Effect of H2O Molecule Adsorption on the Electronic Structure and Optical Properties of the CsI(Na) Crystal
Abstract
1. Introduction
2. Materials and Methods
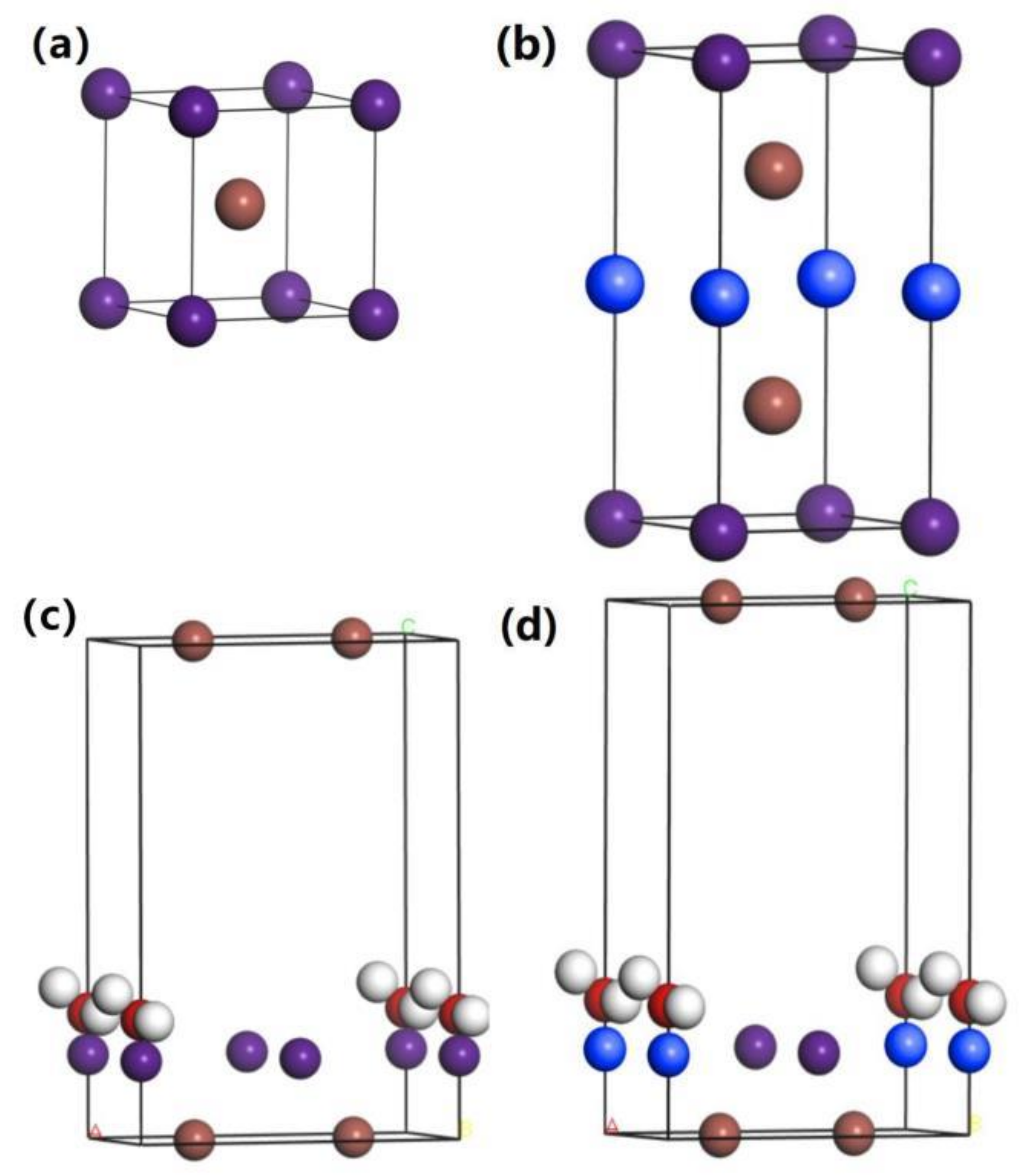
2.1. Theoretical Method
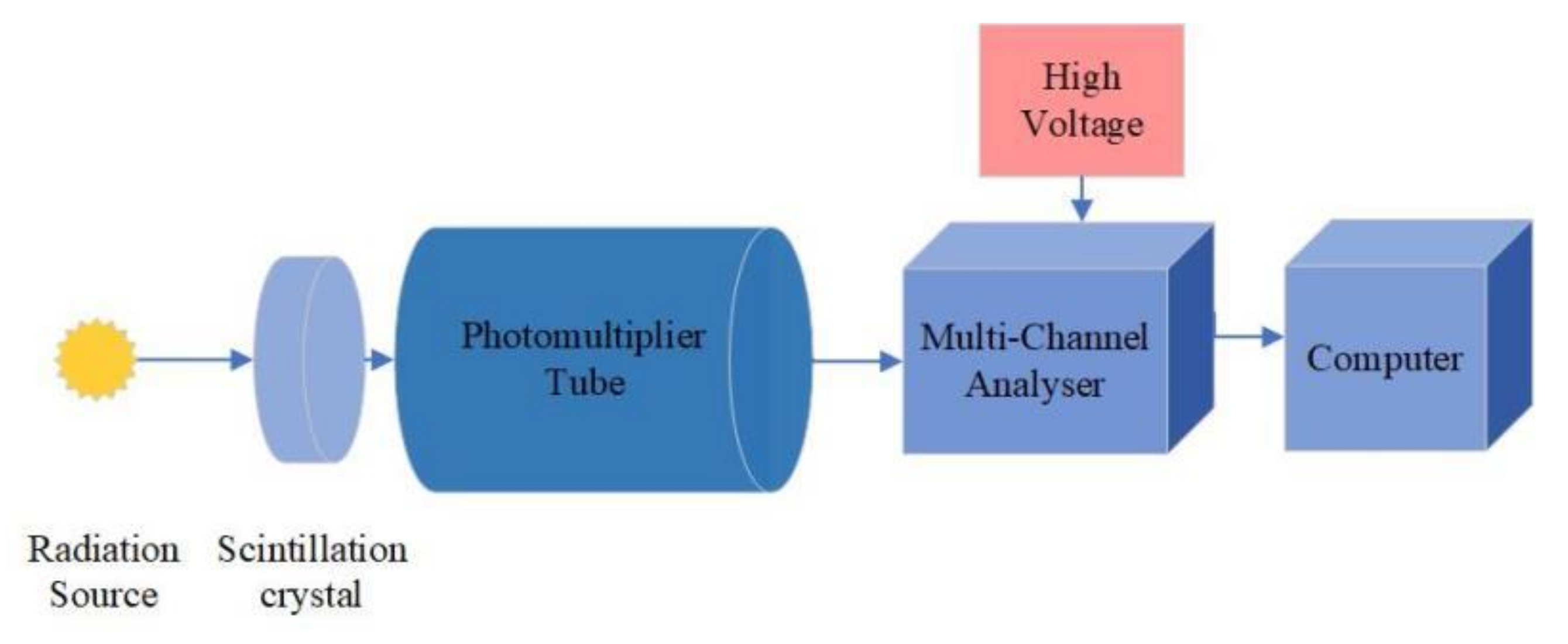
2.2. Experimental Setup
3. Data Analysis
3.1. Energy Spectrum Measurement in Different Relative Humidity Environments
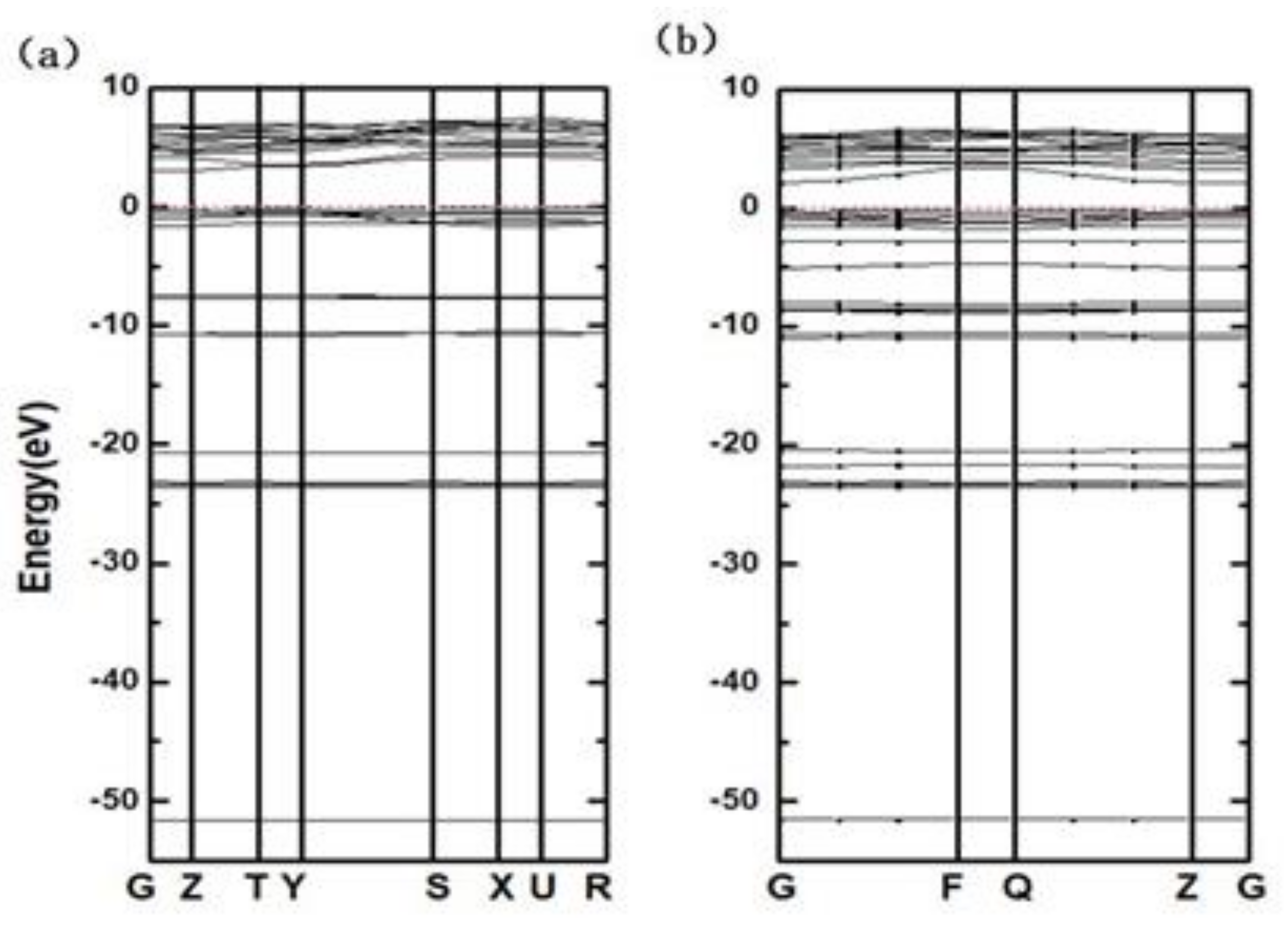
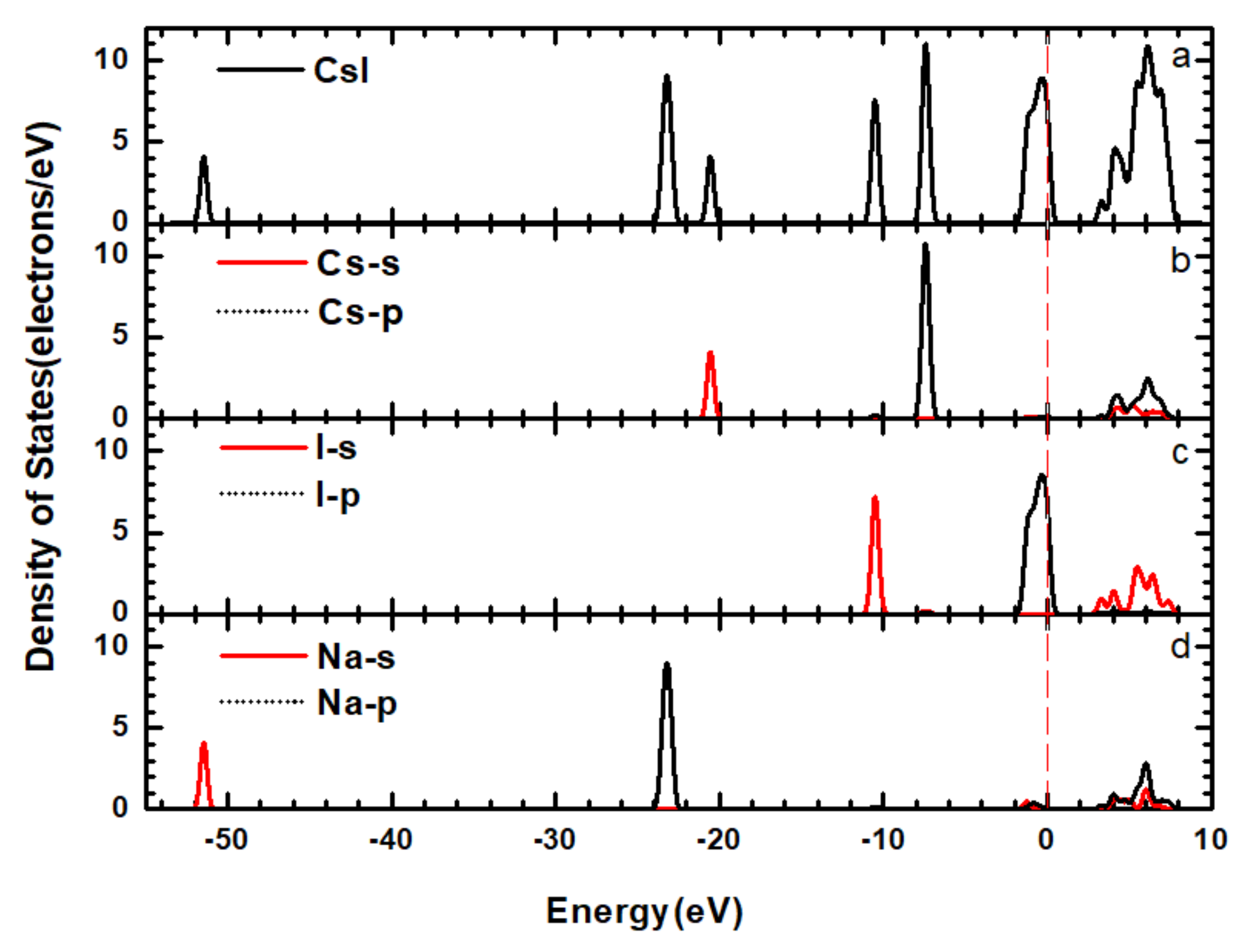
3.2. Band Structure and Electronic Density of States
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menefee, J.; Cho, Y.; Swinehart, C. Sodium Activated Cesium Iodide as a Gamma Ray and Charged Particle Detector. IEEE Trans. Nucl. Sci. 1967, 14, 464–467. [Google Scholar] [CrossRef]
