Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate
Abstract
1. Introduction
2. Chemicals
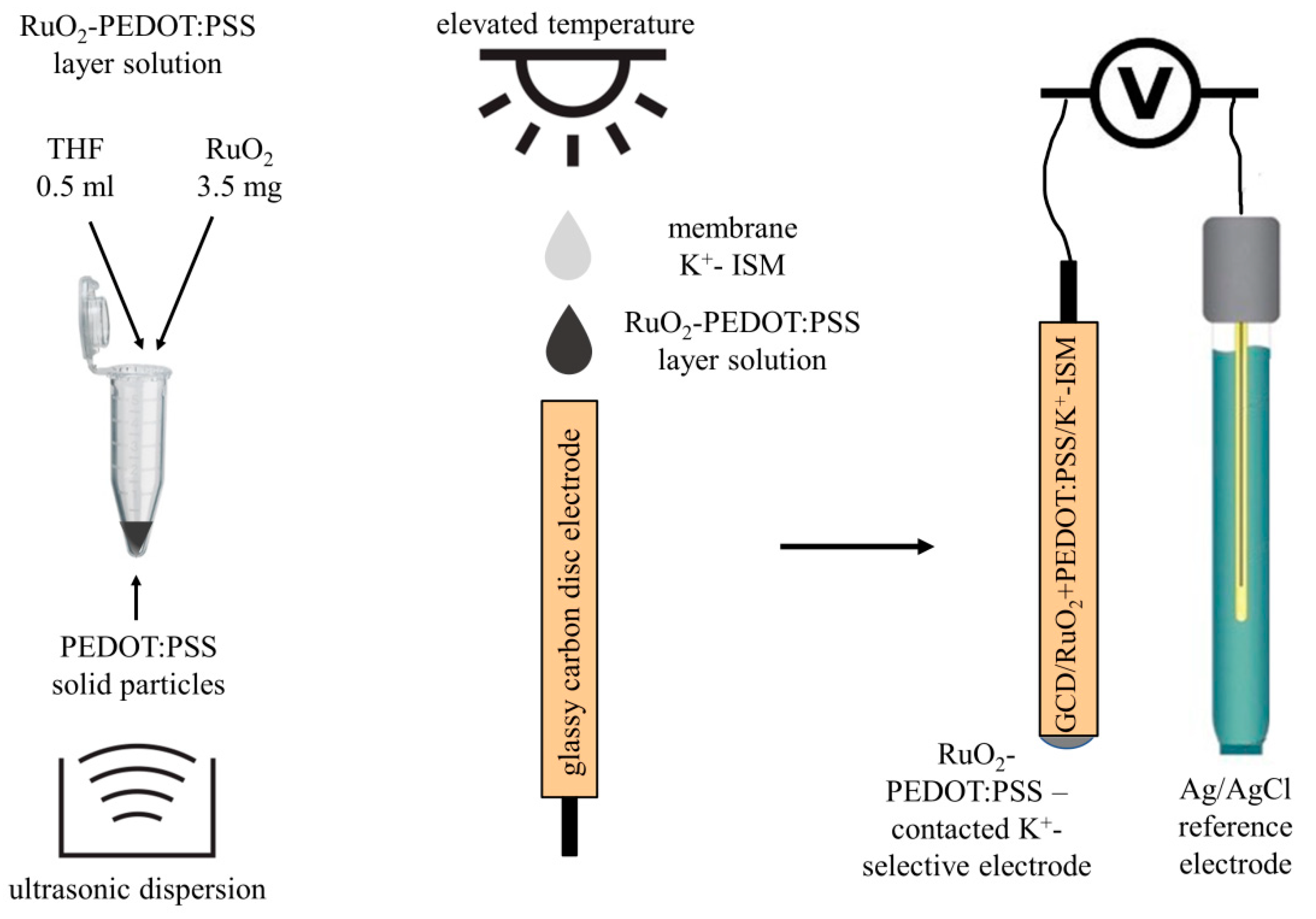
2.1. Layer Hybrid Materials
2.2. Electrodes Preparation
2.3. Conducted Measurements
3. Results
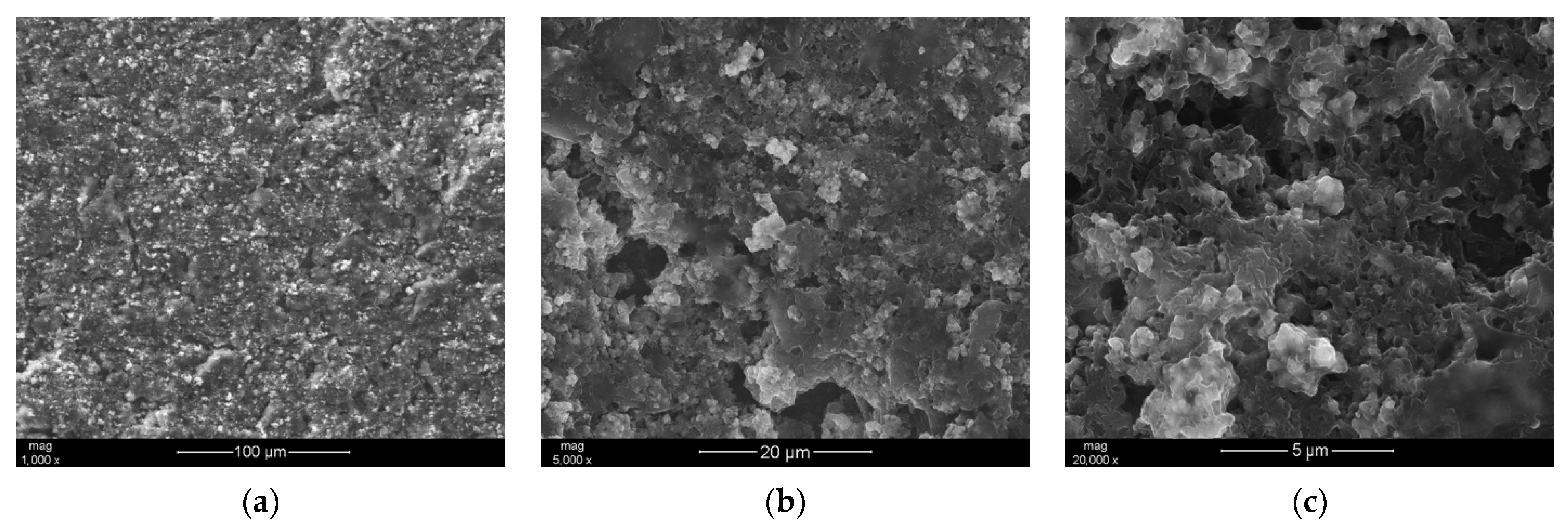
3.1. SEM
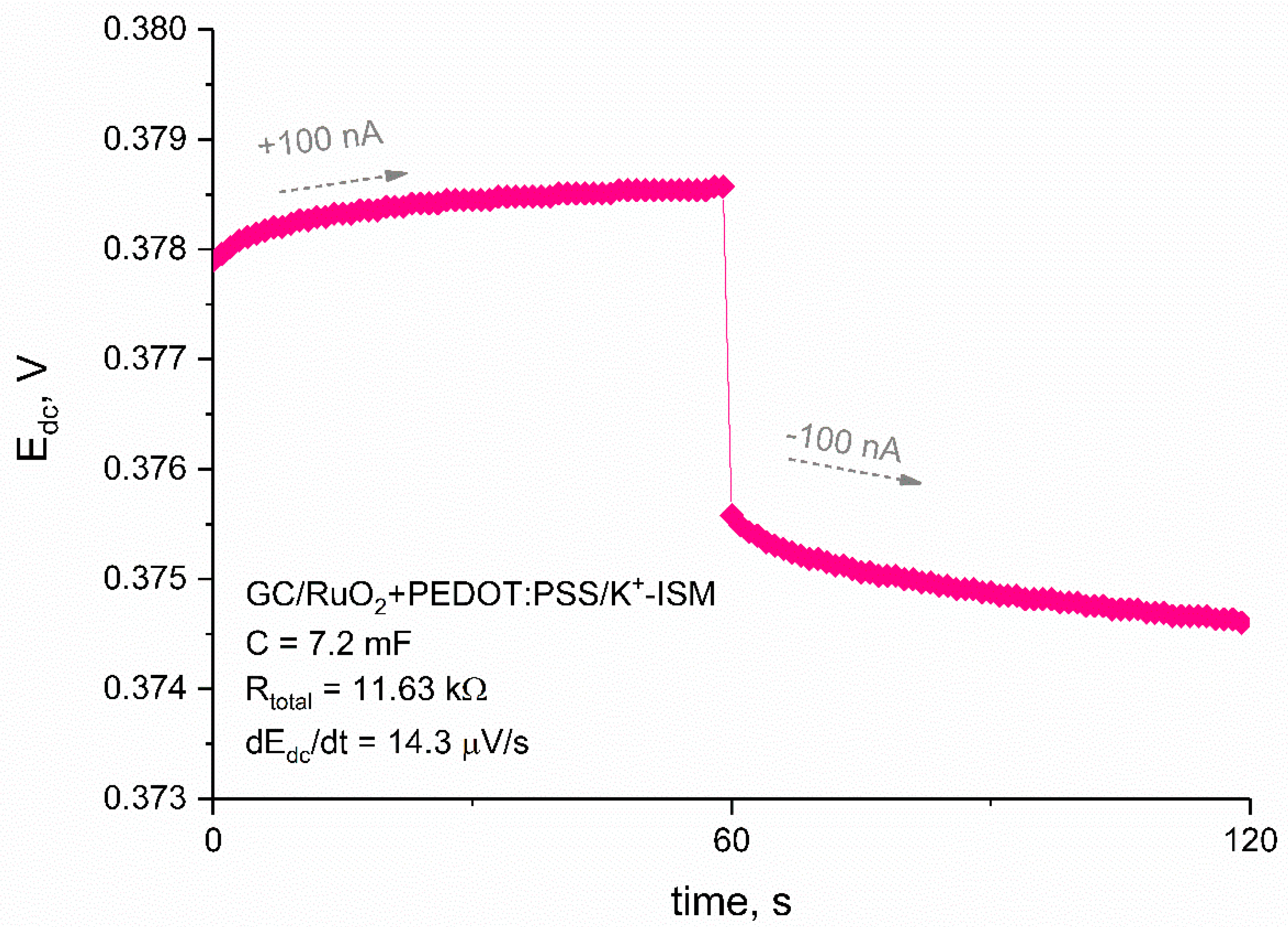
3.2. Chronopotentiometry
3.3. Potentiometry
3.3.1. Potentiometric Response
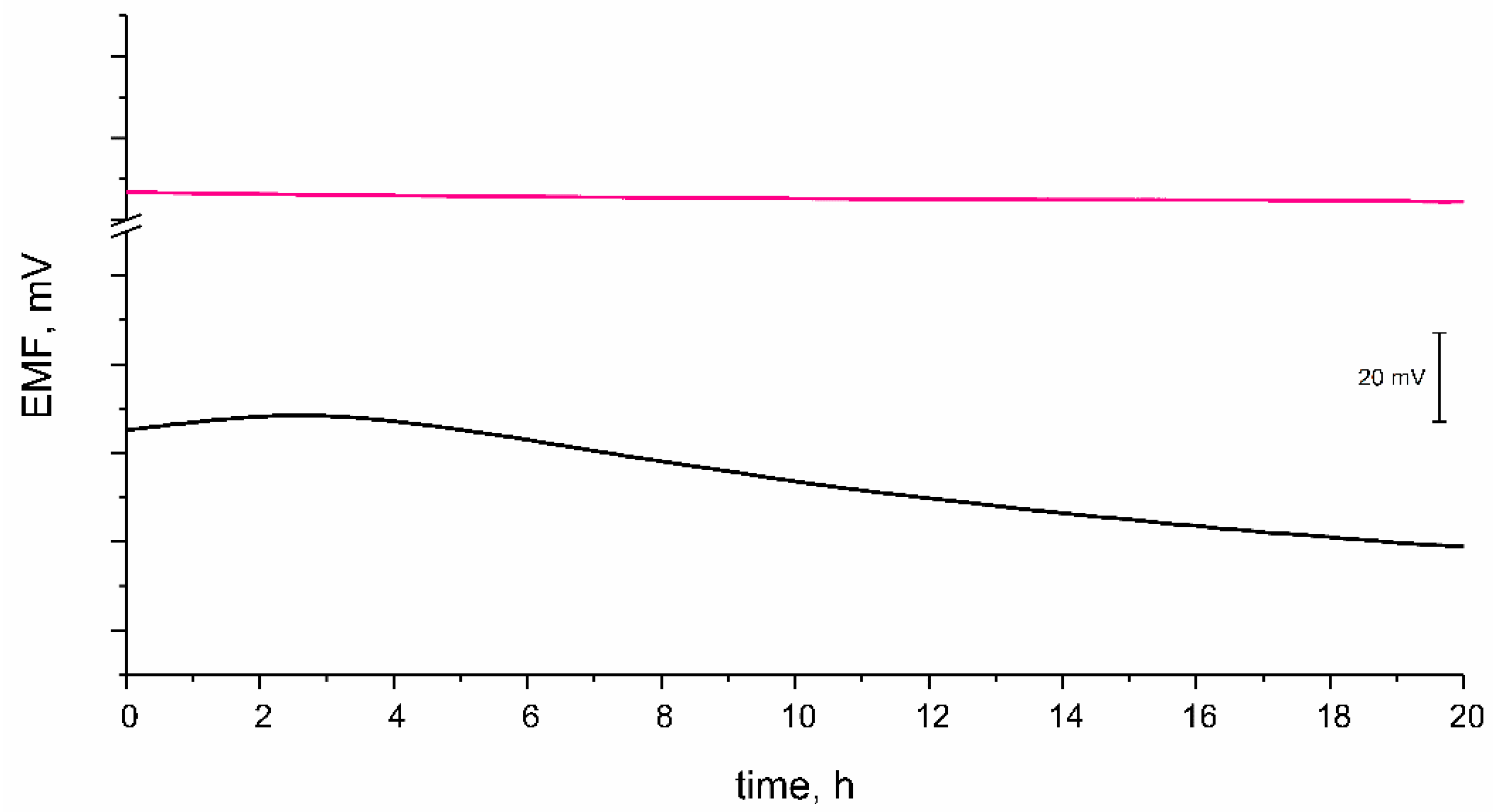
3.3.2. Potential Stability
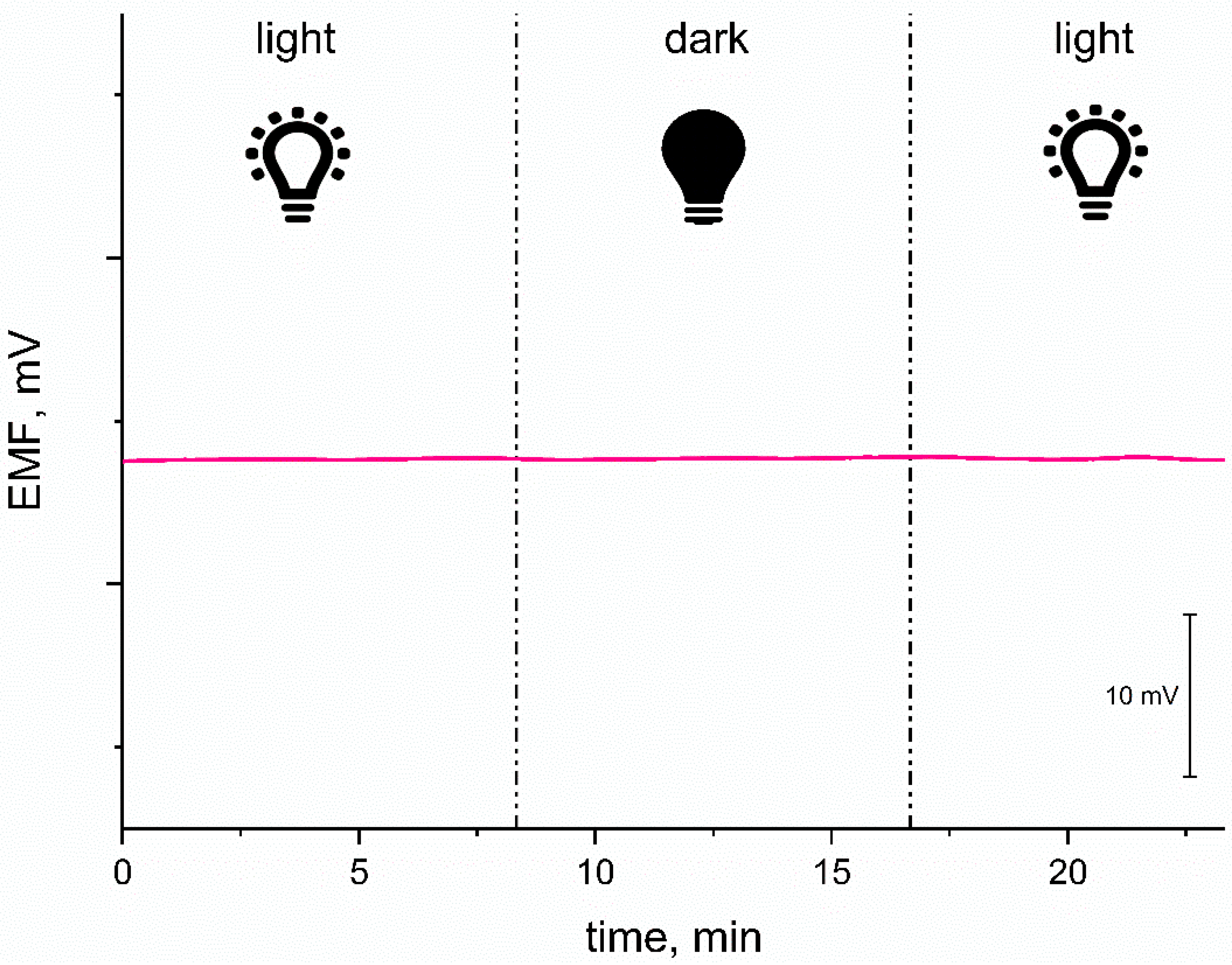
3.3.3. Light Sensitivity Test
3.3.4. pH Sensitivity Test
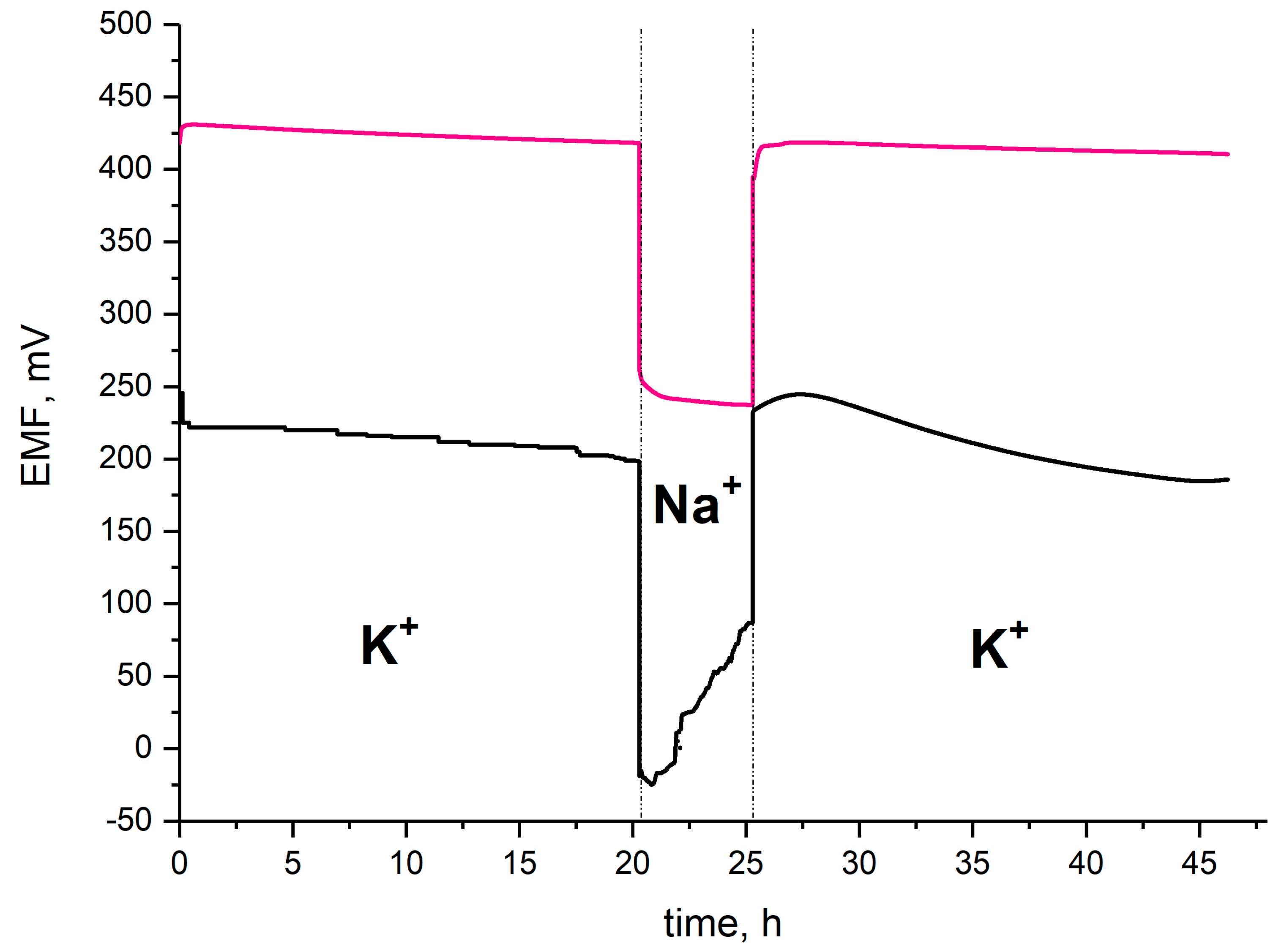
3.3.5. Water Layer Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, E. Ion-Selective Electrodes. Encycl. Anal. Sci. 2019, 231–251. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zdrachek, E.; Bakker, E. Potentiometric Sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef]
- Cattrall, R.W.; Freiser, H. Coated wire ion-selective electrodes. Anal. Chem. 1971, 43, 1905–1906. [Google Scholar] [CrossRef]
