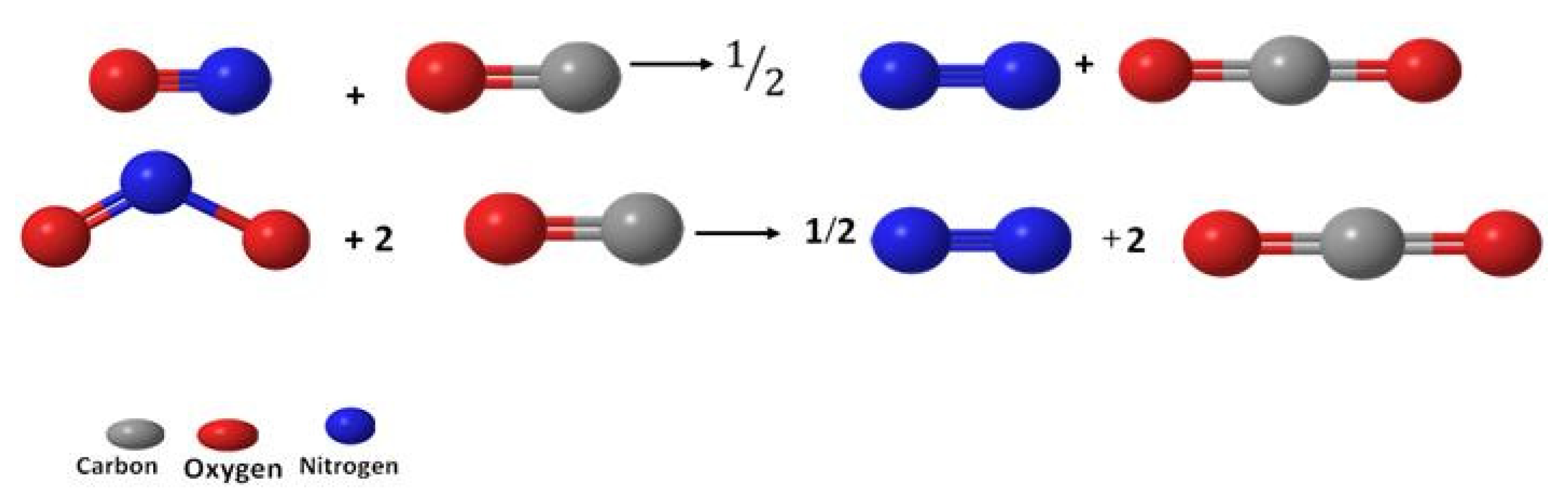
3.1. Structural Characterizations
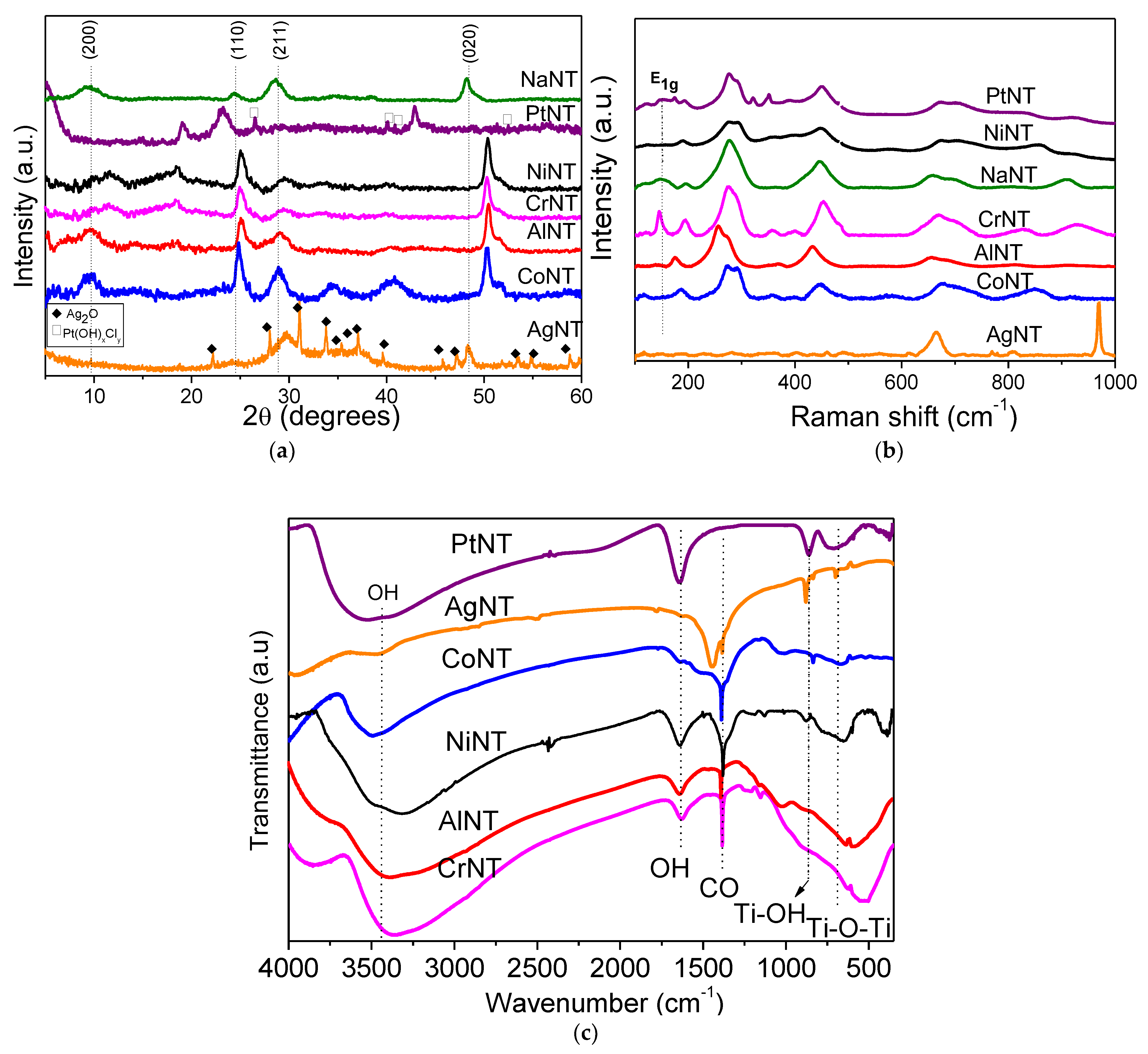
X-ray diffraction (XRD) patterns of the solids are depicted in
Figure 1a. The pristine NaNT shows four diffraction peaks at 2θ equal to 10.1, 24.3, 28.7 and 48.3°, attributed to the 200, 110, 211 and 020 reflection planes, respectively [
25,
26,
27,
28,
29]. All peaks shown in the NaNT diffractogram can be indexed to the
P21/m space group from the monoclinic structure of Na
2Ti
3O
7. According to the findings, the Na
2Ti
3O
7 structure has an edge-shared TiO
6 octahedron in a zigzag-like patterned arrangement, where the Na
+ ions reside between these layers and occupy two different crystallographic sites bound to the oxygen anions [
26,
27].