- Syntfeld-Kazuch, A.; Sibczyński, P.; Moszyński, M.; Gektin, A.V.; Grodzicka, M.; Iwanowska, J.; Szawł, M.; Szczęśniak, T.; Świderski, Ł. Performance of CsI(Na) Scintillators in gamma-Ray Spectrometry. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; Volume N25, pp. 1474–1479. [Google Scholar]
- Kilimchuk, I.V.; Gavrilyuk, V.P.; Grinyov, B.V.; Tarasov, V.A.; Viday, Y.T. Features of scintillation characteristics of CsI:Na and NaI:Tl crystals as the basis for soft gamma radiation detectors. Opt. Mater. 2008, 30, 1800–1802. [Google Scholar] [CrossRef]
- Yang, P.; Harmon, C.D.; Doty, F.P.; Ohlhausen, J.A. Effect of Humidity on Scintillation Performance in Na and Tl Activated CsI Crystals. IEEE Trans. Nucl. Sci. 2014, 61, 1024–1031. [Google Scholar] [CrossRef]
- Shakhova, K.V.; Panova, A.N.; Goriletsky, V.I.; Prikhod’ko, Y.A.; Gavrylyuk, V.P.; Korsunova, S.P.; Kosinov, N.N. Luminescence and scintillation properties of Na-activated CsI—CsBr crystals. Radiat. Meas. 2001, 33, 769–771. [Google Scholar] [CrossRef]
- Keillor, M.E.; Cooper, M.W.; Hayes, J.C.; McIntyre, J.I. Degradation of 81 keV 133Xe gamma-rays into the 31 keV X-ray peak in CsI scintillators. J. Radioanal. Nucl. Chem. 2009, 282, 699–702. [Google Scholar] [CrossRef]
- Fan, L.; Yang, Z.W.; Chen, T.; Li, J.; Li, Y.K.; Cao, Z.R. Influence of air exposure on the structure and properties of cesium iodide film. Acta Phys. Sin. 2014, 63, 146801. [Google Scholar]