- Kisiel, A.; Michalska, A.; Maksymiuk, K. Rectifying effect for ion-selective electrodes with conducting polymer solid contact. Synth. Met. 2018, 246, 246–253. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Nikolskii, B.P.; Materova, E.A. Solid contact in membrane ion-selective electrodes. Ion-Select. Electr. Rev. 1985, 7, 3–39. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Jang, J. Screen-Printable and Flexible RuO2 Nanoparticle-Decorated PEDOT:PSS/Graphene Nanocomposite with Enhanced Electrical and Electrochemical Performances for High-Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 10213–10227. [Google Scholar] [CrossRef]
- Bobacka, J.; Lewenstam, A.; Ivaska, A.; Fin, A. Electrochemical impedance spectroscopy of oxidized poly(3, 4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanal. Chem. 2000, 489, 17–27. [Google Scholar] [CrossRef]
- Veder, J.; De Marco, R.; Clarke, G.; Jiang, P.; Prince, K.; Bakker, E. Water uptake in the hydrophilic poly(3,4-ethylenedioxythiophene):poly-(styrene sulfonate) solid-contact of all-solid-state polymeric ion-selective electrodes. Analyst 2011, 136, 3252–3258. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, A.; Lewenstam, A.; Gao, Z.; Ivaska, A.; Diamond, D. All-solid-state sodium-selective electrode based on a calixarene ionophore in a poly(vinyl chloride) membrane with a polypyrrole solid contact. Anal. Chem. 2005, 64, 2496–2501. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; Pendley, B.D.; Lindner, E. Equilibration Time of Solid Contact Ion-Selective Electrodes. Anal. Chem. 2015, 87, 6654–6659. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Yin, H.; Li, W.; Jiang, Y. Poly(3,4-ethylenedioxythiophene)/MoS2 nanocomposites with enhanced electrochemical capacitance performance. RSC Adv. 2014, 4, 56926–56932. [Google Scholar] [CrossRef]
- Ocaña, C.; Abramova, N.; Bratov, A.; Lindfors, T.; Bobacka, J. Calcium-selective electrodes based on photo-cured polyurethane-acrylate membranes covalently attached to methacrylate functionalized poly(3,4-ethylenedioxythiophene) as solid-contact. Talanta 2018, 186, 279–285. [Google Scholar] [CrossRef]
- Zuliani, C.; Matzeu, G.; Diamond, D. A Liquid-Junction-Free Reference Electrode Based on a PEDOT Solid-Contact and Ionogel Capping Membrane. Talanta 2014, 125, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, Z.