Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review
Abstract
1. Introduction
2. Innovative Process: Sol-Gel



3. Hybrid Materials
3.1. Hybrid Materials: Characterization Performed According to a Multi-Technique Approach
3.2. Hybrid Materials: Thermal Characterization
3.3. Hybrid Materials: Biomedical Applications
4. Preparation and Characterization Details of Hybrid Materials
4.1. Synthesis by Sol-Gel Route of Hybrid Materials
4.2. Coupling of FTIR and TG Measurements for the Assessment of Thermal Decomposition Steps
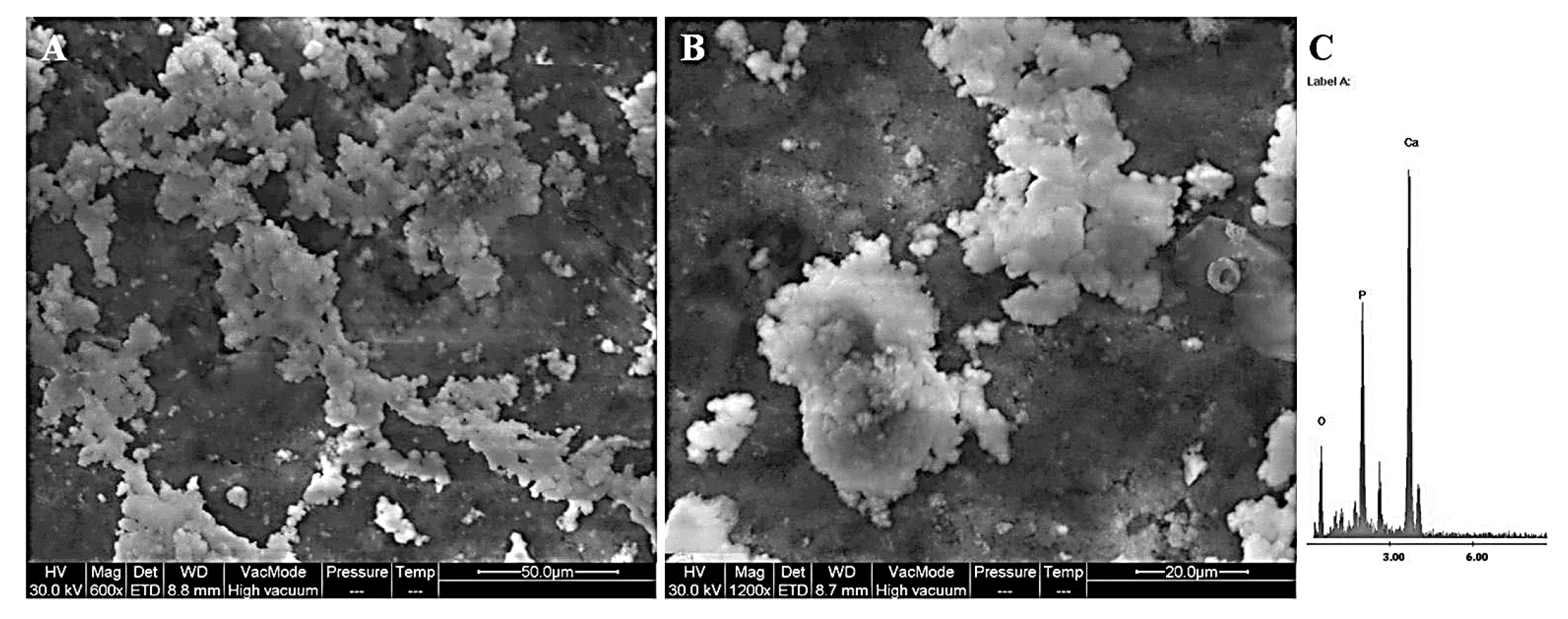
4.3. Structural and Morphological Characterization
4.4. Bioactivity Tests
4.5. Antiradical Capacity
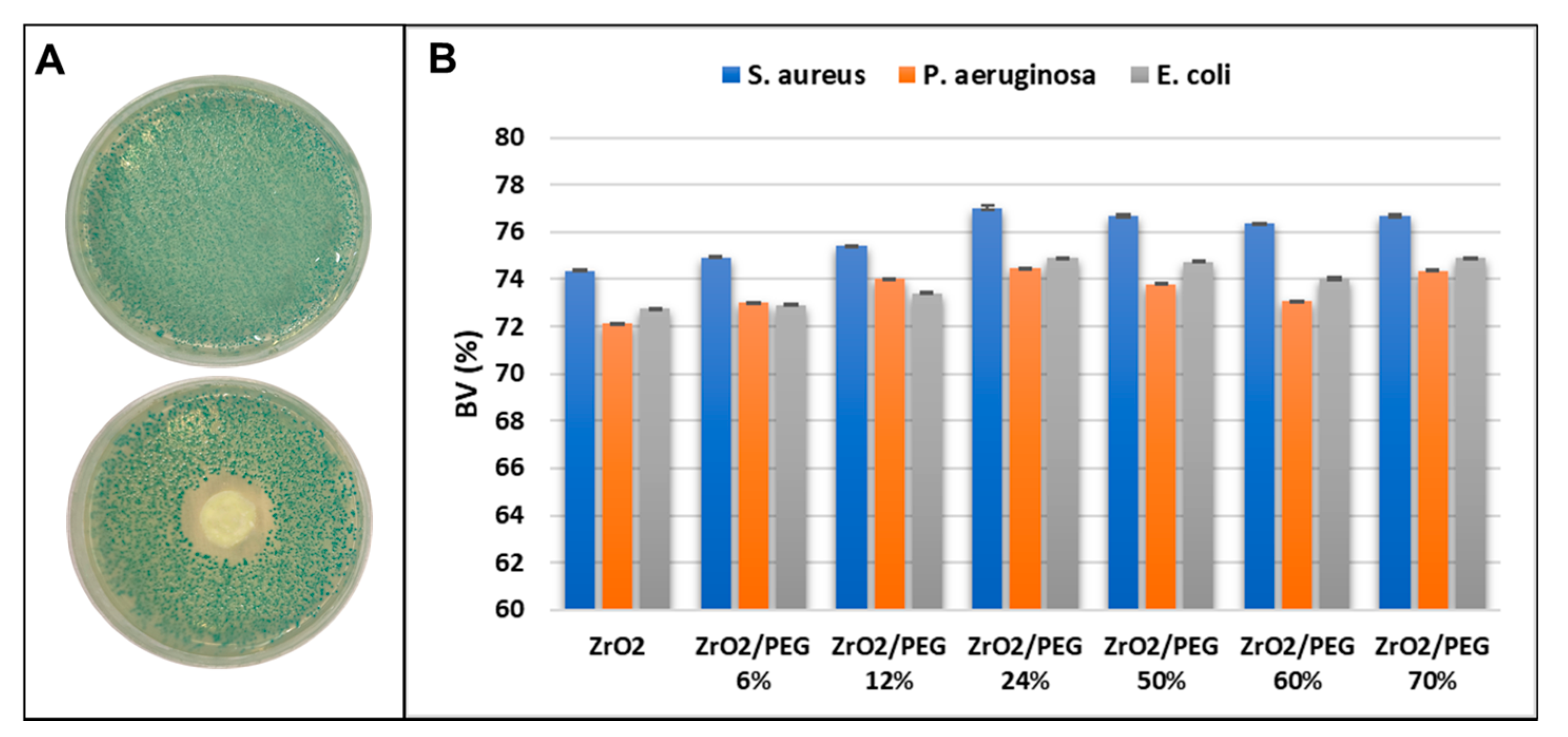
4.6. Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A revies of phatogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, M.J.; Jablonowski, L.J.; Parvizi, J.; Freeman, T.A. The role of oxidative stress in aseptic loosening of total hip arthroplasties. J. Arthroplasty 2014, 29, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, G.M.; Varaprasad, K.; Jayaramudu, T. Chapter 2—Biomaterials: Design, Development and Biomedical Applications A2—Thomas, Sabu. In Nanotechnology Applications for Tissue Engineering; Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 21–44. [Google Scholar] [CrossRef]
- Bizari, D.; Rabiee, M.; Moztarzadeh, F.; Tahriri, M.; Alavi, S.H.; Masaeli, R. Synthesis, characterization and biological evaluation of sol-gel derived nanomaterial in the ternary system 64 % SiO2—31 % CaO—5 % P2O5 as a bioactive glass: In vitro study. Ceram. Silikáty 2013, 57, 201–209. [Google Scholar]
- Li, H.C.; Wang, D.G.; Hu, J.H.; Chen, C.Z. Influence of fluoride additions on biological and mechanical properties of Na2O-CaO-SiO2-P2O5 glass-ceramics. Mater. Sci. Eng. C 2014, 35, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosth etic materials. J. Biomed. Mater. Res. 1972, 2, 117–141. [Google Scholar] [CrossRef]
- Radev, L.; Hristov, V.; Michailova, I.; Fernandes, H.M.V.; Salvado, M.I.M. In vitro bioactivity of biphasic calcium phosphate silicate glass-ceramic in CaO-SiO2-P2O5 system. Process. Appl. Ceram. 2010, 4, 15–24. [Google Scholar] [CrossRef]
- Owens, J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.; Mahapatra, C.; Kim, H.; Jonathan, C.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef]
- Brinker, C.; Scherer, G. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Zhao, L.; Wu, Y.; Chen, S.; Xing, T. Preparation and characterization of cross-linked carboxymethyl chitin porous membrane scaffold for biomedical applications. Carbohydr. Polym. 2015, 126, 150–155. [Google Scholar] [CrossRef]
- Chellappa, M.; Thejaswini, B.; Vijayalakshmi, U. Biocompatibility assessment of SiO2-TiO2 composite powder on MG63 osteoblast cell lines for orthopaedic applications. IET Nanobiotechnol. 2017, 11, 77–82. [Google Scholar] [CrossRef]
- Montazerian, M.; Yekta, B.E.; Marghussian, V.K.; Bellani, C.F.; Siqueira, R.L.; Zanotto, E.D. Bioactivity and cell proliferation in radiopaque gel-derived CaO–P2O5–SiO2–ZrO2 glass and glass–ceramic powders. Mater. Sci. Eng. C 2015, 55, 436–447. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Nahrawy, A.M.; Hammad, A.B.A. Sol-gel synthesis and characterizations of hybrid chitosan-PEG/calcium silicate nanocomposite modified with ZnO-NPs and (E102) for optical and antibacterial applications. Int. J. Biol. Macromol. 2017, 97, 561–567. