Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial
Abstract
1. Introduction
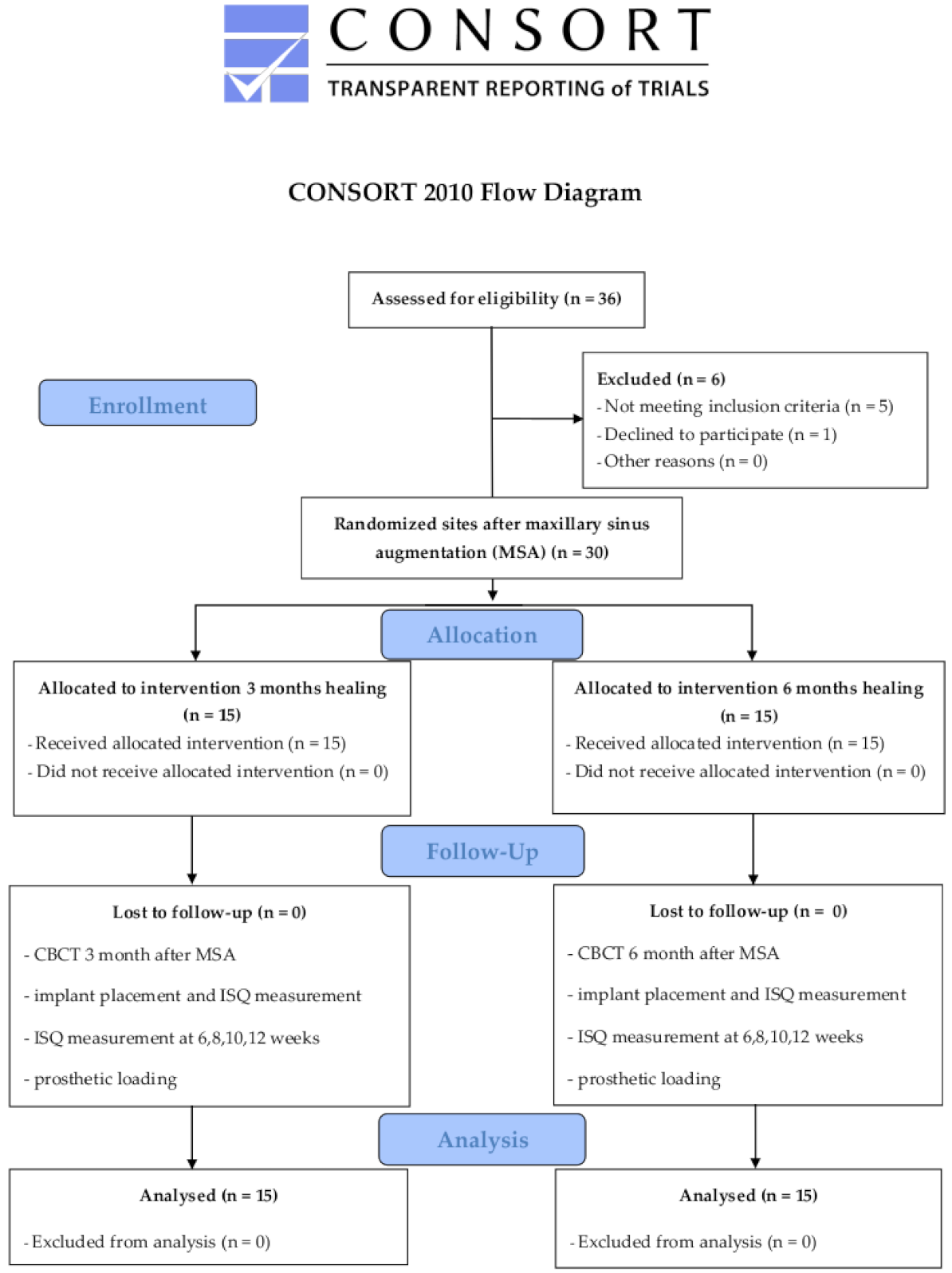
2. Materials and Methods
2.1. Study Protocol
2.2. Sample Size Calculation
2.3. Patients
2.4. Randomization
2.5. A-PRF Preparation
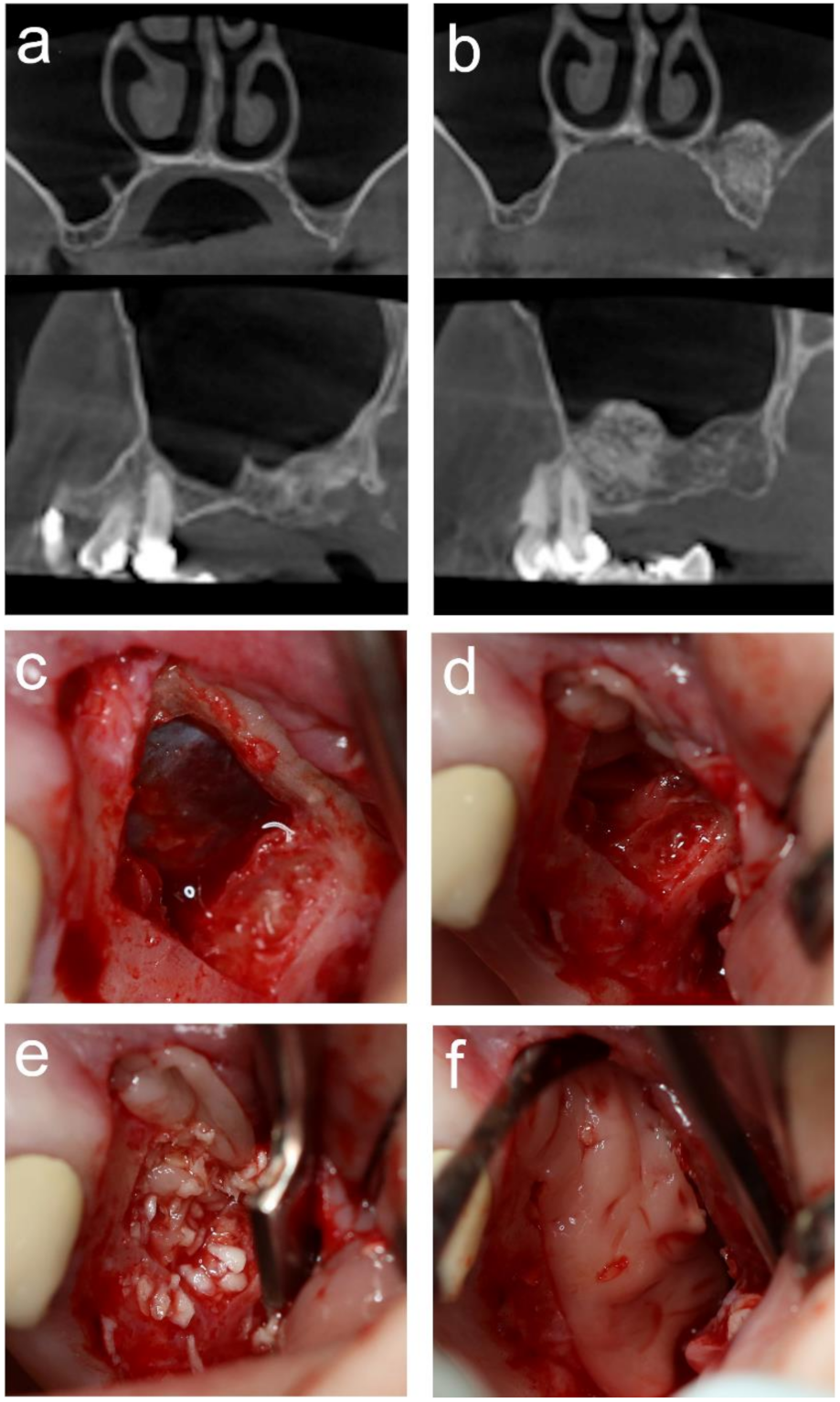
2.6. MSA
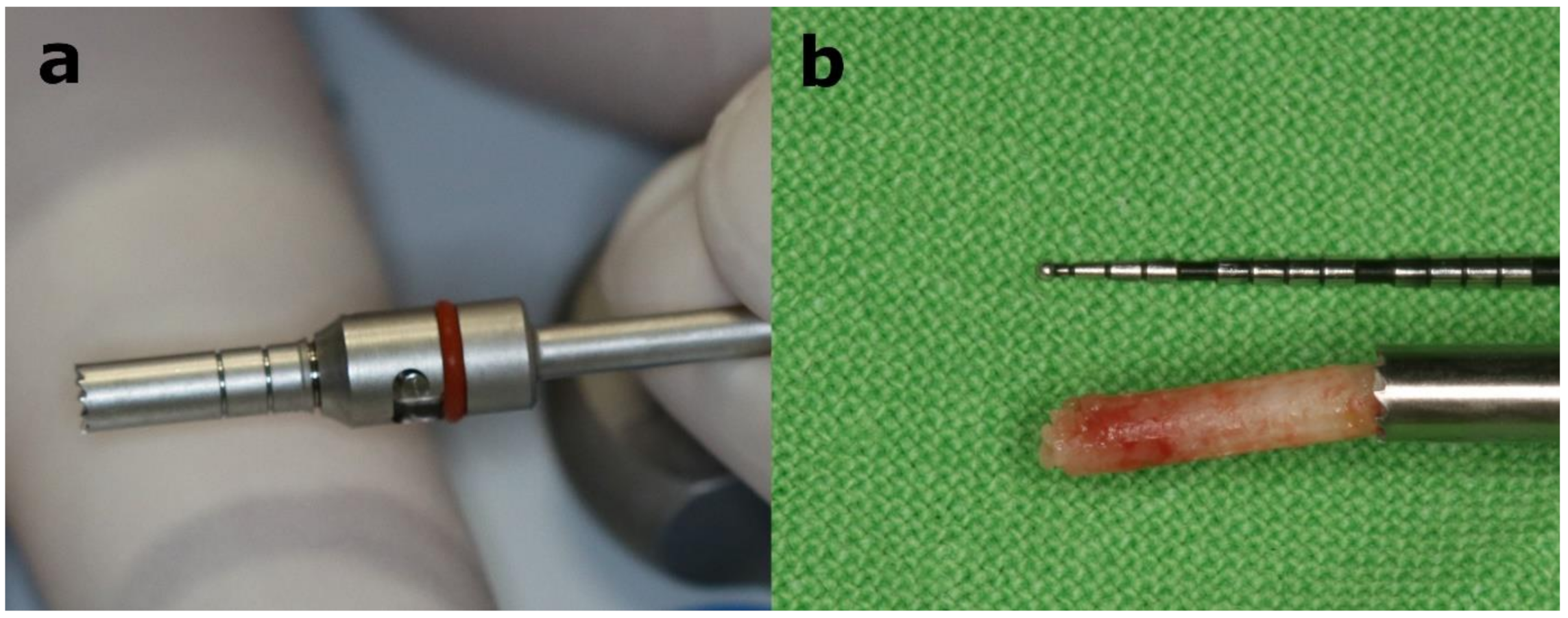
2.7. Implant Placement
2.8. RFA
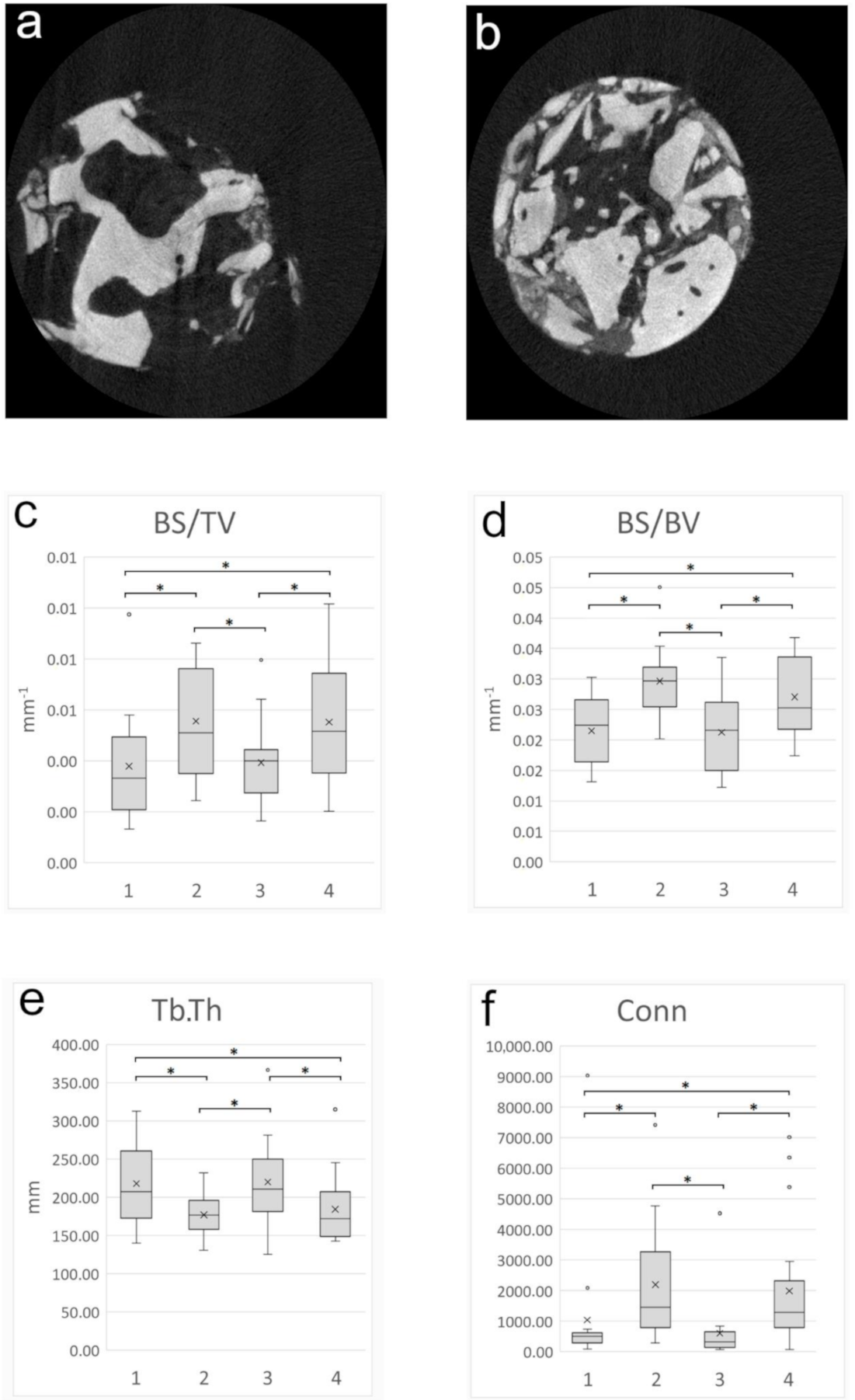
2.9. Micromorphometric Analysis
2.10. Histology and Histomorphometric Analysis
2.11. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. MSA
3.3. Implant Placement
3.4. RFA
3.5. Micromorphometry Analysis
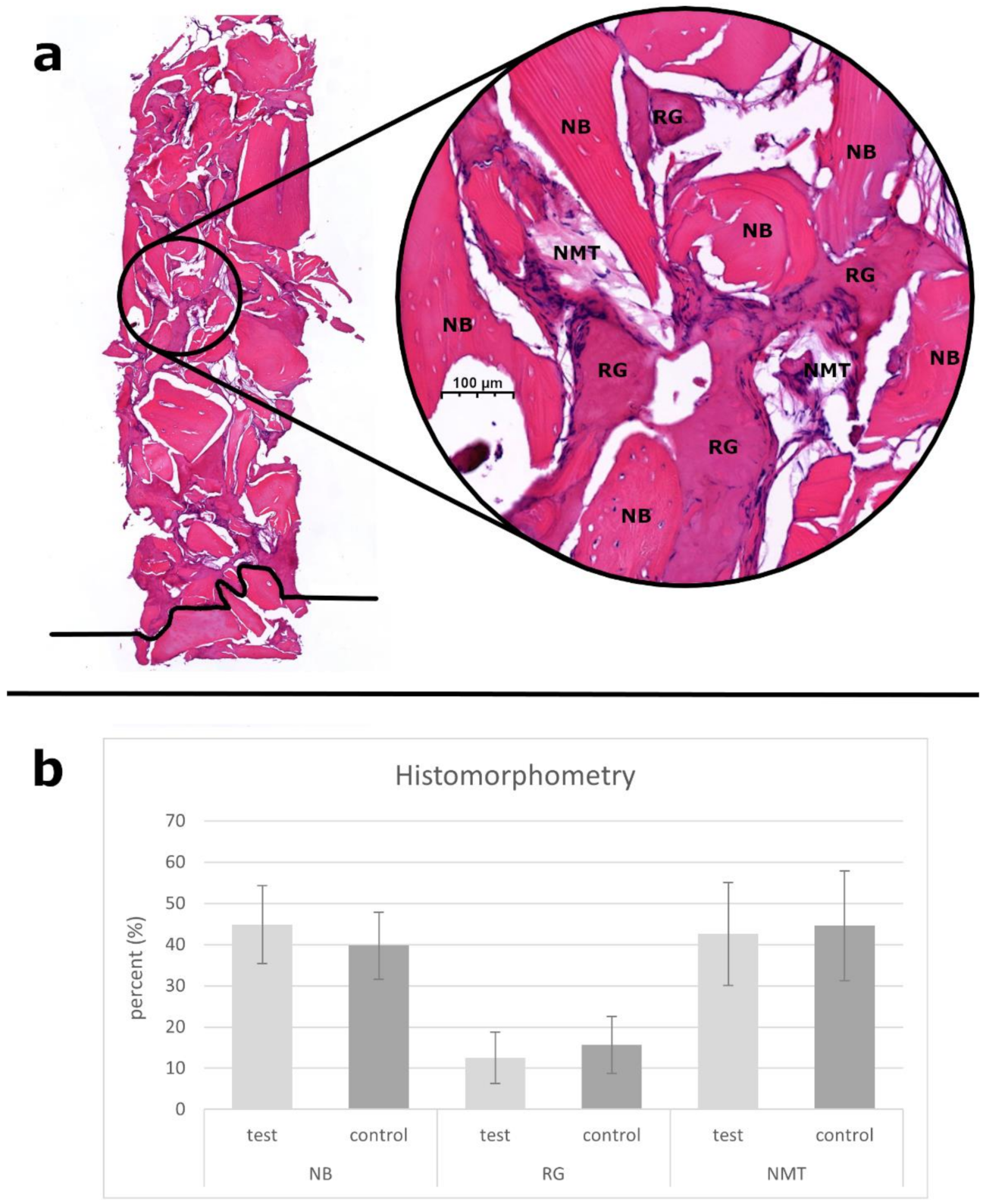
3.6. Histological and Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | autologous bone |
| A-PRF | advanced platelet-rich fibrin |
| BS/BV | bone surface/volume ratio |
| BS/TV | bone surface density |
| BV/TV | bone volume fraction |
| CBCT | cone-beam computed tomography |
| Conn | connectivity |
| ISQ | implant stability quotient |
| MSA | maxillary sinus augmentation |
| NB | newly formed bone |
| NMT | nonmineralized tissue |
| Po(op) | open porosity |
| Po(tot) | total porosity |
| RFA | resonance frequency analysis |
| RG | residual graft particles |
| ROI | region of interest |
| PRF | platelet-rich fibrin |
| SACBA | serum albumin-coated bone allograft |
| SM | Schneiderian membrane |
| Tb.Pf | trabecular bone pattern factor |
| Tb.Sp | trabecular separation |
| Tb.Th | trabecular thickness |
| µCT | micro–computed tomography |
Appendix A
| Abbreviation | Group | N | Mean | Std. Deviation | p-Value |
|---|---|---|---|---|---|
| BV/TV | pristine bone-test | 17 | 18.5008 | 10.1497 | 0.851 # |
| augmented area-test | 17 | 19.1761 | 8.2003 | ||
| pristine bone-control | 17 | 20.4003 | 10.5609 | ||
| augmented area-control | 17 | 21.2211 | 10.0378 | ||
| BS/BV | pristine bone-test | 17 | 0.0215 | 0.0056 | 0.000 # |
| augmented area-test | 17 | 0.0297 | 0.0057 | ||
| pristine bone-control | 17 | 0.0213 | 0.0063 | ||
| augmented area-control | 17 | 0.0270 | 0.0061 | ||
| BS/TV | pristine bone-test | 17 | 0.0038 | 0.0021 | 0.023 ## |
| augmented area-test | 17 | 0.0056 | 0.0022 | ||
| pristine bone-control | 17 | 0.0039 | 0.0016 | ||
| augmented area-control | 17 | 0.0055 | 0.0024 | ||
| Tb.Th | pristine bone-test | 17 | 218.0640 | 52.7430 | 0.017 ## |
| augmented area-test | 17 | 177.0196 | 28.4101 | ||
| pristine bone-control | 17 | 220.2552 | 58.7725 | ||
| augmented area-control | 17 | 184.4137 | 45.6001 | ||
| Tb.Sp | pristine bone-test | 17 | 627.6184 | 238.2173 | 0.615 # |
| augmented area-test | 17 | 569.7924 | 269.8554 | ||
| pristine bone-control | 17 | 542.3623 | 234.8811 | ||
