Thermodynamic Assessment of Bio-Oriented Ti-Ta-Sn System
Abstract
1. Introduction
2. Experimental Information
3. Thermodynamic Models
| Phase/Model | Thermodynamic Parameters | Reference |
|---|---|---|
| Liquid: (Sn,Ta,Ti)1 | [14] | |
| [12] | ||
| [12] | ||
| [13] | ||
| [13] | ||
| bcc: (Sn,Ta,Ti)1(Va)3 | [14] | |
| [12] | ||
| [12] | ||
| [13] | ||
| [13] | ||
| This work | ||
| This work | ||
| This work | ||
| hcp: (Sn,Ta,Ti)1(Va)0.5 | [12] | |
| [13] | ||
| [13] | ||
| [13] | ||
| bct: (Sn,Ta,Ti)1 | [13] | |
| Ti3Sn: (Ta,Ti)3(Sn,Va)1 | [13] | |
| [13] | ||
| This work | ||
| This work | ||
| This work | ||
| Ti2Sn: (Ta,Ti,Va)2(Sn,Va)1 | [13] | |
| [13] | ||
| [13] | ||
| [13] | ||
| [13] | ||
| [13] | ||
| This work | ||
| This work | ||
| This work | ||
| Ti5Sn3: (Ta,Ti)5(Sn)3 | [13] | |
| This work | ||
| This work | ||
| Ti6Sn5: (Ta,Ti)6(Sn)5 | [13] | |
| This work | ||
| This work | ||
| Ti2Sn3: (Ta,Ti)2(Sn)3 | [13] | |
| This work | ||
| This work | ||
| Ta3Sn: (Ta,Ti)3(Sn)1 | [14] | |
| This work | ||
| This work | ||
| Ta2Sn3: (Ta)2(Sn)3 | [14] | |
| Ti36Ta28Sn36: (Ti)0.36(Ta) 0.28(Sn)0.36 | This work |
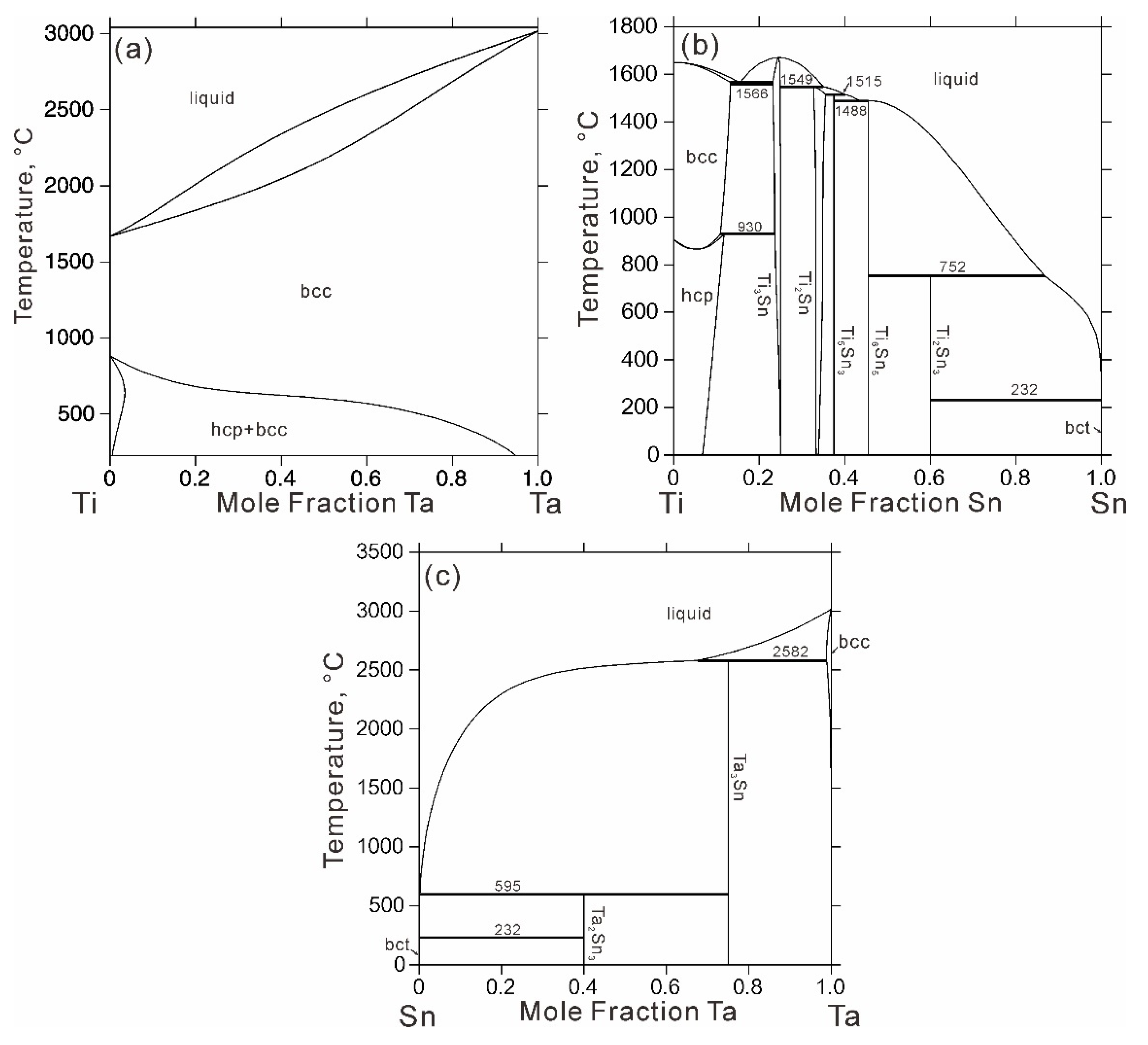