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Marciano, S.; Pacifico, S. TiO2/PCL hybrid materials synthesized via sol-gel technique for biomedical applications. Mater. Sci. Eng. C 2015, 47, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Giovanardi, R.; Veronesi, P. Corrosion behavior and mechanical properties of bioactive sol-gel coatings on titanium implants. Mater. Sci. Eng. C 2014, 43, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Bollino, F. Coatings of titanium substrates with xCaO(1−x)SiO2 sol-gel materials: Characterization, bioactivity and biocompatibility evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Bollino, F.; Gallicchio, M.; Pacifico, S. Biological evaluation of zirconia/PEG hybrid materials synthesized via sol-gel technique. Mater. Sci. Eng. C 2014, 40, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Vichery, C.; Nedelec, J.-M. Bioactive glass nanoparticles: From synthesis to materials design for biomedical applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef]
- Kumar, A.; Murugavel, S.; Aditya, A.; Boccaccini, A.R. Mesoporous 45S5 bioactive glass: Synthesis, in vitro dissolution and biomineralization behavior. J. Mater. Chem. B 2017, 5, 8786–8798. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Mozetic, P.; Rainer, A.; Trombetta, M. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol-gel route: In vitro evaluation. Mater. Sci. Eng. C 2014, 45, 395–401. [Google Scholar] [CrossRef]
- Midha, S.; Kim, T.B.; van den Bergh, W.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Preconditioned 70S30C bioactive glass foams promote osteogenesis in vivo. Acta Biomater. 2013, 9, 9169–9182. [Google Scholar] [CrossRef] [PubMed]
- Khamsehashari, N.; Hassanzadeh-Tabrizi, S.; Bigham, A. Effects of strontium adding on the drug delivery behavior of silica nanoparticles synthesized by P123-assisted sol-gel method. Mater. Chem. Phys. 2018, 205, 283–291. [Google Scholar] [CrossRef]
- Imam, S.S.; Bukhari, S.N.A.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lin, C.; Zhong, W. Sol-gel derived terbium-containing mesoporous bioactive glasses nanospheres: In vitro hydroxyapatite formation and drug delivery. Colloids Surf. B Biointerfaces 2017, 160, 406–415. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Pacifico, S. Modulation of indomethacin release from ZrO2/PCL hybrid multilayers synthesized via sol-gel dip coating. J. Drug Deliv. Sci. Technol. 2015, 26, 10–16. [Google Scholar] [CrossRef]
- Jiménez-Flores, Y.; Suárez-Quezada, M.; Rojas-Trigos, J.B.; Lartundo-Rojas, L.; Suárez, V.; Mantilla, A. Characterization of Tb-doped hydroxyapatite for biomedical applications: Optical properties and energy band gap determination. J. Mater. Sci. 2017, 52, 1–11. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Okilova, M.V.; Tcvetkov, N.Y.; Shakirova, A.I.; Afanasyev, B.V.; Gorin, D.A.; Sukhrukov, G.B. Intracellular Breakable and Ultrasound-Responsive Hybrid Microsized Containers for Selective Drug Release into Cancerous Cells. Part. Part. Syst. Charact. 2017, 34, 1600417. [Google Scholar] [CrossRef]
- De Oliveira, L.F.; Bouchmella, K.; Goncalves, K.A.; Bettini, J.; Kobarg, J.; Cardoso, M.B. Functionalized Silica Nanoparticles As an Alternative Platform for Targeted Drug-Delivery of Water Insoluble Drugs. Langmuir 2016, 32, 3217–3225. [Google Scholar] [CrossRef]
- Naghibi, S.; Madaah Hosseini, H.R.; Faghihi Sani, M.A.; Shokrgozar, M.A.; Mehrjoo, M. Mortality response of folate receptor-activated, PEG-functionalized TiO2 nanoparticles for doxorubicin loading with and without ultraviolet irradiation. Ceram. Int. 2014, 40, 5481–5488. [Google Scholar] [CrossRef]
- Schubert, U. Chemistry and fundamentals of the sol-gel process. In The Sol-Gel Handbook-Synthesis, Characterization and Applications: Synthesis; Academic Press: San Diego, CA, USA, 2015; Volume 3, pp. 1–28. [Google Scholar]
- Milea, C.; Bogatu, C.; Duta, A. The influence of parameters in silica sol-gel process. Bull. Transilv. Univ. Bras. 2011, 4, 53. [Google Scholar]
- Ogoshi, T.; Chujo, Y. Organic–inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces 2005, 11, 539–566. [Google Scholar] [CrossRef]
