Effect of Cement Types and Superabsorbent Polymers on the Properties of Sustainable Ultra-High-Performance Paste
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Material and Ratio Design
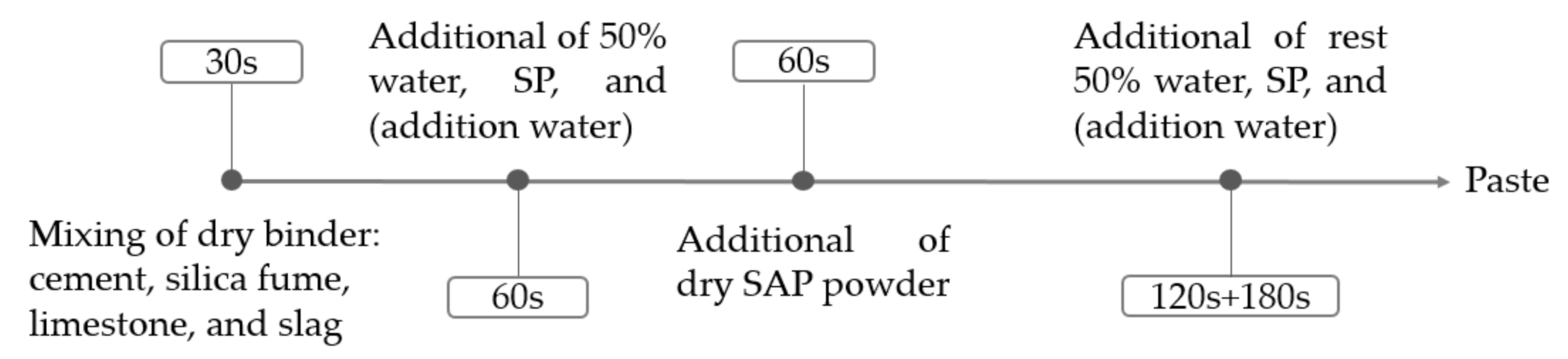
2.2. Mixture Preparation Method
2.3. Experimental Methods
3. Experimental Results
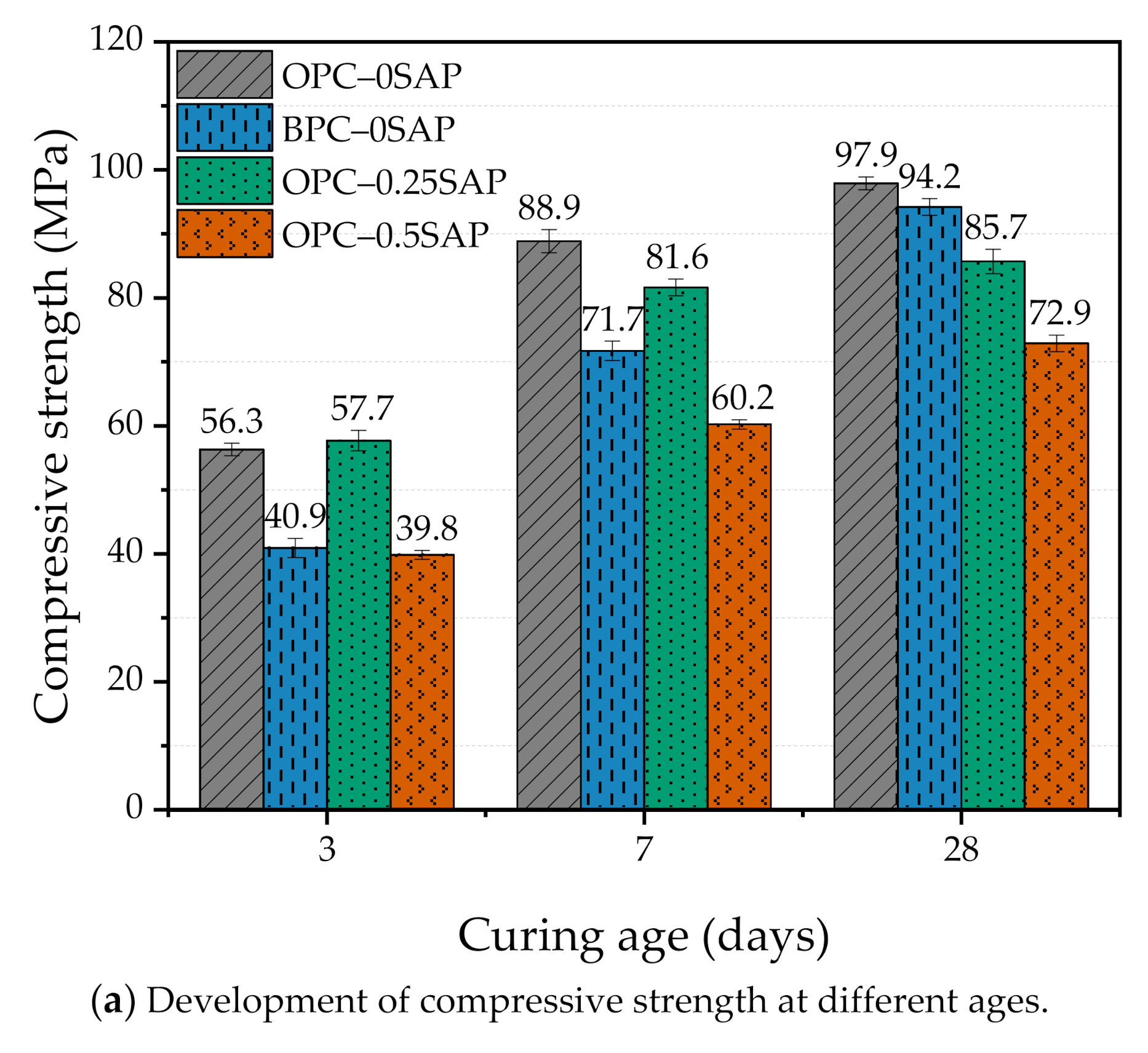