| augmented area-control | 17 | 519.8590 | 242.8923 | ||
| Tb.Pf | pristine bone-test | 17 | 0.0043 | 0.0045 | 0.072 ## |
| augmented area-test | 17 | 0.0078 | 0.0066 | ||
| pristine bone-control | 17 | 0.0054 | 0.0046 | ||
| augmented area-control | 17 | 0.0068 | 0.0050 | ||
| Po(tot) | pristine bone-test | 17 | 81.4992 | 10.1497 | 0.851 # |
| augmented area-test | 17 | 80.8239 | 8.2003 | ||
| pristine bone-control | 17 | 79.5997 | 10.5609 | ||
| augmented area-control | 17 | 78.7789 | 10.0378 | ||
| Po(op) | pristine bone-test | 17 | 81.4899 | 10.1554 | 0.851 # |
| augmented area-test | 17 | 80.8136 | 8.2076 | ||
| pristine bone-control | 17 | 79.5864 | 10.5724 | ||
| augmented area-control | 17 | 78.7685 | 10.0459 | ||
| Conn | pristine bone-test | 17 | 1040.3500 | 2104.7550 | 0.000 ## |
| augmented area-test | 17 | 2197.2400 | 1911.5760 | ||
| pristine bone-control | 17 | 603.6500 | 1045.0600 | ||
| augmented area-control | 17 | 1988.9400 | 2158.4780 |
| First Author | Year of Publication | Study Design | Intervention | Sample Size (MSA) | Biomaterials Used | New Bone Formation % (Mean ± Std. Deviation) | p-Value | Healing Time Prior Implant Placement (Month) |
|---|---|---|---|---|---|---|---|---|
| Pichotano | 2019 | RCT | two-stage MSA | 12 | DBBM + L-PRF | 44.58 ± 13.9% | 0.0087 | 4 |
| 12 | DBBM | 30.02 ± 9.99% | 8 | |||||
| Nizam | 2018 | RCT | two-stage MSA | 13 | DBBM + L-PRF | 21.38 ± 8.78% | n.s. | 6 |
| 13 | DBBM | 21.25 ± 5.59% | 6 | |||||
| Olgun | 2018 | RCT | two-stage MSA | 10 | T-PRF | 16.58 ± 1.05% | 0.611 | 4 |
| 8 | FDBA | 17.28 ± 2.53% | 6 | |||||
| Kilic | 2017 | RCT | two-stage MSA | 9 | ß-TCP | 33.40 ± 10.43% | n.s. | 6 |
| 9 | ß-TCP + PRP | 34.83 ± 10.12% | 6 | |||||
| 8 | ß-TCP + PRF | 32.03 ± 6.34% | ||||||
| Bolukbasi | 2015 | CS | two-stage MSA | 17 | DBBM + PRF | 35.0 ± 8.60% | 0.61 | 6 |
| 15 | DBBM | 32.97 ± 9.71% | 6 | |||||
| Zhang | 2012 | RCT | two-stage MSA | 6 | DBBM + PRF | 18.35 ± 5.62% | 0.138 | 6 |
| 5 | DBBM | 12.95 ± 5.33% | 6 | |||||
| Tatullo | 2012 | RCT | two-stage MSA | 8 | DBBM | 26.44% | n.s. | 3 |
| 12 | DBBM + PRF | 22.79% | 3 | |||||
| 8 | DBBM | 28.7% | n.s. | 4 | ||||
| 12 | DBBM + PRF | 26.15% | 4 | |||||
| 8 | DBBM | 38.97% | n.s. | 5 | ||||
| 12 | DBBM + PRF | 37.06% | 5 | |||||
| Choukroun | 2006 | CS | two-stage MSA | 6 | FDBA + PRF | 20.95% | n.s. | 4 |
| 3 | FDBA | 20.306% | 8 |
References
- Iwanaga, J.; Wilson, C.; Lachkar, S.; Tomaszewski, K.A.; Walocha, J.A.; Tubbs, R.S. Clinical anatomy of the maxillary sinus: Application to sinus floor augmentation. Anat. Cell Biol. 2019, 52, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar]