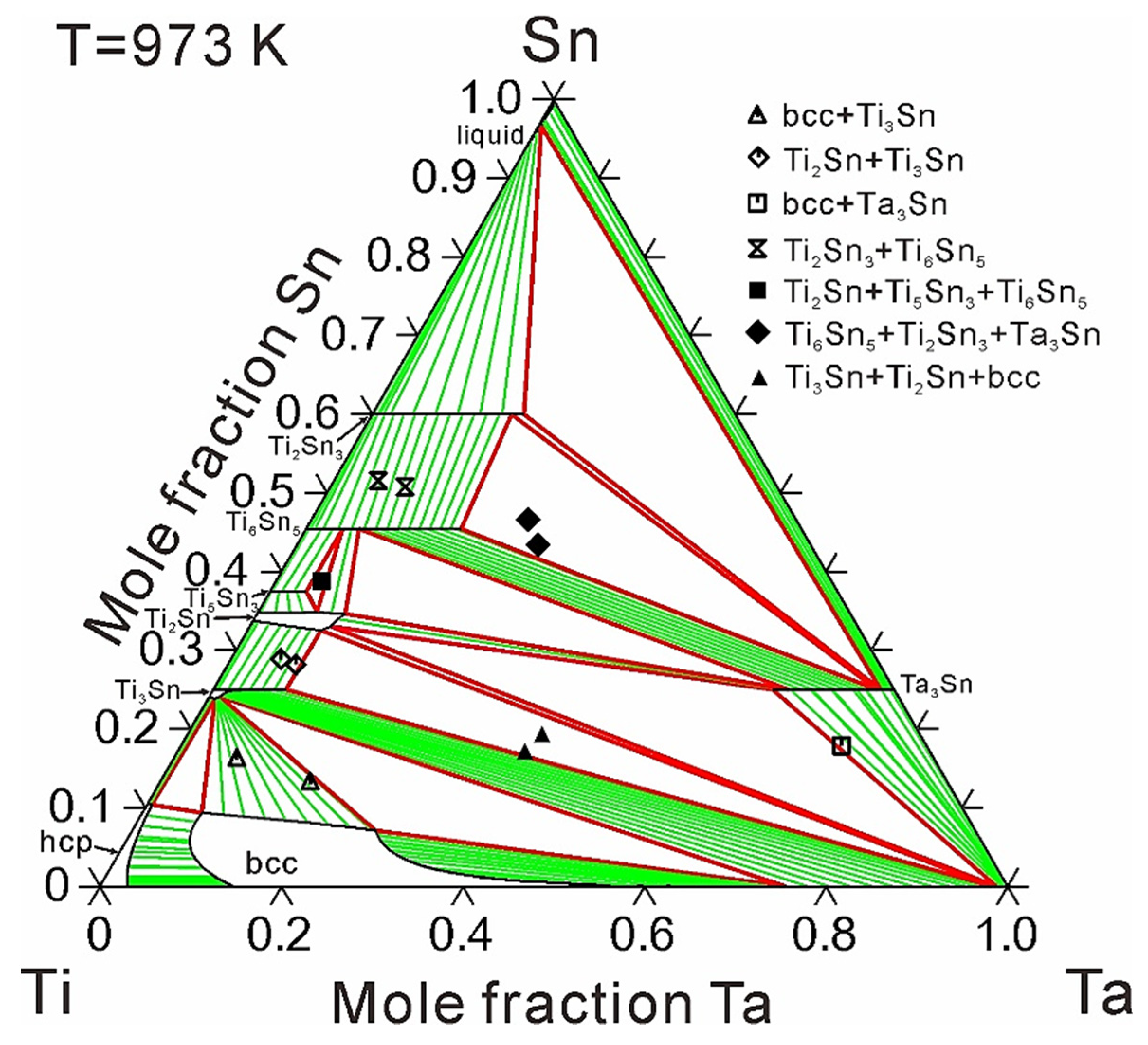
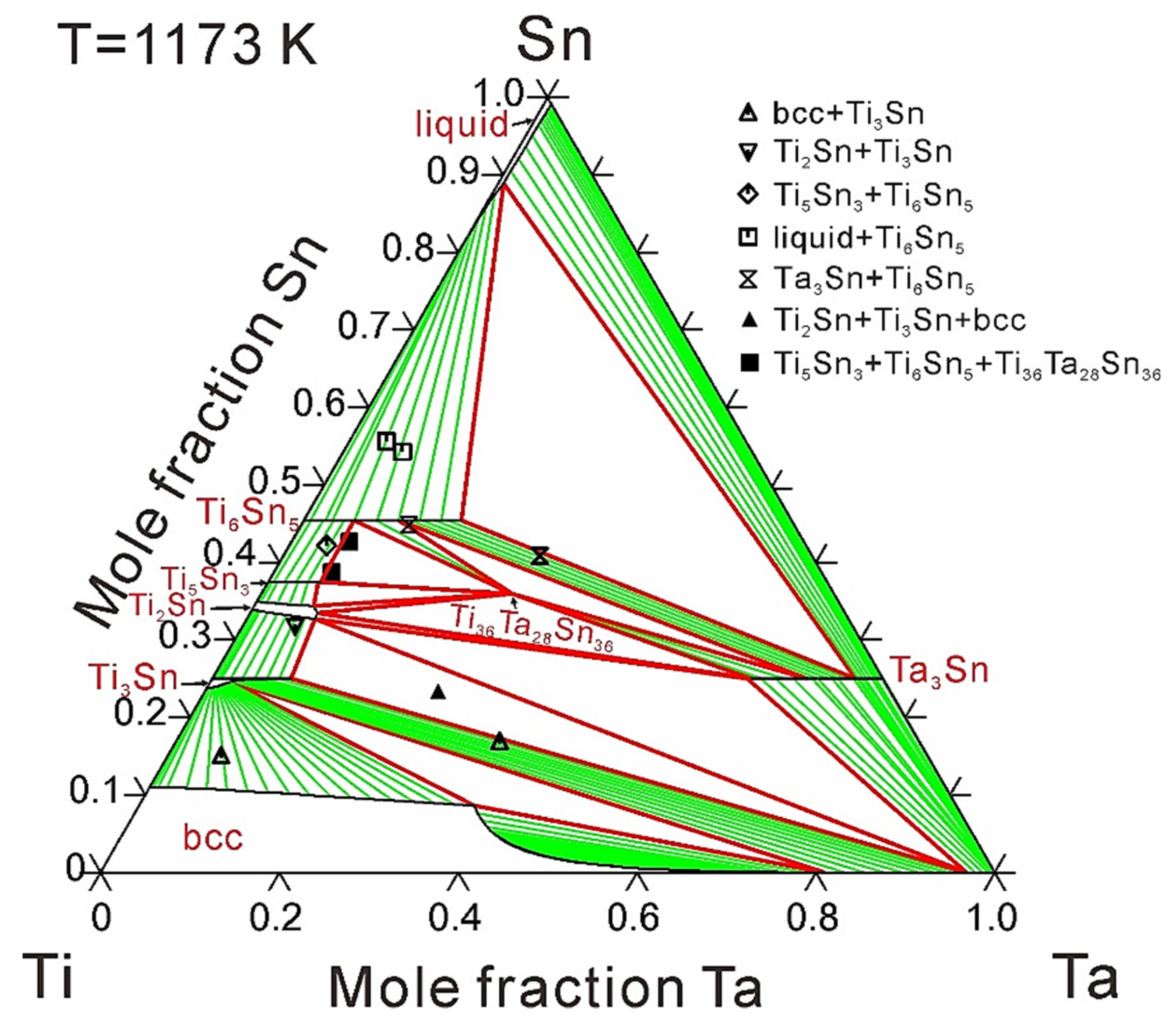
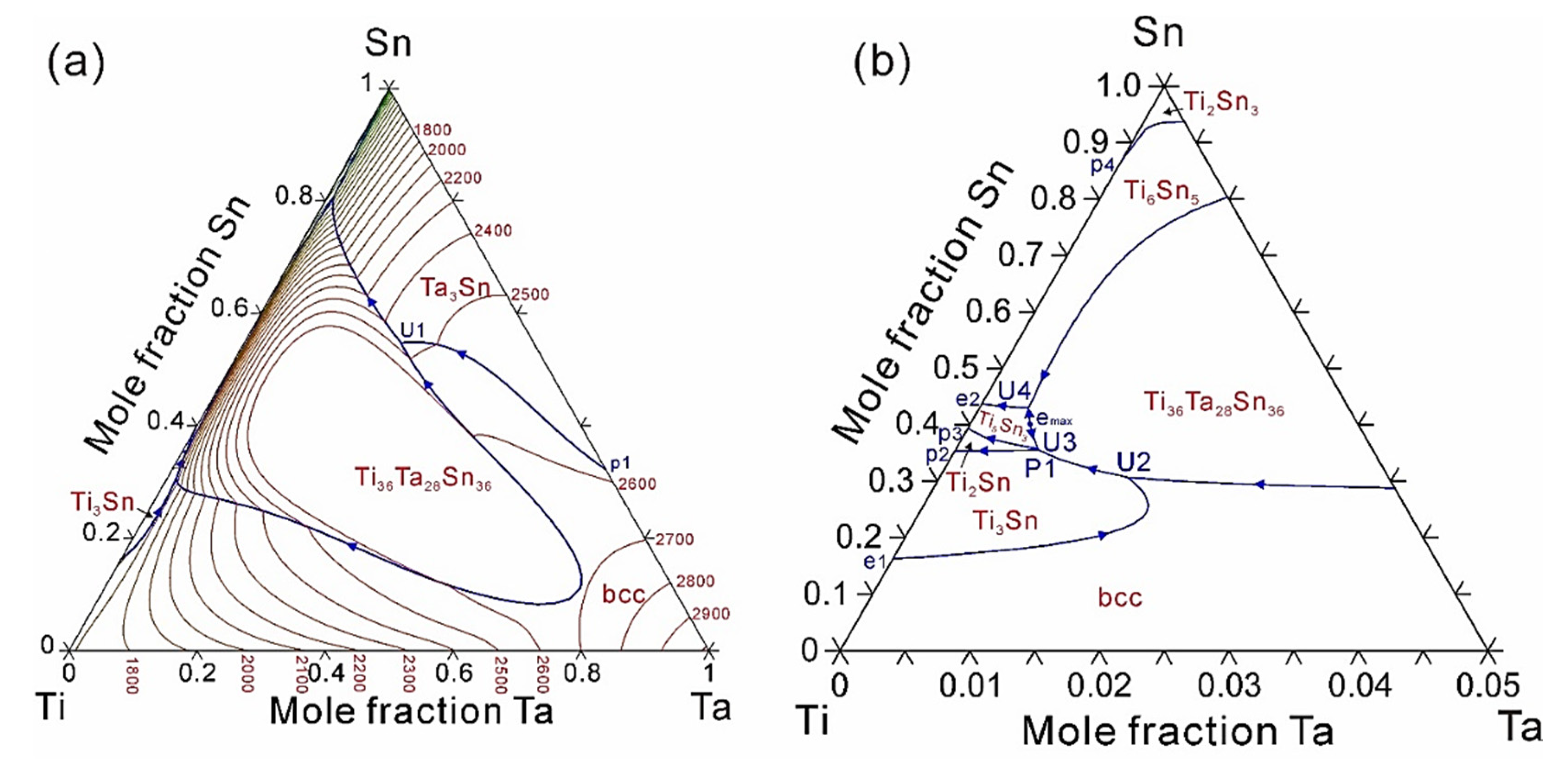
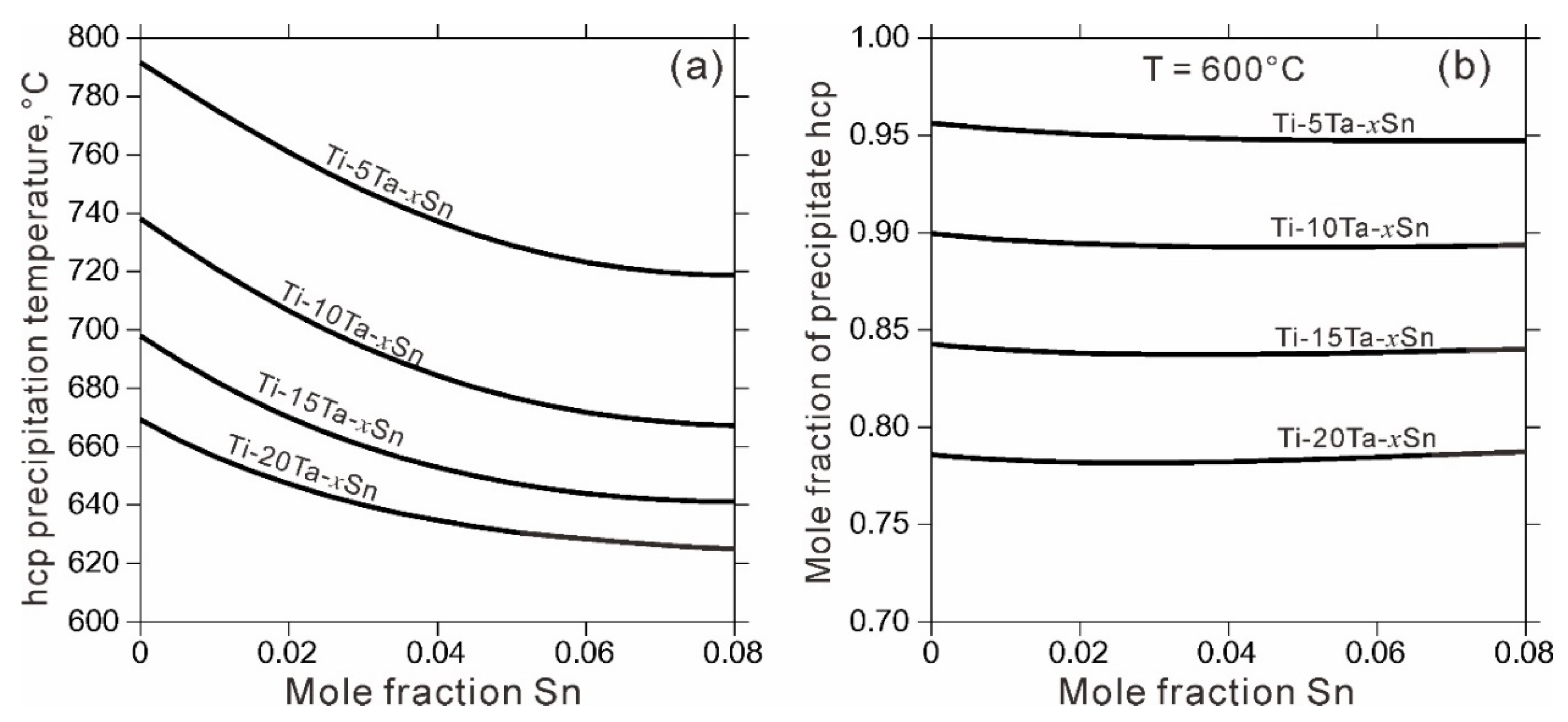
4. Results and Discussion
| Compounds | Temperature, K | Solubilities of the Third Element, at.% | Reference | |
|---|---|---|---|---|
| Ti | Ta | |||
| Ti3Sn | 973 | - | 8.3 | [16] |
| - | 8.0 | This work | ||
| 1173 | - | 9.3 | [17] | |
| - | 9.0 | This work | ||
| Ti2Sn | 973 | - | over 8.8 | [16] |
| - | 9.7 | This work | ||
| 1173 | - | over 7.3 | [17] | |
| - | 7.6 | This work | ||
| Ti5Sn3 | 973 | - | 4.2 | [16] |
| - | 4.0 | This work | ||
| 1173 | - | 6.1 | [17] | |
| - | 6.0 | This work | ||
| Ti6Sn5 | 973 | - | 16.8 | [16] |
| - | 17.0 | This work | ||
| 1173 | - | over 15.5 | [17] | |
| - | 17.6 | This work | ||
| Ta3Sn | 973 | 21.3 | over 10.9 | [16] |
| 20.8 | 11.6 | This work | ||
| 1173 | 24.9 | over 8.8 | [17] | |
| 25.3 | 15.2 | This work | ||
| Type | Invariant Reaction | Temperature, °C | Source |
|---|---|---|---|
| p1 | liquid + bcc = Ta3Sn | 2582 | [14] |
| U1 | liquid + bcc = Ta3Sn + Ti36Ta28Sn36 | 2450 | This work |