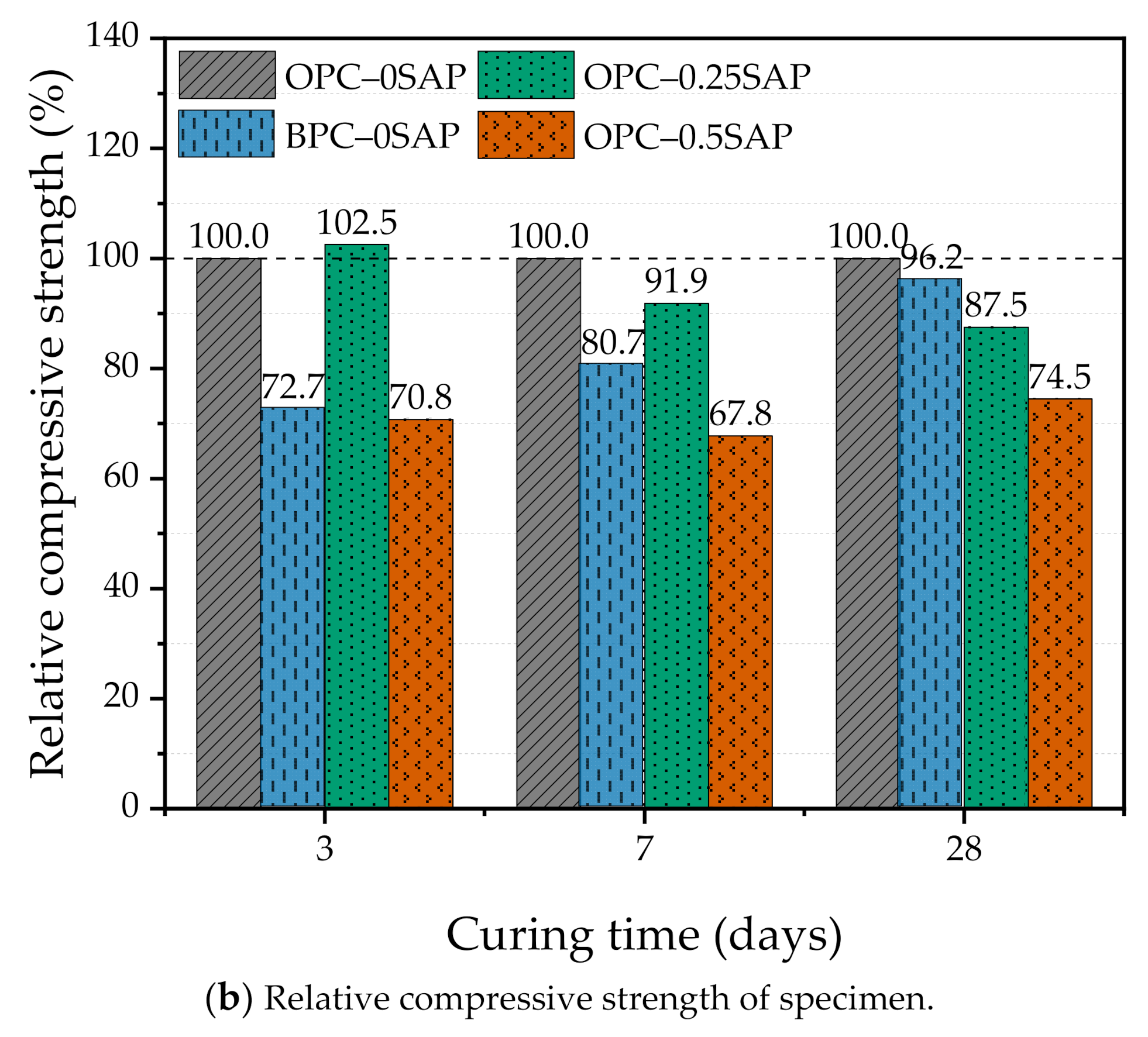
3.1. Compressive Strength
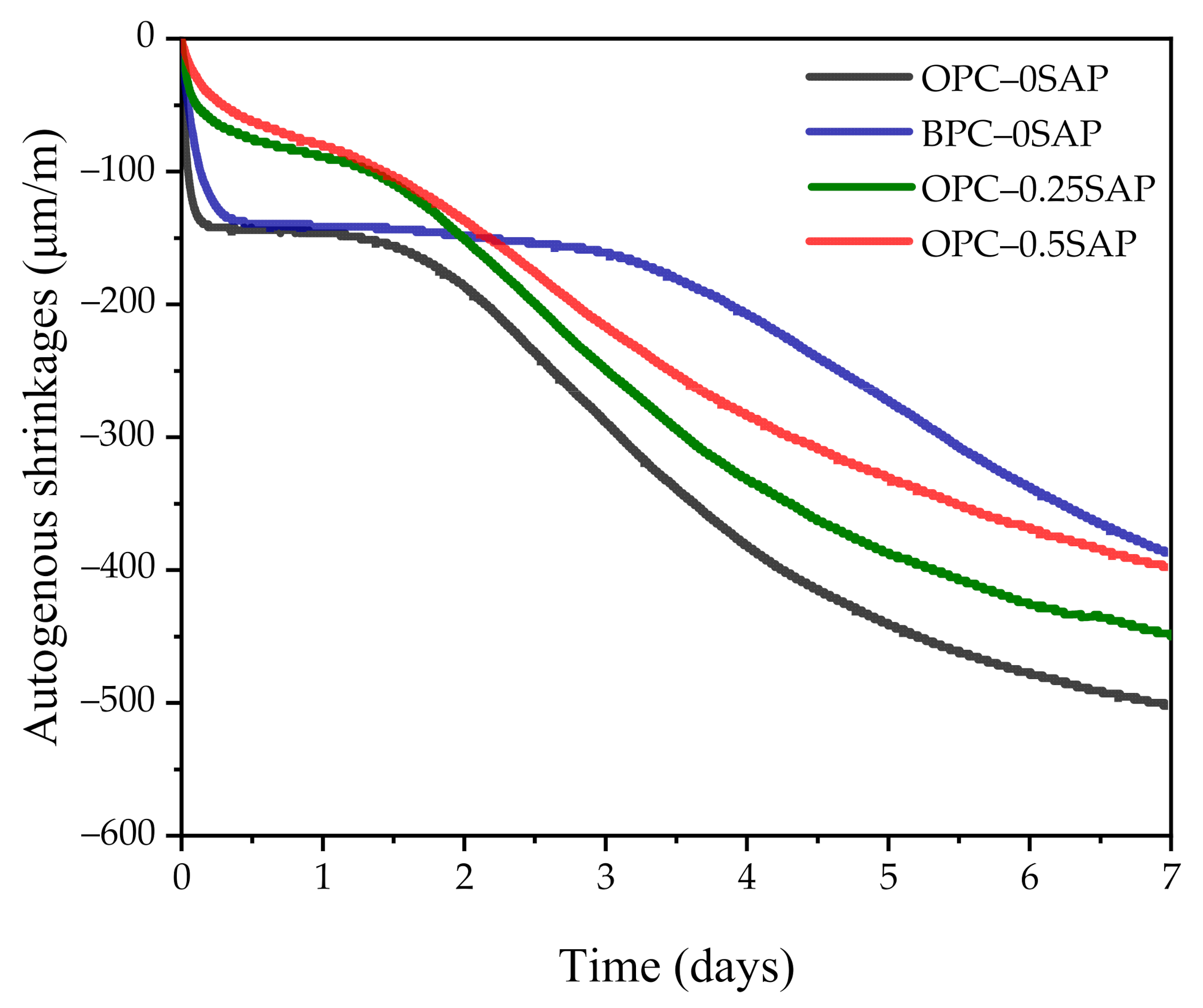
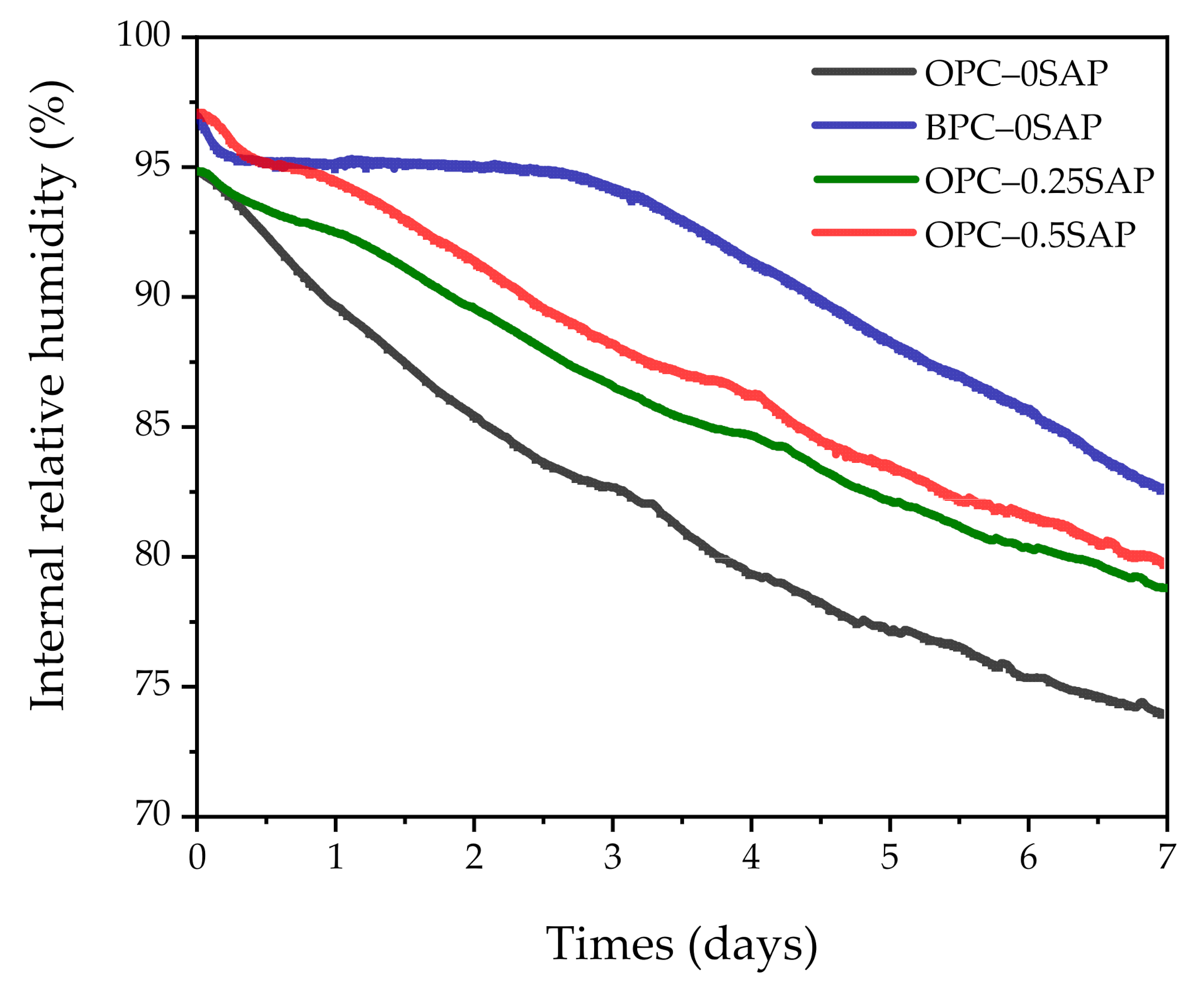
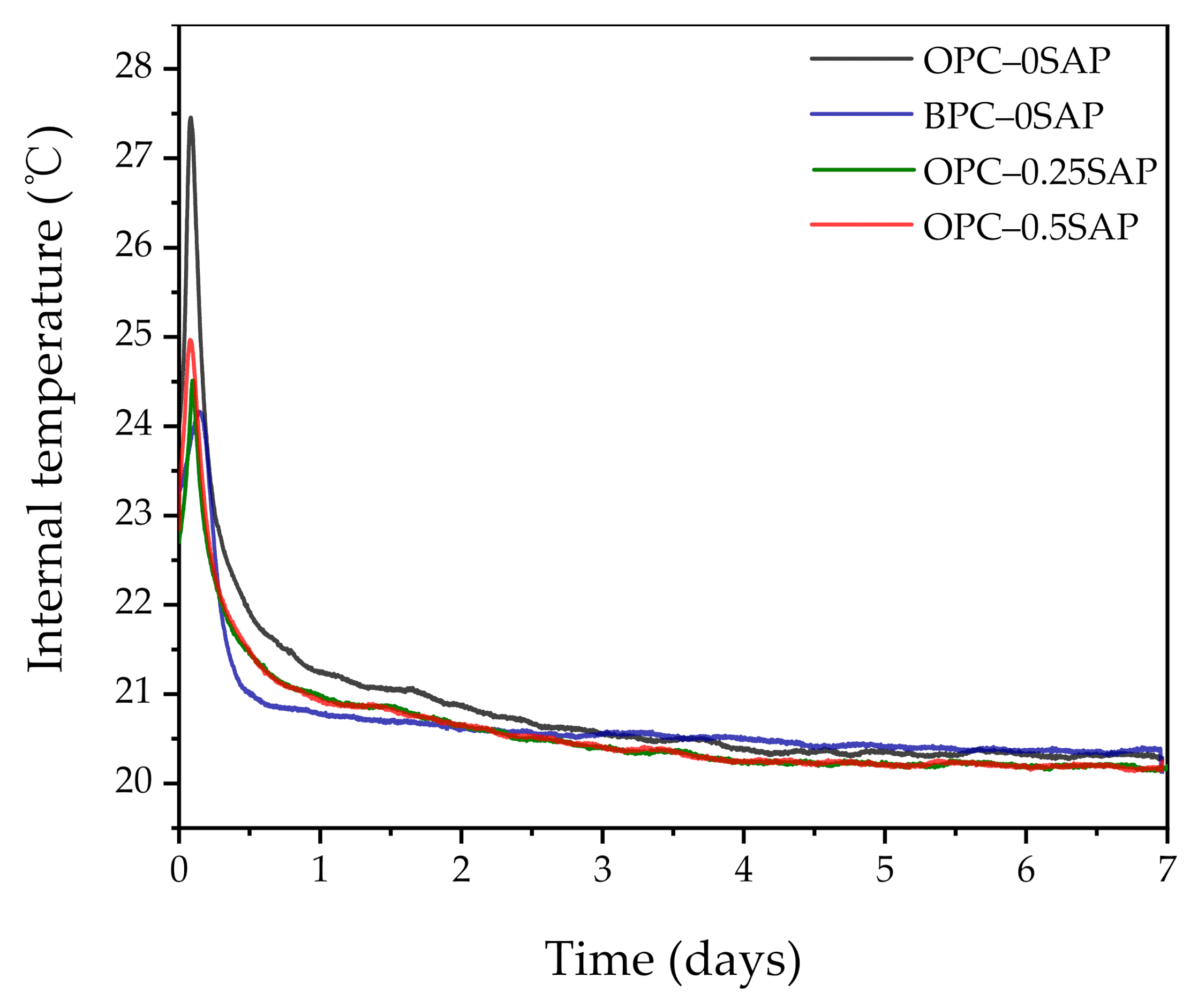
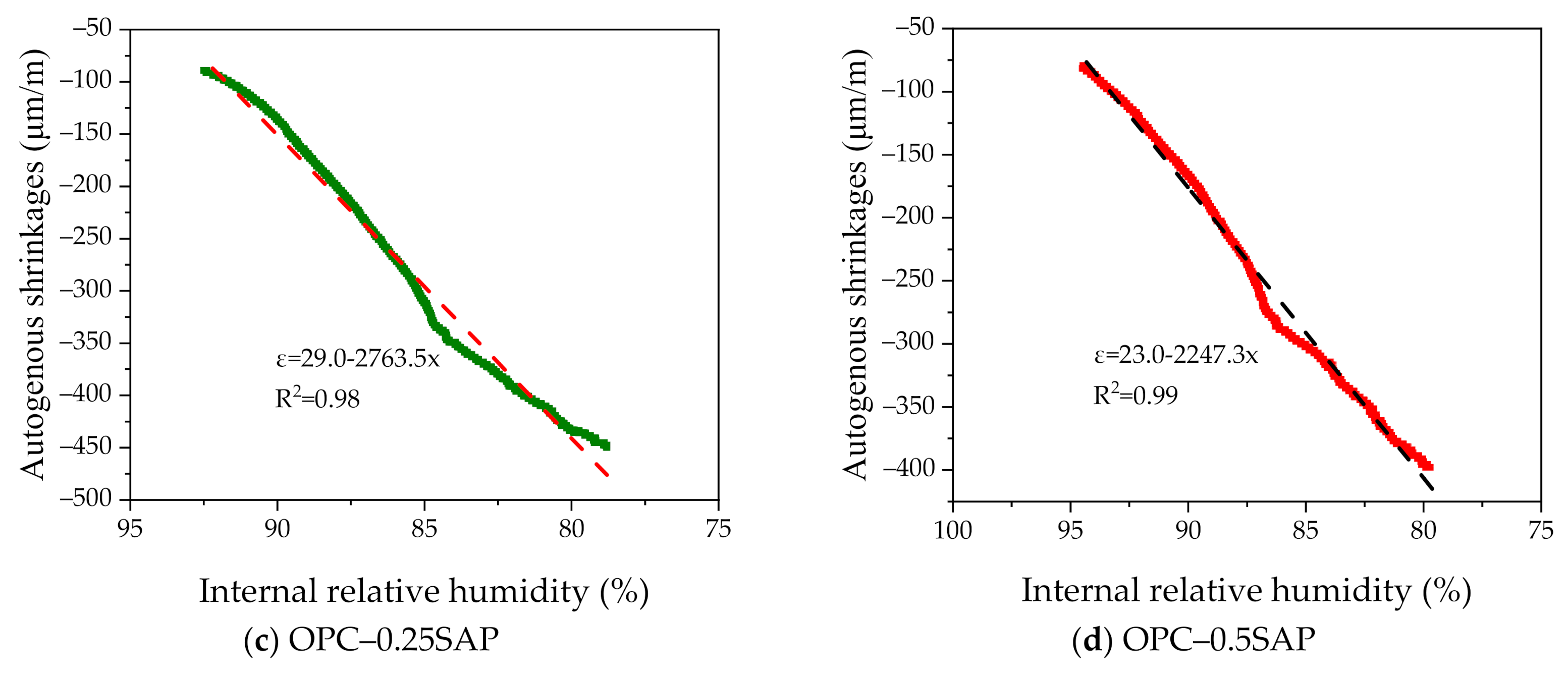
3.2. Autogenous Shrinkage (AS) Coupled with Internal Relative Humidity and Temperature
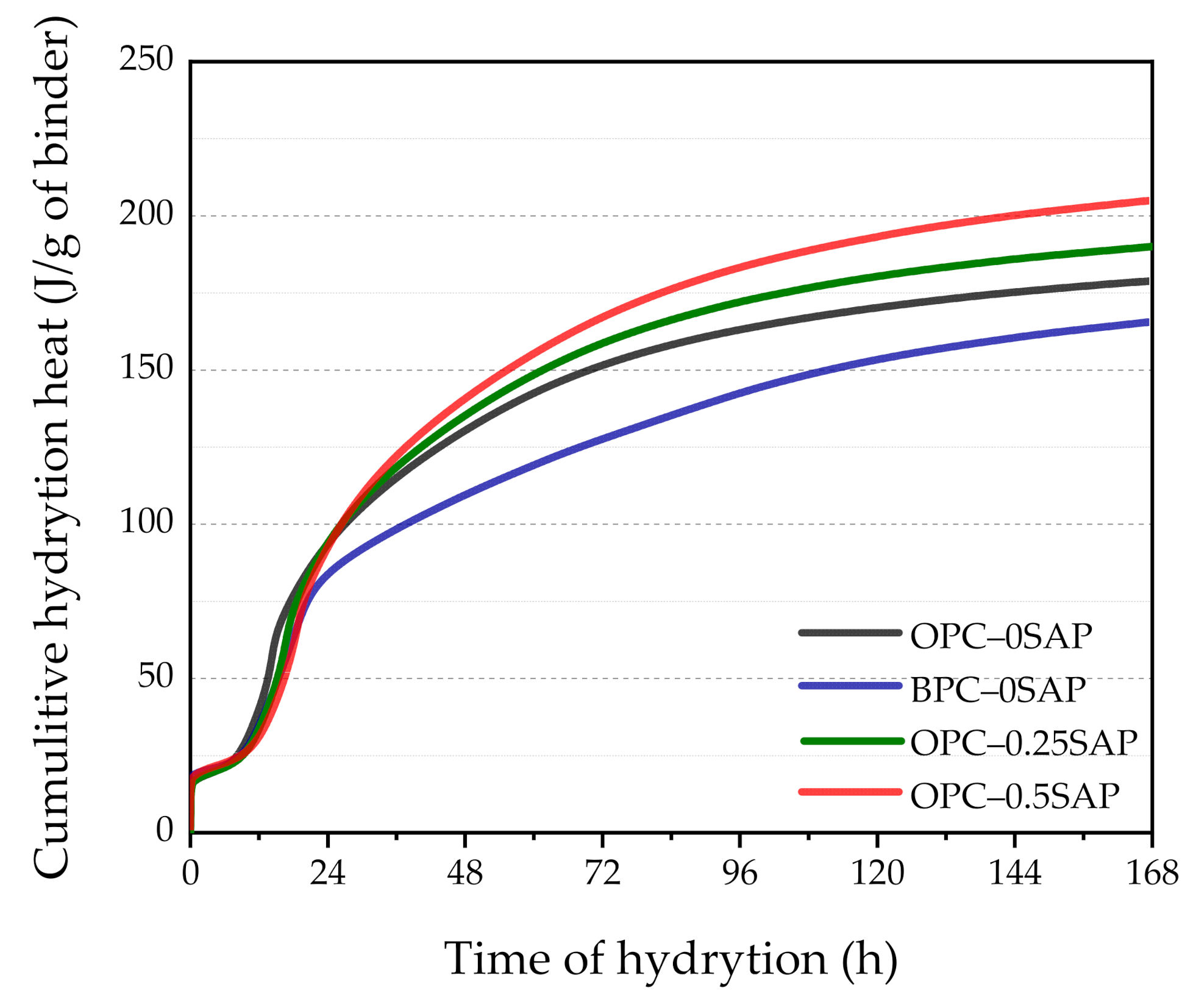