Moreover, the reflection at 2θ = 10.1° (200) is the characteristic peak of the periodic layer structure of the titanate nanotubes possessing an interlayer distance of 0.84 nm (
Table 1). Several studies illustrate the layered structure of the titanate and silicates-based materials [
4,
27]. Compared to NaNT, all solids containing metal impregnated or intercalated into the titanate nanotubes have similar features, being NiNT and AgNT exceptions. Noteworthy, the CoNT experiences a downshift of the diffraction angles of the Na
2Ti
3O
7 phase, which is an indication of the structural disorder of the titanates due to the replacement of the Co metal cations by sodium. Consequently, there is an expansion of the (200) plane favoring the diffusion of the Na+ ions diffusion through the interlayer and facilitates the ion exchange process.
As the layered structure of the titanate nanotubes is composed of the TiO
6 octahedra, this feature allows Na
+ cations to diffuse and easily be exchanged by Al
3+, Cr
3+ and Ni
2+ and Co
3+ ones. Accordingly, the Na/Ti ratios of the MeNT are lower than that of NaNT, which is an indication of the sodium removal of the sodium trititanate structure (
Table 1).
Besides, NiNT solid does not show the Na
2Ti
3O
7 tubular structure probably in reason of the fact that part of the Ni
2+ species might be intercalated into the NTs structure, but most of these species are found in the NiOOH form on the NTs surface. This could be a result of ammonia washing during the synthesis of the solids, in agreement with the findings [
29,
30]. Moreover, the good dispersion of intercalated Pt nanoparticles (decorated) on the nanotubes shows that the impregnation of Pt on NTs does not significantly affect the titanate structure, implying that the scrolled titanate nanosheets remain. However, the (110) peak in PtNT diffractogram shifts to the left compared with the other catalysts due to the presence of intercalated Pt into the NTs structure. In addition, some characteristic peaks of a minor platinum contribution coming from PtO
x, PtO
xCl
y or Pt(OH)
xCl
y species are observed, as found elsewhere [
25,
26].
In the case of AgNT, however, the main reflections at 2θ = 32.4 (111), 38.1 (200) and 55.4° (220) correspond to the Ag
2O cubic structure (space group:
Pn-3m) [
31,
32,
33]. Besides that, the silver incorporation to the NTs results in a solid with high crystallinity due to the organization of the monoclinic structure of the solid. Moreover, both inner and outer surfaces of NTs have negative charges, which allow the metals to be adsorbed on these surfaces [
28,
34,
35].
Meanwhile, the AlNT catalyst exhibits low intensity peaks at 2θ = 15.4° (020) and 32.1° (120), which could be ascribed to orthorhombic oxy-hydroxide boehmite γ-AlOOH structure (space group
Cmcm). This result is in agreement with previous findings on boehmite based materials [
36]. For CoNT, the peaks at 2θ values of 10.6 (003), 19.6 (006), 35.1 (102) and 39.0° (015) may depict the existence of cobalt oxyhydroxide CoOOH (space group of
R3m) as a poor crystallized phase [
2,
37]. Furthermore, all of these extra framework reflections seem to be on the surface of the titanate nanotubes. Therefore, the adsorption of the ions on the outer surfaces is likely whereas that on interlayer is reduced due to the difficulty of the ions to diffuse into the inner surface.
Moreover, CrNT has a very broad peak appearing at 2θ value of 18.4° (003) and another one at 2θ = 12.4° with d = 0.8 nm, which could be indexed to be from the rhombohedral structure of chromium oxyhydroxide α-CrOOH grimaldiite phase (space group:
R-3m) as found elsewhere [
2,
38].
Thus, the ion-exchange of Na
+ (102 pm) ions by a cation possessing lesser ionic radii such as r(Al
3+) = 53 pm, r(Cr
3+) = 84 pm or r(Co
3+) = 61 pm favors the metals incorporation into the NTs structure. This is consistent with the increased interwall distance of the MeNT compared with that of NaNT (
Table 1). In contrast, Ag
+ having a bigger atomic radius of r(Ag
+) = 115 pm may form an extra framework Ag
2O oxide. This also suggests that a possible solid-state reaction between Ag
2O and Na
3Ti
3O
7 may take place.