| U2 | liquid + bcc = Ti3Sn + Ti36Ta28Sn36 | 1628 | This work |
| e1 | liquid = bcc + Ti3Sn | 1566 | [13] |
| p2 | liquid + Ti3Sn = Ti2Sn | 1549 | [13] |
| P2 | liquid + Ti3Sn + Ti36Ta28Sn36 = Ti2Sn | 1542 | This work |
| U3 | liquid + Ti36Ta28Sn36 = Ti2Sn + Ti5Sn3 | 1541 | This work |
| U4 | liquid + Ti36Ta28Sn36 = Ti6Sn5 + Ti5Sn3 | 1519 | This work |
| p3 | liquid + Ti2Sn = Ti5Sn3 | 1515 | [13] |
| e2 | liquid = Ti5Sn3 + Ti6Sn5 | 1488 | [13] |
| p4 | liquid + Ti6Sn5 = Ti2Sn3 | 752 | [13] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banerjee, D.; Williams, J.C. Perspectives on Titanium Science and Technology. Acta Mater. 2013, 61, 844–879. [Google Scholar] [CrossRef]
- Yoshimitsu, O.; Katsuda, S. Biological Safety Evaluation and Surface Modification of Biocompatible Ti–15Zr–4Nb Alloy. Materials 2021, 14, 731. [Google Scholar]
- Kim, H.Y.; Fukushima, T.; Buenconsejo, P.J.S.; Nam, T.H.; Miyazaki, S. Martensitic transformation and shape memory properties of Ti-Ta-Sn high temperature shape memory alloys. Mater. Sci. Eng. A 2011, 528, 7238–7246. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Panicker, A.G.; Acharya, S.; Laxmi, D.V.; Suwas, S.; Chatterjee, K. Study of the influence of Zr on the mechanical properties and functional response of Ti-Nb-Ta-Zr-O alloy for orthopedic applications. Mater. Des. 2019, 164, 107555. [Google Scholar]
- Li, M.J.; Min, X.H.; Yao, K.; Ye, F. Novel insight into the formation of α”-martensite and ω-phase with cluster structure in metastable Ti-Mo alloys. Acta Mater. 2019, 164, 332–333. [Google Scholar] [CrossRef]
- Tong, Y.X.; Guo, B.; Zheng, Y.F.; Chung, C.Y.; Ma, L.W. Effects of Sn and Zr on the microstructure and mechanical properties of Ti-Ta-based shape memory alloys. J. Mater. Eng. Perform. 2011, 20, 762–766. [Google Scholar] [CrossRef]
- Guo, B.; Tong, Y.X.; Chen, F.; Zheng, Y.F.; Li, L.; Chung, C.Y. Effect of Sn addition on the corrosion behavior of Ti-Ta alloy. Mater. Corros. 2012, 63, 259–263. [Google Scholar] [CrossRef]
- Cremasco, A.; Andrade, P.N.; Contieri, R.J.; Lopes, E.S.N.; Afonso, C.R.M.; Caram, R. Correlations between aging heat treatment, ω phase precipitation and mechanical properties of a cast Ti-Nb alloy. Mater. Des. 2011, 32, 2387–2390. [Google Scholar] [CrossRef]
