Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Binder Materials
2.1.2. Alkaline Activators
2.2. Experimental Method
2.2.1. Properties of Alkaline Activators
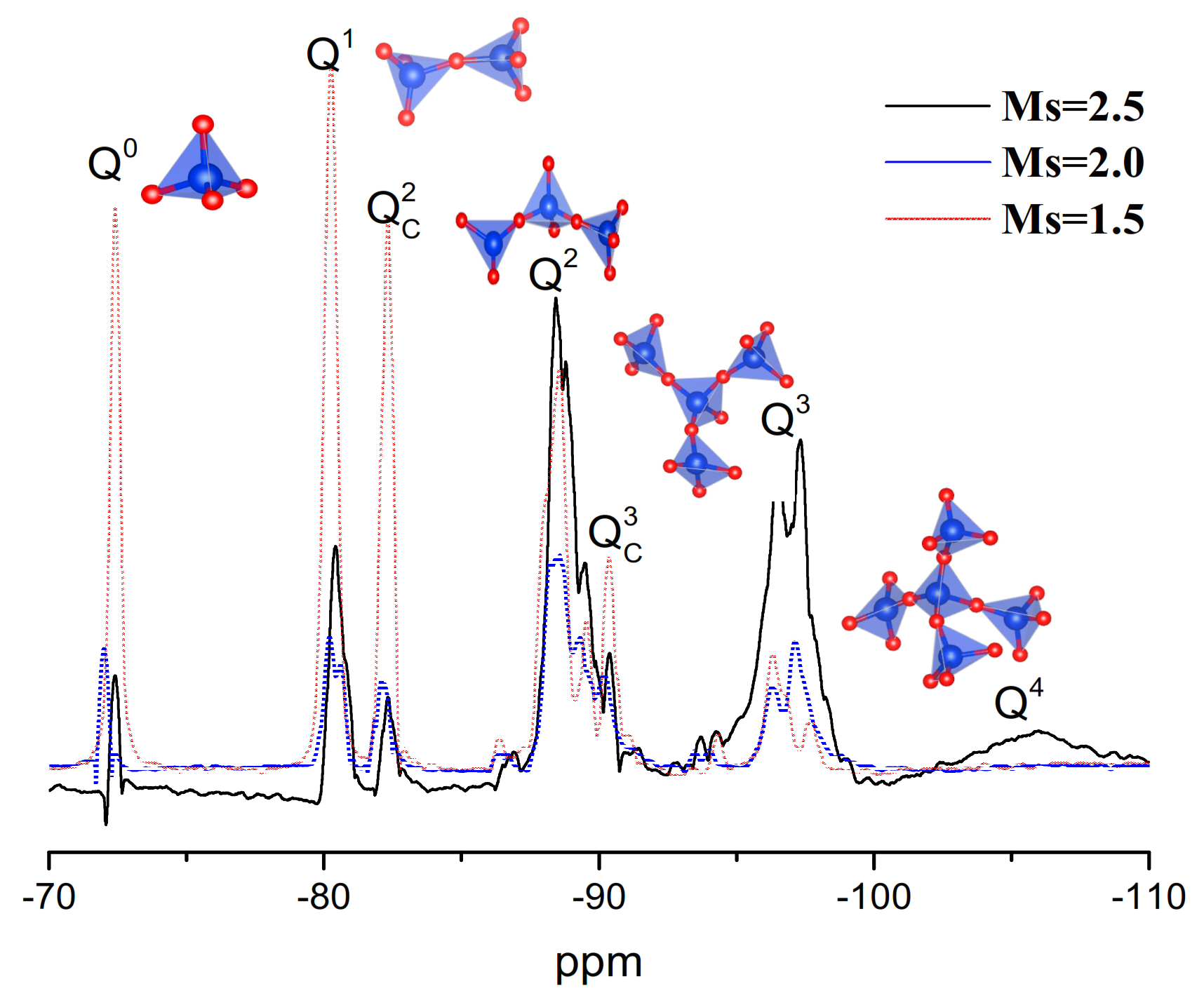
Liquid-State 29Si NMR
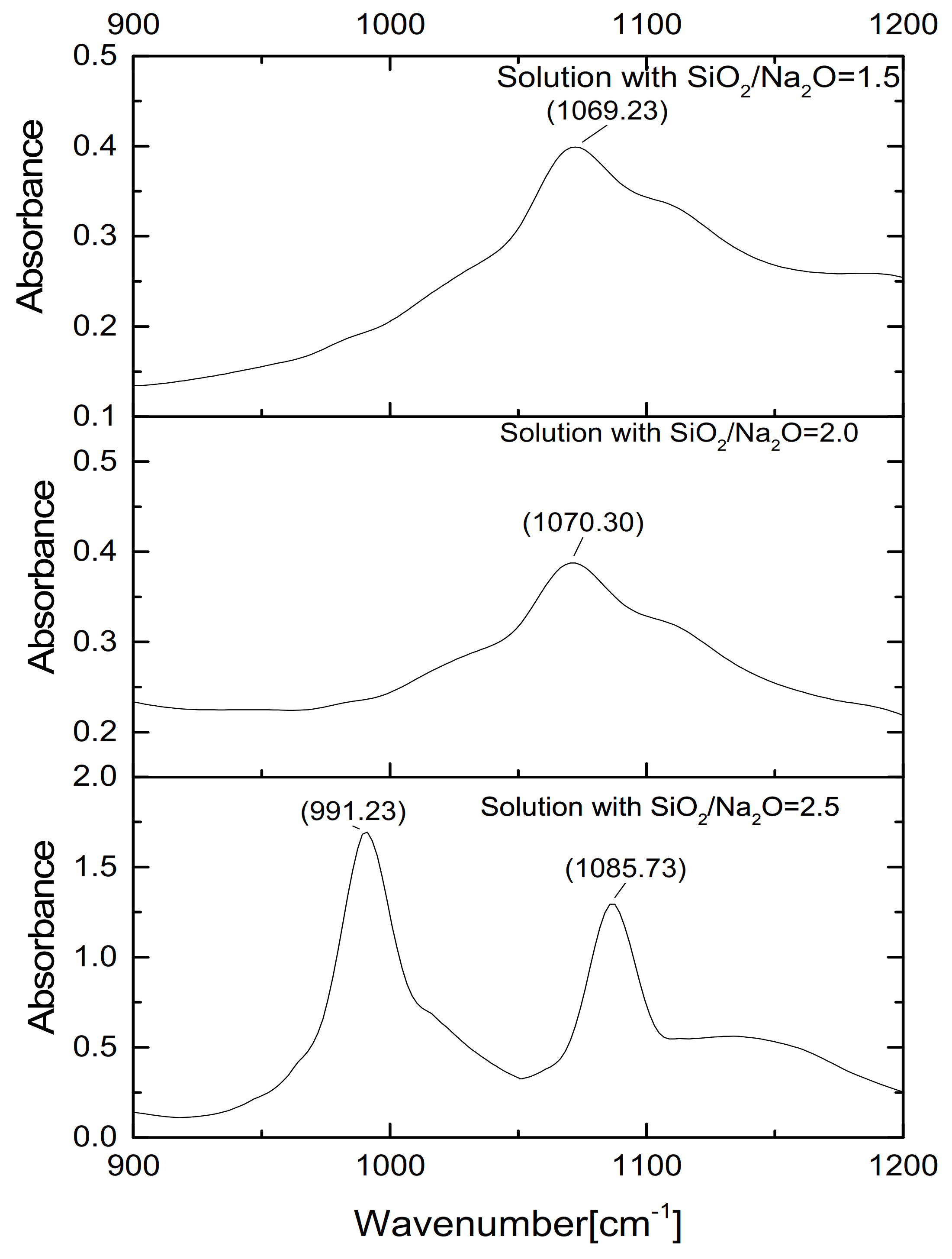
Liquid-State FTIR
Dynamic Light Scattering (DLS) of Alkaline Activator Solution
Gel Permeation Chromatography (GPC) of Alkaline Activators Solution
2.2.2. Preparation and Properties of Geopolymer Pastes
Preparation of Geopolymer Pastes
Curing Conditions
2.2.3. Properties of Hardened Geopolymer Pastes
2.2.4. Microstructure
SEM
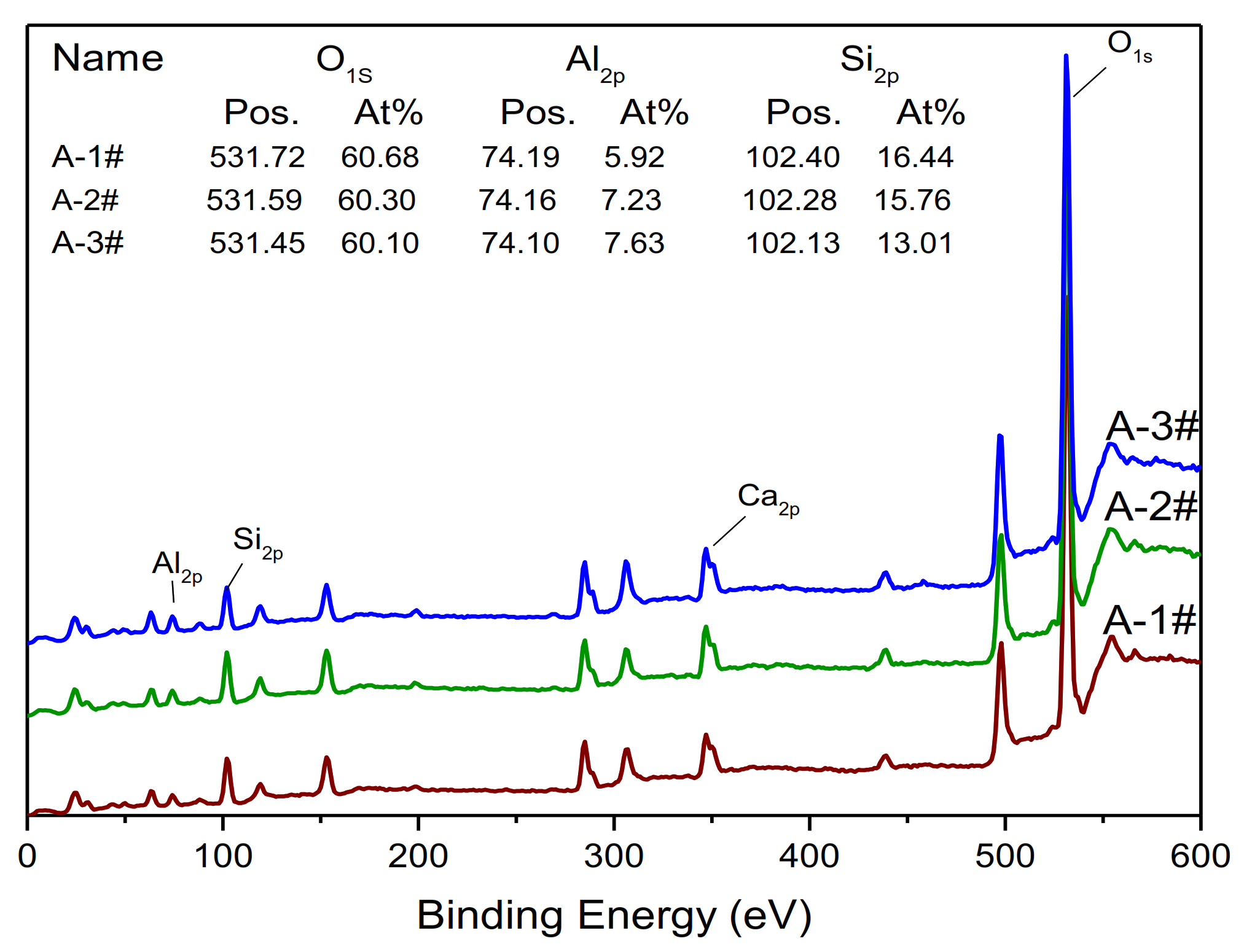
XPS
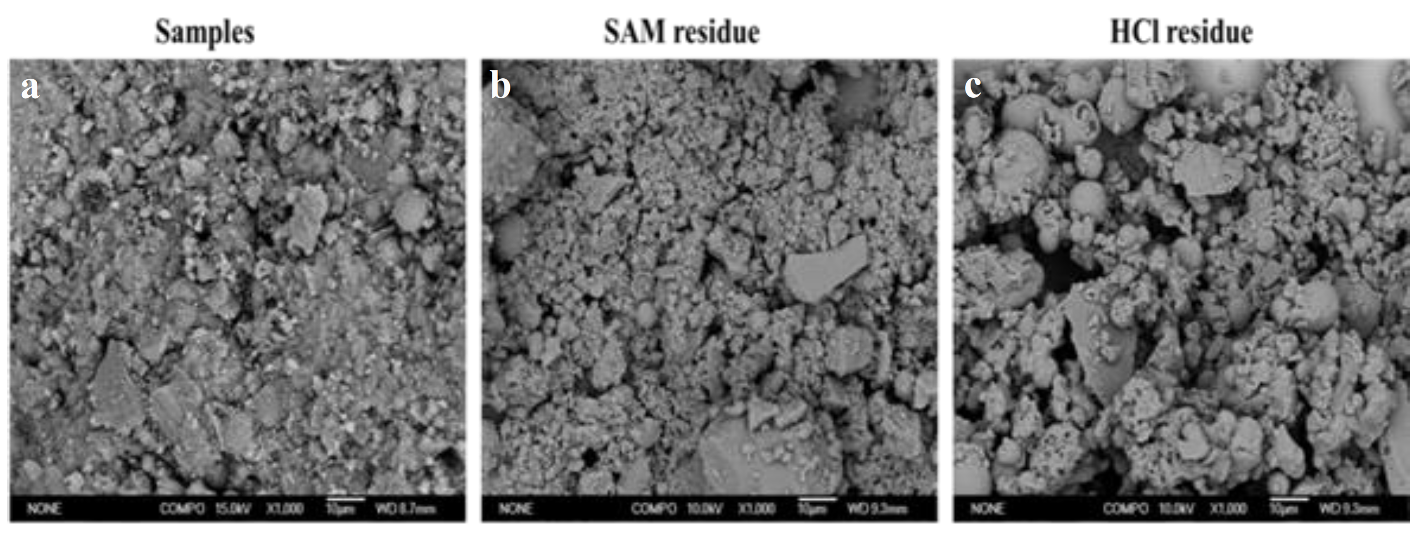
2.2.5. Selective Dissolution
3. Results
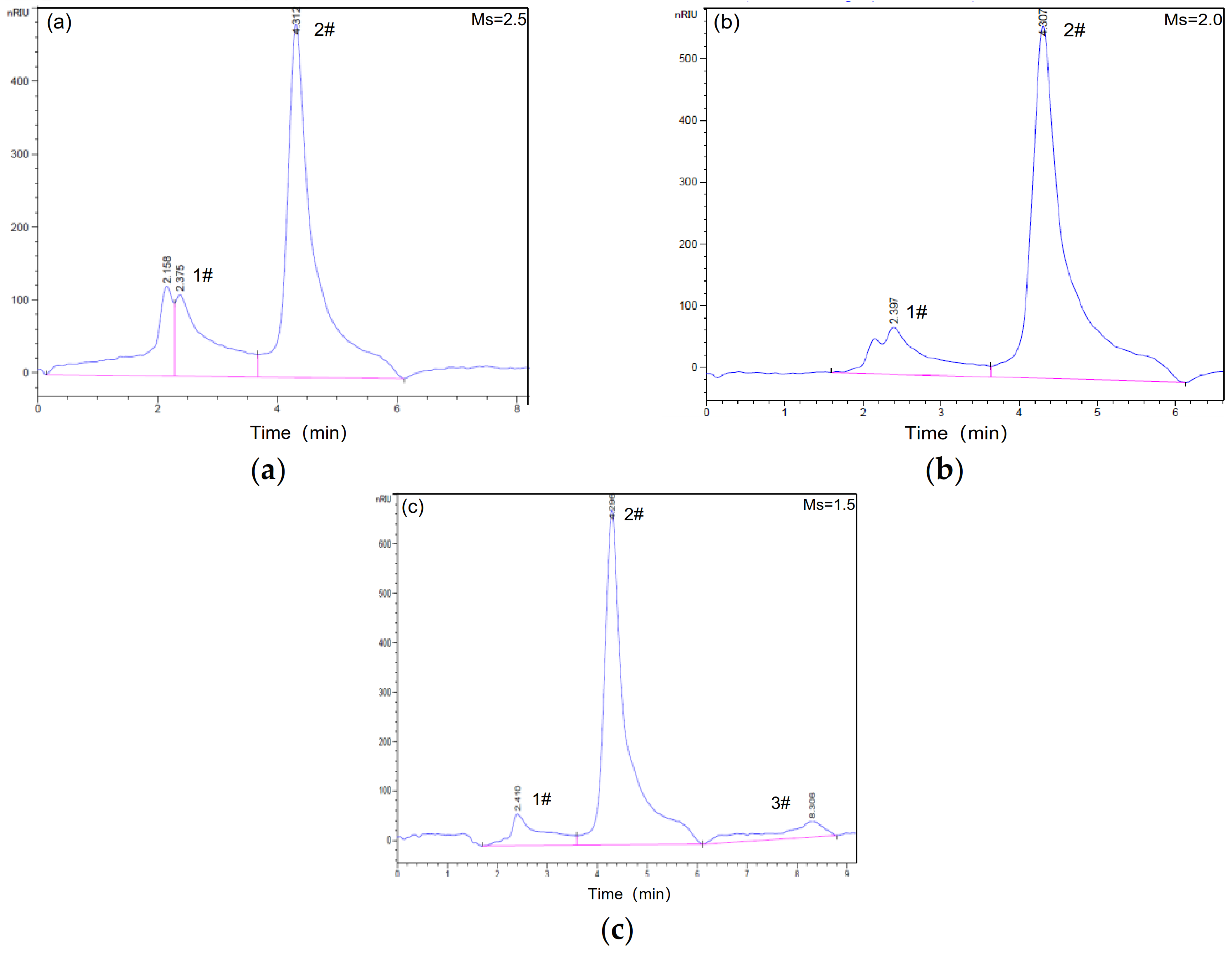
3.1. Inluence of Ms on the Properties of Waterglass
3.1.1. Composition of Waterglass with Different Ms
3.1.2. Properties of Waterglass with Different Ms
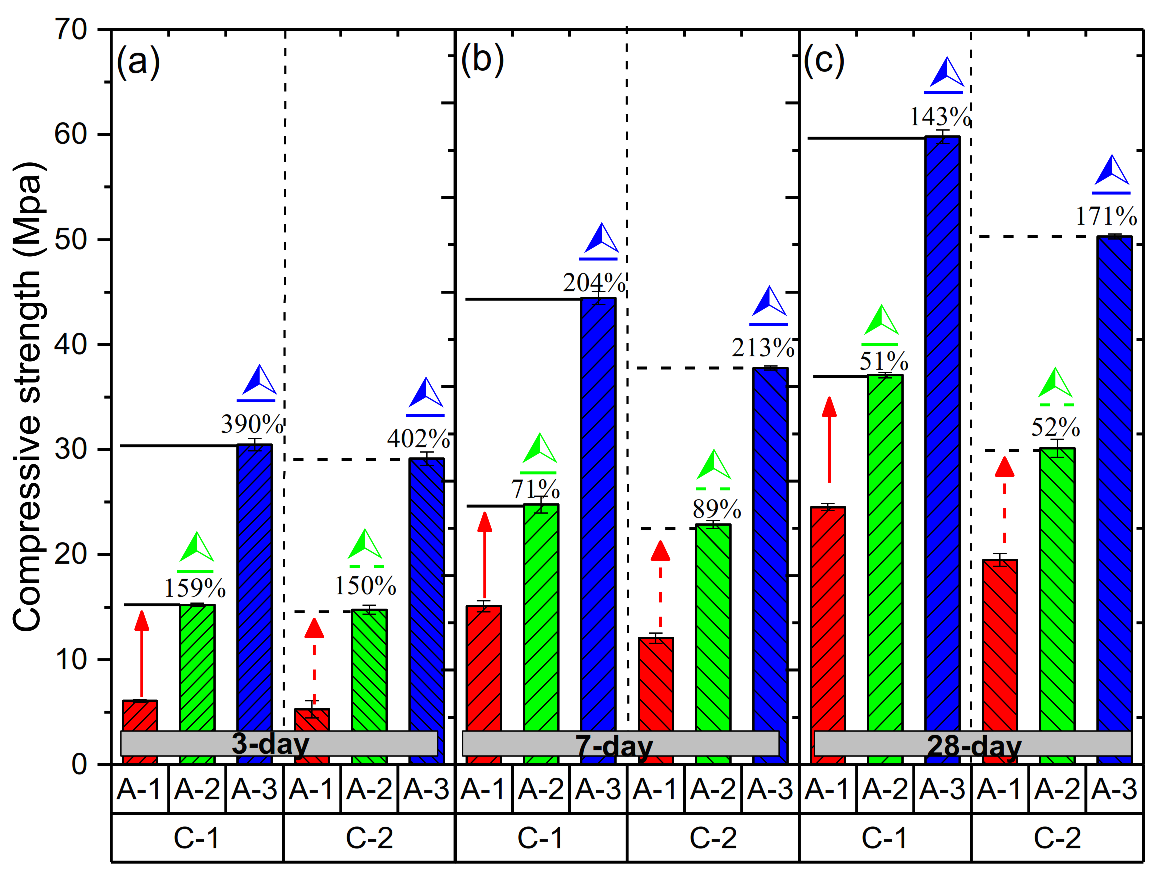
3.2. Mechanical Properties of Geopolymer Hardened Pastes with Different Ms
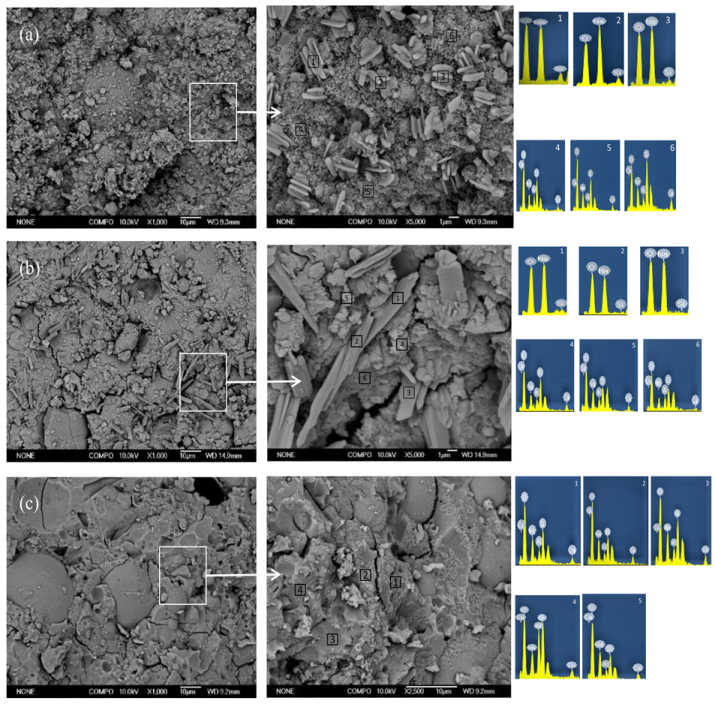
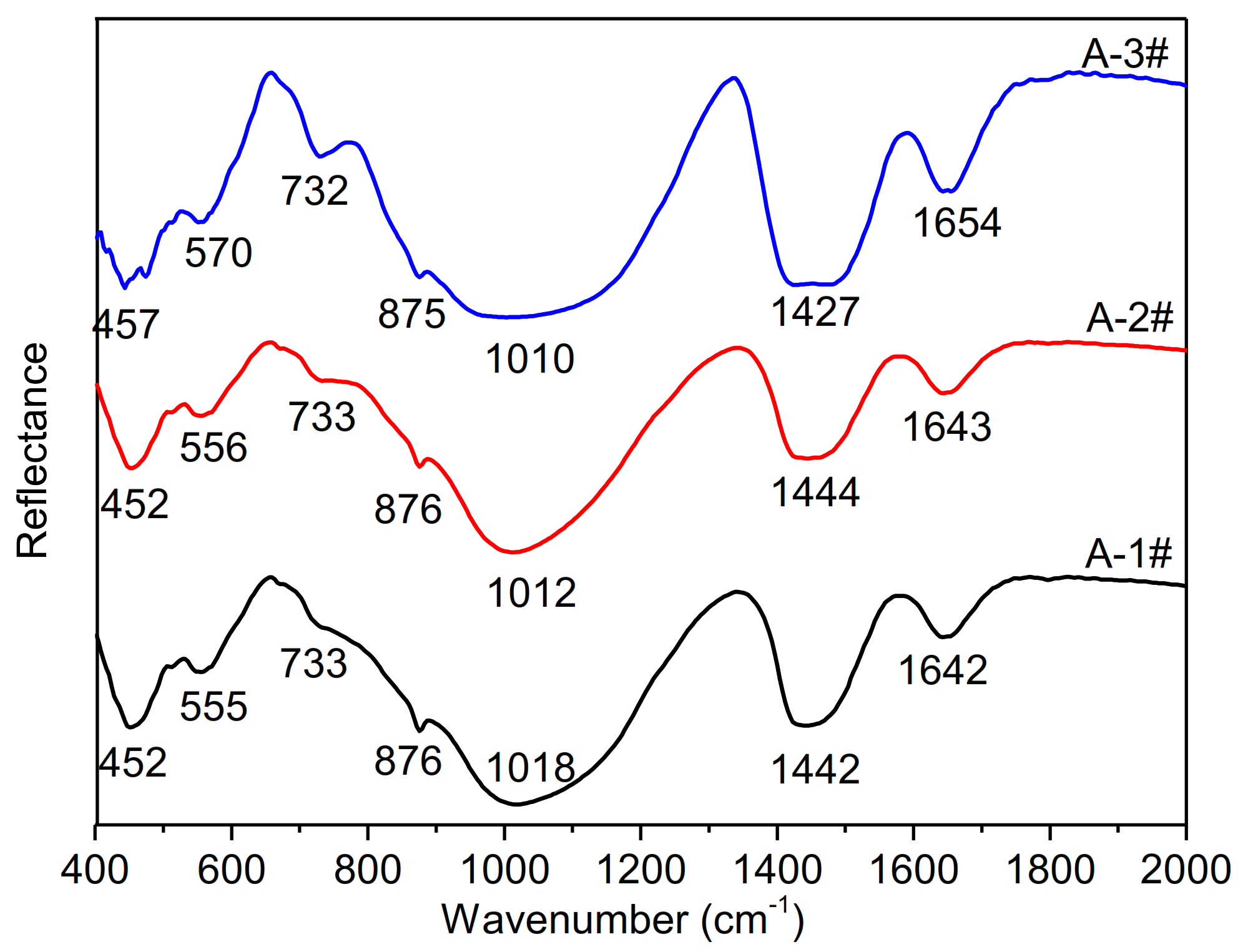
3.3. Development of Amorphous Gel with the Different Ms
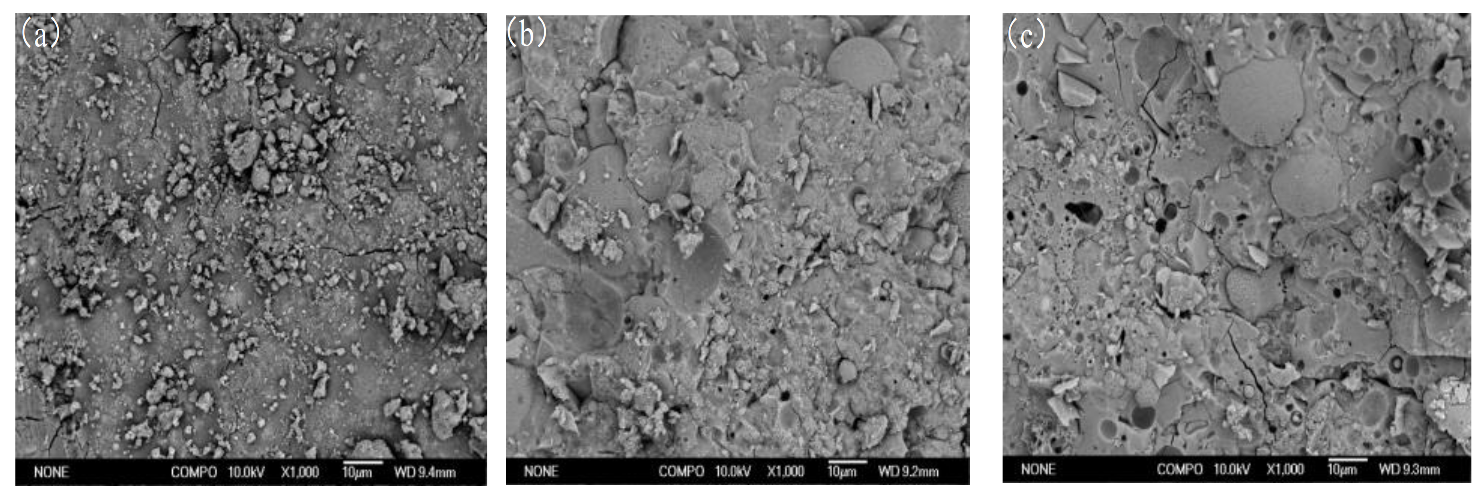
SEM-EDS
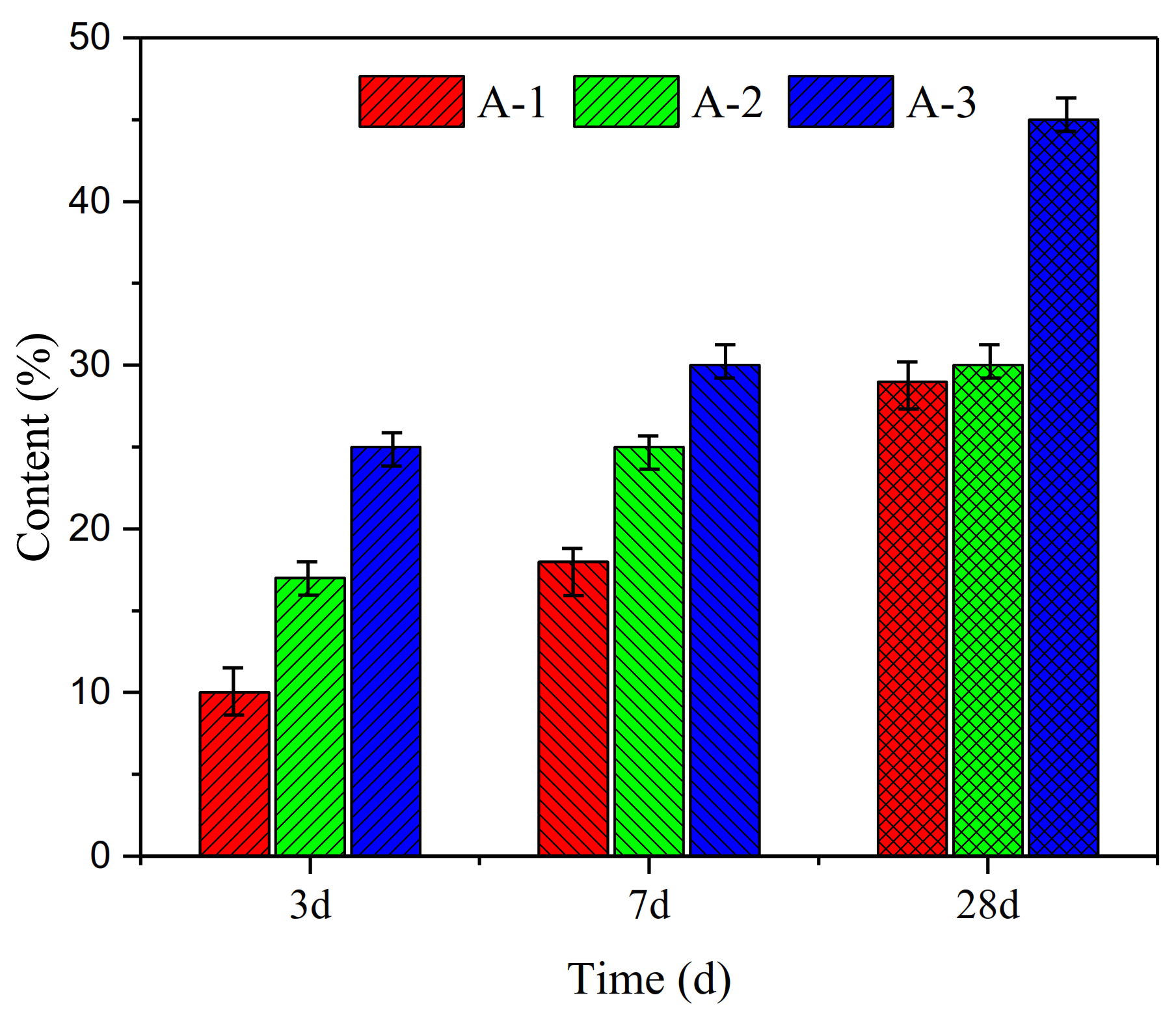
3.4. Relationship Between Waterglass Properties, Gel Content and Compressive Strength