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Poly(3,4-ethylenedioxythiophene) (PEDOT) doped with carbon nanotubes as ion-to-electron transducer in polymer membrane-based potassium ion-selective electrodes. J. Electroanal. Chem. 2009, 633, 246–252. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Ruthenium Dioxide as High-Capacitance Solid-Contact Layer in K+-Selective Electrodes Based on Polymer Membrane. J. Electrochem. Soc. 2019, 166, 1470–1476. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R.; Królicka, A. Poly(3-octylthiophene-2,5-diyl)—Nanosized ruthenium dioxide composite material as solid-contact layer in polymer membrane-based K+-selective electrodes. Electrochim. Acta 2019, 322, 13418. [Google Scholar] [CrossRef]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Ruthenium dioxide nanoparticles as a high-capacity transducer in solid-contact polymer membrane-based pH-selective electrodes. Microchim. Acta 2019, 186, 777. [Google Scholar] [CrossRef] [PubMed]
- Lenar, N.; Paczosa-Bator, B.; Piech, R. Optimization of Ruthenium Dioxide Solid Contact in Ion-Selective Electrodes. Membranes 2020, 10, 182. [Google Scholar] [CrossRef]
- Huang, L.-M.; Lin, H.-Z.; Wen, T.-C.; Gopalan, A. Highly dispersed hydrous ruthenium oxide in poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid) for supercapacitor electrode. Electrochim. Acta 2006, 52, 1058–1063. [Google Scholar] [CrossRef]
- Sellam; Hashmi, S.A. High Rate Performance of Flexible Pseudocapacitors fabricated using Ionic-Liquid-Based Proton Conducting Polymer Electrolyte with Poly(3, 4-ethylenedioxythiophene):Poly(styrene sulfonate) and Its Hydrous Ruthenium Oxide Composite Electrodes. ACS Appl. Mater. Interfaces 2013, 5, 3875–3883. [Google Scholar] [CrossRef]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; De Rooij, N.F.; Pretsch, E. Potential drifts of solid-contacted ion-selective electrodes due to zero-current ion fluxes through the sensor membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Guzinski, M.; Jarvis, J.M.; D’Orazio, P.; Izadyar, A.; Pendley, B.D.; Lindner, E. Solid-Contact pH Sensor without CO2 Interference with a Superhydrophobic PEDOT-C14 as Solid Contact: The Ultimate “Water Layer” Test. Anal. Chem. 2017, 89, 8468–8475. [Google Scholar] [CrossRef] [PubMed]

