Effect of Sintering Process on Ionic Conductivity of Li7-xLa3Zr2-xNbxO12 (x = 0, 0.2, 0.4, 0.6) Solid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Ma, C.; Cheng, A.Y.; Yin, B.K.; Luo, A.J.; Sharafi, C.A. Interfacial stability of Li metal/solid electrolyte elucidated via in situ electron microscopy. Nano. Lett. 2016, 16, 7030–7036. [Google Scholar] [CrossRef]
- Han, F.D.; Zhu, Y.Z.; He, X.F.; Mo, Y.F. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 2016, 6, 1501590. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Cussen, E.J. Structure and ionic conductivity in lithium garnets. J. Mater. Chem. 2010, 20, 5167–5173. [Google Scholar] [CrossRef]
- Huang, M.; Xu, W.; Shen, Y.; Lin, Y.H.; Nan, C.W. X-ray absorption near-edge spectroscopy study on Ge-doped Li7La3Zr2O12: Enhanced ionic conductivity and defect chemistry. Electrochim. Acta 2014, 115, 581–586. [Google Scholar] [CrossRef]
- Lu, X.J.; Yang, D.Y. Preparation of garnet-type Li7−3xAlxLa3Zr2O12 at lower temperature by using powders of mixed pre-treatment conditions. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2023–2027. [Google Scholar] [CrossRef]
- Rettenwander, D.; Wagner, R.; Reyer, A.; Bonta, M.; Chen, L.; Doeff, M.M.; Limbeck, A.; Wilkening, M.; Amthauer, G. Interface instability of Fe-stabilized Li7La3Zr2O12 versus Li metal. J. Phys. Chem. C 2018, 122, 3780–3785. [Google Scholar] [CrossRef]
- Wu, J.F.; Chen, E.Y.; Yu, Y.; Liu, L.; Wu, Y.; Pang, W.K.; Peterson, V.K.; Guo, X. Gallium-doped Li7La3Zr2O12 garnet-type electrolytes with high lithium-ion conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1542–1552. [Google Scholar] [CrossRef]