4. Discussion
5. Conclusions
- With decreasing Ms, branched and agglomerated coordination unit groups of silicon were destroyed with the formation of Si-ONa+ and the electric charge effect of Na+, which produces the linear and circular-chain silicon structures with lower sizes and molecule weight in the Ms = 1.5 waterglass activator.
- With decreasing Ms, the compressive strength of geopolymer hardened pastes significantly increases. The highest value 58.9 MPa was obtained for Ms = 1.5 after the 28 days curing.
- The waterglass with low Ms (Ms = 1.5) results in the linear and circular-chain silicon structures with the lower size and molecule weight, which improve the chances of contact of active component, resulting in the improvement in the formation of the gel structure with low Si/Al ratio and size. This could be the major reason for the highest compressive strength of hardened pastes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favier, A.; Hot, J.; Habert, G.; Roussel, N.; De Lacaillerie, J.-B.D. Flow properties of MK-based geopolymer pastes. A comparative study with standard Portland cement pastes. Soft Matter 2014, 10, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Wang, D.-M.; Xie, F.-Z. Microrheology of geopolymer fresh pastes with different NaOH content at room temperature. Constr. Build. Mater. 2019, 207, 284–290. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Zhou, B.; Gao, H.; Lin, Y. Resistance of Soda Residue-Fly Ash Based Geopolymer Mortar to Acid and Sulfate Environments. Materials 2021, 14, 785. [Google Scholar] [CrossRef]
- Avirneni, D.; Peddinti PR, T.; Saride, S. Durability and long term performance of geopolymer stabilized reclaimed asphalt pavement base courses. Constr. Build. Mater. 2016, 121, 198–209. [Google Scholar] [CrossRef]
- Hýsek, Š.; Frydrych, M.; Herclík, M.; Louda, P.; Fridrichová, L.; Le Van, S.; Le Chi, H. Fire-Resistant Sandwich-Structured Composite Material Based on Alternative Materials and Its Physical and Mechanical Properties. Materials 2019, 12, 1432. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Peters, J.A.H.W. Trends in Global CO2 Emissions. Emissions Database for Global Atmospheric Research Edgar. Environ. Monit. Assess. 2013, 31, 93–106. [Google Scholar]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Gomonsirisuk, K.; Thavorniti, P. Use of Water Glass from Rice Husk and Bagasse Ashes in the Preparation of Fly Ash Based Geopolymer. Key Eng. Mater. 2019, 798, 364–369. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Rossignol, S.; Bonnet, J.P. Effect of alkali cation on irreversible gel formation in basic medium. J. Non-Cryst. Solids 2011, 357, 43–49. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Parameters That Influence Silica Dissolution in Alkaline Media. In Developments in Strategic Materials and Computational Design III; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate: A thermal analysis study. J. Non-Cryst. Solids 2013, 366, 13–21. [Google Scholar] [CrossRef]
- Prud’Homme, E.; Autef, A.; Essaidi, N.; Michaud, P.; Samet, B.; Joussein, E.; Rossignol, S. Defining existence domains in geopolymers through their physicochemical properties. Appl. Clay Sci. 2013, 73, 26–34. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers. Int. J. Miner. Process. 2002, 66, 121–143. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Soro, J.; Rossignol, S. Physical-chemistry of silica/alkaline silicate interactions during consolidation. Part 1: Effect of cation size. J. Non-Cryst. Solids 2012, 358, 81–87. [Google Scholar] [CrossRef]
- Le Losq, C.; Neuville, D.R. Molecular structure, configurational entropy and viscosity of silicate melts: Link through the Adam and Gibbs theory of viscous flow. J. Non-Cryst. Solids 2017, 463, 175–188. [Google Scholar] [CrossRef]
- Goudarzi, N. Silicon-29NMR Spectroscopy Study of the Effect of Tetra phenyl ammonium (TPA) as a Template on Distribution of Silicate Species on Alkaline Aqueous and Alcoholic Silicate Solutions. Appl. Magn. Reson. 2013, 44, 469–478. [Google Scholar] [CrossRef]
