Surface Integrity of Dimethacrylate Composite Resins with Low Shrinkage Comonomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Experimental Composites Resins
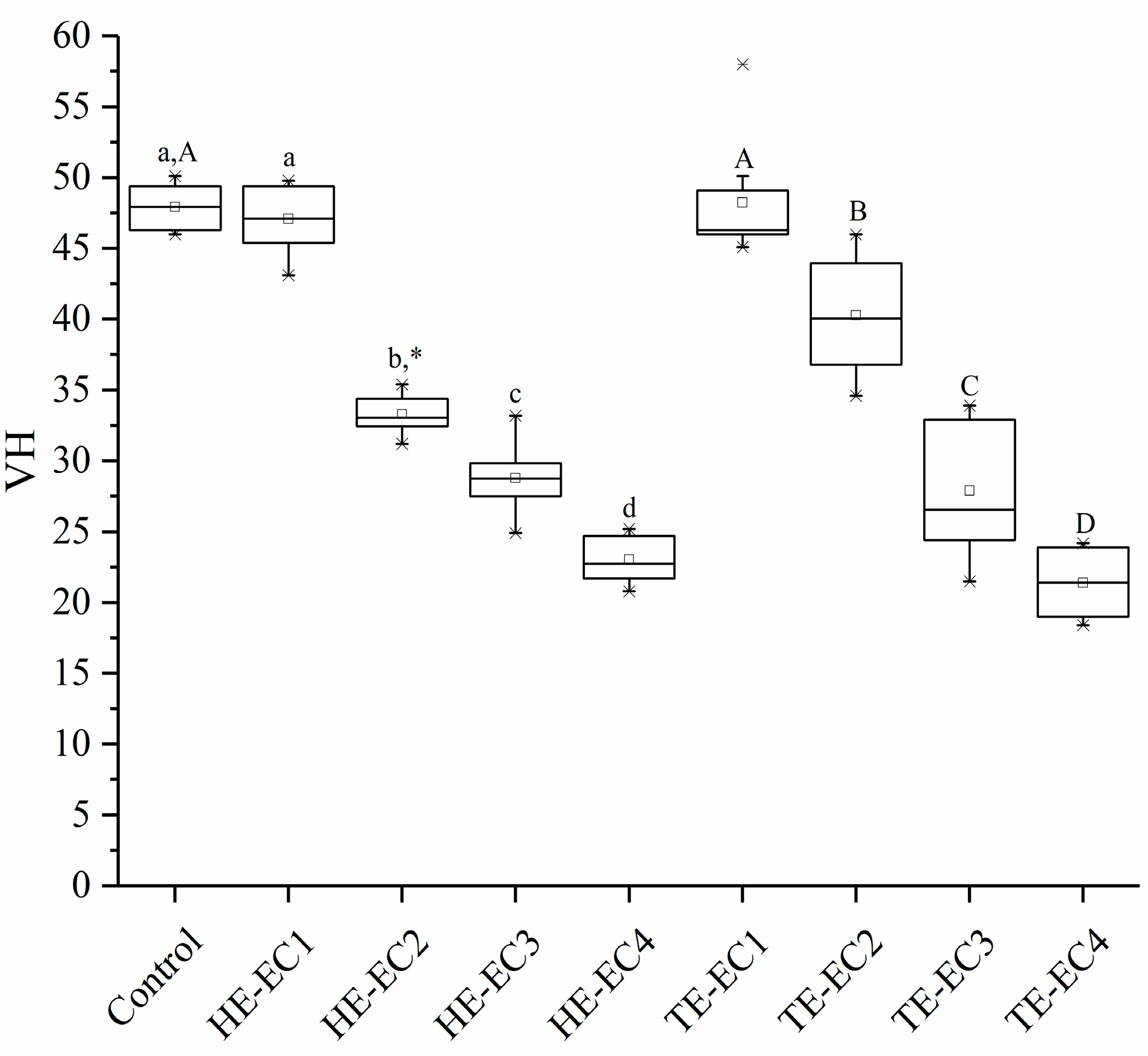
2.3. Surface Hardness
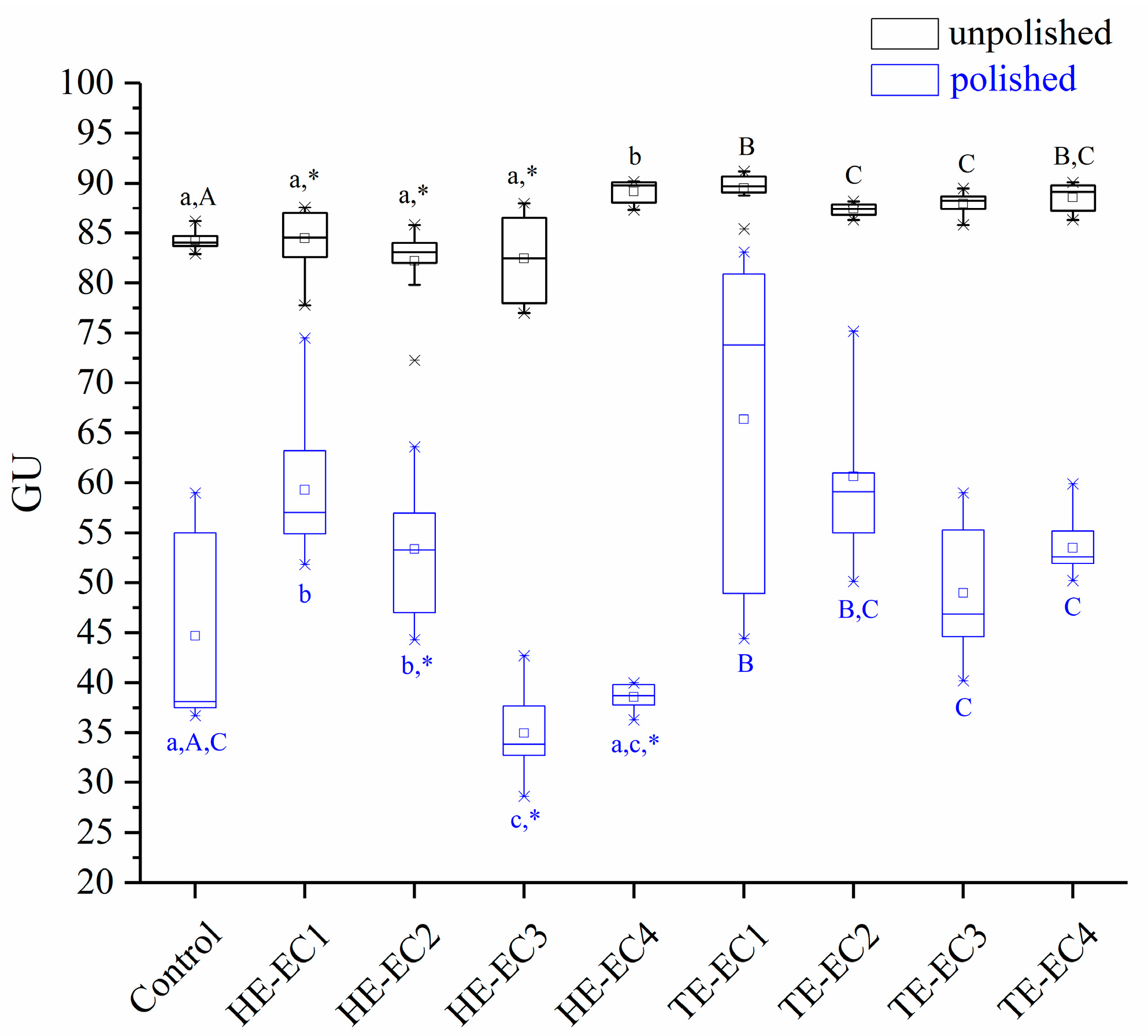
2.4. Surface Gloss
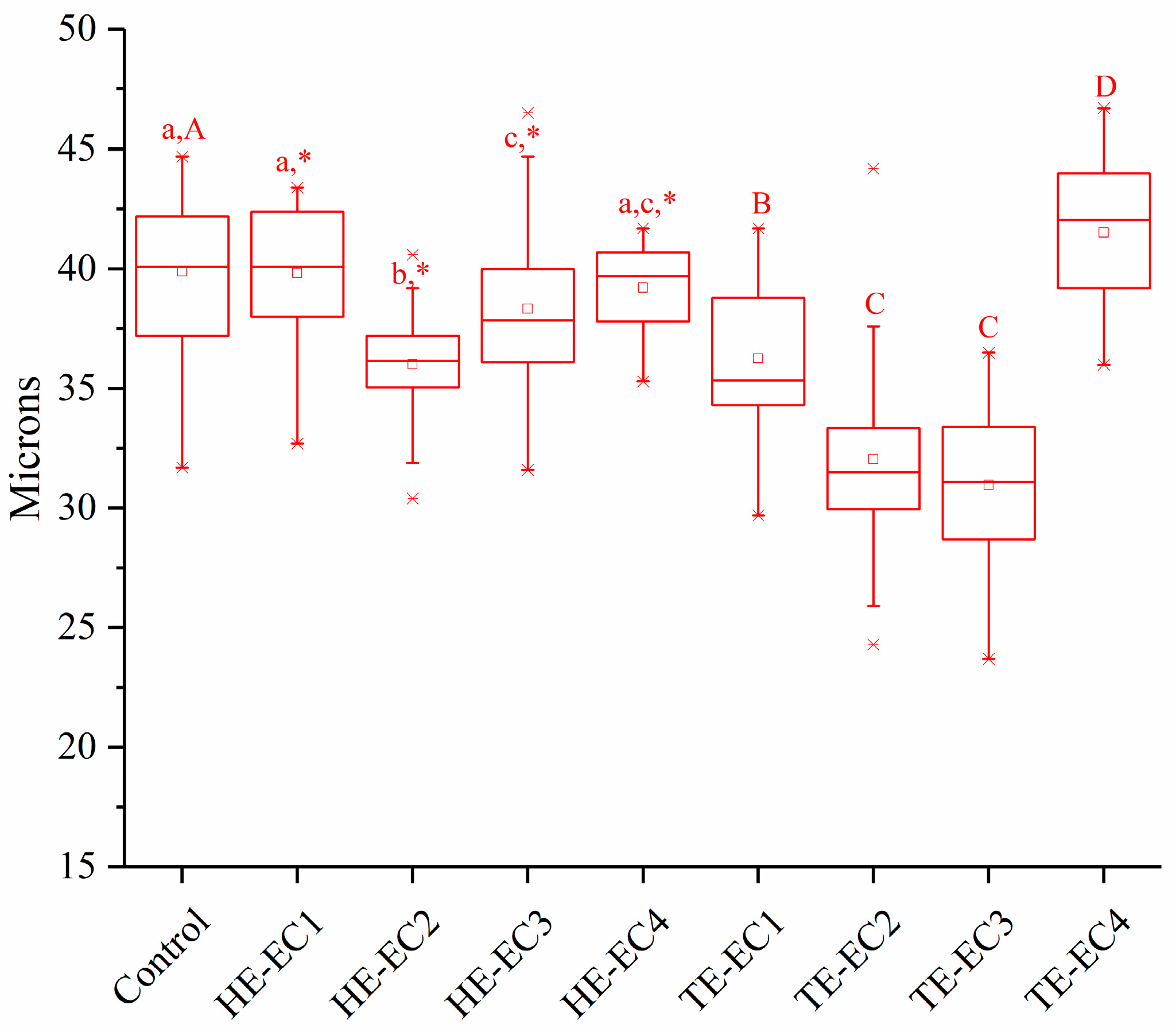
2.5. Two-Body Wear
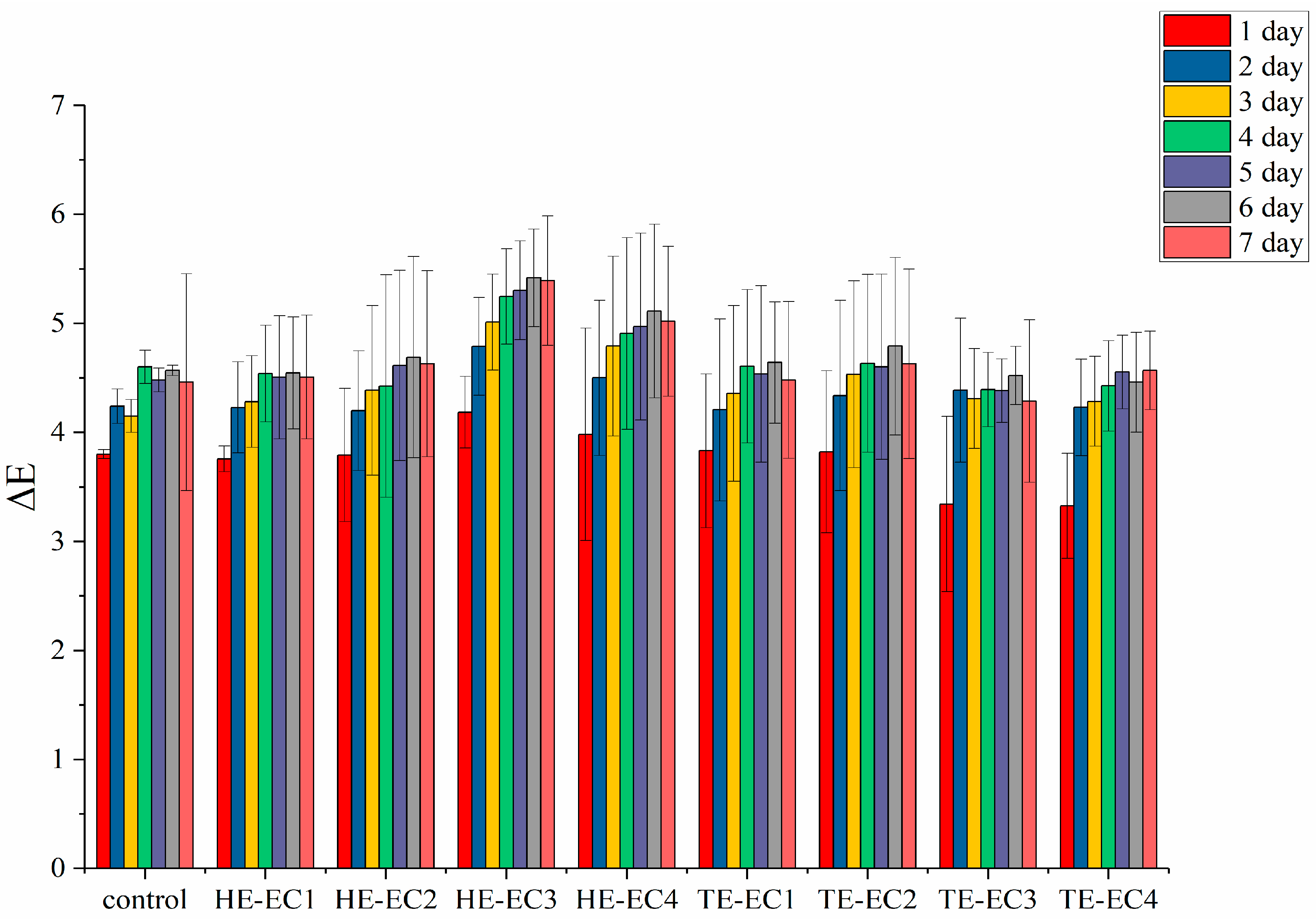
2.6. Color Change (∆E) and Translucency (TP)
2.7. Statistical Analysis
3. Results
3.1. Surface Hardnes
3.2. Surface Gloss
3.3. Wear Depth
3.4. Color Change (∆E) and Translucency (TP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferracane, J.L. Resin Composites-State of art. Dent. Mater. 2010, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ałka, K.; Kleczewska, J.; Sasimowski, E.; Belcarz, A.; Przekora, A. Improved Fracture Toughness and Conversion Degree of Resin-Based Dental Composites after Modification with Liquid Rubber. Materials 2020, 13, 2704. [Google Scholar]
- Liu, D.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDMTA) and its application in dental resin. J. Mater. Sci. Mater. Med. 2013, 24, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Hilton, T.J. Polymerization stress-is it clinically meaningful? Dent. Mater. 2016, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.W.; Lindberg, A. A 15-year randomized controlled study of a reduced shrinkage stress resin composite. Dent. Mater. 