- Buschmann, H.; Berendts, S.; Mogwitz, B.; Janek, J. Lithium metal electrode kinetics and ionic conductivity of the solid lithium ion conductors “Li7La3Zr2O12” and Li7-xLa3Zr2–xTaxO12 with garnet-type structure. J. Power Sources 2012, 206, 236–244. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lai, W. High ionic conductivity lithium garnet oxides of Li7-xLa3Zr2-xTaxO12 compositions. Electrochem. Solid State Lett. 2012, 15, A68–A71. [Google Scholar] [CrossRef]
- Hayamizu, K.; Matsuda, Y.; Matsui, M.; Imanishi, N. Lithium ion diffusion measurements on a garnet-type solid conductor Li6.6La3Zr1.6Ta0.4O12 by using a pulsed-gradient spin-echo NMR method. Solid State Nucl. Magn. Reson. 2015, 70, 21–27. [Google Scholar] [CrossRef]
- Janani, N.; Ramakumar, S.; Kannan, S.; Murugan, R. Optimization of lithium content and sintering aid for maximized Li+ conductivity and density in Ta-doped Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 2039–2046. [Google Scholar] [CrossRef]
- Huang, M.; Mao, S.; Shen, Y.; Nan, C.W.; Munakata, H.; Kanamura, K. Preparation and electrochemical properties of Zr-site substituted Li7La3(Zr2-xMx)O12 (M=Ta, Nb) solid electrolytes. J. Power Sources 2014, 261, 206–211. [Google Scholar] [CrossRef]
- Deviannapoorani, C.; Dhivya, L.; Ramakumar, S.; Murugan, R. Synthesis of garnet structured Li7+xLa3YxZr2-xO12 (x=0-0.4) by modified sol-gel method. J. Sol. Gel. Sci. Technol. 2012, 64, 510–514. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Z.G.; Jin, Y.J.; Ouyang, J.H.; Chen, L.; Wang, Y.J. Effect of sintering process on the microstructure and ionic conductivity of Li7–xLa3Zr2–xTaxO12 ceramics. Ceram. Int. 2019, 45, 18439–18444. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−xLa3 (Zr2−x, Nbx)O12 (x=0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Lee, H.C.; Oh, N.R.; Yoo, A.R.; Kim, Y.; Skamoto, J. Preparation