Raman measurements are performed to investigate the structure of the MeNTs and the electronic interaction between Ti and metals. The region of the Raman spectrum that corresponds to the lattice vibrations is shown in
Figure 1b. Accordingly, NaNT has vibrational modes at around 158 (E1g), 195 (Eg), 276 (Ag), 445 (B1g), 656 (A1g), 696 (Ag) and 908 (Bg) cm
−1 [
25]. The weak bands at 156 and 192 cm
−1 are attributed to the anatase-type structure of TiO
2, whereas the modes at 276 and 656 cm
−1 are due to the Na cation coordinated to the oxygen atom of the framework Ti–O vibration [
25,
35]. At 445 cm
−1, the Ti–O–Ti framework vibration is clearly visible [
35]. Importantly, the band at 908 cm
−1 has previously been correlated with the symmetric stretching vibration mode of a short Ti–O bond of sodium titanate in layer structure and the Ti–O–Na stretching vibration in the interlayer regions of the nanotube walls [
25,
34].
The spectrum of AlNT is very similar to that of NaNT, but a high downshift of the main titanate nanotubes vibrational modes confirm the structural disorder of the solid due to the Al insertion in the NTs structure. Moreover, an absorption band at around 360 cm
−1 evidences the presence of the α-AlOOH particles [
36]. Important is to mention that the band associated with the Ag mode for AlNT splits into two shoulders at 256 and 275 cm
−1, having these modes much lower intensities than those of the original Ag ones. This indicates that the replacement of the Na cation coordinated to the oxygen atom of the framework Ti–O vibration by Al one provokes a hardening of the vibrational modes. These observations are consistent with the XRD results. The modes of CoNT spectrum upshifts comparing with that of NaNT, which indicates the softening of the modes, as Co is incorporated to the NTs structure. Similar to AlNT, the Ag modes split into two new ones illustrating the perturbation of the Ti–O bond by cobalt incorporation. Besides, the appearance of the bands with maxima at 357, 406 and 582 cm
−1 is assigned to the presence of α-CoOOH, as found elsewhere [
37].
Interestingly, the modes of the titanates nanotubes are not affected by the presence of Cr in CrNT compared to NaNT, confirming that the Cr
3+ ion exchange by Na
+ ions, in line with the XRD results. In other words, the titanate nanotubes bands remain virtually unperturbed upon Cr insertion. Moreover, the Raman bands of CrNT become more intense and additional modes at 361, 397 and 827 cm
−1 are due to the ν
1 Cr–O symmetric stretching mode in the α-CrO(OH) structure [
38]. Upon the Ag addition to the NTs, the vibrational properties of the AgNT sample change substantially, as shown in
Figure 1b. The titanate nanotubes bands decreased in intensity and some of them vanished from the spectrum, implying that the phase transformation of the titanate nanotube structure occurs after the silver addition. This feature indicates that silver does not participate in the bonds included in the vibrations associated with titanate nanotubes bands, most probably in the formation of the Ag
2O phase. Based on XRD results, the presence of Ag
2O confirms the crystallization of the AgNT catalyst. Indeed, the modes located at about 561, 700 and 1010 cm
−1 are associated to the Ag–O mode vibration, as reported in the literature [
33].
The Raman spectrum of PtNT exhibits broad bands characteristic of the tubular nanotube with the layered structure of the NTs. Some of these bands depict a blueshift of the modes near 630 cm
−1 due to the Me–O bonds, as a consequence of the intercalation of the ions in the NTs structure. The findings also state that the observed blueshift can be associated with the creation of oxygen vacancies in the NTs structure [
8]. Moreover, two narrow bands appear at around 350–400 cm
−1, which can be ascribed to the formation of the PtO
x, PtO
xCl
y and Pt(OH)
xCl
y phases [
26].
FTIR spectroscopic investigation of the as-synthesized catalysts (
Figure 1c) show the strong absorption band at 3600 cm
−1 corresponding to the stretching vibrations of the OH groups from the Ti–OH bonds of the trititanate structure [
39,
40]. Moreover, these OH groups can be attributed to either physically adsorbed water or the extra framework hydroxides such as α-CrOOH, α-CoOOH, NiOOH, α-AlOOH and Pt species, in agreement with XRD and Raman results. Most likely, the low concentration of OH groups of AgNT is illustrated by the small size of the aforesaid absorption band, which is evident from the XRD measurements. At 1620 cm
−1, the weak infrared bands with bending vibrations are assigned to the OH groups in all solids [
39]. It should be pointed out that the band at 1360–1400 cm
−1 arises from C–O bonds of surface carbonate species during the preparation of the solids. The presence of these surface carbonates will be confirmed later by XPS. Meanwhile, the FTIR spectra depict bands at around 707, 930 and 990 cm
−1, being characteristic of the Ti–OH and Ti–O–Ti bonds from the NTs lattice vibrations [
18,
39]. Moreover, these bands below 500 cm
−1 can be attributed the Me–O vibrations.
3.2. Morphology and Textural Properties of the Catalysts
The surface morphology of the catalysts is examined by SEM micrographs. Appearance of abundant nanorods mostly agglomerated is illustrated in the SEM micrograph of NaNT (
Figure 2a). The EDS spectrum illustrates that the sodium, oxygen and titanium contents on the surface are, respectively, 0.45, 46.7 and 28.7%. This indicates that the hydrothermal preparation method allowed the formation of the Na
2Ti
3O
7 phase, taking into account the XRD and Raman results. Besides, the HRTEM micrograph (
Figure 2b) confirms the existence of the randomly tangled nanotubes with inner empty structure and diameters varying of 3–10 nm and several hundred nanometers of length. These morphological aspects of the NTs are predictable by literature reports using similar hydrothermal synthesis methods [
23,
35].
The SEM micrograph of CoNT consists of disordered agglomerates of particles in the form of platelets (
Figure 2c), although some nanotubes are visible in the edge of the plates. Taking into account the expectations that the ion-exchange of Na by Co results in the substitution of the sodium ions in the NTs structure, the EDS spectrum illustrates that a very low amount of Co ca. 0.7% is on solid surface. Moreover, Co is uniformly distributed throughout the solid surface. This is consistent with the Raman spectrum of the solids because it also indicates that the displacement of the bands caused by Co insertion in the NTs structure. Additionally, the CoNT morphology exhibits entangled nanotubular shaped particles with some black dots representing the Co nanoparticles decorating the nanotubes, as observed by HRTEM micrograph (
Figure 2d). The measured interwall distance is 0.91 nm corresponding to the NTs, demonstrating the successful incorporation of Co into the NTs structure.