2015, 31, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Tosco, V.; Vitiello, F.; Furlani, M.; Gatto, M.L.; Monterubbianesi, R.; Giuliani, A.; Orsini, G.; Putignano, A. Microleakage Analysis of Different Bulk-Filling Techniques for Class II Restorations: µ-CT, SEM and EDS Evaluations. Materials 2021, 14, 31. [Google Scholar] [CrossRef]
- He, J.; Söderling, E.; Lassila, L.V.J.; Vallittu, P.K. Preparation of antibacterial and radio-opaque dental resin with new polymerizable quaternary ammonium monomer. Dent. Mater. 2015, 31, 575–582. [Google Scholar] [CrossRef]
- He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.K.; Lassila, L. The effect of adding a new monomer “Phene” on the polymerization shrinkage reduction of a dental resin composite. Dent. Mater. 2019, 35, 627–635. [Google Scholar] [CrossRef]
- Wang, X.; Huyang, G.; Palagummi, S.V.; Liu, X.; Skrtic, D.; Beauchamp, C.; Bowen, R.; Sun, J. High performance dental resin composites with hydrolytically stable monomers. Dent. Mater. 2018, 34, 228–237. [Google Scholar] [CrossRef]
- Asmussen, E. Factors affecting the color stability of restorative resins. Acta Odontol. Scand. 1983, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Garoushi, S.; Lassila, L.V.; Vallittu, P.K. Influence of nanometer scale particulate fillers on some properties of microfilled composite resin. J. Mater. Sci. Mater. Med. 2011, 22, 1645–1651. [Google Scholar] [CrossRef]