- Zhang, Z.; Zhao, Q.; Li, Y.; Ouyang, X.P. Electronic structure and optical properties of CsI, CsI(Ag), and CsI(Tl). J. Korean Phys. Soc. 2016, 68, 1069–1074. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Assessment and validation of a screened Coulomb hybrid density functional. J. Chem. Phys. 2004, 120, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Heyd, J.; Scuseria, G.E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd—Scuseria—Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.M.; Izmaylov, A.F.; Scalmani, G.; Scuseria, G.E. Can short-range hybrids describe long-range-dependent properties. J. Chem. Phys. 2009, 131, 669–724. [Google Scholar] [CrossRef] [PubMed]
- Pela, R.R.; Marques, M.; Teles, L.K. Comparing LDA-1/2, HSE03, HSE06 and G0W0 approaches for band gap calculations of alloys. J. Phys. Condens. Matter 2015, 27, 505502. [Google Scholar] [CrossRef] [PubMed]
- Goriletsky, V.I.; Grinyov, B.V.; Panova, A.M.; Shakhova, K.V.; Vinograd, E.L.; Korsunov, S.P. Kinetic and scintillation characteristics of CsI(Na) crystals grown under melt mixing. Nucl. Instrum. Methods Phys. Res. 1999, 159, 111–115. [Google Scholar] [CrossRef]
- Crannell, C.J.; Kurz, R.J.; Viehmann, W. Characteristics of cesium iodide for use as a particle discriminator for high-energy cosmic rays. Nucl. Instrum. Methods 1974, 115, 253–261. [Google Scholar] [CrossRef]
- Ribeiro, R.M.; Coutinho, J.; Torres, V.J.B.; Jones, R.; Sque, S.J.; Oeberg, S.; Shaw, M.J.; Briddon, P.R. Ab initio study of CsI and its surface. Phys. Rev. B 2006, 74, 035430. [Google Scholar] [CrossRef]