The ion-exchange process of replacing of Na
+ ions by Cr
3+ gives rise to agglomerated particles of irregular morphology, but some nanorods remain visible (
Figure 2e). Moreover, the EDS spectrum suggests that the Cr content is 1.4% whereas the Na one is lesser than the one in NaNT, in agreement with the low Na/Ti ratio observed in
Table 1. Most likely, the ion-exchange process does not affect the morphology of the solids with CrNT displaying entangled titanate nanotubes formation; however, the length of CrNT becomes short (
Figure 2f).
AlNT shows particles much less morphologically similar to those observed in CrNT, with irregular shapes and the ensemble of nanotubes forming voids between the particles (
Figure 3a). This could be an indication of the formation of large pores surrounded the particles, which is later confirmed by textural properties analyses. Although a certain amount of Na
+ ions is substituted by Al
3+ ones in the NTs structure with the consequent drop of the sodium content, the EDS spectrum reveals that most of the Al content of ca. 1.8% is concentrated on the solid surface. Accordingly, Raman measurements suggest that the structural disorder of the AlNT due to the Al insertion in the NT structure and the presence of the α-AlOOH phase. In particular, HRTEM micrograph of AlNT displays the interlayer distance of the ca. 0.95 nm pointing to the NTs structure along with the nanoparticles (black dots in
Figure 3b) attributed to being boehmite.
Conversely, the SEM micrograph of the AgNT particles significantly differs from those of the other MeNTs. The formation of aggregated particles with a rough surface (
Figure 3c) indicates that these particles are visually bigger than the metal-containing NTs counterparts. Moreover, small voids are observed among the particles representing the shrinkage of the NTs pores, as further seen by textural properties analyses. Moreover, the EDS spectrum confirms the presence of 0.57% of titanium along with 12.0% of Na, 54.6% of Ag and 24.0% of O atoms, respectively. This indicates that silver species are entirely deposited on the nanotubes surfaces and some nanosheets can be formed, as previously indicated by XRD and Raman results. Importantly, the residual sodium content is not negligible as the Ag ions deposited on solid surface. HRTEM micrograph of AGNT is seen in
Figure 3d. The image clearly depicts big particles with a lattice spacing of 0.23 nm corresponding to the (200) plane of Ag
2O, as observed by XRD. These big Ag
2O particles are deposited on the solid surface, but the lattice fringes of the (001) and (020) plane highlight that the monoclinic titanate nanotubes structure remains in the solid, after the ion-exchange process. The lattice fringe of 0.24 nm corresponding to the (111) plane of Ag
2O is clearly visible.
The SEM images of NiNT are illustrated in
Figure 3e,f whereas PtNT micrographs are seen in
Figure 3g,h. As it can be seen, the morphology of the PtNT and NiNT surfaces is very similar to that of NaNT (
Figure 2a). Nevertheless, the densification of the nanotubes can be clearly seen forming plates, which demonstrates that the ionic exchange of Na by Ni and Pt promotes the aggregation of the tubular morphology in a clumped manner. Notably, the Ni/Ti and Pt/Ti ratios measured by EDS are 0.17 and 0.18, respectively. This implies that most of the Na ions (Na/Ti ratio of NaNT is 0.22) are removed during the ionic exchange and impregnation processes, and some Pt and Ni ions are substituted by sodium ions in the layered structure of the PtNT and NiNT.
Furthermore, TEM image of NiNT clearly illustrates that even after the ionic exchange, the tubular morphology is preserved (
Figure 3f), being the bundles of nanotubes entangled with Ni addition. Moreover, the morphology of the PtNT indicates that the tubular multiple-walled like morphology is still retained, but with their sizes are reduced upon Pt incorporation (
Figure 3h).
Moreover, the Ni and Pt nanoparticles are not observable in the framework of the tubular structure indicating that they are well dispersed on both inner and outer surfaces of the nanotubes due to the electrostatic interaction between the negative charges of the NTs sheets and positively charged cations [
29]. Important is to say that the nanoparticles cannot be seen due to the low resolution of the images.
These results are consistent with the XRD, Raman and FTIR measurements that suggest the maintenance of titanate nanotubes layered structures after ion exchange. On the contrary, the NiNT, PtNT and AgNT are exceptions, since their morphology is not similar to that of NaNT.
Nitrogen physisorption curves are assigned to be from type IV isotherms, which is characteristic of mesoporous materials possessing slit-shaped mesopores (
Figure 4a). The H3 hysteresis loops of CrNT, AlNT, NiNT, PtNT and CoNT start at high relative pressure. It can indicate a remarkable porosity of the solids revealing large pore sizes and their pore connectivity, as well. This is reasonable to expect since the findings accord that the isotherms of sodic titanates nanotubes are similar to those of the metal-containing titanate nanotubes [
2,
19].
On the contrary, the appearance of an H4 hysteresis loop for AgNT suggests that the solid has large particles compared to the other MeNT, which is consistent with previous results of SEM-EDS, XRD, TEM and Raman spectroscopy. The BET surface area of NaNT is 189 m
2·g
−1 and the pore volume is of 0.62 cm
3·g
−1, as shown in
Table 2. The textural properties of the MeNT depict significant quantitative differences in the parameters, when the Me are incorporated into NTs structures. Especially for CoNT, NiNT and AlNT, the surface area and pore volume become much higher than that of NaNT, as a result of the increased interwall distance (
Table 1) experienced by the introduction of Al, Ni and Co into the interlayer region.
The results in
Table 2 also illustrate a trend of declining of the textural properties values for PtNT, CrNT and AgNT compared to the Ni, Al and Co substituted titanate nanotubes. This result can be correlated to the formation of nanoparticles on the solid surfaces of PtNT and CrNT.