- Cavalcante, L.M.; Schneider, L.F.; Silikas, N.; Watts, D.C. Surface integrity of solvent-challenged ormocer-matrix composite. Dent. Mater. 2011, 27, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Yap, A.U. Effects of surface finish on indentation modulus and hardness of dental composite restoratives. Dent. Mater. 2005, 21, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Lassila, L.; Säilynoja, E.; Prinssi, R.; Vallittu, P.K.; Garoushi, S. The effect of polishing protocol on surface gloss of different restorative resin composites. Biomater. Investig. Dent. 2020, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Jager, N.; Feilzer, A.J.; Davidson, C.L. The influence of surface roughness on porcelain strength. Dent. Mater. 2000, 16, 381–388. [Google Scholar] [CrossRef]
- Omba, A.; Scotti, N.; Maravić, T.; Mazzoni, A.; Carossa, M.; Breschi, L.; Cadenaro, M. Vickers Hardness and Shrinkage Stress Evaluation of Low and High Viscosity Bulk-Fill Resin Composite. Polymers 2020, 12, 1477. [Google Scholar]
- Kakaboura, A.; Fragouli, M.; Rahiotis, C.; Silikas, N. Evaluation of surface characteristics of dental composites using profilometry, scanning electron, atomic force microscopy and gloss-meter. J. Mater. Sci. Mater. Med. 2007, 18, 155–163. [Google Scholar] [CrossRef]