- Vidal, L.; Gharzouni, A.; Rossignol, S. Alkaline Silicate Solutions: An Overview of their Structure, Reactivity, and Applications. Handb. Sol-Gel Sci. Technol. 2016, 22, 1–24. [Google Scholar]
- Hunt, J.D.; Kavner, A.; Schauble, E.A.; Snyder, D.; Manning, C.E. Polymerization of aqueous silica in H2O–K2O solutions at 25–200 °C and 1 bar to 20 kbar. Chem. Geol. 2011, 283, 162–170. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solid 2009, 355, 245–253. [Google Scholar] [CrossRef]
- Lucas, S.; Tognonvi, M.T.; Gelet, J.L.; Soro, J.; Rossignol, S. Interactions between silica sand and sodium silicate solution during consolidation process. J. Non-Cryst. Solids 2011, 357, 1245–1253. [Google Scholar] [CrossRef]
- GB/T 8077-2012. Method for Testing Uniformity of Concrete Admixture; Chinese Guobiao Standards: Beijing, China, 2012. [Google Scholar]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Co-existence of aluminosilicate and calcium silicate gel characterized through selective dissolution and FTIR spectral subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Chindaprasirt, P. Role of microwave radiation in curing the fly ash geopolymer. Adv. Powder Technol. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Jiang, W.; Roy, D.M. Hydrothermal processing of new fly ash cement. Am. Ceram. Soc. Bull. 1992, 71, 642–647. [Google Scholar]