For AgNT, the deposition of the Ag2O on the NTs surface for the solid might be caused by the strong electronic interactions of the bigger Ag particles and the nanotubes surface rather than the Ag ions bound in the NTs lattices.
Furthermore, the CoNT, AlNT and CrNT have considerably higher pore volume values than that of the NaNT indicating the expansion of the pores due to the metal incorporation into the inner and outer surfaces of the NTs structures. Moreover, all the pore size distribution curves exhibit broad features that can be reasoned as the presence of large mesopores (
Figure 4b). Among the investigated solids, the deposition of the silver oxide particles mainly affects the pores with their consequent blocking and formation of micropores, in line with SEM-EDS and TEM results.
3.3. Electronic States, Reduction Behavior and Acidity of the Catalysts
Electron Paramagnetic Resonance (EPR) spectroscopy is a standard and non-destructive tool to determine the valence state of some elements of the studied catalysts. The EPR spectra of the samples exhibit two asymmetric signals centered at 2000 and 3600 G, which is typical for metal oxides paramagnetic species. Despite the fact that the EPR spectra of titanate nanotubes do not differ in shape, there are considerable differences among them. For instance, EPR line of CoNT is much broader than that of CrNT (
Figure 5a) probably due to the local atomic and electronic structure of Cr in the NTs and interaction mechanisms between spins, as well. Indeed, the broad line shape of CrNT depicts the well asymmetrical defined doublet with
g value of 5.04. It is found that the paramagnetic lines of the EPR spectrum can be undoubtedly assigned to Cr
3+ species (r = 0.61 Å) partially occupying the positions of Ti
4+ in the NTs lattice.
For the sake of comparison, trivalent chromium species in a structurally related Cr-doped TiO
2 solid shows high spin configuration where a substitutional Cr
3+ species occupies the vacant tetragonal Ti
4+ sites environment, which are coordinated by a slightly distorted octahedron of oxygen [
41]. This is corroborated by Raman results, demonstrating the existence of Cr in the NTs framework and CrOOH species.
The Ti
3+ species from NTs are observable through the resonance signal with g values close to 2.0, as found elsewhere [
42,
43]. Moreover, the paramagnetic defects of the foreign species are embedded in the titanate nanotubes structure possessing a lower symmetry [
44]. For, AgNT (
Figure 5a), two isotropic bands doublet signals at around 2500 and 3200 G are ascribable to the isolated Ag
+ ions and Ag coordinated to oxygen on the NTs surface [
45].
For CoNT, the Co
3+ ions are included into the monoclinic layered trititanate structure as a result of the Na
+ substitution in the interlayer region. The
g value of 4.9–5.4 indicates that the solid contains Co
2+ and Co
3+ species. On the bases of the findings reported so far, Co
2+ can be incorporated in the framework of trititanate nanotubes occupying octahedral sites and substituting partially the Ti
4+ions, as well [
46,
47]. Although the differences in the atomic radius of Co
2+, e.g., r = 0.745 Å and Co
3+, e.g., r = 0.61 Å when replacing the Ti
4+, e.g., r = 0.605 Å into the TiO
6 octahedra exists, it is believed that the trivalent one may be visibly incorporated in the NTs framework due to its size being close to that of titanium. In agreement, EDS indicates the surface of the solid has Ti and O in trivial amount besides the Co incorporated in the NTs structure.
The EPR results of AlNT and NiNT depict similar features to those of CoNT with asymmetric signals associated with isolated Me ions. Especially for PtNT, the EPR spectrum has narrow anisotropic EPR signals, which is attributed to the Pt species in accordance with the Pt/TiO
2 samples [
48]. The reports also show that the oxygen vacancies of TiO
2 surfaces are identified with
g values close to 2.0 which is in agreement with our XRD results.
Table 3 summarizes the results of the XPS, data of selected samples. On the basis of the XPS results, all the studied titanate nanotubes possess Ti
4+ on solid surface. The Ti 2
p core level spectra display a doublet Ti 2
p3/2 and Ti 2
p1/2 at two main peaks at 458.7 and 465.1 eV, respectively, typical of Ti
4+ in TiO
2. According to previous literature on titanate nanotubes, these values were also observed for the walls of Ti
3O
72− trititanate structure with cations intercalated [
4,
26]. All of the solids have almost identical binding energies for Ti
2p core level. Moreover, the remaining sodium content still visible on the surface of the NTs with Binding Energy (BE) for Na
1s being 1071.4–1071.6 eV form the remaining Na
+ bonded to titanate structure (
Table 3), in agreement with EDS analyses. Notably, the O 1
s signals have 91% of the contribution (
Figure A2,
Appendix A) appearing at 530.3 eV. This signal is assigned to lattice oxygen in the NTs studied, which is indicative of either lattice oxygen of TiO
2 or sodium trititanate structure, in line with the literature reports [
25,
26]. Moreover, the minor contribution arises at 532.2 eV, which suggests the presence of the oxygen from OH groups of the NTs and the extra framework NiOOH, CoOOH, Pt(OH)Cl
x species as shown in NiNT, CoNT and PtNT, respectively. This agrees with the FTIR spectra that showed the presence of OH groups.
For CoNT samples, the Co 2
p core level has two broad peaks (
Figure 5b) illustrating the typical Co 2
p3/2–Cr 2
p1/2 doublet. The binding energy values for the Co 2
p3/2 and 2
p1/2 core levels are at around 781.0 and 796.0 eV assigning the presence of Co
2+ in tetrahedral coordination state and shake-up satellites, respectively. However, the presence of Co
3+ species cannot be ruled out [
13,
25]. The satellite indeed indicates the Co
2+ greatly interacting with OH groups from CoOOH phase. This is in accordance with XRD and Raman EPR results of the solids.