- He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.K.; Lassila, L. Physicochemical properties of dimethacrylate resin composites with comonomer of Hexa/Tri-ethylene glycol bis(carbamate-isoproply-α-methylstyrene). J. Mech. Behav. Biomed. Mater. 2020, 108, 103832. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA, Bis-GMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [CrossRef]
- Silva, E.M.; Miragaya, L.; Noronha-filho, J.D.; Amaral, C.M.; Poskus, L.T.G. Characterization of an experimental resin composite resin composite organic matrix based on a tri-functional methacrylate monomer. Dent. Mater. J. 2016, 35, 159–165. [Google Scholar] [CrossRef]
- Hahnel, S.; Schultz, S.; Trempler, C.; Ach, B.; Handel, G.; Rosentritt, M. Two-body wear of dental restorative materials. J. Mech. Behav. Biomed. Mater. 2011, 4, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Characterization of fluoride releasing restorative dental materials. Dent. Mater. J. 2018, 37, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Furuse, A.Y.; Gordon, K.; Rodrigues, F.P.; Silikas, N.; Watts, D.C. Colour-stability and gloss-retention of silorane and dimethacrylate composites with accelerated aging. J. Dent. 2008, 36, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Roeder, L.B.; Tate, W.H.; Powers, J.M. Effect