- Wijnen, P.W.J.G.; Beelen, T.P.M.; Rummens, K.P.J.; Saeijs, H.C.P.L.; Van Santen, R.A. Silica gel from water glass: A SAXS study of the formation and ageing of fractal aggregates. J. Appl. Crystallogr. 1991, 24, 759–764. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1707–1713. [Google Scholar] [CrossRef]
- Brough, A.R.; Holloway, M.; Sykes, J.; Atkinson, A. Sodium silicate-based alkali-activated slag mortars: Part II. The retarding effect of additions of sodium chloride or malic acid. Cem. Concr. Res. 2000, 30, 1375–1379. [Google Scholar] [CrossRef]
- Rimer, J.D.; And RF, L.; Vlachos, D.G. Physical Basis for the Formation and Stability of Silica Nanoparticles in Basic Solutions of Monovalent Cations. Langmuir ACS J. Surf. Colloids 2005, 21, 8960–8971. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S. Influence of Slag as Additive on Compressive Strength of Fly Ash-Based Geopolymer. J. Mater. Civil Eng. 2007, 19, 470–474. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Black, L.; Stumm, A.; Garbev, K.; Stemmermann, P.; Hallam, K.R.; Allen, G.C. X-ray photoelectron spectroscopy of the cement clinker phases tricalcium silicate and β-dicalcium silicate. Cem. Concr. Res. 2003, 33, 1561–1565. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, H.H.; Wang, Y.C.; Xu, D.L. Geopolymer Microstructure and Hydration Mechanism of Alkali-Activated Fly Ash-Based Geopolymer. Adv. Mater. Res. 2011, 374, 1481–1484. [Google Scholar] [CrossRef]
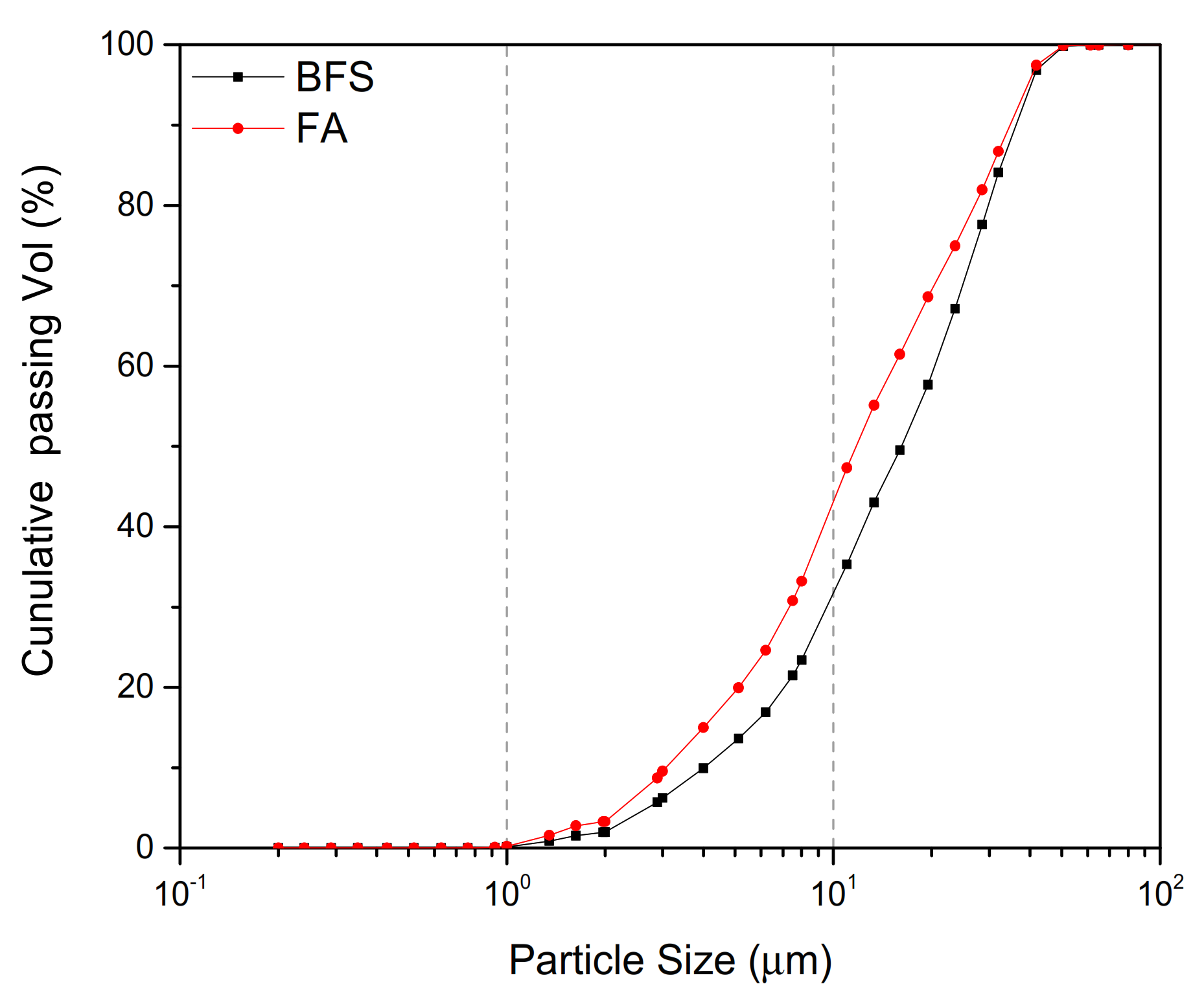



| Chemical Composition/wt % | Fineness/μm | Density/gcm−3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | K2O | LOI | |||
| FA | 5.17 | 43.57 | 38.36 | 0.61 | 5.39 | 0.99 | 0.53 | 0.81 | 2.73 | 10.3 | 2.20 |
| BFS | 40.72 | 24.96 | 12.84 | 0.94 | 0.53 | 1.858 | 0.94 | 0.34 | 4.63 | 11.2 | 1.96 |
| Waterglass/wt% | Sodium Hydroxide/wt% | Ms | |
|---|---|---|---|
| AA-1 | 100 | 0 | 2.5 |
| AA-2 | 95.24 | 4.76 | 2.0 |
| AA-3 | 85.42 | 14.58 | 1.5 |
| Mix | Mass Ratio | ||||
|---|---|---|---|---|---|
| Binder | W/B | Alkaline Activators | |||
| FA | BFS | Ms a | Content b | ||
| A-1# | 0.7 | 0.3 | 0.45 | 2.5 | 30% |
| A-2# | 0.7 | 0.3 | 0.45 | 2.0 | 30% |
| A-3# | 0.7 | 0.3 | 0.45 | 1.5 | 30% |
| Postioion | O/wt% | Na/wt% | Si/wt% | Al/wt% | Ca/wt% | Si/Al | |
|---|---|---|---|---|---|---|---|
| A-1 | 1 | 63.22 | 25.72 | 11.06 | / | / | / |
| 2 | 62.13 | 26.71 | 11.16 | / | / | / | |
| 3 | 58.80 | 26.90 | 14.30 | / | / | / | |
| 4 | 71.88 | 8.02 | 14.32 | 2.06 | 3.72 | 6.75 | |
| 5 | 70.05 | 8.82 | 14.41 | 2.11 | 3.61 | 6.59 | |
| 6 | 70.29 | 8.16 | 15.06 | 2.16 | 3.88 | 6.72 | |