- Tag, X.; Ahmed, T.; Rack, H.J. Phase transformations in Ti–Nb–Ta and Ti–Nb–Ta–Zr alloys. J. Mater. Sci. 2000, 35, 1805–1811. [Google Scholar]
- Peng, Y.B.; Du, Y.; Zhou, P.; Zhang, W.B.; Chen, W.M.; Chen, L.; Wang, S.Q.; Wen, G.H.; Xie, W. CSUTDCC1—A thermodynamic database for multicomponent cemented carbides. Int. J. Refract. Met. Hard Mater. 2014, 42, 57–70. [Google Scholar] [CrossRef]
- Saunders, N. System Ta-Ti. In Thermochemical Database for Light Metal Alloys; Ansara, I., Dinsdale, A.T., Rand, M.H., Eds.; Office for Official Publications of the European Communities: Luxembourg, 1998; Volume 2, pp. 293–296. [Google Scholar]
- Liu, C.; Klotz, U.E.; Uggowitzer, P.J.; Löffler, J.F. Thermodynamic assessment of the Sn-Ti system. Monatsh. Chem. 2005, 136, 1921–1930. [Google Scholar] [CrossRef]
- Wang, J.; Tuo, B.; Hu, X.; Liu, Z. Thermodynamic properties and thermodynamic assessment of Ta-Sn system. Adv. Mater. Res. 2013, 821, 848–853. [Google Scholar] [CrossRef]
- Marker, C.; Shang, S.; Liu, X.L.; Lindwall, G.; Liu, Z.-K. First-principles calculations and thermodynamic modeling of the Sn-Ta system. CALPHAD 2017, 57, 46–54. [Google Scholar] [CrossRef]