3.3. Isothermal Calorimetry
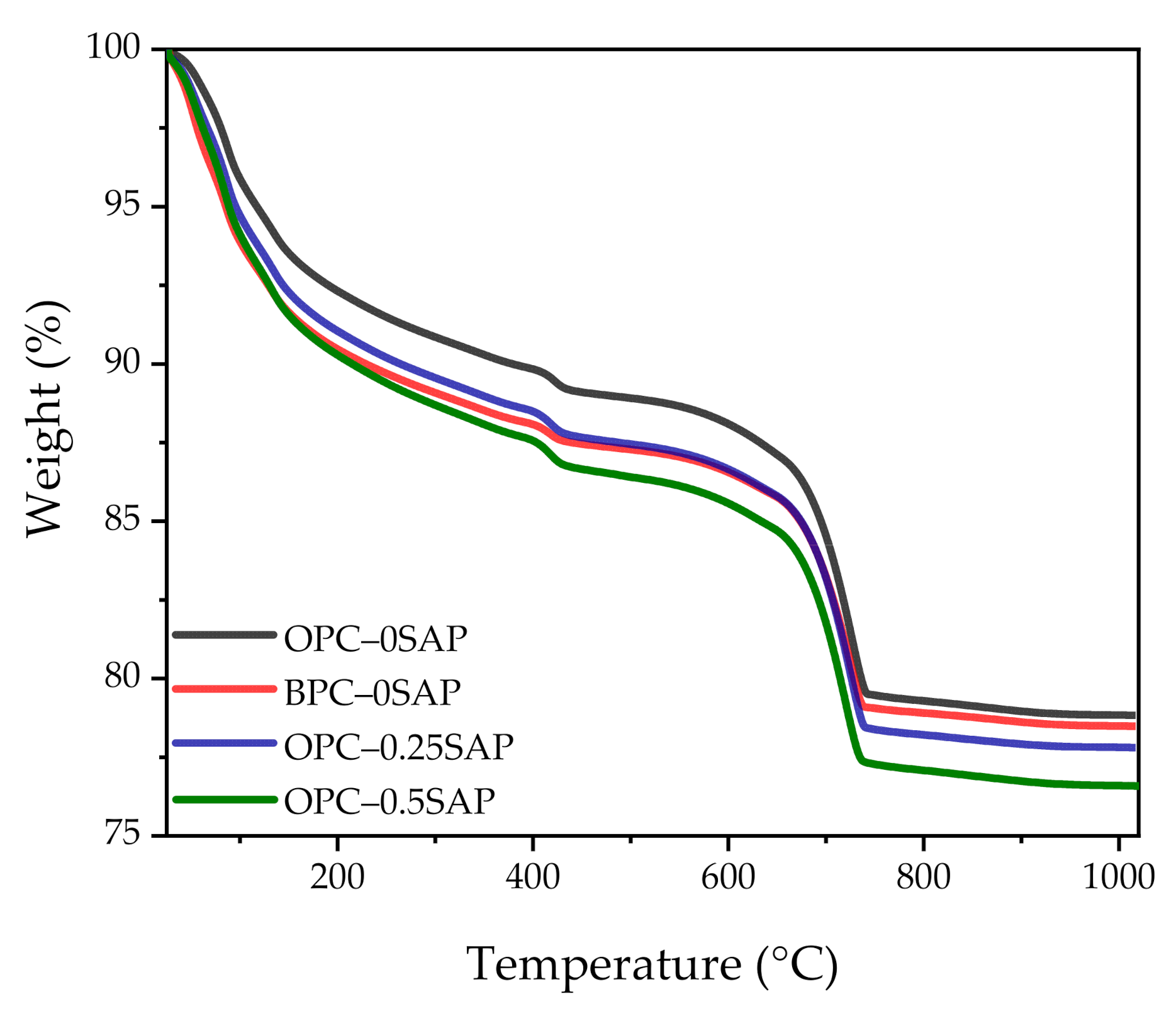
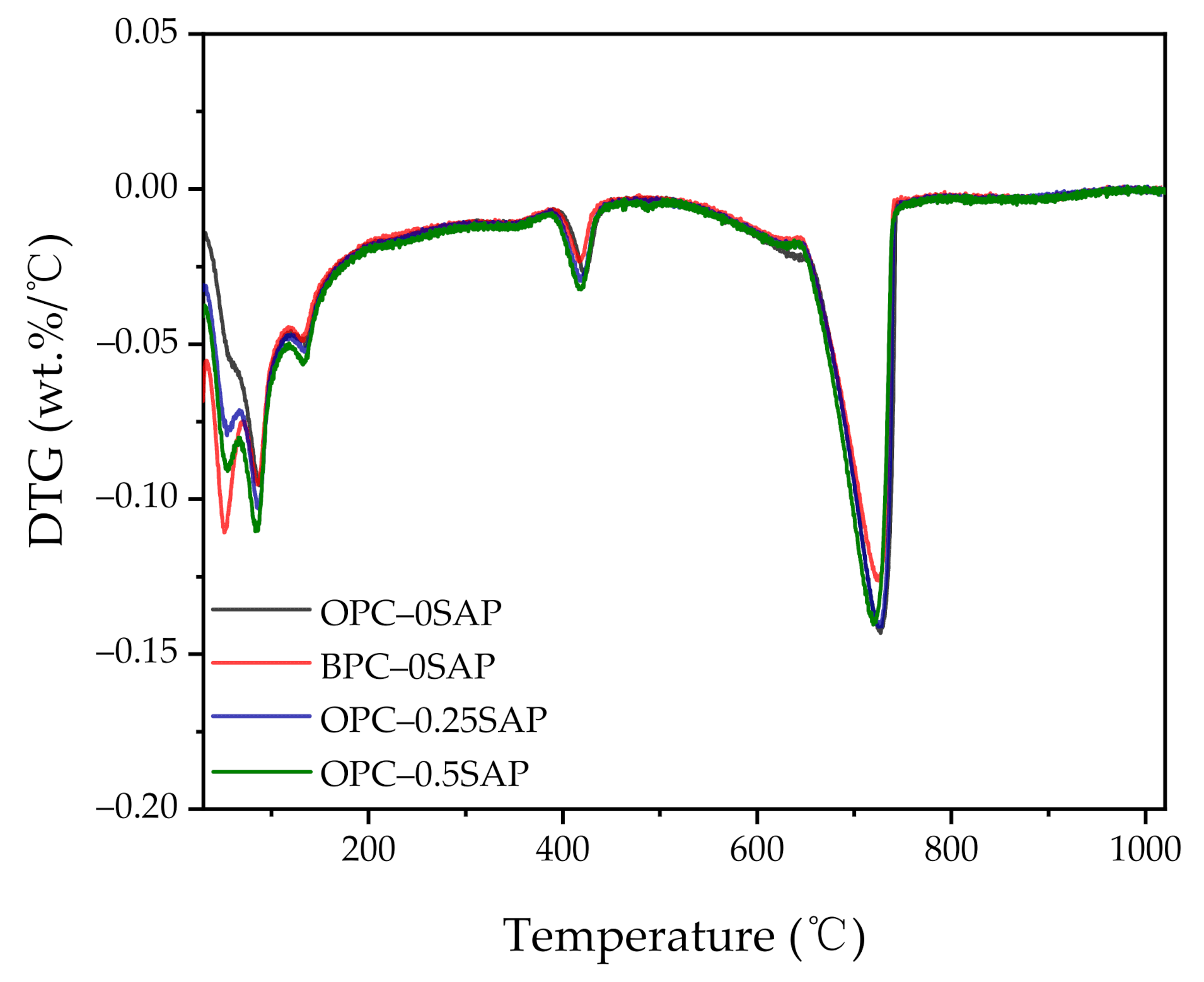
3.4. X-ray Diffraction (XRD) and Thermogravimetric Analysis (TGA)
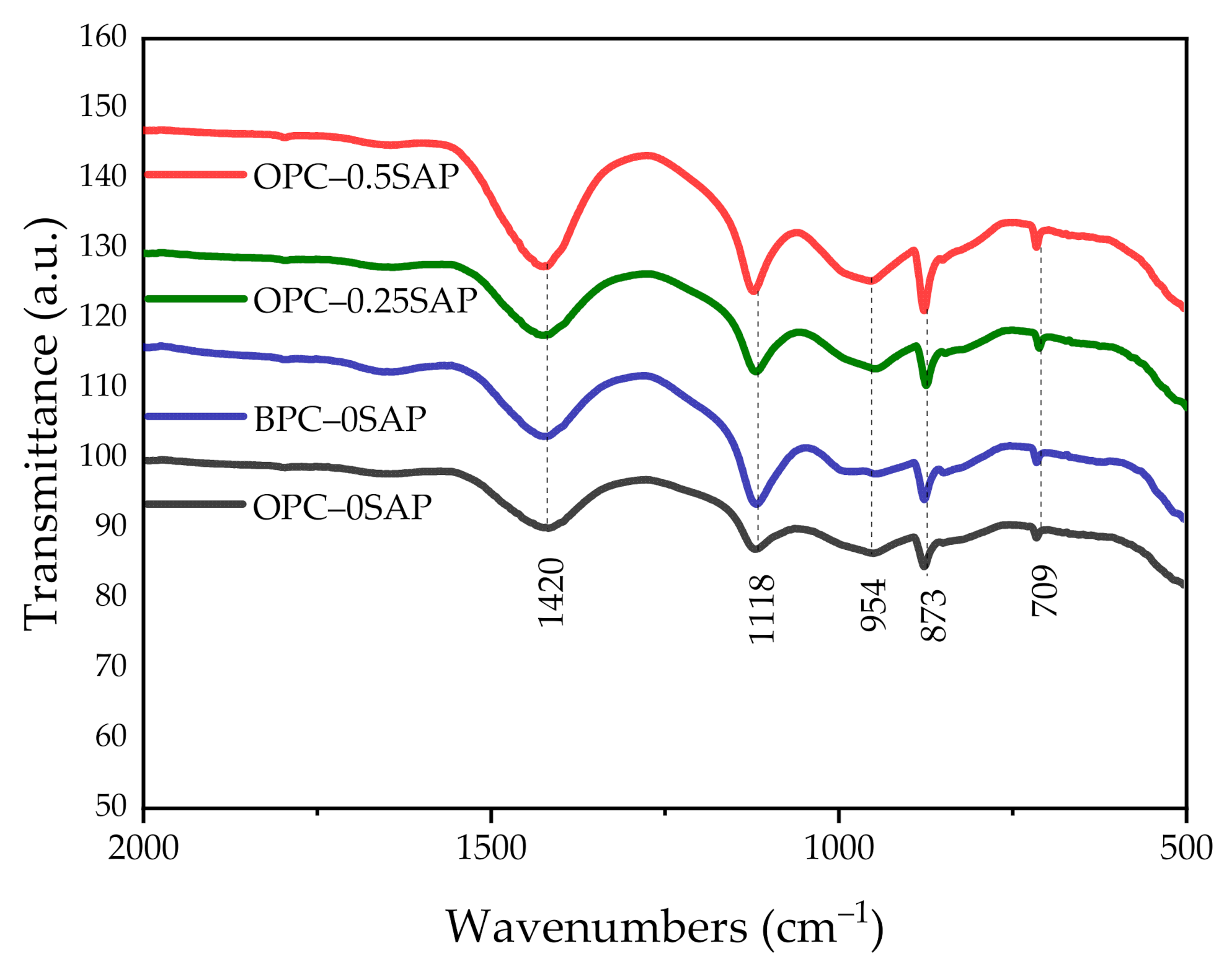
3.5. Attenuated Total Reflectance (ATR)–Fourier-Transform Infrared Spectroscopy (FTIR)
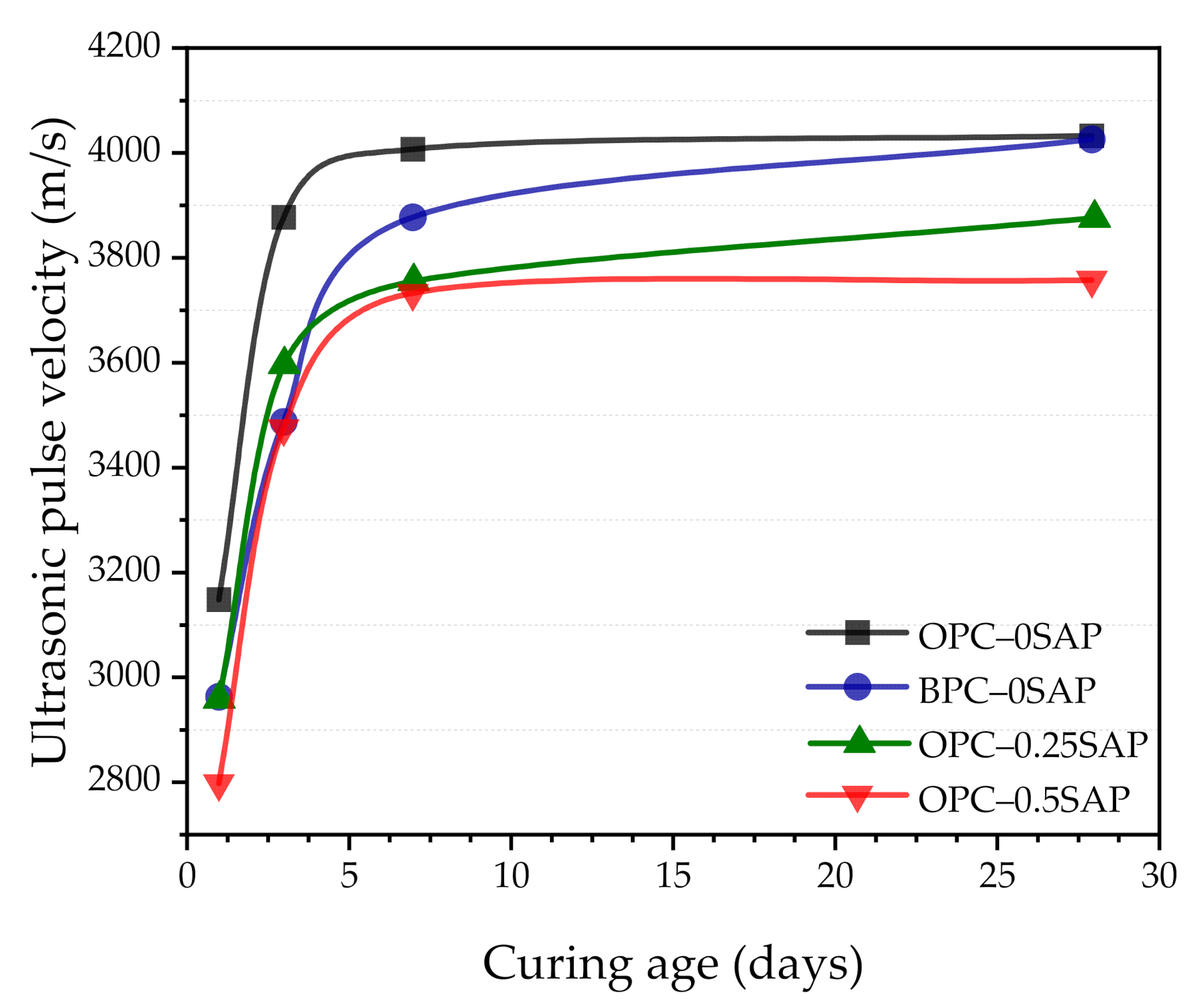