- Pérez-Fonseca, A.A.; Robledo-Ortíz, J.R.; Ramirez-Arreola, D.E.; Ortega-Gudiño, P.; Rodrigue, D.; González-Núñez, R. Effect of hybridization on the physical and mechanical properties of high density polyethylene–(pine/agave) composites. Mater. Des. 2014, 64, 35–43. [Google Scholar] [CrossRef]
- Gomez-Romero, P. Hybrid organic–inorganic materials—in search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Wang, G.-H.; Zhang, L.-M. Using Novel Polysaccharide-Silica Hybrid Material to Construct An Amperometric Biosensor for Hydrogen Peroxide. J. Phys. Chem. B 2006, 110, 24864–24868. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.M.; Vasconcelos, W.L.; Mansur, H.S.; Pereira, M.M. Synthesis and Characterization of Silica-Chitosan Porous Hybrids for Tissue Engineering. J. Key Eng. Mater. 2008, 361–363, 967–970. [Google Scholar] [CrossRef]
- Jose, S.K.; George, A.; Jose, A.; Joseph, C.; Unnikrishnan, N.V.; Biju, P.R. Structural and luminescence characterization of Eu3+/ZnS nanoparticle-doped ZrO2/PEG composites. J. Mater. Sci. Mater. Electron. 2021, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Ahumada, D.; Verastica-Ward, L.J.; Orozco, M.; Vargas-Hernández, D.; Castro-Beltrán, A.; Ramirez-Bon, R.; Alvarado-Beltrán, C.G. In-situ low-temperature synthesis of PS-ZrO2 hybrid films and their characterization for high-k gate dielectric application. Prog. Org. Coat. 2021, 154, 106188. [Google Scholar] [CrossRef]
- Sun, Y.; Mao, Y.; Zhang, C.; Di, N.; Qi, D.; Shentu, B. Thermodynamics and kinetics of Cu2+ adsorption of organic-inorganic hybrid hollow mesoporous silica spheres. J. Sol-Gel Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Evstatieva, Y.; Yordanova, M.; Chernev, G.; Ruseva, Y.; Nikolova, D. Sol-gel immobilization as a suitable technique for enhancement of α-amylase activity of Aspergillus oryzae PP. Biotechnol. Biotechnol. Equip. 2014, 28, 724–732. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, V.P.; Salta, M.M. Alcohol-Aminosilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Mortar. J. Electrochem. Soc. 2014, 161, 349–362. [Google Scholar] [CrossRef]
- Xue, J.M.; Shi, M. PLGA/mesoporous silica hybrid structure for controlled drug release. J. Control. Release 2004, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Krasia-Christoforou, T. Organic–inorganic polymer hybrids: Synthetic strategies and applications. In Hybrid and Hierarchical Composite Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–63. [Google Scholar]
- Castrillo, P.; Olmos, D.; Amador, D.; González-Benito, J. Real dispersion of isolated fumed silica nanoparticles in highly filled PMMA prepared by high energy ball milling. J. Colloid Interface Sci. 2007, 308, 318–324. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol-gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Zadeh, M.A.; Van Der Zwaag, S.; Garcia, S. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: A review. Self-Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef]
- Druart, M.E.; Recloux, I.; Thai, T.T.; Ershov, S.; Snyders, R.; Olivier, M.G. Impact of the addition of cerium salts (Ce(III) and Ce(IV)) on formation and ageing of a silica sol-gel layer. Surf. Coat. Technol. 2016, 304, 40–50. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, T.; Akiyama, K.; Hakozaki, T.; Shino, M.; Adachi, K. Synthesis of cellulose/silica gel polymer hybrids via in-situ hydrolysis method. Polym. Bull. 2017, 74, 4997–5009. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol− gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Loy, D.A.; Shea, K.J. Bridged polysilsesquioxanes. Highly porous hybrid organic-inorganic materials. Chem. Rev. 1995, 95, 1431–1442. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Rejab, M.R.; Hamdan, M.H.; Quanjin, M.; Siregar, J.P.; Bachtiar, D.; Muchlis, Y. Historical Development of Hybrid Materials. Mat. Sci. Mat. Eng. 2020, 4, 445–455. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Mozzati, M.C.; Ferrara, C.; Mustarelli, P. ZrO2/PEG hybrid nanocomposites synthesized via sol-gel: Characterization and evaluation of the magnetic properties. J. Non-Cryst. Solids 2015, 413, 1–7. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Veronesi, P.; Lamanna, G. Influence of PCL on mechanical properties and bioactivity of ZrO2-based hybrid coatings synthesized by sol-gel dip coating technique. Mater. Sci. Eng. C 2014, 39, 344–351. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Bioencapsulation within synthetic polymers (Part 1): Sol-gel encapsulated biologicals. Trends Biotechnol. 2000, 18, 282–296. [Google Scholar] [CrossRef]
- Wojcik, A.; Klein, L. Organic-inorganic gels based on silica and multifunctional acrylates. J. Sol-Gel Sci. Technol. 1994, 2, 115–120. [Google Scholar] [CrossRef]
- Gill, I. Bio-doped nanocomposite polymers: Sol−gel bioencapsulates. Chem. Mater. 2001, 13, 3404–3421. [Google Scholar] [CrossRef]
- Sur, G.; Mark, J. Elastomeric networks cross-linked by silica or titania fillers. Eur. Polym. J. 1985, 21, 1051–1052. [Google Scholar] [CrossRef]
- Uilk, J.M.; Mera, A.E.; Fox, R.B.; Wynne, K.J. Hydrosilation-cured poly (dimethylsiloxane) networks: Intrinsic contact angles via dynamic contact angle analysis. Macromolecules 2003, 36, 3689–3694. [Google Scholar] [CrossRef]
- Zanoaga, M.; Tanasa, F. Antimicrobial reagents as functional finishing for textiles intended for biomedical applications. I. Synthetic organic compounds. Chem. J. Mold. 2014, 9, 14–32. [Google Scholar] [CrossRef]
- Atal, M.K.; Saini, A.; Jat, S.K.; Rathore, K.S.; Dhayal, V. Synthesis and characterization of oxime-modified phenylimido vanadium(V) isopropoxide and their hydrolytic study. J. Sol-Gel Sci. Technol. 2017, 83, 281–290. [Google Scholar] [CrossRef]
- Lee, E.J.; Huh, B.K.; Kim, S.N.; Lee, J.Y.; Park, C.G.; Mikos, A.G.; Choy, Y.B. Application of materials as medical devices with localized drug delivery capabilities for enhanced wound repair. Prog. Mater. Sci. 2017, 89, 92–410. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Mozzati, M.C.; Ferrara, C.; Mustarelli, P. Structure and magnetic properties of SiO2/PCL novel sol-gel organic-hybrid materials. J. Solid State Chem. 2013, 203, 92–99. [Google Scholar] [CrossRef]
- Catauro, M.; Scolaro, C.; Dal Poggetto, G.; Pacifico, S.; Visco, A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers 2020, 12, 978. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Ryczkowski, J.; Pasieczna-Patkowska, S.; Schroeder, G. Photoacoustic infrared spectroscopic studies of silica gels with organically functionalized surface. Spectrosc. Lett. 2016, 49, 529–534. [Google Scholar] [CrossRef]
- Akhter, T.; Saeed, S.; Siddiqi, H.M.; Park, O.O.; Ali, G. Synthesis and characterization of novel coatable polyimide-silica nanocomposites. J. Polym. Res. 2013, 21, 332. [Google Scholar] [CrossRef]
- Catauro, M.; Dell’Era, A.; Vecchio Ciprioti, S. Synthesis, structural, spectroscopic and thermoanalytical study of sol-gel derived SiO2-CaO-P2O5 gel and ceramic materials. Thermochim. Acta 2016, 625, 20–27. [Google Scholar] [CrossRef]
- Hong, S.-G.; Huang, S.-C. Crystallization properties of polyhydroxybutyrate with modified silicas. J. Polym. Res. 2015, 22, 61. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Su, Q.; Zheng, J. Use of unmodified SiO2 as nanofiller to improve mechanical properties of polymer-based nanocomposites. Compos. Sci. Technol. 2013, 89, 52–60. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Khan, A.; Asiri, A.M.; Rub, M.A.; Azum, N.; Khan, A.A.P.; Khan, S.B.; Rahman, M.M.; Khan, I. Synthesis, characterization of silver nanoparticle embedded polyaniline tungstophosphate-nanocomposite cation exchanger and its application for heavy metal selective membrane. Compos. Part B Eng. 2013, 45, 1486–1492. [Google Scholar] [CrossRef]