- Wang, J. Study on Phase Diagrams, Thermodynamics of Phase Equilibria and Alloy Design of Titanium Alloys. Ph.D. Thesis, Central South University, Changsha, China, 2015. [Google Scholar]
- Wang, J.; Liu, L.; Zhang, X.; Wang, X.; Bai, W. Isothermal section of the Ti-Ta-Sn ternary system at 1173 K. J. Phase Equilib. Diff. 2014, 39, 273–280. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. CALPHAD 1991, 15, 317–425. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, P.; Du, Y.; Chang, K. Thermodynamic evaluation of the C-Ta-Ti system and extrapolation to the C-Ta-Ti-N system. Int. J. Refract. Met. Hard Mater. 2013, 40, 36–42. [Google Scholar] [CrossRef]
- Hayes, F. System Sn-Ti. In Thermochemical Database for Light Metal Alloys; Ansara, I., Dinsdale, A.T., Rand, M.H., Eds.; Office for Official Publications of the European Communities: Luxembourg, 1998; Volume 2, pp. 284–290. [Google Scholar]
- Yin, F.; Tedenac, J.C.; Gascoin, F. Thermodynamic modelling of the Ti–Sn system and calculation of the Co–Ti–Sn system. CALPHAD 2007, 31, 370–379. [Google Scholar] [CrossRef]
- Basile, F. Crystallographic study and supraconductive properties of compounds V3Sn and Ta2Sn3. Ann. Chim. 1971, 6, 241–244. [Google Scholar]