- Raghoebar, G.M.; Onclin, P.; Boven, G.C.; Vissink, A.; Meijer, H.J.A. Long-term effectiveness of maxillary sinus floor augmentation: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: Anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Angelo, T.; Marcel, W.; Andreas, K.; Izabela, S. Biomechanical Stability of Dental Implants in Augmented Maxillary Sites: Results of a Randomized Clinical Study with Four Different Biomaterials and PRF and a Biological View on Guided Bone Regeneration. BioMed Res. Int. 2015, 2015, 850340. [Google Scholar] [CrossRef]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary Sinus Augmentation With Different Biomaterials: A Comparative Histologic and Histomorphometric Study in Man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Vinci, R.; Capparè, P.; Gherlone, E.; Romanos, G. Calvarial versus iliac crest for autologous bone graft material for a sinus lift procedure: A histomorphometric study. Int. J. Oral Maxillofac. Implant. 2007, 22, 527–532. [Google Scholar]
- Klijn, R.J.; Meijer, G.J.; Bronkhorst, E.M.; Jansen, J.A. Sinus Floor Augmentation Surgery Using Autologous Bone Grafts from Various Donor Sites: A Meta-Analysis of the Total Bone Volume. Tissue Eng. Part. B Rev. 2010, 16, 295–303. [Google Scholar] [CrossRef]
- Precheur, H.V. Bone Graft Materials. Dent. Clin. N. Am. 2007, 51, 729–746. [Google Scholar] [CrossRef]
- Stacchi, C.; Orsini, G.; Di Iorio, D.; Breschi, L.; Di Lenarda, R. Clinical, Histologic, and Histomorphometric Analyses of Regenerated Bone in Maxillary Sinus Augmentation Using Fresh Frozen Human Bone Allografts. J. Periodontol. 2008, 79, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Soardi, C.M.; Spinato, S.; Zaffe, D.; Wang, H.L. Atrophic maxillary floor augmentation by mineralized human bone allograft in sinuses of different size: An histologic and histomorphometric analysis. Clin. Oral Implant. Res. 2010, 22, 560–566. [Google Scholar] [CrossRef]
- Kolerman, R.; Nissan, J.; Rahmanov, M.; Vered, H.; Cohen, O.; Tal, H. Comparison between mineralized cancellous bone allograft and an alloplast material for sinus augmentation: A split mouth histomorphometric study. Clin. Implant. Dent. Relat. Res. 2017, 19, 812–820. [Google Scholar] [CrossRef]
- Avila, G.; Neiva, R.; Misch, C.E.; Galindo-Moreno, P.; Benavides, E.; Rudek, I.; Wang, H.L. Clinical and histologic outcomes after the use of a novel allograft for maxillary sinus augmentation: A case series. Implant. Dent. 2010, 19, 330–341. [Google Scholar] [CrossRef]