- Mohseni, M.; Bastani, S.; Jannesari, A. Influence of silane structure on curing behavior and surface properties of sol-gel based UV-curable organic–inorganic hybrid coatings. Prog. Org. Coat. 2014, 77, 1191–1199. [Google Scholar] [CrossRef]
- Rahimi, H.; Mozaffarinia, R.; Hojjati Najafabadi, A. Corrosion and Wear Resistance Characterization of Environmentally Friendly Sol-gel Hybrid Nanocomposite Coating on AA5083. J. Mater. Sci. Technol. 2013, 29, 603–608. [Google Scholar] [CrossRef]
- Qian, X.; Song, L.; Hu, Y.; Yuen, R.K.K. Thermal degradation and flammability of novel organic/inorganic epoxy hybrids containing organophosphorus-modified oligosiloxane. Thermochim. Acta 2013, 552, 87–97. [Google Scholar] [CrossRef]
- Brusciotti, F.; Snihirova, D.V.; Xue, H.; Montemor, M.F.; Lamaka, S.V.; Ferreira, M.G.S. Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar] [CrossRef]
- Oberdisse, J.; Hellweg, T. Structure, interfacial film properties, and thermal fluctuations of microemulsions as seen by scattering experiments. Adv. Colloid. Interface Sci. 2017, 247, 354–362. [Google Scholar] [CrossRef]
- Samet, L.; Ben Nasseur, J.; Chtourou, R.; March, K.; Stephan, O. Heat treatment effect on the physical properties of cobalt doped TiO2 sol-gel materials. Mater. Charact. 2013, 85, 1–12. [Google Scholar] [CrossRef]
- Yaghtin, M.; Taghvaei, A.H.; Hashemi, B.; Janghorban, K. Effect of heat treatment on magnetic properties of iron-based soft magnetic composites with Al2O3 insulation coating produced by sol-gel method. J. Alloys Compd. 2013, 581, 293–297. [Google Scholar] [CrossRef]
- Boukerika, A.; Guerbous, L. Annealing effects on structural and luminescence properties of red Eu3+-doped Y2O3 nanophosphors prepared by sol-gel method. J. Lumin. 2014, 145, 148–153. [Google Scholar] [CrossRef]
- Shao, G.N.; Imran, S.M.; Jeon, S.J.; Engole, M.; Abbas, N.; Salman Haider, M.; Kang, S.J.; Kim, H.T. Sol-gel synthesis of photoactive zirconia–titania from metal salts and investigation of their photocatalytic properties in the photodegradation of methylene blue. Powder Technol. 2014, 258, 99–109. [Google Scholar] [CrossRef]
- Scalera, F.; Gervaso, F.; Sanosh, K.P.; Sannino, A.; Licciulli, A. Influence of the calcination temperature on morphological and mechanical properties of highly porous hydroxyapatite scaffolds. Ceram. Int. 2013, 39, 4839–4846. [Google Scholar] [CrossRef]
- Bollino, F.; Armenia, E.; Tranquillo, E. Zirconia/hydroxyapatite composites synthesized via sol-gel: Influence of hydroxyapatite content and heating on their biological properties. Materials 2017, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Renella, R.A.; Papale, F. Sol-gel synthesis of SiO2-CaO-P2O5 glasses: Influence of the heat treatment on their bioactivity and biocompatibility. Ceram. Int. 2015, 41, 12578–12588. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol-gel method: Dissolution behaviour and biological properties after crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef]
- Catauro, M.; Renella, R.; Papale, F.; Ciprioti Vecchio, S. Investigation of bioactivity, biocompatibility and thermal behavior of sol-gel silica glass containing a high PEG percentage. Mater. Sci. Eng. C 2016, 61, 51–55. [Google Scholar] [CrossRef]
- Kumar, R.; Münstedt, H. Polyamide/silver antimicrobials: Effect of crystallinity on the silver ion release. Polym. Int. 2005, 54, 1180–1186. [Google Scholar] [CrossRef]
- Mavropoulos, E.; Costa, A.M.; Costa, L.T.; Achete, C.A.; Mello, A.; Granjeiro, J.M.; Rossi, A.M. Adsorption and bioactivity studies of albumin onto hydroxyapatite surface. Colloids Surf. B Biointerfaces 2011, 83, 1–9. [Google Scholar] [CrossRef]