The Ni 2p core level spectrum for NiNT presents the main Ni 2p3/2 peak at 855.9 eV corresponding to the Ni2+ state. This indicates that the structure of the trititanate nanotubes is preserved with Ni intercalated into the titanate interlayer. The Ni 2p3/2 shake up satellite peak 861.2 eV suggests the presence of Ni2+ in NiOOH, as observed by XRD and EPR results.
The Pt 4
f core level spectrum for the PtNT sample exhibits an asymmetric Pt 4f
7/2 peak that can be decomposed into two contributions at 72.5 eV (88%) and 75.3 eV (12%), as shown in
Figure 5b. The components at 72.5 and 75.3 eV arise from Pt
2+ and Pt
4+ species, respectively, in either Pt oxides or Pt(OH)Cl
x form [
13,
25,
49]. More importantly, the Cl
2p core level spectrum for PtNT shows the doublet Cl 2
p3/2 and Cl 2
p1/2 at 199.0 and 200.6 eV, respectively; this is typical of Pt(OH)Cl
x species [
13,
49].
The presence of Ni2+ ions as intercalated nickel species is confirmed in NiNT spectrum whereas PtNT shows Pt species in two valence state such as Pt2+ and Pt4+ ions from PtO and Pt(OH)Clx, respectively. In the case of CoNT, the Co2+ species are found by XPS. This is expected taking into consideration the ion-exchange and impregnation processes used to obtain the titanate nanotubes.
The TPR measurements of fresh NiNT, PtNT and CoNT were previously published [
4,
25]. As a summary in
Table 4, the TPR pattern of NiNT depicts a broad signal in the 400–600 °C range, which is associated with NiO strongly interacting with the NTs support during the reduction of the solid, as shown previously [
4]. Contrary, the CoNT exhibits a less reducible species at temperatures as low as 200 °C. This indicates that some Co
2+ species are not incorporated into the NTs and low interacting with the NTs support whereas the peak at high temperatures suggests the reduction of the Co nanoparticles highly dispersed on solid surface forming the surface like Co
3O
4 phase.
In the case of TPR curve of PtNT, two reduction stages are observed at 200 °C and up to 700 °C, most probably due to the reduction of the oxychlorinated platinum species. Presumably, the second stage can be attributed to the consecutive reduction of the [PtO
xCl
y] or PtCl
6− to PtO
x and the further reduction of these species, as found elsewhere [
25,
26].
The acidity measurements by TPD-pyridine reveals that the amount of the acid sites in NaNT is about 7 μmol Py gcat
−1 mostly having weak acidity. Contrary, PtNT has the acid sites of medium to strong strength possessing the amount of ca. 261 μmol Py gcat
−1 (
Table 4). This is attributed to the surface Pt species such as oxidized PtO
x (PtO or PtO
2) and chlorine (Pt(OH)
xCl
y and PtO
xCl
y) species [
4,
25]. When comparing PtNT and NiNT catalysts, the amount of acid sites decreases with NiNT having the minor amount of acid sites due to the weak acidity of the Ni
2+ species. Thus, acidity measurements by pyridine-TPD follows the order: CoNT < NiNT < PtNT.
The basicity of the fresh titanates based-solids is investigated by the CO
2-TPD. For CoNT, the first band is located at 100–300 °C (
Table 4) being related to the decomposition of the as-synthesized titanate nanotubes into TiO
2 species, and possibly the adsorption of CO
2 on the weak acid sites of the samples. The second peak spans from 300 to 500 °C being related to the CO
2 adsorption on the medium-strength basic sites, arising from NaO
x species TiO
2 or CoTiO
3 phases [
25]. The NiNT catalyst has low intensity desorption peak at temperatures as low as 300 °C due to the decomposition of the titanate and the formation of weak basic sites, as well. At temperatures superior to 300 °C, the medium strength basic sites are observed accounting for the low interaction of Ti with NiO. In the case of PtNT, a peak in the 100–250 °C range is due to NaO
x presence and the nanotubes decomposition [
4]. The second peak arises in the 300–500 °C range assigning to the CO
2 adsorption on weak Pt or Ti sites.
All these facts are evidenced by the EPR, XRD and Raman measurements of the solids shown in the previous section.
3.4. Catalytic Results
The catalytic evaluation of the solids in the selective reduction of NO
x by CO (CO-SCR) reaction is carried out in the temperature range of 50−550 °C. At temperatures lower than 100 °C, NO
x conversions inferior to 37% are seen for all titanate nanotubes catalysts (
Figure 6). Importantly, preliminary investigations without using catalysts in blank runs afforded less than 5% of conversion at 100 °C. As NO
x can be physically adsorbed on the surface of the solids at low temperatures [
6,
48], the subsequent increase of the activity is observed at higher temperatures.
The NaNT sample possessing Ti
4+ centers from Na
2Ti
3O
7 phase is inactive in the reaction, independently of the temperature evaluated. As the solids have residual alkali sodium contents, it can be seen that the presence of Na does not affect the catalytic performance during the CO-SCR reaction. In contrast to NaNT, the metal-containing titanate nanotubes are actives in the reaction, suggesting that the metals incorporated in the NTs work as active sites for the acid-base or redox reaction. It is noteworthy that AgNT, AlNT and CrNT exhibit similar behavior with conversions below 15% in all temperature range. Moreover, literature reports show that the catalyst having sites for NO
x adsorption on the alkali elements such as Na, protonic nanotubes and alkaline earth are active in the reaction [
50,
51,
52,
53]
, but the presence of a metal is needed.