of a Li7La3Zr1.5Nb0.5O12 garnet solid electrolyte ceramic by using Sol-gel powder synthesis and hot pressing and its characterization. J. Korean Phys. Soc. 2018, 73, 1535–1540. [Google Scholar] [CrossRef]
- Imagawa, H.; Ohta, S.; Kihira, Y.; Asaoka, T. Garnet-type Li6.75La3Zr1.75Nb0.25O12 synthesized by coprecipitation method and its lithium ion conductivity. Solid State Ion. 2014, 262, 609–612. [Google Scholar] [CrossRef]
- David, I.N.; Thompson, T.; Wolfenstine, J.; Allen, J.L.; Sakamoto, J. Microstructure and Li-ion conductivity of hot-pressed cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 1209–1214. [Google Scholar] [CrossRef]
- Huang, X.; Song, Z.; Xiu, T.P.; Badding, M.E.; Wen, Z.Y. Sintering, micro-structure and Li+ conductivity of Li7−xLa3 Zr2−xNbxO12 /MgO (x=0.2–0.7) Li-Garnet composite ceramics. Ceram. Int. 2019, 45, 56–63. [Google Scholar] [CrossRef]
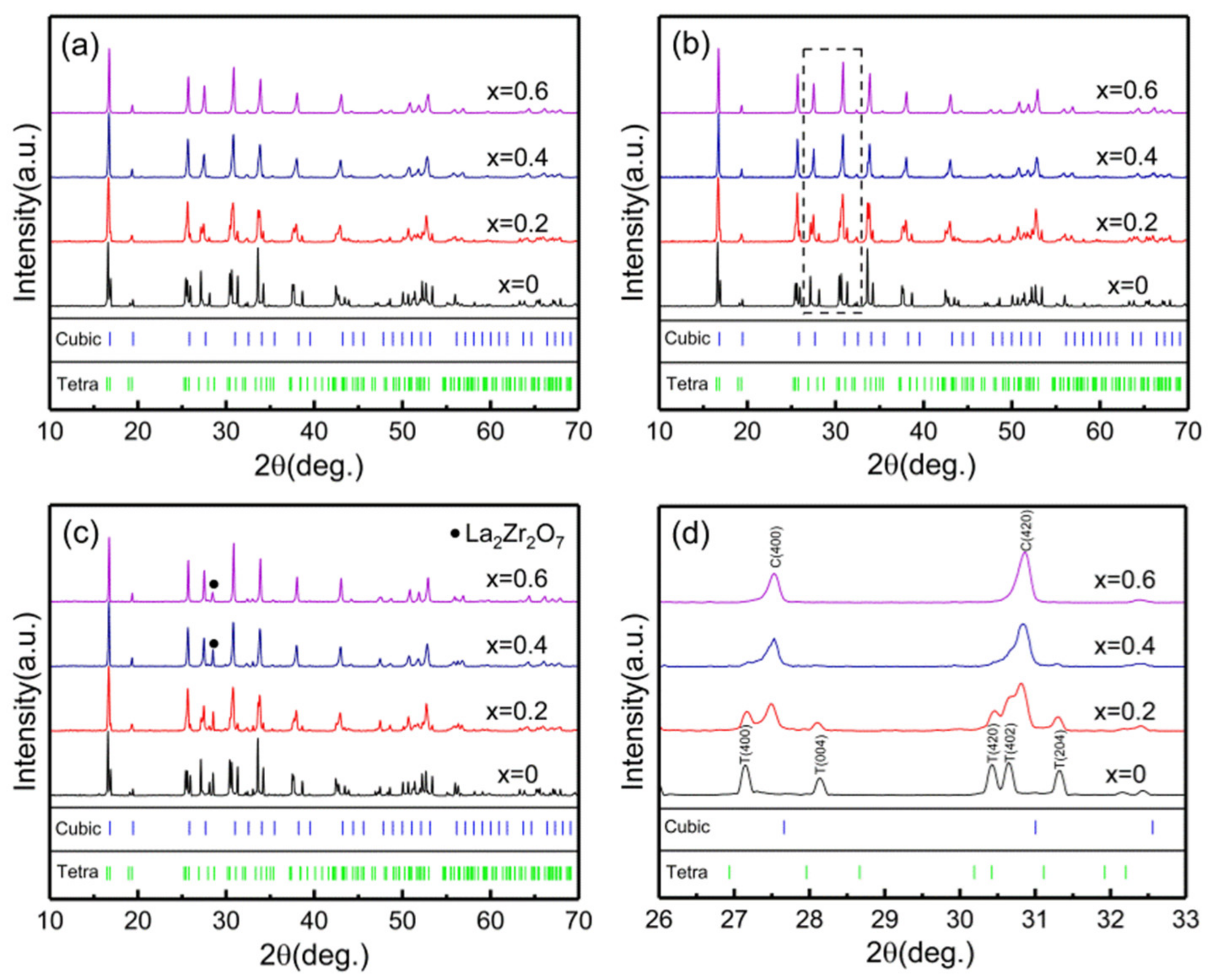
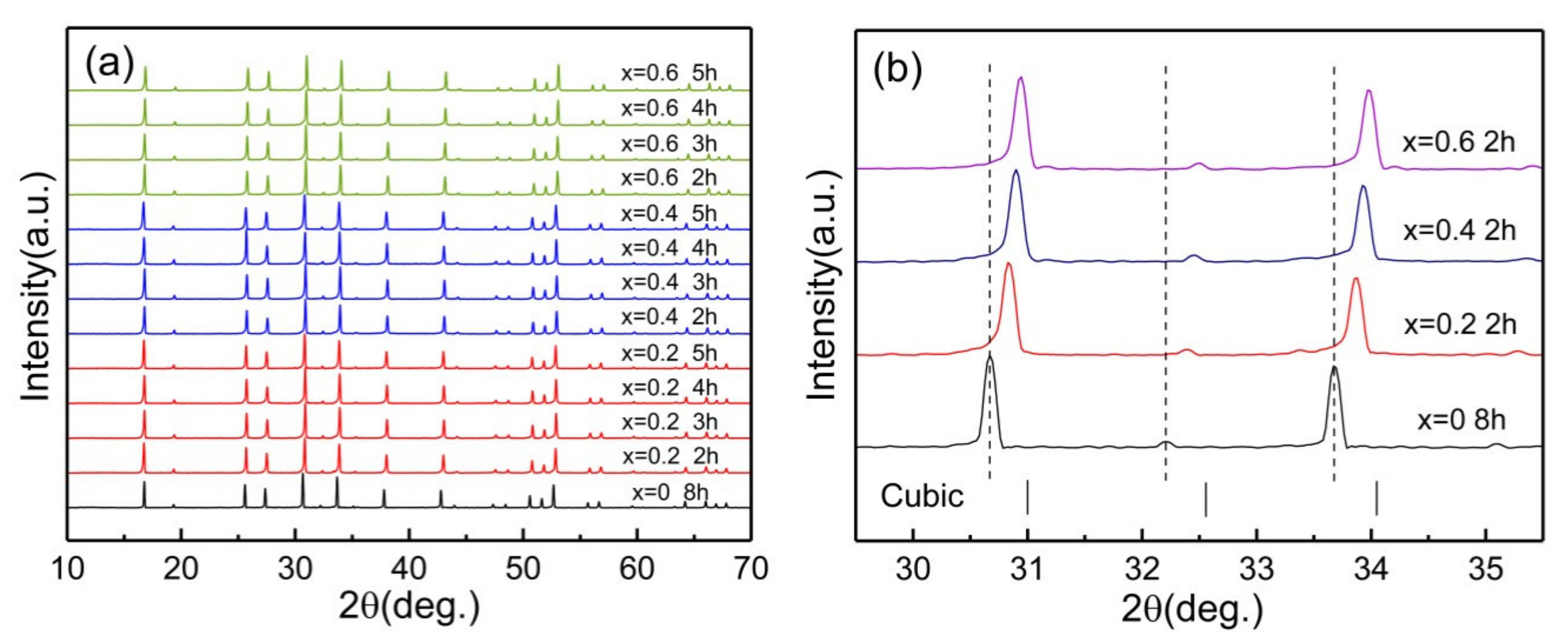
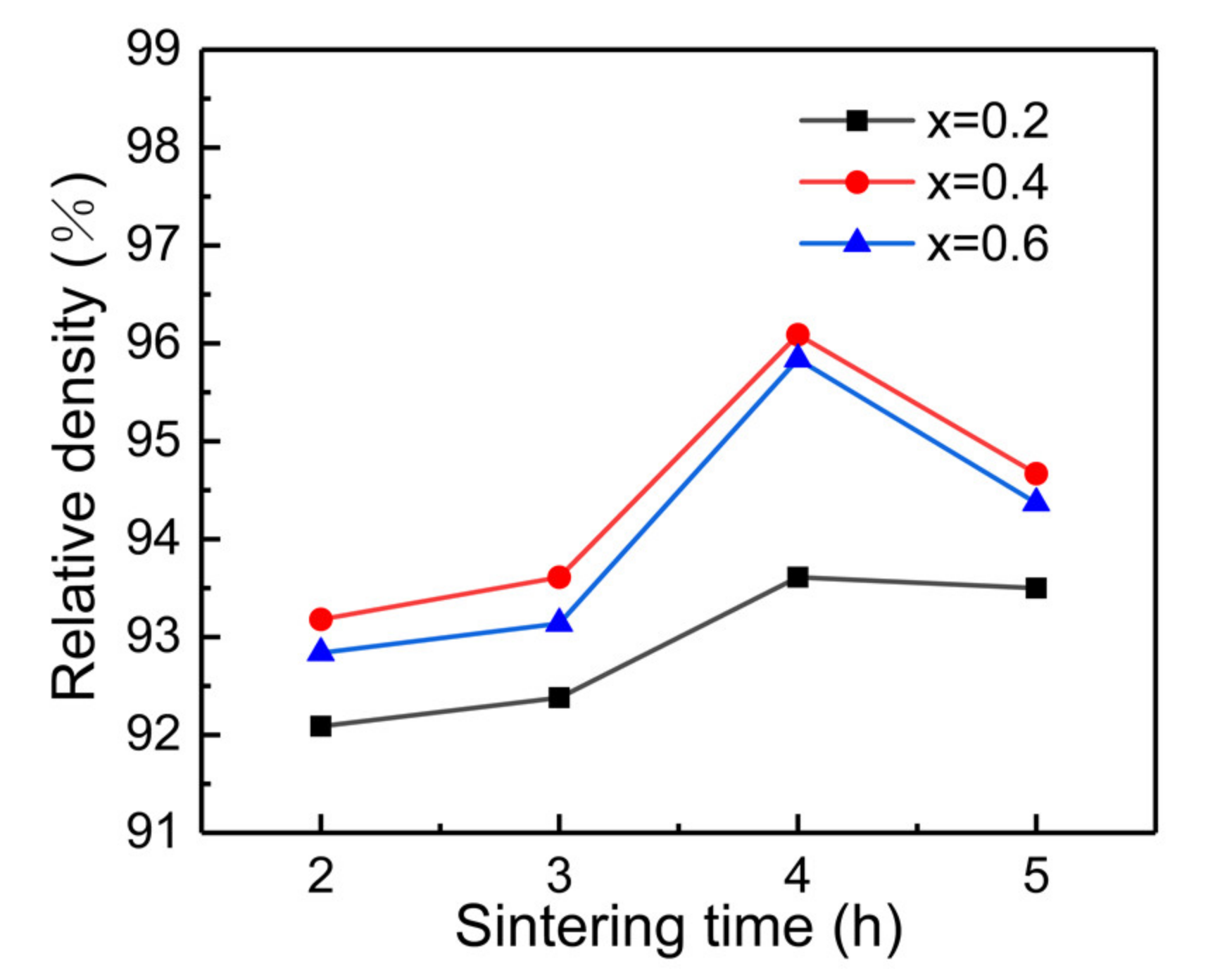
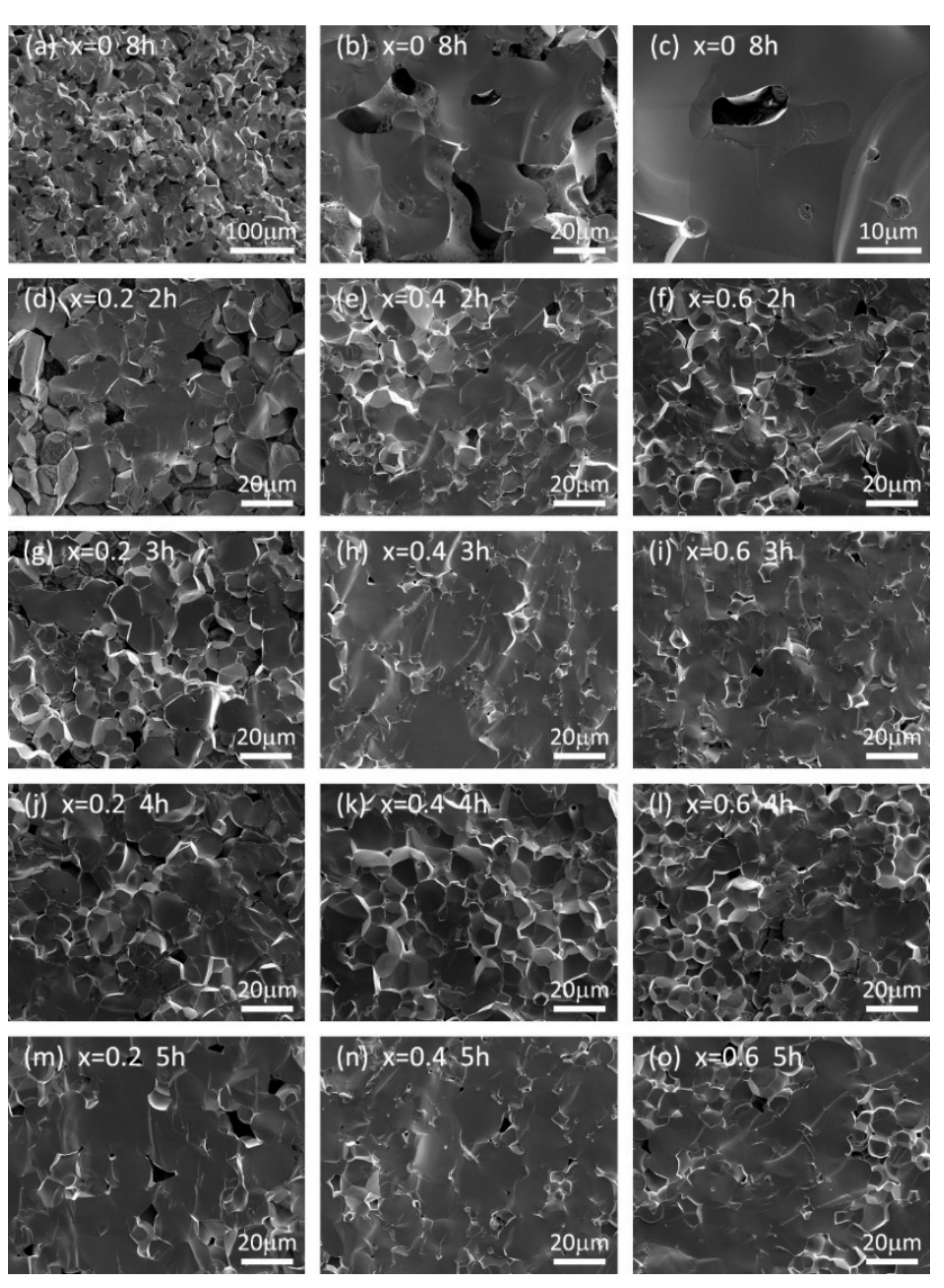
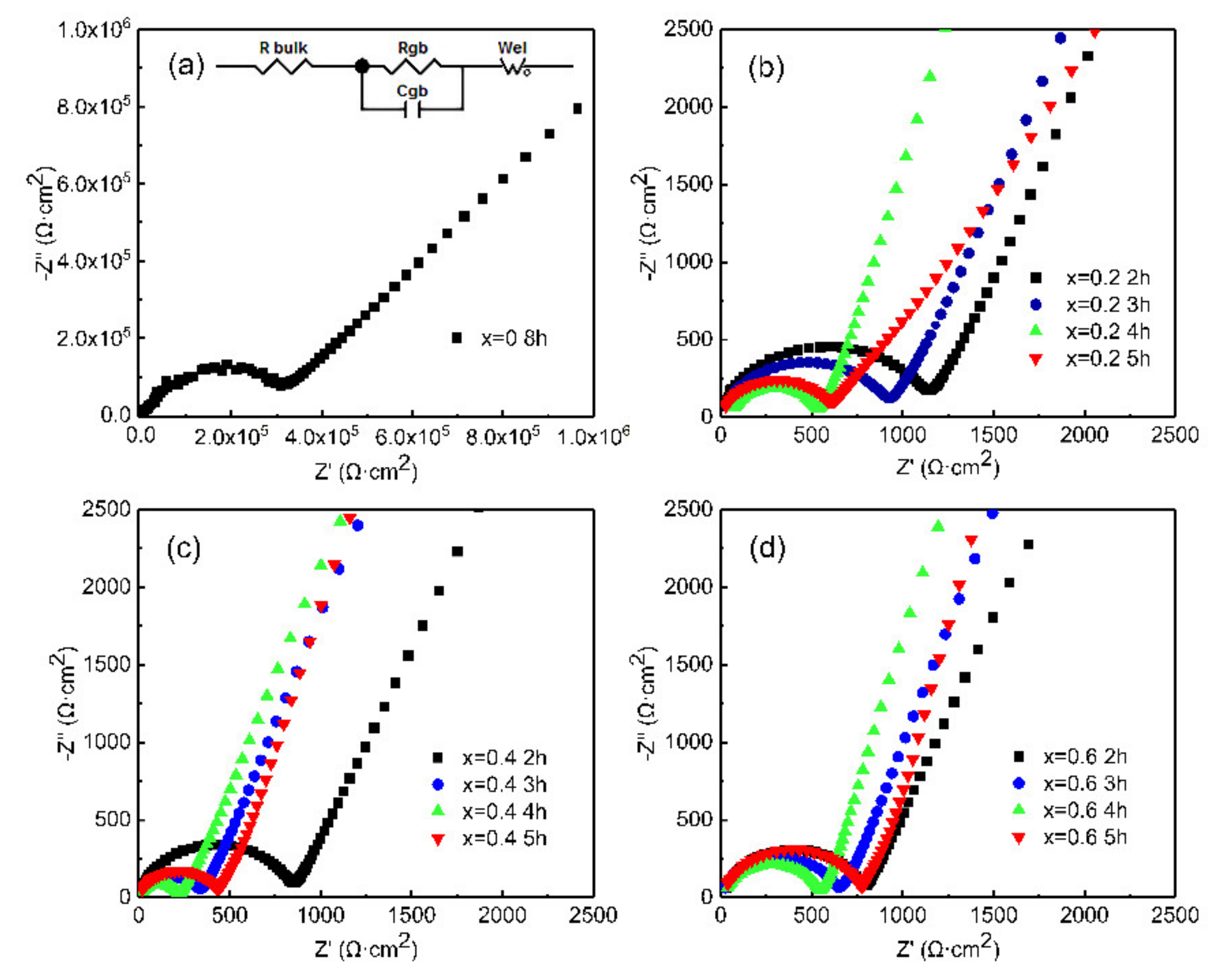
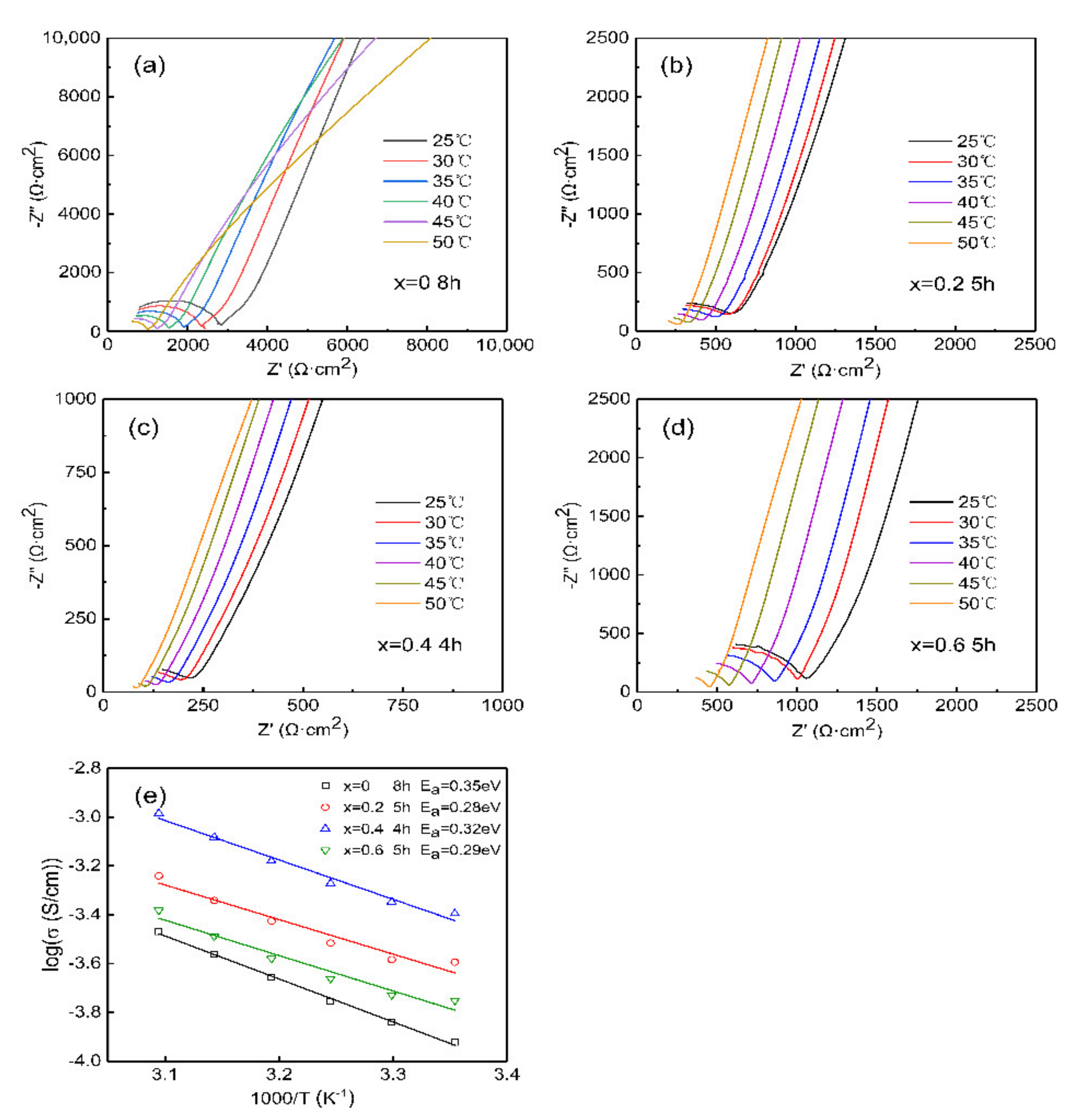
| Composition Sintering Time (h) | Conductivity (S·cm−1) | Composition Sintering Time (h) | Conductivity (S·cm−1) | Composition Sintering Time (h) | Conductivity (S·cm−1) |
|---|---|---|---|---|---|
| x = 0.2, 2 h | 1.09 × 10−4 | x = 0.4, 2 h | 1.56 × 10−4 | x = 0.6, 2 h | 1.49 × 10−4 |
| x = 0.2, 3 h | 1.43 × 10−4 | x = 0.4, 3 h | 3.56 × 10−4 | x = 0.6, 3 h | 1.92 × 10−4 |
| x = 0.2, 4 h | 2.37 × 10−4 | x = 0.4, 4 h | 3.86 × 10−4 | x = 0.6, 4 h | 2.36 × 10−4 |