- Radev, L. Influence of thermal treatment on the structure and in vitro bioactivity of sol-gel prepared CaO-SiO2-P2O5 glass-ceramics. Process. Appl. Ceram. 2014, 8, 155–166. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Dell’Era, A.; Vecchio Ciprioti, S. Pure Al2O3·2SiO2 synthesized via a sol-gel technique as a raw material to replace metakaolin: Chemical and structural characterization and thermal behavior. Ceram. Int. 2016, 42, 16303–16309. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Vecchio Ciprioti, S. Investigation on bioactivity, biocompatibility, thermal behavior and antibacterial properties of calcium silicate glass coatings containing Ag. J. Non-Cryst. Solids 2015, 422, 16–22. [Google Scholar] [CrossRef]
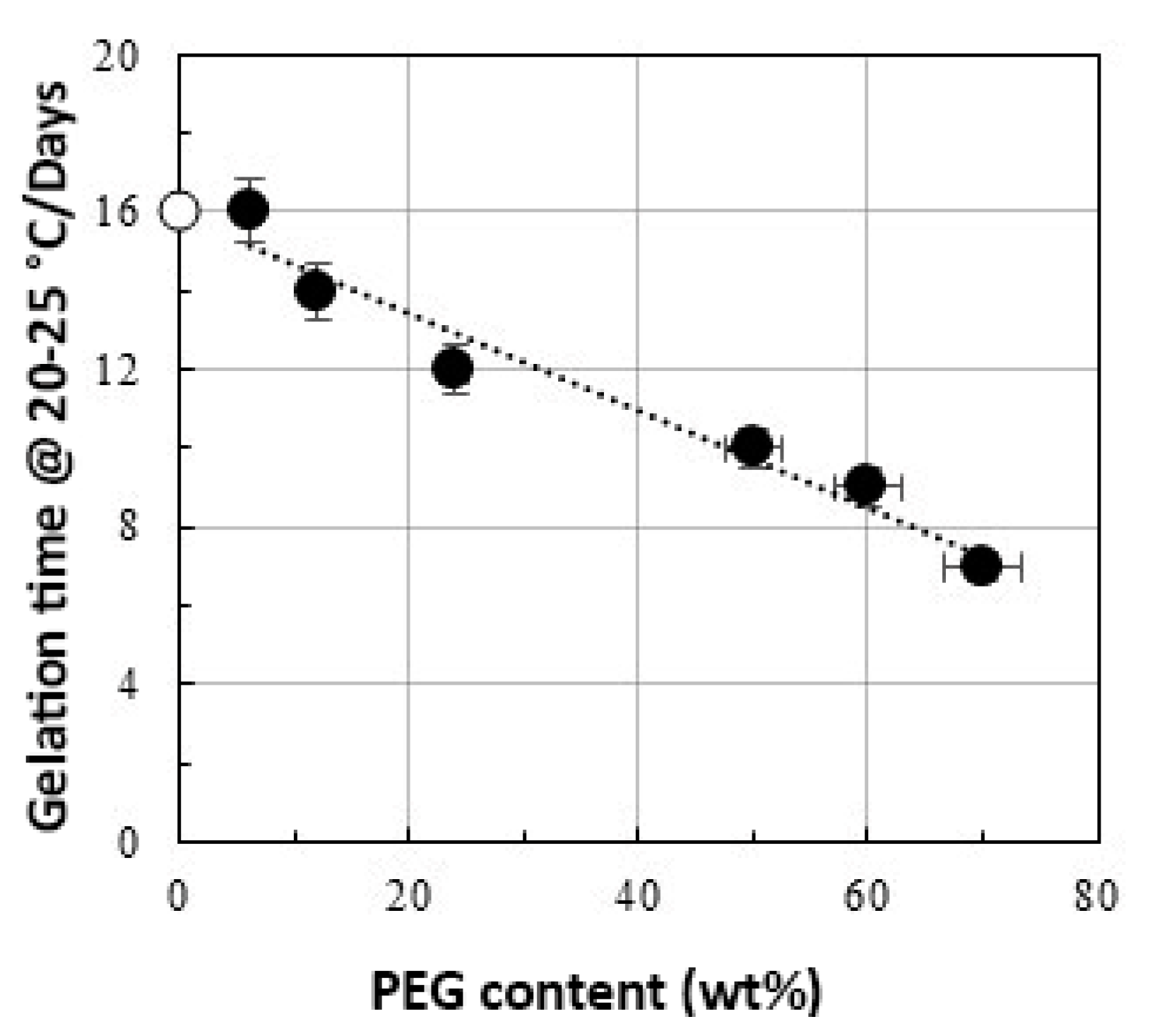
- Vecchio Ciprioti, S.; Bollino, F.; Tranquillo, E.; Catauro, M. Synthesis, thermal behavior and physicochemical characterization of ZrO2/PEG inorganic/organic hybrid materials via sol-gel technique. J. Therm. Anal. Calorim. 2017, 130, 535–540. [Google Scholar] [CrossRef]
- Vecchio Ciprioti, S.; Catauro, M. Synthesis, structural and thermal behavior study of four Ca-containing silicate gel-glasses: Activation energies of their dehydration and dehydroxylation processes. J. Therm. Anal. Calorim. 2016, 123, 2091–2101. [Google Scholar] [CrossRef]
- Vecchio Ciprioti, S.; Catauro, M.; Bollino, F.; Tuffi, R. Thermal behavior and dehydration kinetic study of SiO2/PEG hybrid gel glasses. Polym. Eng. Sci. 2017, 57, 606–612. [Google Scholar] [CrossRef]
- De Angelis Curtis, S.; Kubiak, M.; Kurdziel, K.; Materazzi, S.; Vecchio, S. Crystal structure and thermoanalytical study of a cadmium(II) complex with 1-allylimidazole. J. Anal. Appl. Pyrol. 2010, 87, 175–179. [Google Scholar] [CrossRef]
- De Angelis Curtis, S.; Kurdziel, K.; Materazzi, S.; Vecchio, S. Crystal structure and thermoanalytical study of cobalt(II) and nickel(II) complexes with 2,2′-bis-(4,5-dimethylimidazole). Thermochim. Acta 2010, 510, 75–81. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S.; Wo, L.W.; De Angelis Curtis, S. Thermoanalytical studies of imidazole-substituted coordination compounds: Mn(II)-complexes of bis(1-methylimidazol-2-yl)ketone. J. Therm. Anal. Calorim. 2011, 103, 59–64. [Google Scholar] [CrossRef]
- Materazzi, S.; Vecchio, S.; Wo, L.W.; De Angelis Curtis, S. TG-MS and TG-FTIR studies of imidazole-substituted coordination compounds: Co(II) and Ni(II)-complexes of bis(1-methylimidazol-2-yl)ketone. Thermochim. Acta 2012, 543, 183–187. [Google Scholar] [CrossRef]
- Meng, S.; Mansouri, J.; Ye, Y.; Chen, V. Effect of templating agents on the properties and membrane distillation performance of TiO2-coated PVDF membranes. J. Membr. Sci. 2014, 450, 48–59. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Z.; Bai, Y.; Zhou, Y.; Chen, X. Effective Control of Enzyme Activity Based on a Subtle Nanoreactor: A Promising Strategy for Biomedical Applications in the Future. ACS Appl. Nano. Mater. 2018, 1, 302–309. [Google Scholar] [CrossRef]
- Savage, T.J.; Dunphy, D.R.; Harbaugh, S.; Kelley-Loughnane, N.; Harper, J.C.; Brinker, C.J. Influence of Silica Matrix Composition and Functional Component Additives on the Bioactivity and Viability of Encapsulated Living Cells. ACS Biomater. Sci. Eng. 2015, 1, 1231–1238. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.P. Optical properties of functional hybrid organic–inorganic nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Gill, J.; Orsat, V.; Kermasha, S. Optimization of encapsulation of a microbial laccase enzymatic extract using selected matrices. Process. Biochem. 2018, 65, 55–61. [Google Scholar] [CrossRef]