- Okamoto, H. Sn-Ta (Tin-Tantalum) system. J. Phase Equilibria 2003, 24, 484. [Google Scholar] [CrossRef]
- Andersson, J.O.; Guillermet, A.F.; Hillert, M.; Jansson, B.; Sundman, B. A compound-energy model of ordering in a phase with sites of different coordination numbers. Acta Metall. 1986, 34, 437–445. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 1948, 40, 345–348. [Google Scholar] [CrossRef]
- Hillert, M.; Staffansson, L.I. The regular solution model for stoichiometric phases and ionic melts. Acta Chem. Scand. 1970, 24, 3618–3626. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc databank system. CALPHAD 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Aguilar, C.; Aguirre, T.; Martínez, C.; Barbieri, F.D.; Martín, F.S.; Salinas, V.; Alfonso, I. Improving the mechanical strength of ternary beta titanium alloy (Ti-Ta-Sn) foams using a bimodal microstructure. Mater. Des. 2020, 195, 108945. [Google Scholar] [CrossRef]

| Phase | Designation | Prototype | Pearson Symbol | Space Group |
|---|---|---|---|---|
| α-Ti | hcp | Mg | hP2 | P63/mmc |
| β-Ti | bcc | W | cI2 | Im3m |
| β-Ta | bcc | W | cI2 | Im3m |