 ) and GCD/K+-ISM (●) electrodes measured after 24, 48, and 72 h of conditioning (n = 3) with error bars depicting repeatability of potentiometric response.
) and GCD/K+-ISM (●) electrodes measured after 24, 48, and 72 h of conditioning (n = 3) with error bars depicting repeatability of potentiometric response.
 ) and GCD/K+-ISM (●) electrodes measured after 24, 48, and 72 h of conditioning (n = 3) with error bars depicting repeatability of potentiometric response.
) and GCD/K+-ISM (●) electrodes measured after 24, 48, and 72 h of conditioning (n = 3) with error bars depicting repeatability of potentiometric response.

| Electrode | Capacitance Value (μF) | Potential Drift (μV/s) | Reference |
|---|---|---|---|
| GCD/PEDOT(CNT)/K+-ISM | 83 | 12 | [18] |
| GCD/RuO2⋅xH2O/K+-ISM | 1233 | 81 | [19] |
| GCD/PEDOT:PSS/K+-ISM | 204 | 4.9 | [9] |
| GCD/PEDOT(Cl)/K+-ISM | 300 | 3.3 | [9] |
| GCD/RuO2 + POT/K+-ISM | 1167 | 86 | [21] |
| GCD/POT/K+-ISM | 1.25 | 798 | [21] |
| GC/RuO2 + PEDOT:PSS/K+-ISM | 7200 | 14.3 | this work |
| Group of Electrodes | Slope (mV/dec) | Standard Potential (mV) | Linear Range (M) |
|---|---|---|---|
| GC/RuO2 + PEDOT:PSS/K+-ISM | 58.93 ± 0.49 | 382 ± 3 | 10−1–10−6 |
| GC/K+-ISM | 59.31 ± 0.61 | 343 ± 15 | 10−1–10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenar, N.; Piech, R.; Paczosa-Bator, B. Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate. Materials 2021, 14, 1891. https://doi.org/10.3390/ma14081891
Lenar N, Piech R, Paczosa-Bator B. Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate. Materials. 2021; 14(8):1891. https://doi.org/10.3390/ma14081891
Chicago/Turabian StyleLenar, Nikola, Robert Piech, and Beata Paczosa-Bator. 2021. "Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate" Materials 14, no. 8: 1891. https://doi.org/10.3390/ma14081891
APA StyleLenar, N., Piech, R., & Paczosa-Bator, B. (2021). Potentiometric Sensor with High Capacity Composite Composed of Ruthenium Dioxide and Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate. Materials, 14(8), 1891. https://doi.org/10.3390/ma14081891