Further reaction temperature increase from 100–250 °C gives distinct behavior for MeNTs solids studied. The literature reports show that the CO-SCR reaction initially proceeds by the simultaneous oxidation of NO to surface nitrates as strong oxidants and oxidation of the CO to surface oxygenates [
14,
15]. This next step is the reaction between these surface intermediates that leads to the formation of NCO and CN species, and finally, the latter species are converted into N
2; the CO is oxidized to CO
2 at relatively mild temperatures. Interestingly,
Figure 6 shows that NO
x decomposition of all the abovementioned MeNTs is very low at temperatures as low as 200 °C, with CoNT, PtNT and NiNT being exceptions. As the reaction proceeds, the catalytic activities suddenly depict a huge upward trend for CoNT, PtNT and NiNT, when the reaction temperature rises above 250 °C. For instance, the NO
x conversion on PtNT is 25% at 100 °C and reaches 92% at 200 °C indicating the moderate temperatures causes an improvement of the catalytic properties. Contrary, the activities of AgNT, AlNT and CrNT achieve a plateau with low conversions below 10% in all temperature ranges.
Importantly, the methods of salt addition to nanotubes, i.e., ion exchange and wet impregnation affect the catalytic performance of the solids. Even though PtNT is prepared via wet impregnation method, the surface acidities of both PtOx and chlorined Pt entities on the tubular NTs play an important role on the catalytic activity, especially the strong adsorption capability of Pt sites toward CO and NOx reactants at low temperatures.
Subsequent temperature increase from 250 to 550 °C results in CoNT, PtNT and NiNT conversions close to 55% at 550 °C, in opposite to NaNT, AlNT, AgNT and CrNT that experience poor performance. Taking into account the presence of the nanotube structures possessing the Na
2Ti
3O
7 phase in these solids (identified later by XRD and Raman of the spent samples), it can be inferred that the sodic phase is thermally transformed to transition states among the trititanate, anatase TiO
2 and traces of rutile TiO
2 phases at about 400 °C [
25].
Besides, the catalytic behavior of AlNT is apparently different from that of NaNT with NO
x conversion of 6% at 550 °C. Such a behavior is considered to be caused by the Al centers incorporated to the titanate nanotubes that promote the CO-SCR reaction. However, a large amount of Na ions on solid surface (
Table 1) leads to lower accessibility of the Al species, thus resulting in a very low performance of the AlNT. Notably, the AlOOH phase onto the NTs surface does not improve the catalytic performance of the solid. To verify this assumption, XRD, FTIR and Raman of the spent solids demonstrate that most of the Na ions remain on AlNT surface, while boehmite is deposited on the solid surface.
CrNT has a NOx conversion of ca. 15%, which is considered to be close to that of AlNT and AgNT. Despite the fact that both AlNT and CrNT display almost identical interplanar distance, most of the Cr3+ ions are out of NTs lattice forming the well disperse CrOOH oxide. Thus, more Cr3+ appears somewhat exposed on surface, as observed from the results of XRD, SEM-EDS, EPR and Raman spectroscopy. It is interesting to note that these trivalent chromium species in extra lattices are assumed to be responsible for the modest catalytic performance of the solids, in comparison with the AgNT, and AlNTs analogues.
It is worthy to mention that a NOx conversion of 7% is accomplished by AgNT, which is indeed two times lesser than that observed in the case of CoNT. The exposition of the Ag2O nanoparticles low interacting with the NTs surface (SEM, HRTEM and XRD) may result in a boost of the catalytic performance of the solid. These data nicely demonstrate that the Ag+ species located on solid surface assists interacting with the CO and NOx molecules; thus, Ag species are not able to maintain the catalytic performance.
The presence of Co incorporated into the NTs structure depicts a significant influence on the activity of the CoNT catalyst. Even with a small amount of cobalt, e.g., 1.0 wt%, the NO
x conversion is noticeably showing a clear increase of two times more than that of AlNT and AgNT. The entire inclusion of Co into the NT structure produces Co
3+ sites accessible to the reactants and plays an important role in improving the NO
x conversion. Conversely, the interaction of Co active sites is inefficient to retain the conversion level. Thereby, the modest performance of CoNT toward NO
x and CO is found. In reference to the literature for other Co-based catalysts for SCR reactions [
54], the CH
4-SCR studies on Co-based zeolites demonstrate the thermodynamic equilibrium allows high NO conversions to NO
2 by O
2 below 400 °C, although the catalyst is needed to the reaction occurrence.
Furthermore, the catalytic performance of the NTs is greatly improved when nickel is incorporated to the NTs. In line with expectations, note that the beneficial effects of the Ni phase on NTs at around 300 °C are those of increasing the dispersion of the titanate nanotube (further shown by TEM and FTIR measurements of the spent solids) and promoting the reduction of Ni
2+ sites during the range of temperature studied (
Table 4, TPR results). Most probably, the reaction environment provides the reduction of Ni species on the defect sites of the NTs, facilitating the coordination of NO
x or CO molecule to a Ni Lewis acid sites and thus improving the catalytic performance. In contrast, isolated NiOOH alone and those incorporated to the NTs sites in NiNT have low acidity (
Table 4, acidity measurements) implying in the low efficiency of these Ni sites to convert NO
x at temperatures lower than 200 °C (
Figure 6). In line with these results, the Ni
2+ is reduced above 550 °C, facilitating the transformation of the Na
2Ti
3O
7 and NiOOH phases to anatase and their corresponding NiO sites to increase the catalytic performance.
Notably, PtNT reaches the maximum values of NO
x conversion at temperatures superior to 200 °C followed by NiNT. The surface acidities of the well dispersed PtO
x and chlorinated Pt entities on the tubular NTs (latter seen by TEM) give the highest acidity for the former, as shown in
Table 3. Thereby, this results in high NO
x conversion within lower temperature region. On the basis CO-TPSR lean trap NO
x measurements for Pt-based samples, the CO reacts with NO
x forming CO
2 and NO, along with trace amounts of N
2 above 330 °C simultaneously [
3]. Moreover, our previous studies on optimizing the selective catalytic reduction of NO by CO reaction in the presence of distinct oxygen concentrations have demonstrated that the catalysts are more tolerant to the presence of oxygen in concentrations as low as 1000 ppm, which inhibit the oxidation of the CO by oxygen [
55]. In agreement, the CO
2-TPD profile indicates that the PtNT catalyst could avoid the oxidation of CO, since the Pt sites have a low affinity for this molecule maintaining the CO as a reducing agent to react with NO
x during the CO-SCR reaction.