- Meyer, H.; Entel, P.; Hafner, J. Physisorption of water on salt surfaces. Surf. Sci. 2001, 488, 177–192. [Google Scholar] [CrossRef]
- Sebastiani, D.; Site, L.D. Adsorption of water molecules on flat and stepped nickel surfaces from first principles. J. Chem. Theor. Comput. 2005, 1, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Liang, B.; Liu, S.; Guo, L.N.; Chen, D.J.; Liu, Y.; Zhong, Z.Y.; Xiong, L.F. Study of humidity on the structure and optical properties of cesium iodide thin film. J. Phys. Conf. Ser. 2015, 680, 012022. [Google Scholar] [CrossRef]
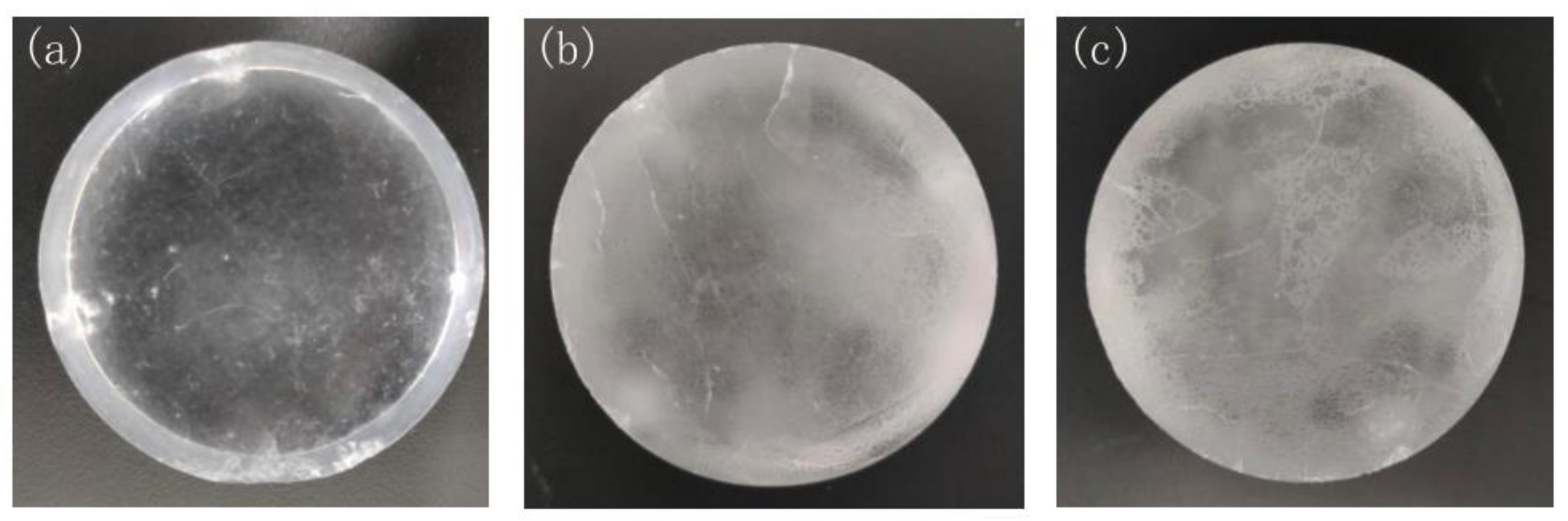
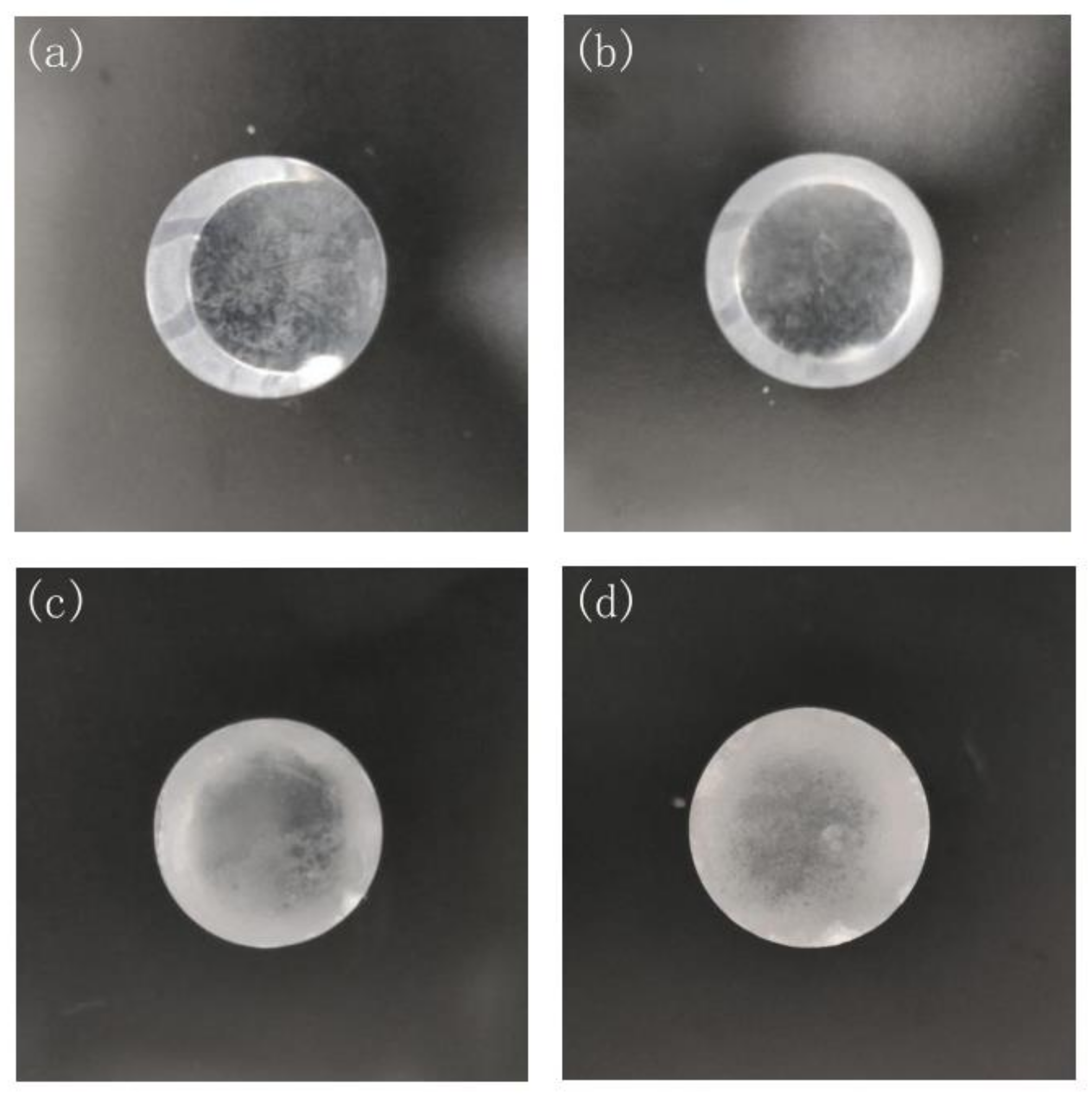

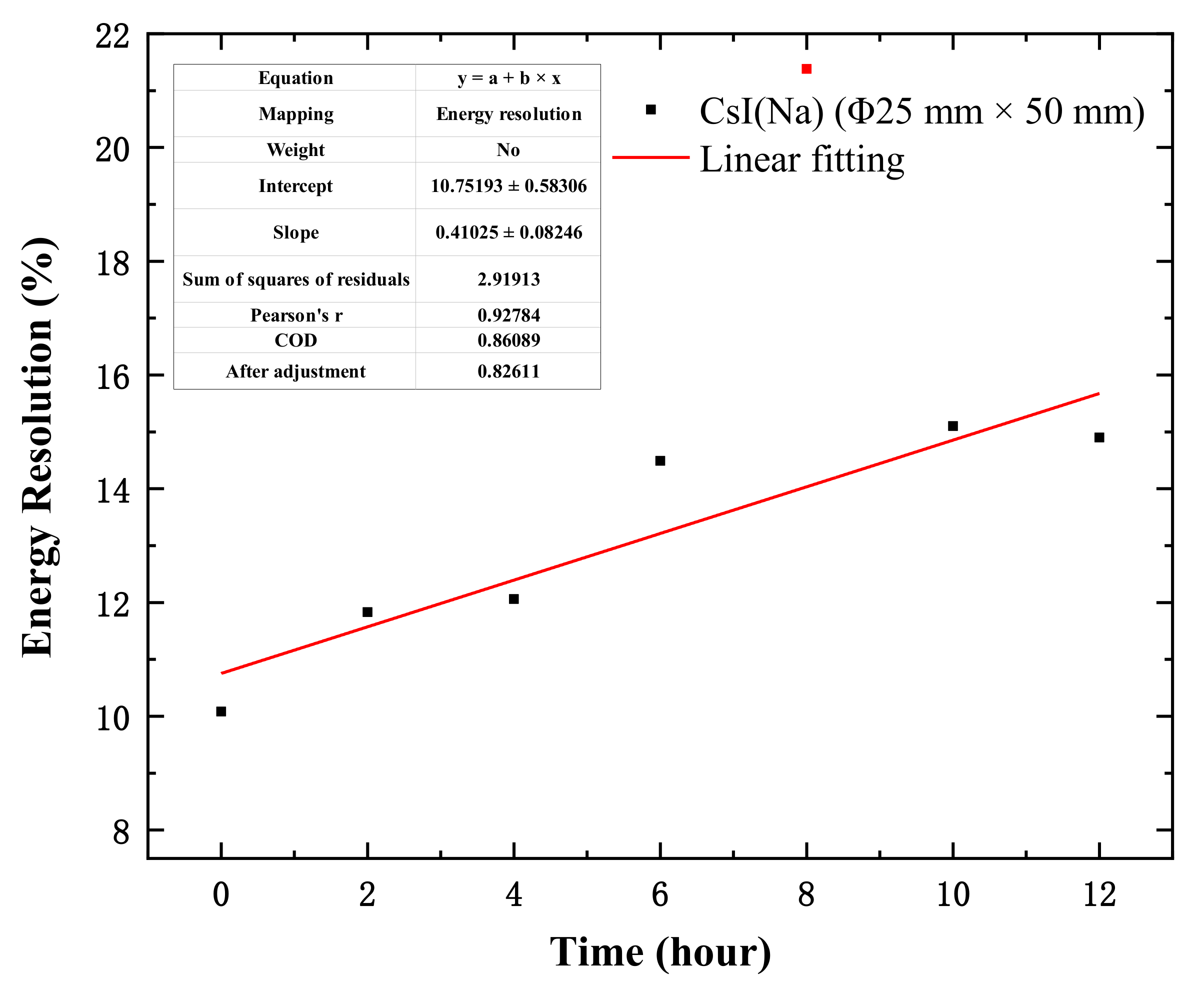
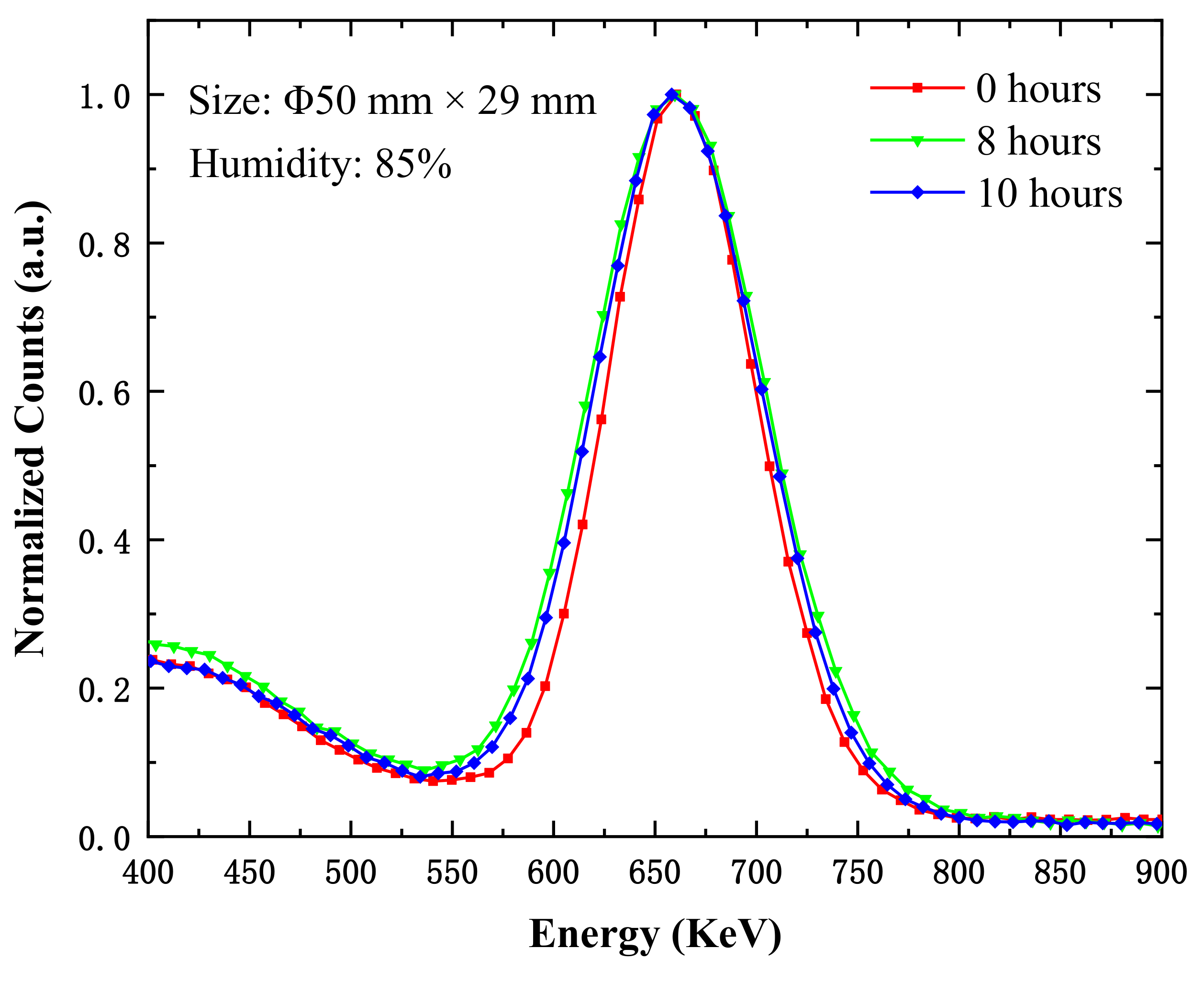

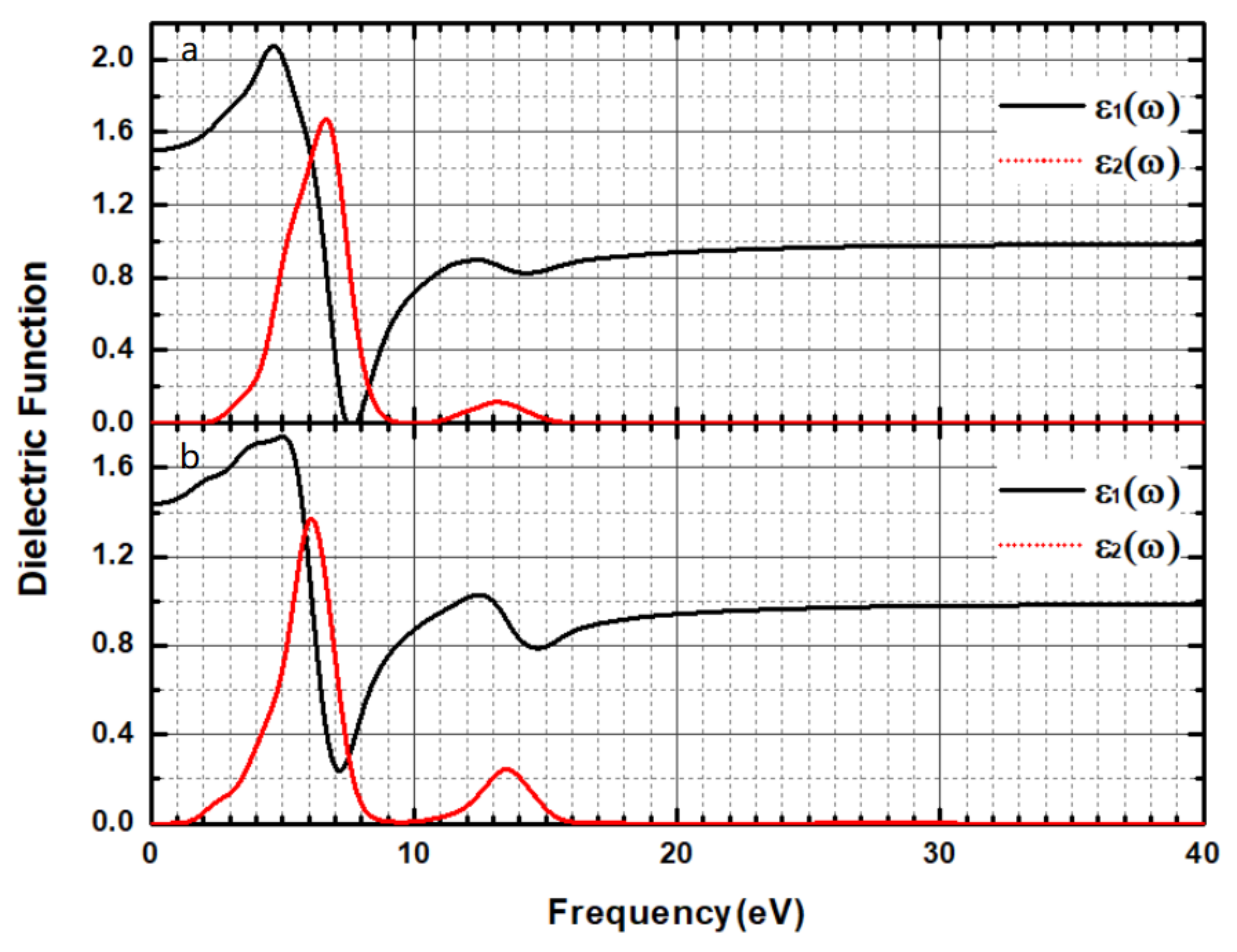
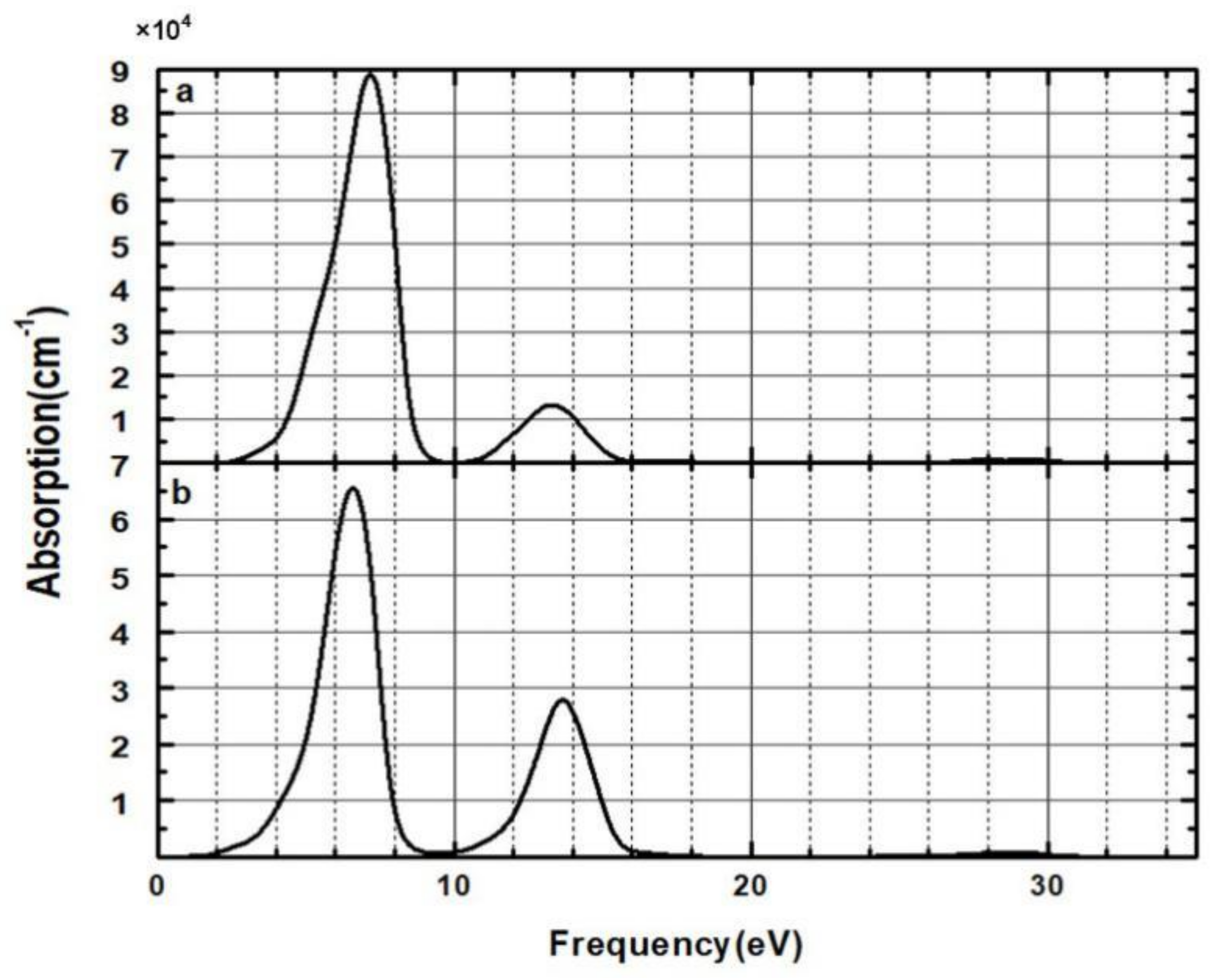