- Weszl, M.; Skaliczki, G.; Cselenyák, A.; Kiss, L.; Major, T.; Schandl, K.; Bognár, E.; Stadler, G.; Peterbauer, A.; Csönge, L.; et al. Freeze-dried human serum albumin improves the adherence and proliferation of mesenchymal stem cells on mineralized human bone allografts. J. Orthop. Res. 2011, 30, 489–496. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Szabó, T.; Szigyártó, I.C.; Toró, I.; Vámos, B.; Hornyák, I.; Renner, K.; Klára, T.; Szabó, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 126–132. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Vácz, G.; Toró, I.; Szabó, T.; May, Z.; Duarte, M.; Hornyák, I.; Szabó, B.T.; Dobó-Nagy, C.; Doros, A.; et al. Remineralization of demineralized bone matrix in critical size cranial defects in rats: A 6-month follow-up study. J. Biomed. Mater. Res Part B Appl. Biomater. 2015, 104, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Simonffy, L.; Minya, F.; Trimmel, B.; Lacza, Z.; Dobo-Nagy, C. Albumin-Impregnated Allograft Filling of Surgical Extraction Sockets Achieves Better Bone Remodeling Than Filling with Either Blood Clot or Bovine Xenograft. Int. J. Oral Maxillofac. Implant. 2020, 35, 297–304. [Google Scholar] [CrossRef]
- Marton, K.; Tamas, S.B.; Orsolya, N.; Bela, C.; Ferenc, D.; Peter, N.; Csaba, D.N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. [Google Scholar] [CrossRef]
- Klára, T.; Csönge, L.; Janositz, G.; Csernátony, Z.; Lacza, Z. Albumin-coated structural lyophilized bone allografts: A clinical report of 10 cases. Cell Tissue Bank 2013, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Chou, T.M.; Chang, H.P.; Wang, J.C. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung Med. Sci. 2020, 36, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Cassetta, M.; Pacifici, A.; Stefanelli, L.V.; Scacco, S.; Dipalma, G.; Pacifici, L.; Inchingolo, F. Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: Clinical and histological evaluations. Int. J. Med. Sci. 2012, 9, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Aalam, A.A.; Miron, R.J. Platelet Rich Fibrin “PRF” and Regenerative Medicine: ‘The Low-Speed Concept’. In Mscs and Innovative Biomaterials in Dentistry; Tatullo, M., Ed.; Humana Press Inc.: Totowa, NJ, USA, Stem Cell Biology and Regenerative Medicine; 2017; pp. 21–42. [Google Scholar]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. the CONSORT group 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Butz, F.; Baachle, M.; Ofer, M.; Marquardt, K.; Kohal, R.J. Sinus Augmentation with Bovine Hydroxyapatite/ Synthetic Peptide in a Sodium Hyaluronate Carrier (PepGen P-15 Putty): A Clinical Investigation of Different Healing Times. Int. J. Oral Maxillofac. Implant. 2011, 26, 1317–1323. [Google Scholar]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implant. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Vercellotti, T.; De Paoli, S.; Nevins, M. The Piezoelectric Bony Window Osteotomy and Sinus Membrane Elevation: Introduction of a New Technique for Simplification of the Sinus Augmentation Procedure. Int. J. Periodontics Restor. Dent. 2001, 21, 560–567. [Google Scholar]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Gundersen, H.; Boyce, R.; Nyengaard, J.; Odgaard, A. The connEulor: Unbiased estimation of connectivity using physical disectors under projection. Bone 1993, 14, 217–222. [Google Scholar] [CrossRef]
- Tatum Jr, O.H.; Lebowitz, M.S.; Tatum, C.A.; Borgner, R.A. Sinus augmentation. Rationale, development, long-term results. N. Y. State Dent. J. 1993, 59, 43–48. [Google Scholar]
- Schlegel, K.A.; Wiltfang, J.; Lutz, R.; Schmitt, C.; Most, T. Hard tissue augmentation-Material selection. Implantologie 2016, 24, 7–15. [Google Scholar]
- Al-Moraissi, E.; Alkhutari, A.; Abotaleb, B.; Altairi, N.; Del Fabbro, M. Do osteoconductive bone substitutes result in similar bone regeneration for maxillary sinus augmentation when compared to osteogenic and osteoinductive bone grafts? A systematic review and frequentist network meta-analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Danesh Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2016, 52, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gede, N.; Hegyi, P.; Szakács, Z.; Mezey, G.; Varga, E.; Kivovics, M.; Hanák, L.; Rumbus, Z.; Szabó, G. Relative performance of various biomaterials used for maxillary sinus augmentation. A Bayesian network meta-analysis. Clin. Oral Implant. Res. 2021, 32, 135–153. [Google Scholar] [CrossRef]
- Pichotano, E.C.; Molon, R.S.; Souza, R.V.; Austin, R.S.; Marcantonio, E.; Zandim-Barcelos, D.L. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 253–262. [Google Scholar] [CrossRef]
- Horváthy, D.B.; Simon, M.; Schwarz, C.M.; Masteling, M.; Vácz, G.; Hornyák, I.; Lacza, Z. Serum albumin as a local therapeutic agent in cell therapy and tissue engineering. Biofactors 2017, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Kivovics, M.; Szabó, B.T.; Németh, O.; Iványi, D.; Trimmel, B.; Szmirnova, I.; Orhan, K.; Mijiritsky, E.; Szabó, G.; Dobó-Nagy, C. Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft. J. Clin. Med. 2020, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J. Cranio-Maxillofac. Surg. 2012, 40, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, N.; Ersanli, S.; Keklikoglu, N.; Basegmez, C.; Ozdemir, T. Sinus Augmentation With Platelet-Rich Fibrin in Combination With Bovine Bone Graft Versus Bovine Bone Graft in Combination with Collagen Membrane. J. Oral Implant. 2015, 41, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.C.; Gungormus, M.; Parlak, S.N. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 959–967. [Google Scholar] [CrossRef]