Effect of the SO2 and Water Vapor Poisons on the Catalytic Performance
To further demonstrate the physicochemical properties of the solids on the catalytic performance, the evaluation of the temperature reaction as a function of reaction time is shown in
Figure 7a. The NaNT, CrNT, AgNT and AlNT catalysts give overall unsatisfactory results with low conversions in 600 min. of reaction because of their lack of structural stability. In consideration of the constraints of the catalysts, no further experiments are pursued using these MeNTs.
Therefore, the catalytic runs are carried out with the NiNT, PtNT and CoNT catalysts. The data clearly indicate that the solids have distinct behavior by using the SO
2 and water vapor poisons (
Figure 7a). At the early stages of the reaction, activity increases by increasing the reaction time using water vapor as a poison with NO
x conversion rising from 21% in 300 min to 30% at the end of the reaction for CoNT. Likewise, NO
x conversions over NiNT and PtNT are enhanced to values up to 40% in 150 min, and the value greatly increases to 100% running the reaction beyond this time in 600 min.Most of the findings state that the SCR reactions in the presence of water vapor poison are inhibited since the referred vapor can compete by the active sites of the catalysts through competitive adsorption causing a decay in the NO
x conversion at relatively low concentrations, e.g.,1–5% of H
2O [
56,
57,
58].
On the contrary, some reports show the role of water in SCR reaction the amount of water does not further retard the SCR reaction since new hydroxyl Brønsted acid site may be created in reason of the adsorption and decomposition of water on the solid surface [
57]. In response to the contradiction, the current result illustrates that the expected NTs performance with NO
x conversion is superior to 20%, even upon steam introduction (
Figure 7a). Thus, moisture interacts with the surface of the NTs and modifies the surface-active sites and the distribution of Lewis and Brønsted acid sites, as found elsewhere [
59]. Meanwhile, a steep plateau in the NO
x conversion is seen in CoNT catalyst with prolonged reaction time due to Co sites oxidation by water vapor. Furthermore, the activity of NiNT and PtNT catalysts appears to be less affected by water vapor reaching up to 90% of conversion after 600 min of reaction. A consequence is that the structure of these trititanate catalysts is maintained after the catalytic test, as further demonstrated by spent catalyst characterizations.
PtNT and NiNT catalysts are found to be very tolerant to SO2 in CO-SCR at low temperatures. However, CoNT is less active than Pt and Ni, being limited to its low resistance against sulfur poisoning, as illustrated by the poisoning experiments. Thus, PtNT and NiNT have a NOx conversion (by ~3.0–93%) within 300 min and upper times values give the complete conversion in the presence of SO2.
The abovementioned results encourage us to investigate the SCR performance of the most active solids regarding their tolerance of SO
2 and H
2O. When water vapor is introduced, the adsorption of H
2O on the active metal sites may occur, and the Lewis acid sites might be converted into Brønsted acid sites via bonding of a water molecule, in line with the findings [
60]. The literature reports also show that the SO
2 might be harmful to the metal active sites in reason of the permanent and irreversible SO
2 adsorption on the metal, besides the competitive adsorption of NO
x, CO and SO
2 for the active sites [
15,
56]. Another fact is that SO
2 addition may form elemental sulfur, which probably could cover the active metal surface and block the pores causing damage in the catalyst structure [
8,
61]. Therefore, the NiNT deactivation is observed, when the catalyst is poisoned by SO
2 (
Figure 7b). Besides, it can be inferred that the adsorption ability of SO
2 on the Lewis and Brønsted sites, i.e., formed by water, is much higher than that of NO
x and CO; hence SO
2 may not occupy the Ni sites of NiNT leading to the catalyst deactivation. Contrary, CoNT and PtNT are resistant against SO
2 deactivation, under the same conditions during the catalytic run. These results confirm that incorporation of Co on the NTs layered structure and the dispersion of Pt on the NTs surface provide active redox sites to the reaction. However, the well-dispersed Co in low interaction with the NTs support may provide unstable sites during the reaction, resulting in the facile poison by both water vapor and SO
2 (
Figure 7b) with the consequent limit catalytic performance of the CoNT catalyst.
PtNT catalyst has the best performance in CO-SCR at low temperatures, when water is introduced in 200 min and then cut off, and simultaneously, SO
2 is introduced in the reaction whilst NiNT has low resistance against sulfur poisoning (
Figure 7b). It can be understood that both NiNT and CoNT are extremely sensitive to the poisons, implying that the catalytic activity declined due to the lattice Co
2+ ions oxidation by SO
2 into the NTs structure and sulfur decomposition on Ni
2+ sites, as well.
Our results show the reaction occurrence at lower temperature, most probably due to the formation of surface isocyanate species (NCO) on the decoration of the Pt nanoparticles on the NTs structure already at 150 °C. In the meantime, the PtNT catalyst undergoes the complete NOx conversion above to 200 °C, due to the lower acidity and reduction of the Pt2+ sites compared to NiNT and CoNT. It is worth noticing that PtNT reduction occurs in two steps, 140–280 and 280–470 °C, respectively, attributed to the reduction of PtOx species (TPR and XPS results).
When correlating with the catalytic behavior of the PtNT, one can observe that the lower conversion of NiNT may be a result of the sintering of the Ni nanoparticles and preferential CO decomposition [
4] during the reaction. On the contrary, an enhancement of the PtNT activity is seen at 200 °C towards the poisons due to the Pt species stability into the NT structure, as further seen by spent catalysts characterizations. In comparison with the other solids, PtNT achieves the intrinsic rate and turnover frequency values of 3 × 10
−10 mol m
−2 s
−1 and 0.56 h
−1, respectively, after 6 h of exposure to the SO
2 and water vapor poisons. This illustrates the influence of the Pt species in the NTs resulting in a suitable catalyst for CO-SCR reaction.