, Z.; Lojkowski, W.; Manna, I. Microstructure and mechanical properties of nano-Y2O3 dispersed ferritic alloys synthesized by mechanical alloying and consolidated by hydrostatic extrusion. Mater. Sci. Eng. A 2013, 580, 231–241. [Google Scholar] [CrossRef]
- Dash, M.K.; Mythili, R.; Ravi, R.; Sakthivel, T.; Dasgupta, A.; Saroja, S.; Bakshi, S.R. Microstructure and mechanical properties of oxide dispersion strengthened 18Cr-ferritic steel consolidated by spark plasma sintering. Mater. Sci. Eng. A 2018, 736, 137–147. [Google Scholar] [CrossRef]
- Shi, Y.; Lu, Z.; Yu, L.; Xie, R.; Ren, Y.; Yang, G. Microstructure and tensile properties of Zr-containing ODS-FeCrAl alloy fabricated by laser additive manufacturing. Mater. Sci. Eng. A 2020, 774, 138937. [Google Scholar] [CrossRef]
- Kürnsteiner, P.; Wilms, M.B.; Weisheit, A.; Barriobero-Vila, P.; Gault, B.; Jägle, E.A.; Raabe, D. In-process Precipitation During Laser Additive Manufacturing Investigated by Atom Probe Tomography. Microsc. Microanal. 2017, 23, 694–695. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wang, C.; Shen, H.; Zhang, H. Feasibility of using Y2Ti2O7 nanoparticles to fabricate high strength oxide dispersion strengthened Fe–Cr–Al steels. Mater. Des. 2015, 88, 862–870. [Google Scholar] [CrossRef]
- Li, S.F.; Zhou, Z.J.; Wang, P.H.; Sun, H.Y.; Wang, M.; Zhang, G.M. Long-term thermal-aging stability of a 16Cr-oxide dispersion strengthened ferritic steel at 973 K. Mater. Des. 2016, 90, 318–329. [Google Scholar] [CrossRef]
- Ma, S.; Li, A.; Zhou, S.; Yang, Y.; Wang, S.; Liu, M. Microstructure and mechanical properties of nickel strengthened by Y2O3 through rock-milling and spark plasma sintering. J. Alloys Compd. 2018, 750, 911–916. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Yu, L.; Li, H.; Liu, Y. Effects of Ti addition on microstructure and mechanical property of spark-plasma-sintered transformable 9Cr-ODS steels. Fusion Eng. Des. 2018, 135, 88–94. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Influence of Zr addition on the microstructures and mechanical properties of 14Cr ODS steels. Mater. Sci. Eng. A 2017, 695, 66–73. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Wang, D.; Zhang, Z.; Han, Y.; Liu, C. Structure and composition of oxides in FeCrAl ODS alloy with Zr addition. Mater. Sci. Technol. 2017, 33, 1790–1795. [Google Scholar] [CrossRef]
- Xia, Y.P.; Wang, X.P.; Zhuang, Z.; Sun, Q.X.; Zhang, T.; Fang, Q.F.; Hao, T.; Liu, C.S. Microstructure and oxidation properties of 16Cr–5Al–ODS steel prepared by sol–gel and spark plasma sintering methods. J. Nucl. Mater. 2013, 432, 198–204. [Google Scholar] [CrossRef]
- Seol, J.B.; Haley, D.; Hoelzer, D.T.; Kim, J.H. Influences of interstitial and extrusion temperature on grain boundary segregation, Y−Ti−O nanofeatures, and mechanical properties of ferritic steels. Acta Mater. 2018, 153, 71–85. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Boulnat, X.; Sallez, N.; Dadé, M.; Borbély, A.; Béchade, J.L.; de Carlan, Y.; Malaplate, J.; Bréchet, Y.; de Geuser, F.; Deschamps, A.; et al. Influence of oxide volume fraction on abnormal growth of nanostructured ferritic steels during non-isothermal treatments: An in situ study. Acta Mater. 2015, 97, 124–130. [Google Scholar] [CrossRef]
- Zhong, S.Y.; Ribis, J.; Lochet, N.; de Carlan, Y.; Klosek, V.; Mathon, M.H. Influence of nano-particle coherency degree on the coarsening resistivity of the nano-oxide particles of Fe–14Cr–1W ODS alloys. J. Nucl. Mater. 2014, 455, 618–623. [Google Scholar] [CrossRef]
- Lin, J.B.Q. Review and Analysis of Powder Prior Boundary (PPB) Formation in Powder Metallurgy Processes for Nickel-based Super Alloys. J. Powder Metall. Min. 2015, 4, 2. [Google Scholar] [CrossRef]
- Klueh, R.L.; Shingledecker, J.P.; Swindeman, R.W.; Hoelzer, D.T. Oxide dispersion-strengthened steels: A comparison of some commercial and experimental alloys. J. Nucl. Mater. 2005, 341, 103–114. [Google Scholar] [CrossRef]