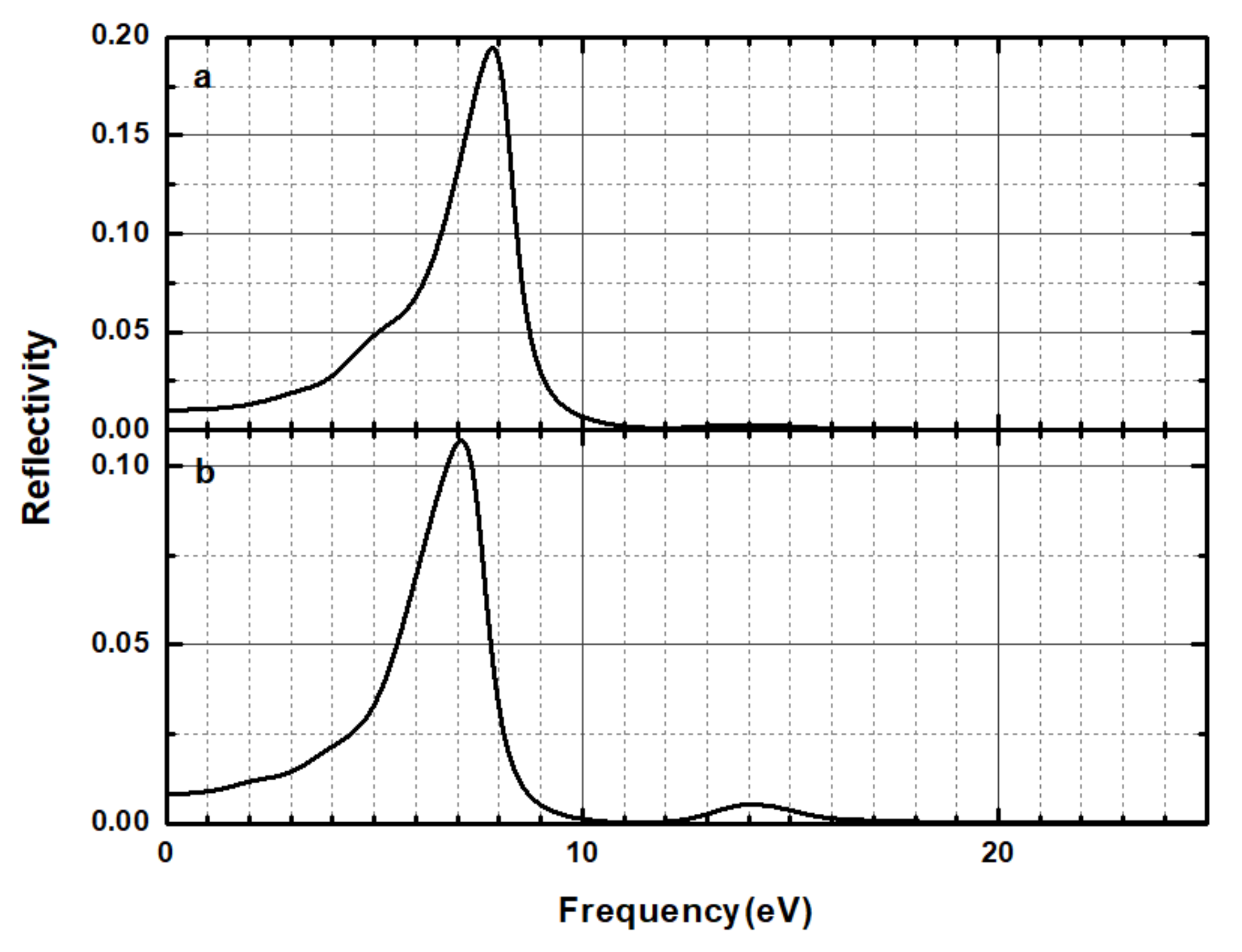
| Time (h) | 0 | 3 | 6 | 9 | 12 | 24 | 27 | 30 | 33 | 36 |
| Energy resolution (%) | 11.26 | 11.19 | 10.59 | 11.12 | 11.76 | 10.84 | 10.79 | 10.54 | 10.96 | 11.11 |
| Material | Adsorption Energy | RO-H | AH-O-H |
|---|---|---|---|
| CsI | 0.1239 eV | 0.973 Å | 103.946° |
| CsI(Na) | −0.5344 eV | 0.999 Å | 105.111° |
| H2O | – | 0.973 Å | 103.946° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zheng, H.; Jiang, T.; Liu, B.; Zhang, J.; Yuan, H.; Liu, F.; Fan, X.; Ouyang, X. Effect of H2O Molecule Adsorption on the Electronic Structure and Optical Properties of the CsI(Na) Crystal. Materials 2021, 14, 1720. https://doi.org/10.3390/ma14071720
Liu F, Zheng H, Jiang T, Liu B, Zhang J, Yuan H, Liu F, Fan X, Ouyang X. Effect of H2O Molecule Adsorption on the Electronic Structure and Optical Properties of the CsI(Na) Crystal. Materials. 2021; 14(7):1720. https://doi.org/10.3390/ma14071720
Chicago/Turabian StyleLiu, Fang, Hao Zheng, Tianze Jiang, Bin Liu, Jianming Zhang, Hang Yuan, Fengcheng Liu, Xiaoxue Fan, and Xiaoping Ouyang. 2021. "Effect of H2O Molecule Adsorption on the Electronic Structure and Optical Properties of the CsI(Na) Crystal" Materials 14, no. 7: 1720. https://doi.org/10.3390/ma14071720
APA StyleLiu, F., Zheng, H., Jiang, T., Liu, B., Zhang, J., Yuan, H., Liu, F., Fan, X., & Ouyang, X. (2021). Effect of H2O Molecule Adsorption on the Electronic Structure and Optical Properties of the CsI(Na) Crystal. Materials, 14(7), 1720. https://doi.org/10.3390/ma14071720