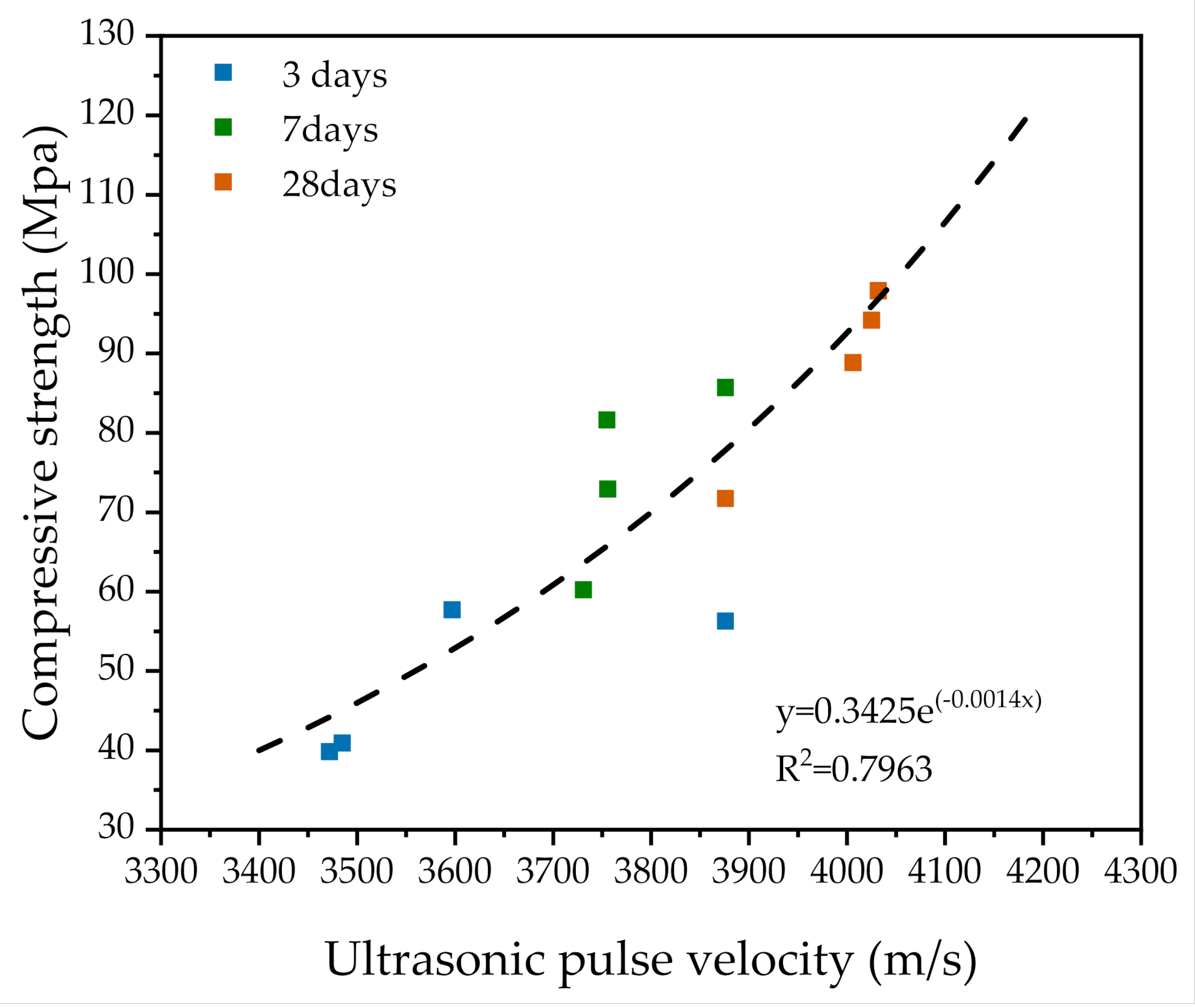
3.6. Ultrasonic Pulse Velocity (UPV)
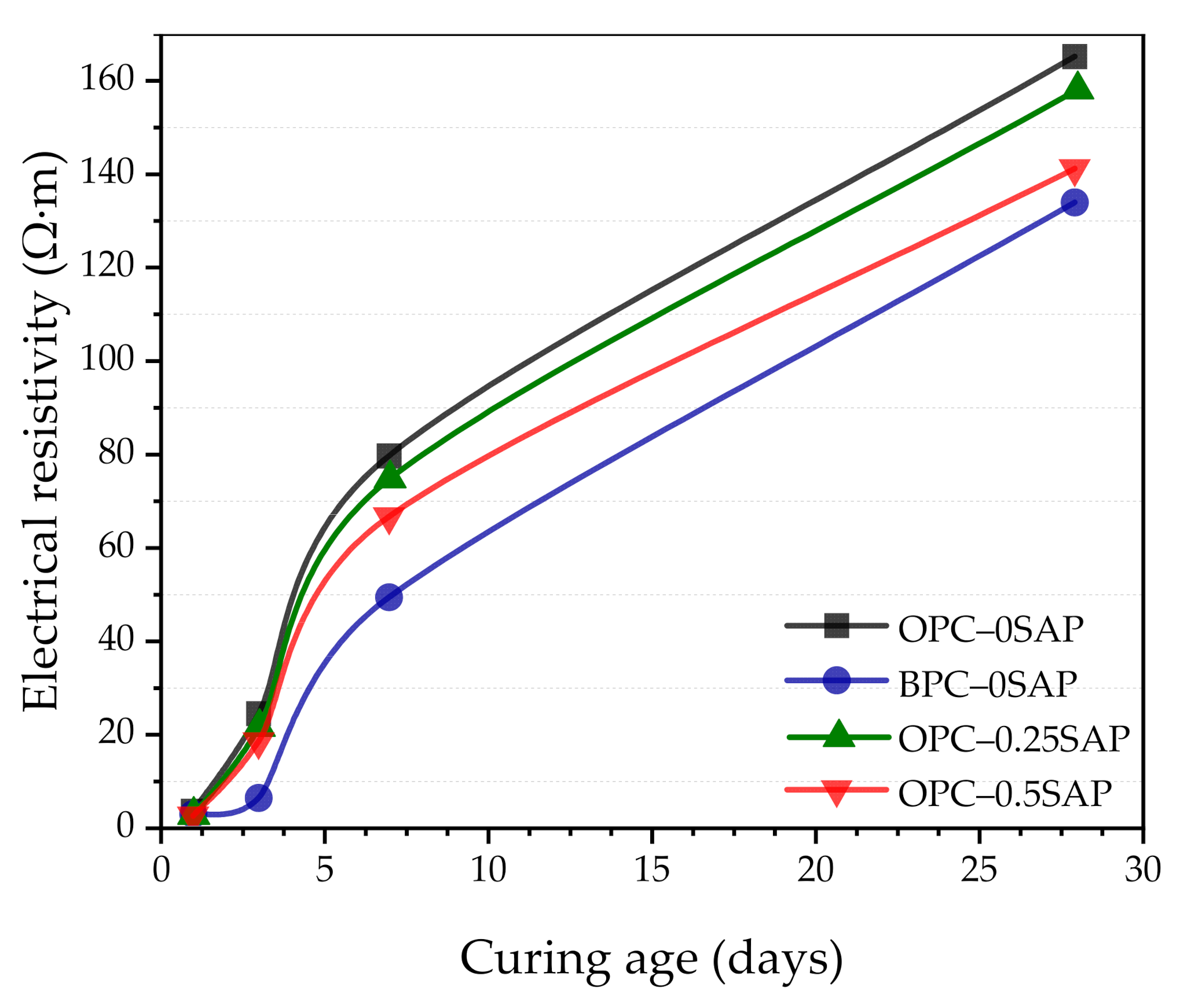
3.7. Electrical Resistivity
4. Discussion
5. Conclusions
- (i)
- At the early age of 3 days, the strength of OPC–0.25SAP samples was 2.5% higher than that of the control specimen. At the later age of 28 days, the compressive strengths of OPC–0.25SAP and OPC–0.5SAP were 12.5% and 25.5% lower than that of the control specimen, OPC–0SAP. This was due to the water release cavities created by SAP. In contrast, the strength of BPC–0SAP developed slowly at an early age, while the strength was similar to that of the control specimen, OPC–0SAP, at the later age of 28 days.