- Kumar, D.; Prakash, U.; Dabhade, V.V.; Laha, K.; Sakthivel, T. High yttria ferritic ODS steels through powder forging. J. Nucl. Mater. 2017, 488, 75–82. [Google Scholar] [CrossRef]
- Ordás, N.; Gil, E.; Cintins, A.; de Castro, V.; Leguey, T.; Iturriza, I.; Purans, J.; Anspoks, A.; Kuzmin, A.; Kalinko, A. The role of yttrium and titanium during the development of ODS ferritic steels obtained through the STARS route: TEM and XAS study. J. Nucl. Mater. 2018, 504, 8–22. [Google Scholar] [CrossRef]
- Rieken, J.R.; Anderson, I.E.; Kramer, M.J.; Odette, G.R.; Stergar, E.; Haney, E. Reactive gas atomization processing for Fe-based ODS alloys. J. Nucl. Mater. 2012, 428, 65–75. [Google Scholar] [CrossRef]
- Nagini, M.; Vijay, R.; Rajulapati, K.V.; Reddy, A.V.; Sundararajan, G. Microstructure–mechanical property correlation in oxide dispersion strengthened 18Cr ferritic steel. Mater. Sci. Eng. A 2017, 708, 451–459. [Google Scholar] [CrossRef]
- Warren, R.; Ingesten, N.G.; Winberg, L.; Rönnhult, T. Particle Surfaces and Prior Particle Boundaries in Hf Modified PM Astroloy. Powder Metall. 2013, 27, 141–146. [Google Scholar] [CrossRef]
- Karlsson, H.; Nyborg, L.; Berg, S. Surface chemical analysis of prealloyed water atomised steel powder. Powder Metall. 2005, 48, 51–58. [Google Scholar] [CrossRef]
- Cintins, A.; Anspoks, A.; Purans, J.; Kuzmin, A.; Timoshenko, J.; Vladimirov, P.; Gräning, T.; Hoffmann, J. ODS steel raw material local structure analysis using X-ray absorption spectroscopy. IOP Conf. Ser. Sci. Eng. 2015, 77, 012029. [Google Scholar] [CrossRef]
- Zhang, B.W.; Liu, G.; Han, K. The Fe-Y (iron-yttrium) system. J. Phase Equilibria 1992, 13, 304–308. [Google Scholar] [CrossRef]
- Gao, X.; Ren, H.; Wang, H.; Chen, S. Activity coefficient and solubility of yttrium in Fe-Y dilute solid solution. J. Rare Earths 2016, 34, 1168–1172. [Google Scholar] [CrossRef]
- Kotan, H.; Darling, K.A.; Scattergood, R.O.; Koch, C.C. Influence of Zr and nano-Y2O3 additions on thermal stability and improved hardness in mechanically alloyed Fe base ferritic alloys. J. Alloys Compd. 2014, 615, 1013–1018. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; De Geuser, F.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, L.; Zhang, H.; Sun, Z. Segregation effects of Y, Ti, Cr and Si on the intergranular fracture of niobium. J. Alloys Compd. 2017, 711, 637–642. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Z.; Li, M.; Wang, M.; Zhang, G. Microstructure characterization and tensile properties of 18Cr–4Al-oxide dispersion strengthened ferritic steel. J. Alloys Compd. 2015, 648, 39–45. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Ma, Z.; Li, H.; Wu, J. Microstructure and tensile properties of a 14Cr ODS ferritic steel. Mater. Sci. Eng. A 2017, 100, 347–350. [Google Scholar] [CrossRef]
- Sun, Z.; Bei, H.; Yamamoto, Y. Microstructural control of FeCrAl alloys using Mo and Nb additions. Mater. Charact. 2017, 132, 126–131. [Google Scholar] [CrossRef]


| O | Cr | Al | Ti | Y | Zr | Fe | |
|---|---|---|---|---|---|---|---|
| A | 0.04 | 22 | 5 | 0.11 | 0.11 | 0.1 | Bal. |
| B | 0.16 | 22 | 5 | 0.12 | 0.10 | 0.1 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yan, Y.; Zhai, Y.; Qin, W.; Che, H.; Wang, T.; Cao, R. Effect of Oxygen Content on Microstructure and Tensile Properties of a 22Cr-5Al ODS Steel. Materials 2021, 14, 2241. https://doi.org/10.3390/ma14092241
Zhang Y, Yan Y, Zhai Y, Qin W, Che H, Wang T, Cao R. Effect of Oxygen Content on Microstructure and Tensile Properties of a 22Cr-5Al ODS Steel. Materials. 2021; 14(9):2241. https://doi.org/10.3390/ma14092241
Chicago/Turabian StyleZhang, Yukun, Yingjie Yan, Yazhong Zhai, Wei Qin, Hongyan Che, Tiejun Wang, and Rui Cao. 2021. "Effect of Oxygen Content on Microstructure and Tensile Properties of a 22Cr-5Al ODS Steel" Materials 14, no. 9: 2241. https://doi.org/10.3390/ma14092241
APA StyleZhang, Y., Yan, Y., Zhai, Y., Qin, W., Che, H., Wang, T., & Cao, R. (2021). Effect of Oxygen Content on Microstructure and Tensile Properties of a 22Cr-5Al ODS Steel. Materials, 14(9), 2241. https://doi.org/10.3390/ma14092241