| A-2 | 1 | 61.71 | 25.87 | 12.43 | / | / | / |
| 2 | 63.40 | 26.06 | 10.54 | / | / | / | |
| 3 | 61.83 | 27.41 | 10.76 | / | / | / | |
| 4 | 68.13 | 9.14 | 14.35 | 3.25 | 5.13 | 4.25 | |
| 5 | 68.59 | 8.82 | 13.82 | 3.29 | 5.37 | 4.05 | |
| 6 | 68.35 | 9.05 | 14.20 | 3.30 | 5.10 | 4.15 | |
| A-3 | 1 | 69.91 | 10.34 | 11.23 | 4.45 | 4.08 | 2.43 |
| 2 | 71.89 | 9.27 | 10.15 | 3.38 | 5.32 | 2.89 | |
| 3 | 72.06 | 10.01 | 9.93 | 3.28 | 4.73 | 2.91 | |
| 4 | 69.33 | 10.25 | 12.06 | 3.37 | 5.00 | 3.45 | |
| 5 | 70.73 | 12.27 | 10.34 | 2.73 | 3.93 | 3.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Rehemituli, R.; Zhang, X. Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure. Materials 2021, 14, 2227. https://doi.org/10.3390/ma14092227
Wang Z, Rehemituli R, Zhang X. Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure. Materials. 2021; 14(9):2227. https://doi.org/10.3390/ma14092227
Chicago/Turabian StyleWang, Zhipu, Rezeye Rehemituli, and Xiaolei Zhang. 2021. "Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure" Materials 14, no. 9: 2227. https://doi.org/10.3390/ma14092227
APA StyleWang, Z., Rehemituli, R., & Zhang, X. (2021). Study on the Compressive Strength of Alkali Activated Fly Ash and Slag under the Different Silicate Structure. Materials, 14(9), 2227. https://doi.org/10.3390/ma14092227