- (ii)
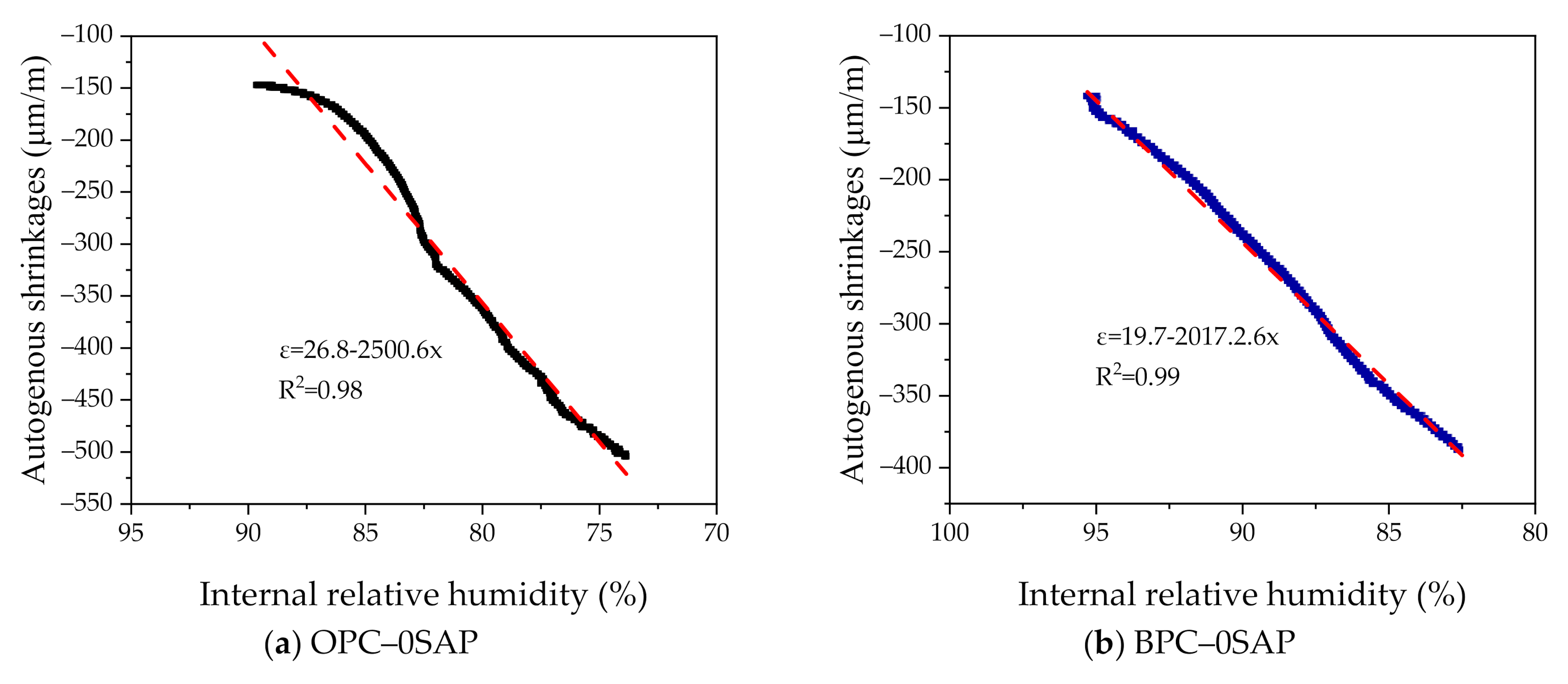
- For the case of OPC–0.25SAP and BPC–0SAP, compared to the control group, the reduction in the AS sample was greater than the strength. However, for the OPC–0.5SAP sample, AS reduction was lower than the strength. Moreover, AS showed a linear relationship with the internal relative humidity. The coefficients of determination between AS and the internal relative humidity were higher than 98%. The reduction in internal relative humidity was the main reason for AS in the SUHPP.
- (iii)
- In terms of hydration heat, the additional water entrained by SAP increased the effective w/c and reduced the initial concentration of the pore solution, resulting in a delayed and blunter main peak of 2.5–4.5 h. The higher w/c increased the degree of hydration, and the cumulative hydration heat at 72 h was significantly higher than that of the control group. The mixture doped with BPC released 15.9% lower cumulative hydration heat at 72 h compared to the control group due to the slow rate of C2S development responses.
- (iv)
- TGA showed the following sequence of CH content: CHOPC-0.5SAP > CHOPC-0.25SAP > CHOPC–0SAP > CHBPC–0SAP. When the SAP content increased, the CH content also increased, indicating that the addition of SAP increased hydration. The samples with BPC had a lower CH content compared to the control group. The analysis results of CH from TGA showed agreement with those of the XRD. Moreover, TGA analysis showed that the trend of combined water was similar to CH. The combined water content was much lower than the theoretical maximum of combined water for the full hydration of 1 g cement. In addition, the ATR–FTIR results showed that the Si-O stretching vibration of C-S-H was enhanced when the SAP content increased.
- (v)
- In terms of UPV, the samples showed faster growth in 3 days because they were able to reach 86.3%–96.1%. For all specimens, there was an exponential correlation between compressive strength and UPV. UPV is a non-destructive test method for evaluating the strength development of SUHPP.
