Water-Tree Resistant Characteristics of Crosslinker-Modified-SiO2/XLPE Nanocomposites
Abstract
1. Introduction
2. Experimental Schemes
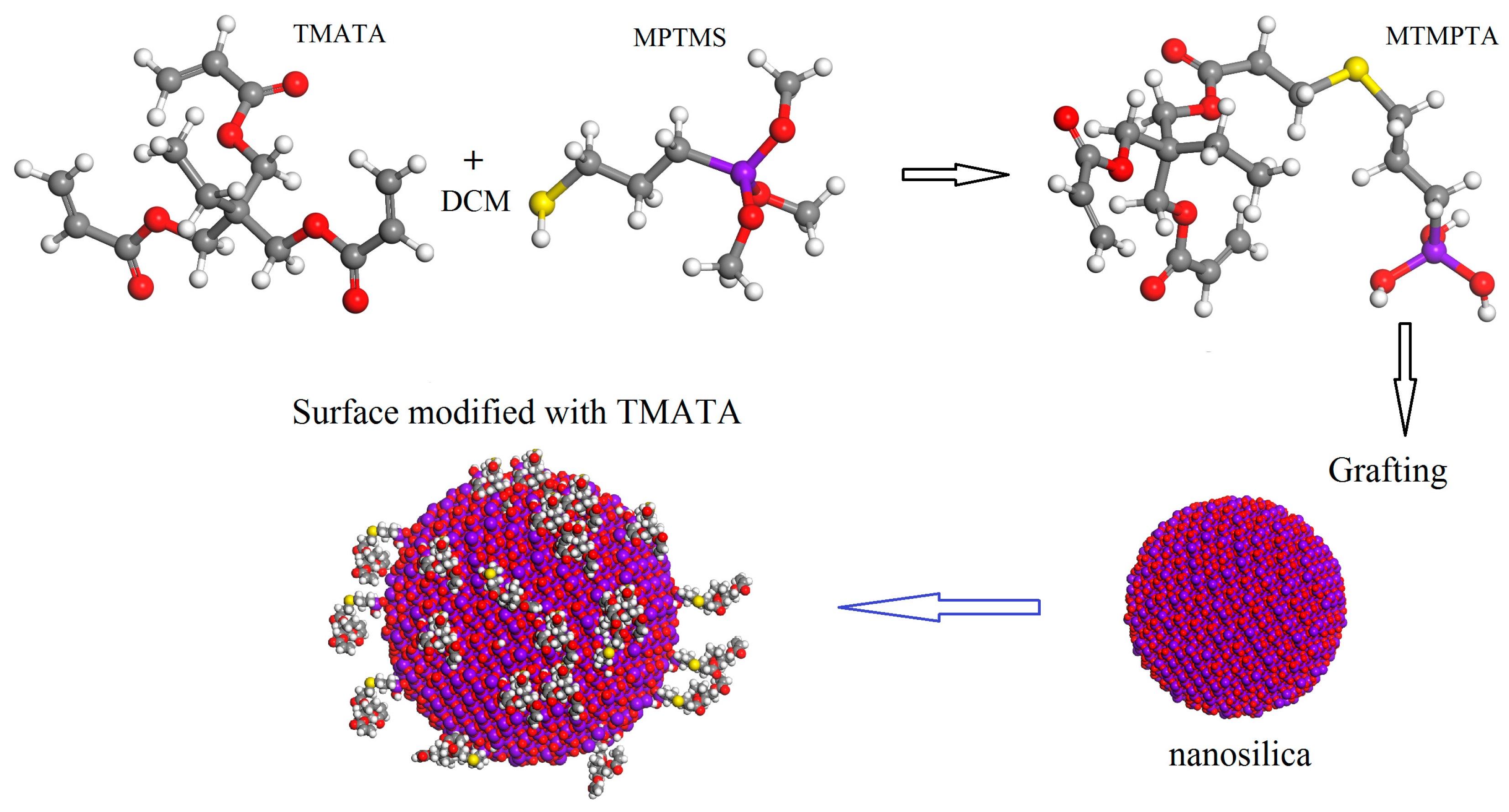
2.1. Materials Preparation