of finishing and polishing procedures on the surface roughness of packable composites. Oper. Dent. 2000, 25, 534–543. [Google Scholar] [PubMed]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Yap, A.U.; Sau, C.W.; Lye, K.W. Effects of finishing/polishing time on surface characteristics of tooth-coloured restoratives. J. Oral Rehabil. 1998, 25, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bijelic-Donova, J.; Garoushi, S.; Lassila, L.V.; Vallittu, P.K. Oxygen inhibition layer of composite resins: Effects of layer thickness and surface layer treatment on the interlayer bond strength. Eur. J. Oral Sci. 2015, 123, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lee, Y.K. Influence of color parameters of resin composites on their translucency. Dent. Mater. 2008, 24, 1236–1242. [Google Scholar] [CrossRef]
- Alkhadim, Y.K.; Hulbah, M.J.; Nassar, H.M. Color shift, solor stability, and post-polishing surface roughness of esthetic resin composites. Materials 2020, 13, 1376. [Google Scholar] [CrossRef]
- Lassila, L.V.; Nagas, E.; Vallittu, P.K.; Garoushi, S. Translucency of flowable bulk-filling composites of various thicknesses. Chin. J. Dent. Res. 2012, 15, 31–35. [Google Scholar]
- Garoushi, S.; Lassila, L.; Hatem, M.; Shembesh, M.; Baady, L.; Salim, Z.; Vallittu, P. Influence of staining solutions and whitening procedures on discoloration of hybrid composite resins. Acta Odontol. Scand. 2013, 71, 144–150. [Google Scholar] [CrossRef] [PubMed]