- (vi)
- In terms of electrical resistivity, the OPC–0.25SAP and OPC–0.5SAP samples showed lower electrical resistivity than the control group due to the increased porosity from SAP. The BPC–0SAP sample showed lower resistivity in the early stage. However, from 3 to 28 days, the electrical resistivity of BPC–0SAP showed a higher increment than other specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Khayat, K.H.; Shi, C. Effect of superabsorbent polymer characteristics on rheology of ultra-high performance concrete. Cem. Concr. Compos. 2020, 112, 103636. [Google Scholar] [CrossRef]
- Heikal, M.; El Aleem, S.A.; Morsi, W. Characteristics of blended cements containing nano-silica. HBRC J. 2013, 9, 243–255. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Costa, H.; Júlio, E.; Portugal, A.; Durães, L. Effect of supplementary cementitious materials on autogenous shrinkage of ultra-high performance concrete. Constr. Build. Mater. 2016, 127, 43–48. [Google Scholar] [CrossRef]
- Yazıcı, H.; Yiğiter, H.; Karabulut, A.Ş.; Baradan, B. Utilization of fly ash and ground granulated blast furnace slag as an alternative silica source in reactive powder concrete. Fuel 2008, 87, 2401–2407. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Liu, F.; Wan, C.; Pu, X. Preparation of Ultra-High Performance Concrete with common technology and materials. Cem. Concr. Compos. 2012, 34, 538–544. [Google Scholar] [CrossRef]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. The effect of superabsorbent polymer on various scale of pore structure in ultra-high per-formance concrete. Constr. Build. Mater. 2018, 172, 29–40. [Google Scholar] [CrossRef]
- Hisseine, O.A.; Soliman, N.A.; Tolnai, B.; Tagnit-Hamou, A. Nano-engineered ultra-high performance concrete for controlled autogenous shrinkage using nanocellulose. Cem. Concr. Res. 2020, 137, 106217. [Google Scholar] [CrossRef]
- Li, L.; Dabarera, A.G.; Dao, V. Time-zero and deformational characteristics of high performance concrete with and without superabsorbent polymers at early ages. Constr. Build. Mater. 2020, 264, 120262. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, Y.; Fang, G.; Wang, X.; Zhang, M. Internal curing of alkali-activated fly ash-slag pastes using superabsorbent polymer. Cem. Concr. Res. 2019, 116, 179–190. [Google Scholar] [CrossRef]
- Bentur, A.; Igarashi, S.-I.; Kovler, K. Prevention of autogenous shrinkage in high-strength concrete by internal curing using wet lightweight aggregates. Cem. Concr. Res. 2001, 31, 1587–1591. [Google Scholar] [CrossRef]
- De Meyst, L.; Kheir, J.; Filho, J.R.T.; Van Tittelboom, K.; De Belie, N. The Use of superabsorbent polymers in high performance concrete to mitigate autogenous shrinkage in a large-scale demonstrator. Sustain. J. Rec. 2020, 12, 4741. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Piérard, J.; Pollet, V.; Cauberg, N. In Mitigating autogenous shrinkage in HPC by internal curing using superabsorbent polymers. In Proceedings of the International RILEM Conference on Volume Changes of Hardening Concrete: Testing and Mitigation, Lyngby, Denmark, 20–23 August 2006; pp. 97–106. [Google Scholar]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Shrinkage characteristics of heat-treated ultra-high performance concrete and its mitigation using superabsorbent polymer based internal curing method. Cem. Concr. Compos. 2018, 89, 130–138. [Google Scholar] [CrossRef]
- Liu, J.; Farzadnia, N.; Shi, C.; Ma, X. Shrinkage and strength development of UHSC incorporating a hybrid system of SAP and SRA. Cem. Concr. Compos. 2019, 97, 175–189. [Google Scholar] [CrossRef]
- Craeye, B.; Geirnaert, M.; De Schutter, G. Super absorbing polymers as an internal curing agent for mitigation of early-age cracking of high-performance concrete bridge decks. Constr. Build. Mater. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Zohourian, M.M.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451-477. [Google Scholar]
- Stefan, L.; Boulay, C.; Torrenti, J.-M.; Bissonnette, B.; Benboudjema, F. Influential factors in volume change measurements for cementitious materials at early ages and in isothermal conditions. Cem. Concr. Compos. 2018, 85, 105–121. [Google Scholar] [CrossRef]
- Liu, J.; Shi, C.; Ma, X.; Khayat, K.H.; Zhang, J.; Wang, D. An overview on the effect of internal curing on shrinkage of high performance cement-based materials. Constr. Build. Mater. 2017, 146, 702–712. [Google Scholar] [CrossRef]
- Snoeck, D.; Jensen, O.; De Belie, N. The influence of superabsorbent polymers on the autogenous shrinkage properties of cement pastes with supplementary cementitious materials. Cem. Concr. Res. 2015, 74, 59–67. [Google Scholar] [CrossRef]
- Mönnig, S. Superabsorbing Additions in Concrete: Applications, Modelling and Comparison of Different Internal Water Sources. Master’s Thesis, Universität Stuttgart, Stuttgart, Germany, 2009. [Google Scholar]
- Song, C.; Choi, Y.C.; Choi, S. Effect of internal curing by superabsorbent polymers—Internal relative humidity and autogenous shrinkage of alkali-activated slag mortars. Constr. Build. Mater. 2016, 123, 198–206. [Google Scholar] [CrossRef]
- Beushausen, H.; Gillmer, M.; Alexander, M. The influence of superabsorbent polymers on strength and durability properties of blended cement mortars. Cem. Concr. Compos. 2014, 52, 73–80. [Google Scholar] [CrossRef]
- Naqi, A.; Jang, J.G. Recent Progress in green cement technology utilizing low-carbon emission fuels and raw materials: A review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- Yang, L.; Shi, C.; Wu, Z. Mitigation techniques for autogenous shrinkage of ultra-high-performance concrete—A review. Compos. Part B Eng. 2019, 178, 107456. [Google Scholar] [CrossRef]
- Kang, S.-H.; Hong, S.-G.; Moon, J. Absorption kinetics of superabsorbent polymers (SAP) in various cement-based solutions. Cem. Concr. Res. 2017, 97, 73–83. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using Portions of Prisms Broken in Flexure); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. Standard Test Method for Autogenous Strain of Cement Paste and Mortar; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Lin, R.-S.; Park, K.-B.; Wang, X.-Y.; Zhang, G.-Y. Increasing the early strength of high-volume Hwangtoh–Cement systems using bassanite. J. Build. Eng. 2020, 30, 101317. [Google Scholar] [CrossRef]
- Kacimi, L.; Simon-Masseron, A.; Salem, S.; Ghomari, A.; Derriche, Z. Synthesis of belite cement clinker of high hydraulic re-activity. Cem. Concr. Res. 2009, 39, 559–565. [Google Scholar] [CrossRef]
- Koumpouri, D.; Angelopoulos, G. Effect of boron waste and boric acid addition on the production of low energy belite cement. Cem. Concr. Compos. 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Jin, M.; Wu, Y.; Zhou, Y.; Tang, S. Hydration, shrinkage, pore structure and fractal dimension of silica fume modified low heat Portland cement-based materials. Constr. Build. Mater. 2021, 272, 121952. [Google Scholar] [CrossRef]
- Zhang, G.-Z.; Cho, H.-K.; Wang, X.-Y. Effect of nano-silica on the autogenous shrinkage, strength, and hydration heat of ultra-high strength concrete. Appl. Sci. 2020, 10, 5202. [Google Scholar] [CrossRef]
- Darquennes, A.; Staquet, S.; Delplancke-Ogletree, M.-P.; Espion, B. Effect of autogenous deformation on the cracking risk of slag cement concretes. Cem. Concr. Compos. 2011, 33, 368–379. [Google Scholar] [CrossRef]
- Duran-Herrera, A.; De-León-Esquivel, J.; Bentz, D.; Valdez-Tamez, P. Self-compacting concretes using fly ash and fine limestone powder: Shrinkage and surface electrical resistivity of equivalent mortars. Constr. Build. Mater. 2019, 199, 50–62. [Google Scholar] [CrossRef]