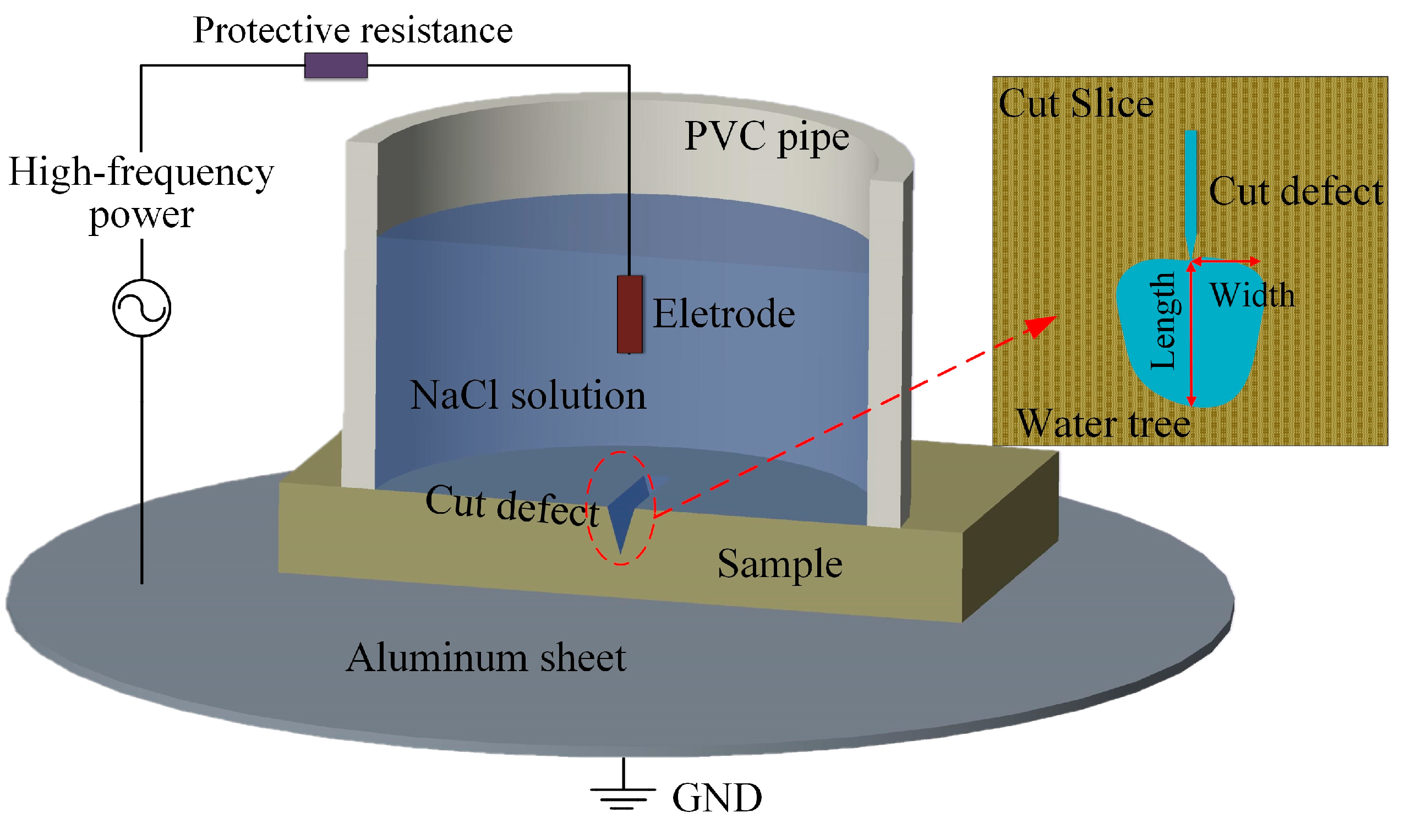
2.2. Characterization and Testing Methodology
3. Results and Discussion