- Barbosa, A.D.S.; Silva, M.A.D.O.; Carvalho, N.B.; Mattedi, S.; Iglesias, M.A.; Fricks, A.T.; Lima, Á.S.; Franceschi, E.; Soares, C.M.F. Immobilization of lipase by encapsulation in silica aerogel. Química Nova 2014, 37, 969–976. [Google Scholar] [CrossRef]
- Das, T.K.; Khan, I.; Rousseau, D.L.; Friedman, J.M. Preservation of the native structure in myoglobin at low pH by sol−gel encapsulation. J. Am. Chem. Soc. 1998, 120, 10268–10269. [Google Scholar] [CrossRef]
- Yang, H.-H.; Zhu, Q.-Z.; Qu, H.-Y.; Chen, X.-L.; Ding, M.-T.; Xu, J.-G. Flow injection fluorescence immunoassay for gentamicin using sol-gel-derived mesoporous biomaterial. Anal. Biochem. 2002, 308, 71–76. [Google Scholar] [CrossRef]
- Nadgir, M.M.; Coffey, A.; Murari, B.M. Modified sol-gel processed silica matrix for gel electrophoresis applications. J. Sol-Gel Sci. Technol. 2017, 83, 55–164. [Google Scholar] [CrossRef]
- Wang, C.; He, C.; Tong, Z.; Liu, X.; Ren, B.; Zeng, F. Combination of adsorption by porous CaCO3 microparticles and encapsulation by polyelectrolyte multilayer films for sustained drug delivery. Int. J. Pharm. 2006, 308, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Bhattacharyya, S.; Ducheyne, P. Silicon oxide based materials for controlled release in orthopedic procedures. Adv. Drug Deliv. Rev. 2015, 94, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Naghipoor, J.; Rabczuk, T. A mechanistic model for drug release from PLGA-based drug eluting stent: A computational study. Comput. Biol. Med. 2017, 90, 15–22. [Google Scholar] [CrossRef]
- Grassi, M.; Grassi, G. Mathematical modelling and controlled drug delivery: Matrix systems. Curr. Drug. Deliv. 2005, 2, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Kiwilsza, A.; Milanowski, B.; Druzbicki, K.; Jenczyk, J.; Jarek, M.; Mielcarek, J.; Lulek, J.; Pajzderska, A.; Wasicki, J. Molecular dynamics and the dissolution rate of nifedipine encapsulated in mesoporous silica. Micropor. Mesopor. Mater. 2017, 250, 186–194. [Google Scholar] [CrossRef]
- Kempahanumakkagari, S.; Kumar, V.; Samaddar, P.; Kumar, P.; Ramakrishnappa, T.; Kim, K.-H. Biomolecule-embedded metal-organic frameworks as an innovative sensing platform. Biotechnol. Adv. 2018, 36, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide–drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 112–126. [Google Scholar] [CrossRef]
- Hakeem, A.; Zahid, F.; Zhan, G.; Yi, P.; Yang, H.; Gan, L.; Yang, X. Polyaspartic acid-anchored mesoporous silica nanoparticles for pH-responsive doxorubicin release. Int. J. Nanomed. 2018, 13, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple magnetite nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence imaging, and drug delivery. J. Am. Chem. Soc. 2009, 132, 552–557. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Blumen, S.R.; Cheng, K.; MacPherson, M.B.; Alexeeva, V.; Lathrop, S.A.; Beuschel, S.L.; Steinbacher, J.L.; Butnor, K.J.; Ramos-Niño, M.E. Increased efficacy of doxorubicin delivered in multifunctional microparticles for mesothelioma therapy. Int. J. Cancer. 2011, 129, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, D.; Bravman, J.C.; Rothbard, J.; Klein, R.L. Bioactive Material Delivery Systems Comprising Sol-Gel Compositions. US Patent Application 20070071789, 16 August 2007. [Google Scholar]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef]
- Zhuravlev, L. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Tiwari, A.; Raj, B. Reactions and Mechanisms in Thermal Analysis of Advanced Materials; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Georgieva, I.; Danchova, N.; Gutzov, S.; Trendafilova, N. DFT modeling, UV-Vis and IR spectroscopic study of acetylacetone-modified zirconia sol-gel materials. J. Mol. Model. 2012, 18, 2409–2422. [Google Scholar] [CrossRef] [PubMed]