- Xuan, M.-Y.; Han, Y.; Wang, X.-Y. The Hydration, mechanical, autogenous shrinkage, durability, and sustainability properties of cement–limestone–slag ternary composites. Sustain. J. Rec. 2021, 13, 1881. [Google Scholar] [CrossRef]
- Jang, J.; Lee, H. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]
- Justs, J.; Wyrzykowski, M.; Winnefeld, F.; Bajare, D.; Lura, P. Influence of superabsorbent polymers on hydration of cement pastes with low water-to-binder ratio. J. Therm. Anal. Calorim. 2014, 115, 425–432. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Liu, Z.; Liu, Y.; Hu, S. Early-state water migration characteristics of superabsorbent polymers in cement pastes. Cem. Concr. Res. 2019, 118, 25–37. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Q.; Lou, X.; Bao, X.; Zhang, B.; Wang, J.; Zhang, X. Solution calorimetry to assess effects of water-cement ratio and low temperature on hydration heat of cement. Constr. Build. Mater. 2021, 269, 121222. [Google Scholar] [CrossRef]
- Lin, R.-S.; Lee, H.-S.; Han, Y.; Wang, X.-Y. Experimental studies on hydration–strength–durability of limestone-cement-calcined Hwangtoh clay ternary composite. Constr. Build. Mater. 2021, 269, 121290. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Trezza, M.; Lavat, A. Analysis of the system 3CaO Al2O3–CaSO4· 2H2O–CaCO3–H2O by FT-IR spectroscopy. Cem. Concr. Res. 2001, 31, 869–872. [Google Scholar] [CrossRef]
- Hughes, T.L.; Methven, C.M.; Jones, T.G.; Pelham, S.E.; Fletcher, P.; Hall, C. Determining cement composition by Fourier transform infrared spectroscopy. Adv. Cem. Based Mater. 1995, 2, 91–104. [Google Scholar] [CrossRef]
- Lin, R.-S.; Wang, X.-Y.; Lee, H.-S.; Cho, H.-K. Hydration and microstructure of cement pastes with calcined hwangtoh clay. Materials 2019, 12, 458. [Google Scholar] [CrossRef]
- Mollah, M.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Wehbe, Y.; Ghahremaninezhad, A. Combined effect of shrinkage reducing admixtures (SRA) and superabsorbent polymers (SAP) on the autogenous shrinkage, hydration and properties of cementitious materials. Constr. Build. Mater. 2017, 138, 151–162. [Google Scholar] [CrossRef]
- Lin, R.-S.; Han, Y.; Wang, X.-Y. Macro–meso–micro experimental studies of calcined clay limestone cement (LC3) paste sub-jected to elevated temperature. Cem. Concr. Compos. 2021, 116, 103871. [Google Scholar] [CrossRef]
- Shariq, M.; Prasad, J.; Masood, A. Studies in ultrasonic pulse velocity of concrete containing GGBFS. Constr. Build. Mater. 2013, 40, 944–950. [Google Scholar] [CrossRef]
- Van Breugel, K.; Fraaij, A. Experimental Study on Ultrasonic Pulse Velocity Evaluation of the Microstructure of Cementitious Material at Early Age; Delft University of Technology: Delft, The Netherlands, 2001. [Google Scholar]
- Ye, G.; Lura, P.; Van Breugel, K.; Fraaij, A. Study on the development of the microstructure in cement-based materials by means of numerical simulation and ultrasonic pulse velocity measurement. Cem. Concr. Compos. 2004, 26, 491–497. [Google Scholar] [CrossRef]
- Huang, H.; Gao, X.; Wang, H.; Ye, H. Influence of rice husk ash on strength and permeability of ultra-high performance concrete. Constr. Build. Mater. 2017, 149, 621–628. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Zhou, S.; Chen, E.; Tang, S. Energy saving benefit, mechanical performance, volume stabilities, hydration properties and products of low heat cement-based materials. Energy Build. 2018, 170, 157–169. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X.; Qin, L. Mathematical modeling of accelerated carbonation curing of Portland cement paste at early age. Cem. Concr. Res. 2019, 120, 187–197. [Google Scholar] [CrossRef]
- Neithalath, N.; Weiss, J.; Olek, J. Characterizing Enhanced Porosity Concrete using electrical impedance to predict acoustic and hydraulic performance. Cem. Concr. Res. 2006, 36, 2074–2085. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Zhou, S.; Chen, E.; Tang, S. Mechanical properties, long-term hydration heat, shinkage behavior and crack resistance of dam concrete designed with low heat Portland (LHP) cement and fly ash. Constr. Build. Mater. 2018, 187, 1073–1091. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Dong, Y.; Chen, E.; Tang, S. Environmental evaluation, hydration, pore structure, volume deformation and abrasion resistance of low heat Portland (LHP) cement-based materials. J. Clean. Prod. 2018, 203, 540–558. [Google Scholar] [CrossRef]
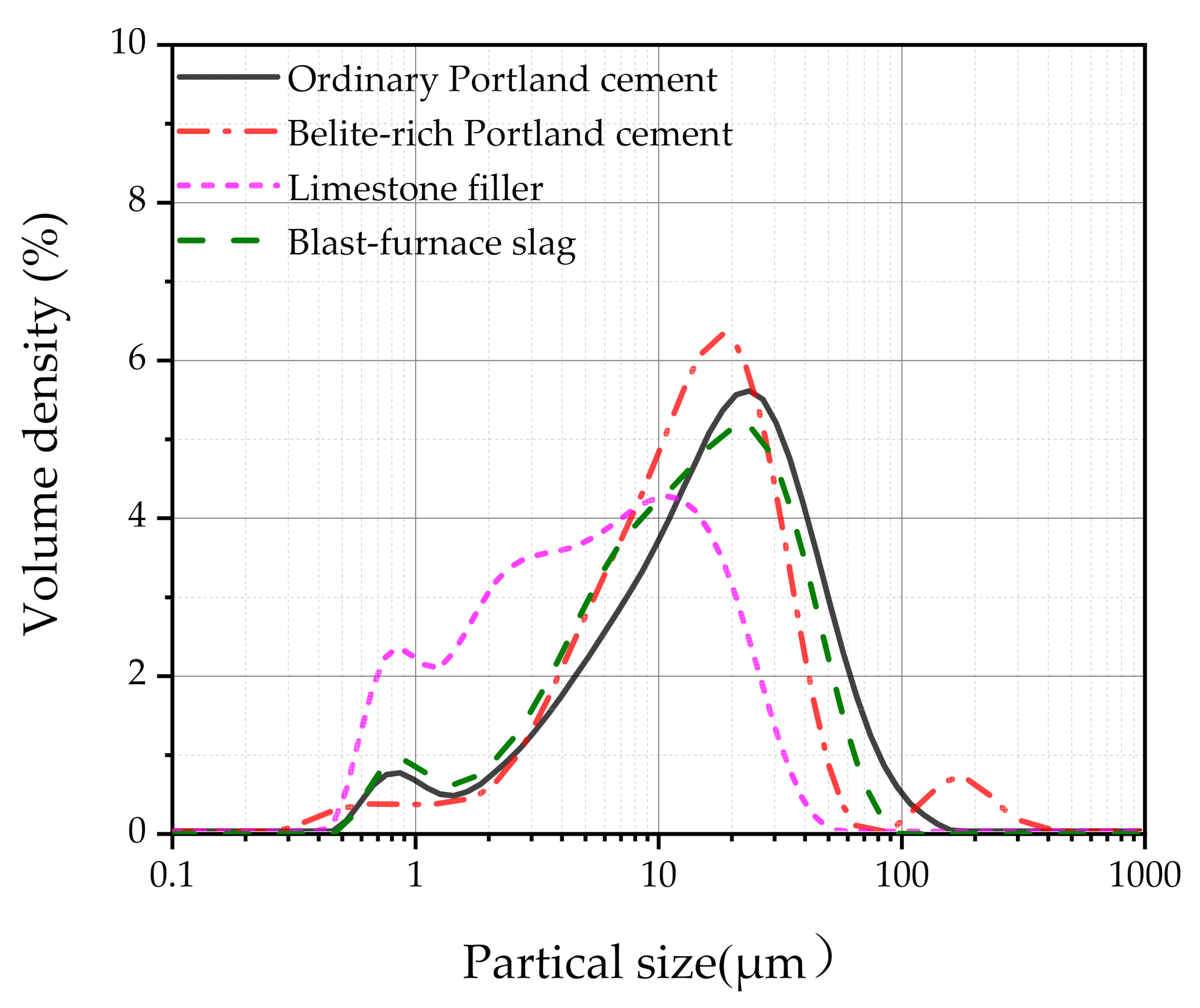



| Oxides | Ordinary Portland Cement (%) | Belite-Rich Portland Cement (%) | Silica Fume (%) | Limestone Filler (%) | Blast-Furnace Slag (%) |
|---|---|---|---|---|---|
| CaO | 64.7 | 63.27 | 0.57 | 59.19 | 38.29 |
| SiO2 | 21.1 | 26.65 | 94.52 | – | 36.12 |
| Al2O3 | 5.07 | 2.36 | 0.73 | 0.21 | 14.82 |
| Fe2O3 | 3.14 | 2.85 | 0.22 | – | 0.47 |
| MgO | 0.89 | 0.96 | 0.49 | 0.45 | 6.49 |
| Na2O | 0.19 | – | – | – | 0.06 |
| TiO2 | 0.22 | – | – | – | 0.62 |
| SO3 | 1.61 | 2.12 | 0.26 | – | 1.61 |
| Loss onignition | 2.32 | 0.89 | 1.59 | 39.21 | 1.16 |
| Mineralogical Composition | OPC (%) | BPC (%) |
|---|---|---|
| 3CaO·SiO2 (C3S) | 59.89 | 29.06 |
| 2CaO·SiO2 (C2S) | 15.31 | 54.48 |
| 3CaO·Al2O3 (C3A) | 8.12 | 1.43 |
| 4CaO·AlO3·Fe2O3 (C4AF) | 9.56 | 8.67 |
| Number | Binders (%) | SAP (%) | Water (%) | Additional Water (%) | SP (%) | |||
|---|---|---|---|---|---|---|---|---|
| Cement (OPC/BPC) | Silica Fume | Limestone | Slag | |||||
| OPC–0SAP | 50 | 10 | 20 | 20 | 0 | 20 | 0 | 1.2 |
| BPC–0SAP | 50 | 10 | 20 | 20 | 0 | 20 | 0 | 1.2 |
| OPC–0.25SAP | 50 | 10 | 20 | 20 | 0.25 | 20 | 2.5 | 1.2 |
| OPC–0.5SAP | 50 | 10 | 20 | 20 | 0.5 | 20 | 5 | 1.2 |
| NO. | Experimental Method | Total Number of Test Samples | Standard Deviation | Sample Size | Test Time |
|---|---|---|---|---|---|
| 1 | Compressive strength | 36 | Within ±0.05% of the indicated load | 50 × 50 × 50 mm | 3, 7, and 28 days |
| 2 | Autogenous shrinkage coupled with relative humidity and temperature | 4 | 0.001 μm/m; ±0.5% RH; ±0.1 °C | Ø29 × 430 mm | 1–7 days |
| 3 | Isothermal calorimetry | 4 | ±20 μW | 5 g paste | 72 h |
| 4 | X-ray diffraction | 4 | λ = 1.5406 Å 2θ = 0.013° | Powder | 28 days |
| 5 | Thermogravimetric analysis | 4 | 0.1 μg | Powder | 28 days |
| 6 | Attenuated total reflectance–Fourier-transform infrared spectroscopy | 4 | ±0.01 cm−1 | Powder | 28 days |
| 7 | Ultrasonic pulse velocity | 36 | 0.5 μs | 50 × 50 × 50 mm | 1, 3, 7, and 28 days |
| 8 | Electrical resistivity | 12 | ±0.2 to ±2 kΩ cm | Ø100 × 200 mm | 1, 3, 7, and 28 days |
| Samples | CH (g/g) | Combined Water (g/g) |
|---|---|---|
| OPC–0SAP | 3.44% | 7.90% |
| BPC–0SAP | 2.99% | 7.62% |
| OPC–0.25SAP | 3.95% | 8.38% |
| OPC–0.5SAP | 4.32% | 8.94% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, M.-Y.; Wang, Y.-S.; Wang, X.-Y.; Lee, H.-S.; Kwon, S.-J. Effect of Cement Types and Superabsorbent Polymers on the Properties of Sustainable Ultra-High-Performance Paste. Materials 2021, 14, 1497. https://doi.org/10.3390/ma14061497
Xuan M-Y, Wang Y-S, Wang X-Y, Lee H-S, Kwon S-J. Effect of Cement Types and Superabsorbent Polymers on the Properties of Sustainable Ultra-High-Performance Paste. Materials. 2021; 14(6):1497. https://doi.org/10.3390/ma14061497
Chicago/Turabian StyleXuan, Mei-Yu, Yi-Sheng Wang, Xiao-Yong Wang, Han-Seung Lee, and Seung-Jun Kwon. 2021. "Effect of Cement Types and Superabsorbent Polymers on the Properties of Sustainable Ultra-High-Performance Paste" Materials 14, no. 6: 1497. https://doi.org/10.3390/ma14061497
APA StyleXuan, M.-Y., Wang, Y.-S., Wang, X.-Y., Lee, H.-S., & Kwon, S.-J. (2021). Effect of Cement Types and Superabsorbent Polymers on the Properties of Sustainable Ultra-High-Performance Paste. Materials, 14(6), 1497. https://doi.org/10.3390/ma14061497