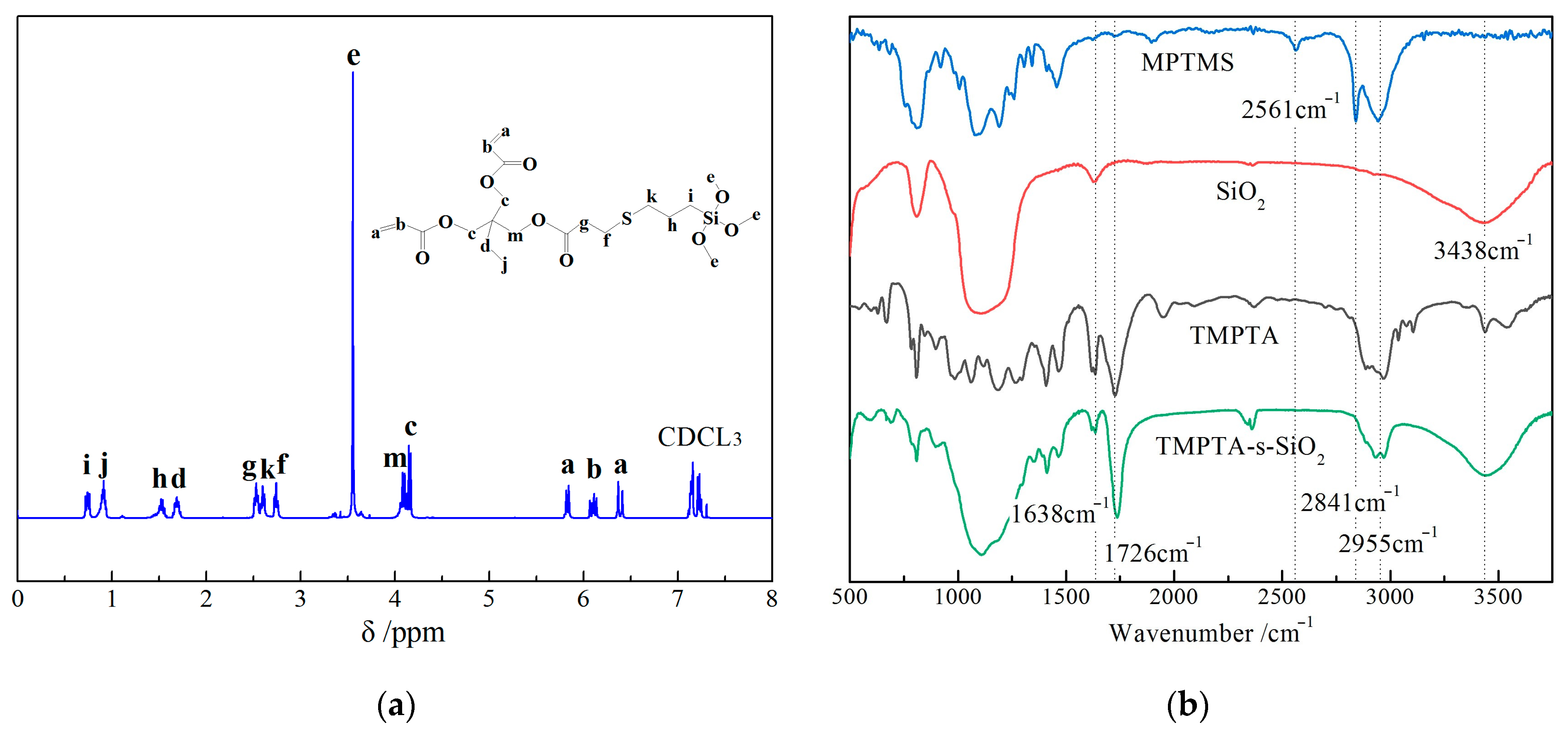
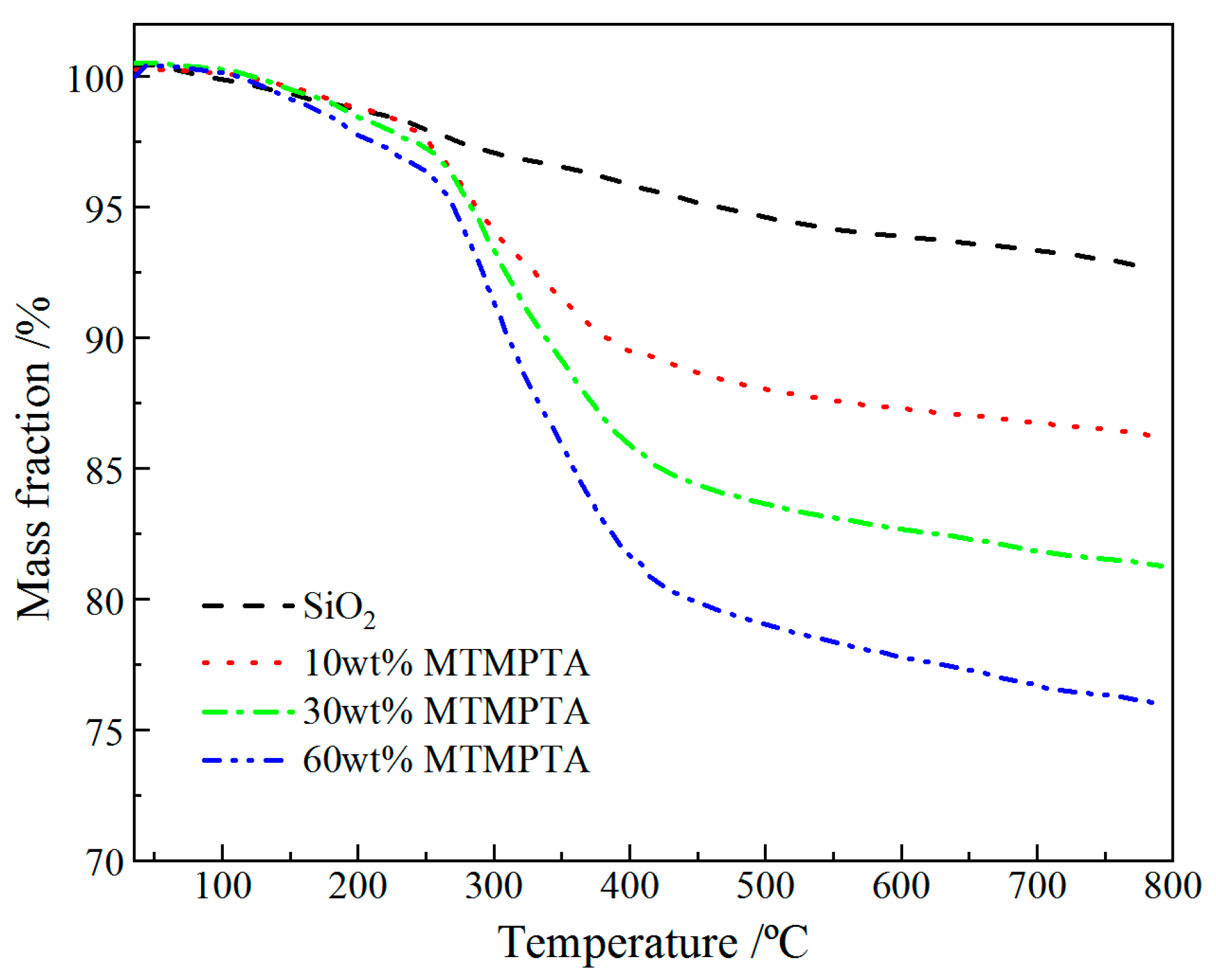