- Karakhanov, E.; Kardasheva, Y.S.; Maksimov, A.; Predeina, V.; Runova, E.; Utukin, A. Macrocomplexes on the basis of functionalized polyethylene glycols and copolymers of ethylene oxide and propylene oxide: Synthesis and catalysis. J. Mol. Catal. A Chem. 1996, 107, 235–240. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Catauro, M.; Bollino, F.; Papale, F. Biocompatibility improvement of titanium implants by coating with hybrid materials synthesized by sol-gel technique. J. Biomed. Mater. Res. A 2014, 102, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and characterization of ZrO2 nanoparticles-antimicrobial activity and their prospective role in dental care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Milazzo, M.; Blanco, I.; Vecchio Ciprioti, S. Structure, drug absorption, bioactive and antibacterial properties of sol-gel SiO2/ZrO2 materials. Ceram. Int. 2020, 46, 29459–29465. [Google Scholar] [CrossRef]
- Tranquillo, E.; Barrino, F.; Dal Poggetto, G.; Blanco, I. Sol-gel Synthesis of Silica-Based Materials with Different Percentages of PEG or PCL and High Chlorogenic Acid Content. Materials 2019, 12, 155. [Google Scholar] [CrossRef] [PubMed]







| Organic-Inorganic Hybrids from Sol-Gel | Properties | Application | Authors |
|---|---|---|---|
| Silica/Chitosan/HRP | Spherical particles 100–200 nm diameter High porosity | Biosensor | G.-H. Wang and L.-M. Zhang, 2006 [37] |
| Silica/Chitosan | Macroporous scaffold 100–500 µm pore size | Bone tissue engineering | Reis et al., 2007 [38] |
| ZrO2/PEG/Eu3+ (4.5 wt%)/ZnS (2–3.5 wt%) | Amorphous nanocrystals 12 nm | Optical devices | Jose et al., 2021 [39] |
| PU/HBNPSi | Homogeneous structure | Flame retardant | Shen et al., 2021 [40] |
| HMSs (hollow mesoporous silica spheres) | Spherical particles (400–421 nm) 2.8–3.9 nm | Cu2+ adsorption and electrostatic interaction | Sun et al., 2020 [41] |
| Silica/Starch/PEO or Calcium alginate | Matrix | Bioencapsulation of microbial cells | Evstatieva et al., 2014 [42] |
| Alcohol-Aminosilicate | Matrix | Anticorrosion | Figueira et al., 2014 [43] |
| PLGA/mesoporous silica | Microspheres (20 µm) | Drug delivery | Xue and Shi, 2004 [44] |
| Dehydration | Decomposition | |||||
|---|---|---|---|---|---|---|
| Material | ΔT (°C) | Ton (°C) | Δm (%) | ΔT (°C) | Ton (°C) | Δm (%) |
| ZrO2 | 15–200 200–400 1 | 82 255 1 | 19.7 9.9 1 | 400–600 | 461 | 6.6 |
| ZrO2/PEG6% | 16–191 | 79 | 13.8 | 200–350 400–600 | 299 488 | 16.9 8.2 |
| ZrO2/PEG12% | 15–188 | 77 | 13.5 | 200–350 400–600 | 296 491 | 19.0 8.1 |
| ZrO2/PEG24% | 15–189 | 74 | 11.4 | 200–350 | 291 | 28.3 |
| ZrO2/PEG50% | 16–188 | 74 | 11.5 | 200–350 | 287 | 40.8 |
| ZrO2/PEG60% | 15–195 | 72 | 11.7 | 200–350 | 274 | 48.5 |
| ZrO2/PEG70% | 16–188 | 81 | 11.6 | 200–350 | 278 | 56.1 |
| Wavelenght (cm−1) | Attribution of Each FTIR Band |
|---|---|
| 3440 | –OH stretching |
| 2870 | C–H stretching of PEG |
| 1585 and 1377 | C=O vibrations of AcAc |
| 1529 and 1280 | C–C vibrations |
| 1454 | C–H asymmetric bending |
| 1425 | C–H symmetric bending |
| 1250 | Alcohol C–O stretching |
| 1104 | Ethereal C–O–C stretching |
| 1026 and 931 | C–C–H bending and C–C stretching |
| 654 | Zr–OH stretching |
| 460 | Zr–O–Zr stretching |
| 422 | Zr–O–AcAc vibrations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Ciprioti, S.V. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. https://doi.org/10.3390/ma14071788
Catauro M, Ciprioti SV. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials. 2021; 14(7):1788. https://doi.org/10.3390/ma14071788
Chicago/Turabian StyleCatauro, Michelina, and Stefano Vecchio Ciprioti. 2021. "Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review" Materials 14, no. 7: 1788. https://doi.org/10.3390/ma14071788
APA StyleCatauro, M., & Ciprioti, S. V. (2021). Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials, 14(7), 1788. https://doi.org/10.3390/ma14071788