| x = 0.2, 5 h | 2.49 × 10−4 | x = 0.4, 5 h | 2.62 × 10−4 | x = 0.6, 5 h | 2.42 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Wu, Z.; Zhang, C. Effect of Sintering Process on Ionic Conductivity of Li7-xLa3Zr2-xNbxO12 (x = 0, 0.2, 0.4, 0.6) Solid Electrolytes. Materials 2021, 14, 1671. https://doi.org/10.3390/ma14071671
Ni L, Wu Z, Zhang C. Effect of Sintering Process on Ionic Conductivity of Li7-xLa3Zr2-xNbxO12 (x = 0, 0.2, 0.4, 0.6) Solid Electrolytes. Materials. 2021; 14(7):1671. https://doi.org/10.3390/ma14071671
Chicago/Turabian StyleNi, Lei, Zhigang Wu, and Chuyi Zhang. 2021. "Effect of Sintering Process on Ionic Conductivity of Li7-xLa3Zr2-xNbxO12 (x = 0, 0.2, 0.4, 0.6) Solid Electrolytes" Materials 14, no. 7: 1671. https://doi.org/10.3390/ma14071671
APA StyleNi, L., Wu, Z., & Zhang, C. (2021). Effect of Sintering Process on Ionic Conductivity of Li7-xLa3Zr2-xNbxO12 (x = 0, 0.2, 0.4, 0.6) Solid Electrolytes. Materials, 14(7), 1671. https://doi.org/10.3390/ma14071671