| Ti3Sn | Ti3Sn | Ni3Sn | hP8 | P41212 |
| β-Sn | bct | β-Sn | - | I41/amd |
| α-Sn | diamond | C(diamond) | cF8 | Fd3m |
| Ti2Sn | Ti2Sn | Ni2In | hP6 | P63/mmc |
| Ti5Sn3 | Ti5Sn3 | Mn5Si3 | hP16 | P63/mcm |
| Ti6Sn5 | Ti6Sn5 | Ti6Sn5 | oI44 | Immm |
| Ti2Sn3 | Ti2Sn3 | - | oC40 | Cmca |
| Ta3Sn | Ta3Sn | Cr3Si | cP8 | Pm3n |
| Ta2Sn3 | Ta2Sn3 | CuMg2 | oF48 | Fddd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Peng, Y.; Li, T.; Yan, L.; He, S.; Xu, T. Thermodynamic Assessment of Bio-Oriented Ti-Ta-Sn System. Materials 2021, 14, 1568. https://doi.org/10.3390/ma14061568
Yan L, Peng Y, Li T, Yan L, He S, Xu T. Thermodynamic Assessment of Bio-Oriented Ti-Ta-Sn System. Materials. 2021; 14(6):1568. https://doi.org/10.3390/ma14061568
Chicago/Turabian StyleYan, Lifang, Yingbiao Peng, Tao Li, Lianwu Yan, Shiwen He, and Tao Xu. 2021. "Thermodynamic Assessment of Bio-Oriented Ti-Ta-Sn System" Materials 14, no. 6: 1568. https://doi.org/10.3390/ma14061568
APA StyleYan, L., Peng, Y., Li, T., Yan, L., He, S., & Xu, T. (2021). Thermodynamic Assessment of Bio-Oriented Ti-Ta-Sn System. Materials, 14(6), 1568. https://doi.org/10.3390/ma14061568