- Nizam, N.; Eren, G.; Akcalı, A.; Donos, N. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: A split-mouth histological and histomorphometric study. Clin. Oral Implant. Res. 2018, 29, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Olgun, E.; Ozkan, S.Y.; Atmaca, H.T.; Yalim, M.; Hendek, M.K. Comparison of the clinical, radiographic, and histological effects of titanium-prepared platelet rich fibrin to allograft materials in sinus-lifting procedures. J. Investig. Clin. Dent. 2018, 9, e12347. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Herrera-Vizcaino, C.; Al-Maawi, S.; Lorenz, J.; Miron, R.J.; Nelson, K.; Schwarz, F.; Choukroun, J.; Sader, R. Fifteen Years of Platelet Rich Fibrin in Dentistry and Oromaxillofacial Surgery: How High is the Level of Scientific Evidence? J. Oral Implant. 2018, 44, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Krummenauer, F.; Büchter, A.; Kleinheinz, J.; Neukam, F.; Petrin, G.; Schlegel, K.A.; Weingart, D.; Wagner, W. Multicenter Randomized Clinical Trial: Early Loading of Implants in Maxillary Bone. Clin. Implant. Dent. Relat. Res. 2012, 15, 625–636. [Google Scholar] [CrossRef]
- Huang, H.-M.; Chee, T.-J.; Lew, W.-Z.; Feng, S.-W. Modified surgical drilling protocols influence osseointegration performance and predict value of implant stability parameters during implant healing process. Clin. Oral Investig. 2020, 24, 3445–3455. [Google Scholar] [CrossRef]
- Balleri, P.; Cozzolino, A.; Ghelli, L.; Momicchioli, G.; Varriale, A. Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading: A pilot study. Clin. Implant. Dent. Relat. Res. 2002, 4, 128–132. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontollgy 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Andersson, P.; Pagliani, L.; Verrocchi, D.; Volpe, S.; Sahlin, H.; Sennerby, L. Factors Influencing Resonance Frequency Analysis (RFA) Measurements and 5-Year Survival of Neoss Dental Implants. Int. J. Dent. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, G.; Eb, H. The clinical significance of implant stability quotient (ISQ) measurements: A literature review. J. Oral Biol. Craniofacial Res. 2020, 10, 629–638. [Google Scholar] [CrossRef]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.-P.; Haiat, G. Biomechanical determinants of the stability of dental implants: Influence of the bone–implant interface properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical behaviours of the bone–implant interface: A review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef] [PubMed]