| Resin Matrix | Components (%) | ||||
|---|---|---|---|---|---|
| Bis-GMA | TEGDMA | HE/TE-Phene | DMAEMA | CQ | |
| Control | 49.3 | 49.3 | 0 | 0.7 | 0.7 |
| HE/TE-EC1 | 44.3 | 44.3 | 10 | 0.7 | 0.7 |
| HE/TE-EC2 | 39.3 | 39.3 | 20 | 0.7 | 0.7 |
| HE/TE-EC3 | 34.3 | 34.3 | 30 | 0.7 | 0.7 |
| HE/TE-EC4 | 29.3 | 29.3 | 40 | 0.7 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.; Lassila, L. Surface Integrity of Dimethacrylate Composite Resins with Low Shrinkage Comonomers. Materials 2021, 14, 1614. https://doi.org/10.3390/ma14071614
He J, Garoushi S, Säilynoja E, Vallittu P, Lassila L. Surface Integrity of Dimethacrylate Composite Resins with Low Shrinkage Comonomers. Materials. 2021; 14(7):1614. https://doi.org/10.3390/ma14071614
Chicago/Turabian StyleHe, Jingwei, Sufyan Garoushi, Eija Säilynoja, Pekka Vallittu, and Lippo Lassila. 2021. "Surface Integrity of Dimethacrylate Composite Resins with Low Shrinkage Comonomers" Materials 14, no. 7: 1614. https://doi.org/10.3390/ma14071614
APA StyleHe, J., Garoushi, S., Säilynoja, E., Vallittu, P., & Lassila, L. (2021). Surface Integrity of Dimethacrylate Composite Resins with Low Shrinkage Comonomers. Materials, 14(7), 1614. https://doi.org/10.3390/ma14071614