| Abbreviation | Variable | Description | Standard Unit |
|---|---|---|---|
| BV/TV | Bone volume fraction | Relative volume of calcified tissue in the selected volume of interest (VOI). | % |
| BS/TV | Bone surface density | The ratio of surface area to total volume measured in 3D, within the VOI. | mm−1 |
| BS/BV | Bone surface / volume ratio | Surface to volume ratio of calcified tissue or “specific surface” is useful for characterising the complexity and thickness of structures. | mm−1 |
| Tb.Th | Trabecular thickness | Mean thickness of trabeculae, assessed using direct 3D methods. | mm |
| Tb.Sp | Trabecular separation | Trabecular separation is essentially the thickness of the spaces as defined by binarisation within the VOI. | mm |
| Tb.Pf | Trabecular bone pattern factor | This is an index of connectivity of trabecular bone; it calculates an index of relative convexity or concavity of the total bone surface, on the principle that concavity indicates connectivity (and the presence of “nodes”), and convexity indicates isolated disconnected structures (struts). | 1/mm |
| Po(tot) | Total porosity | Total porosity is the volume of all open plus closed pores as a percent of the total VOI volume. | % |
| Po(op) | Open porosity | Percent open porosity is the volume of open pores as a percent of the total VOI volume. | % |
| Conn. | Connectivity | One useful and fast algorithm for calculating the Euler connectivity in 3D is the “Conneulor”. It measures what might be called “redundant connectivity”, the degree to which parts of the object are multiply connected. It is a measure of how many connections in a structure can be severed before the structure falls into two separate pieces. | none |
| Patient | Age (years) | Gender | Maxillary Sinus | Residual Ridge Height | Maxillary Sinus Width | Healing Protocol | Implants Position (FDI) | Position of Bone Core Biopsy (FDI) |
|---|---|---|---|---|---|---|---|---|
| 1 | 41 | F | R | 4.7 mm | 13.9 mm | 6 months | 14, 16 | 16 |
| 2 | 43 | F | L | 4.2 mm | 14.8 mm | 6 months | 25, 26 | 26 |
| 3 | 54 | F | R | 4.8 mm | 13.8 mm | 6 months | 14, 16 | 16 |
| L | 4.5 mm | 13.4 mm | 3 months | 24, 26 | 26 | |||
| 4 | 63 | F | R | 2.2 mm | 14.1 mm | 3 months | 15, 16 | 16 |
| 5 | 56 | M | L | 4.6 mm | 16.2 mm | 3 months | 26 | 26 |
| 6 | 46 | F | R | 1.9 mm | 15.6 mm | 6 months | 16 | 16 |
| 7 | 60 | F | L | 3.8 mm | 12.7 mm | 6 months | 25, 26 | 26 |
| 8 | 51 | F | R | 2.3 mm | 14.7 mm | 3 months | 15, 16 | 15, 16 |
| 9 | 62 | F | L | 3.7 mm | 14.8 mm | 6 months | 25, 26 | 26 |
| 10 | 67 | M | R | 4.5 mm | 13.8 mm | 6 months | 17 | 17 |
| 11 | 63 | M | L | 1.6 mm | 16.5 mm | 3 months | 24, 26 | 26 |
| 12 | 60 | F | R | 3.3 mm | 11.8 mm | 6 months | 16 | 16 |
| 13 | 68 | F | R | 1.9 mm | 15.3 mm | 3 months | 14, 16 | 16 |
| 14 | 60 | M | L | 4.2 mm | 15.2 mm | 6 months | 26, 27 | 26, 27 |
| 15 | 56 | F | L | 3.9 mm | 14.5 mm | 3 months | 26 | 26 |
| 16 | 63 | F | R | 1.9 mm | 14.6 mm | 6 months | 15, 16 | 16 |
| L | 2.5 mm | 14.7 mm | 3 months | 24, 26 | 26 | |||
| 17 | 61 | M | L | 1.2 mm | 15.2 mm | 3 months | 26 | 26 |
| 18 | 46 | M | R | 3.1 mm | 14.9 mm | 3 months | 14, 17 | 17 |
| L | 3.4 mm | 15.2 mm | 6 months | 24, 26 | 26 | |||
| 19 | 41 | F | L | 4.2 mm | 15.7 mm | 3 months | 26, 27 | 26 |
| 20 | 68 | F | L | 4.1 mm | 15.5 mm | 6 months | 15, 17 | 17 |
| R | 3.3 mm | 14.6 mm | 3 months | 24, 27 | 27 | |||
| 21 | 53 | F | R | 1.7 mm | 16.9 mm | 6 months | 15, 16 | 16 |
| 22 | 54 | M | R | 2.4 mm | 15.9 mm | 3 months | 15, 16 | 16 |
| 23 | 61 | M | L | 1.9 mm | 15.8 mm | 3 months | 25, 26 | 25, 26 |
| 24 | 56 | M | R | 2.4 mm | 16.8 mm | 6 months | 15, 16 | 16 |
| 25 | 64 | F | R | 4.3 mm | 14.4 mm | 3 months | 16 | 16 |
| 26 | 51 | F | L | 3.6 mm | 13.2 mm | 6 months | 26, 27 | 26, 27 |
| Group | N | Mean | Std. Deviation | p-Value | |
|---|---|---|---|---|---|
| ISQ week 0 | test | 24 | 68.92 | 7.56 | 0.105 # |
| control | 25 | 72.20 | 6.30 | ||
| ISQ week 6 | test | 25 | 68.52 | 7.35 | 0.003## |
| control | 24 | 74.22 | 4.52 | ||
| ISQ week 8 | test | 26 | 72.00 | 7.16 | 0.041## |
| control | 25 | 75.70 | 4.76 | ||
| ISQ week 10 | test | 26 | 74.26 | 5.79 | 0.501 ## |
| control | 24 | 75.74 | 4.93 | ||
| ISQ week 12 | test | 26 | 75.96 | 4.75 | 0.345 ## |
| control | 24 | 76.96 | 4.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trimmel, B.; Gyulai-Gaál, S.; Kivovics, M.; Jákob, N.P.; Hegedűs, C.; Szabó, B.T.; Dobó-Nagy, C.; Szabó, G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials 2021, 14, 1810. https://doi.org/10.3390/ma14071810
Trimmel B, Gyulai-Gaál S, Kivovics M, Jákob NP, Hegedűs C, Szabó BT, Dobó-Nagy C, Szabó G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials. 2021; 14(7):1810. https://doi.org/10.3390/ma14071810
Chicago/Turabian StyleTrimmel, Bálint, Szabolcs Gyulai-Gaál, Márton Kivovics, Noémi Piroska Jákob, Csaba Hegedűs, Bence Tamás Szabó, Csaba Dobó-Nagy, and György Szabó. 2021. "Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial" Materials 14, no. 7: 1810. https://doi.org/10.3390/ma14071810
APA StyleTrimmel, B., Gyulai-Gaál, S., Kivovics, M., Jákob, N. P., Hegedűs, C., Szabó, B. T., Dobó-Nagy, C., & Szabó, G. (2021). Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials, 14(7), 1810. https://doi.org/10.3390/ma14071810