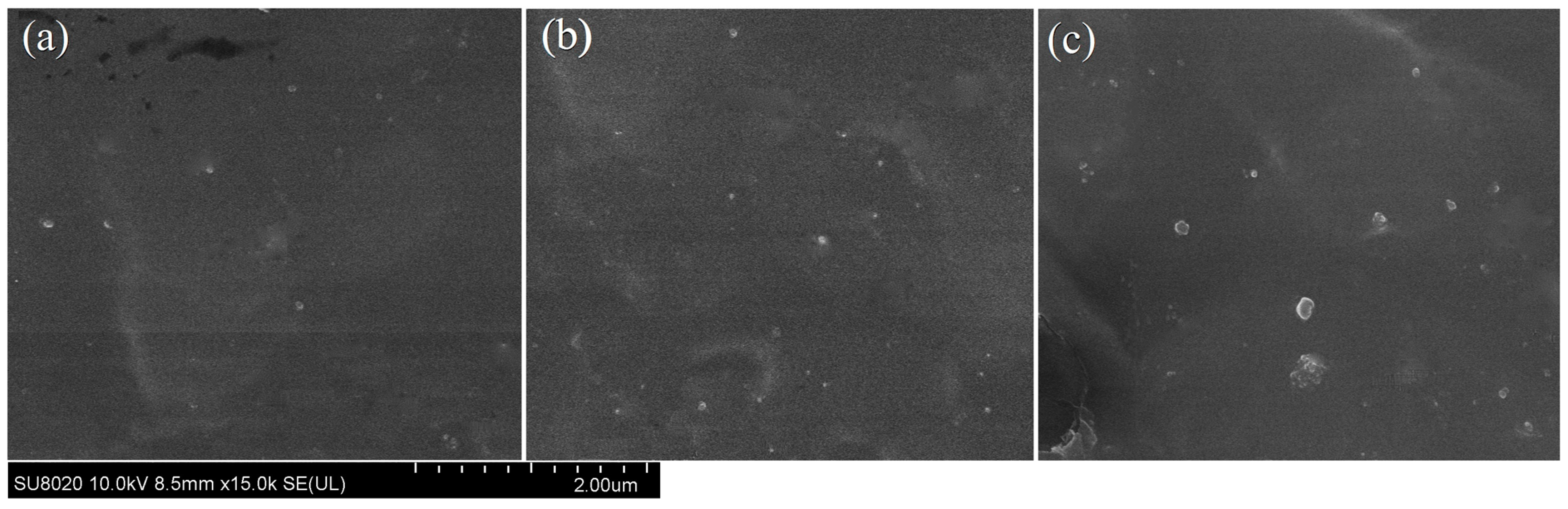
3.1. Material Characterization
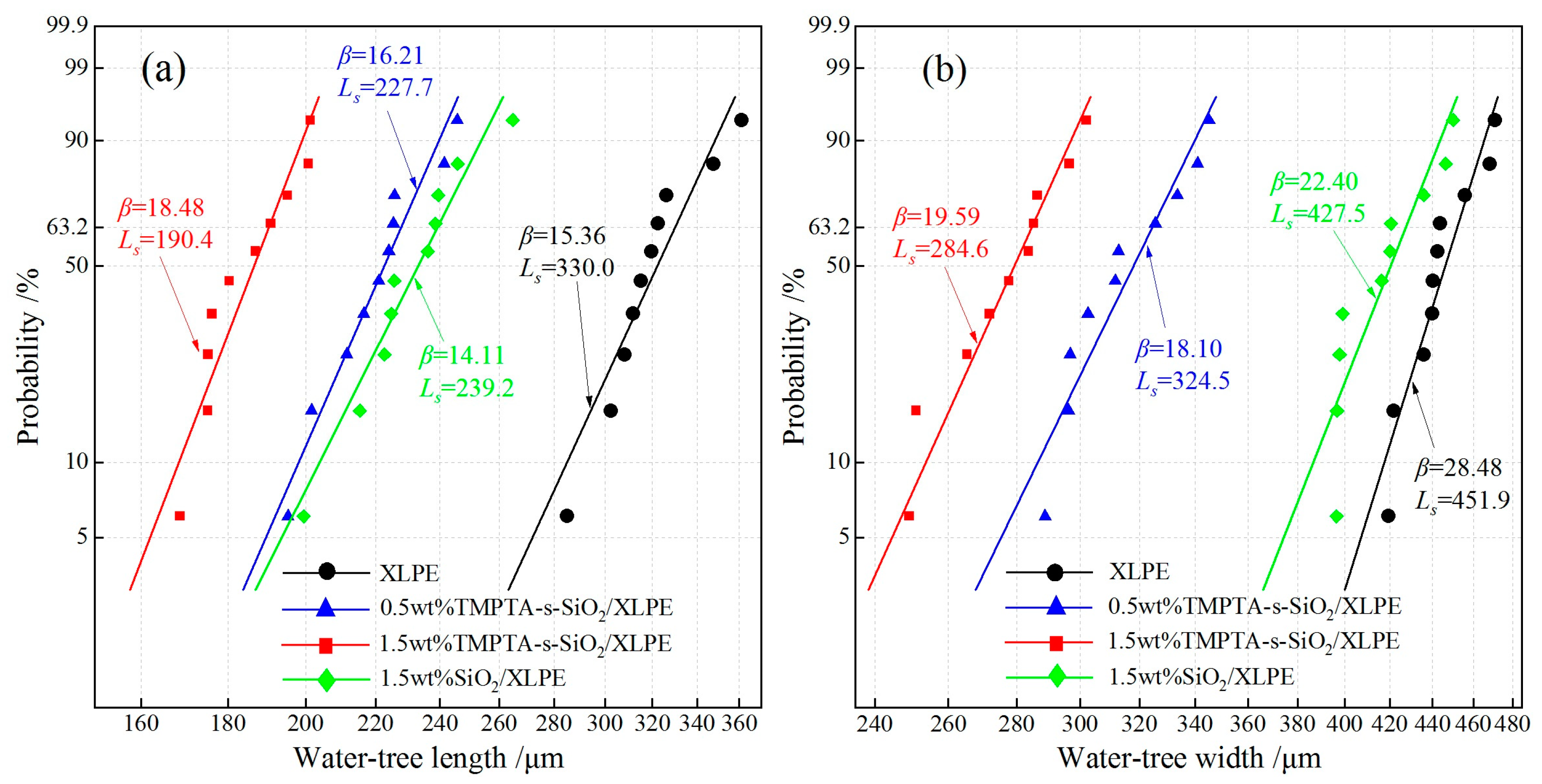
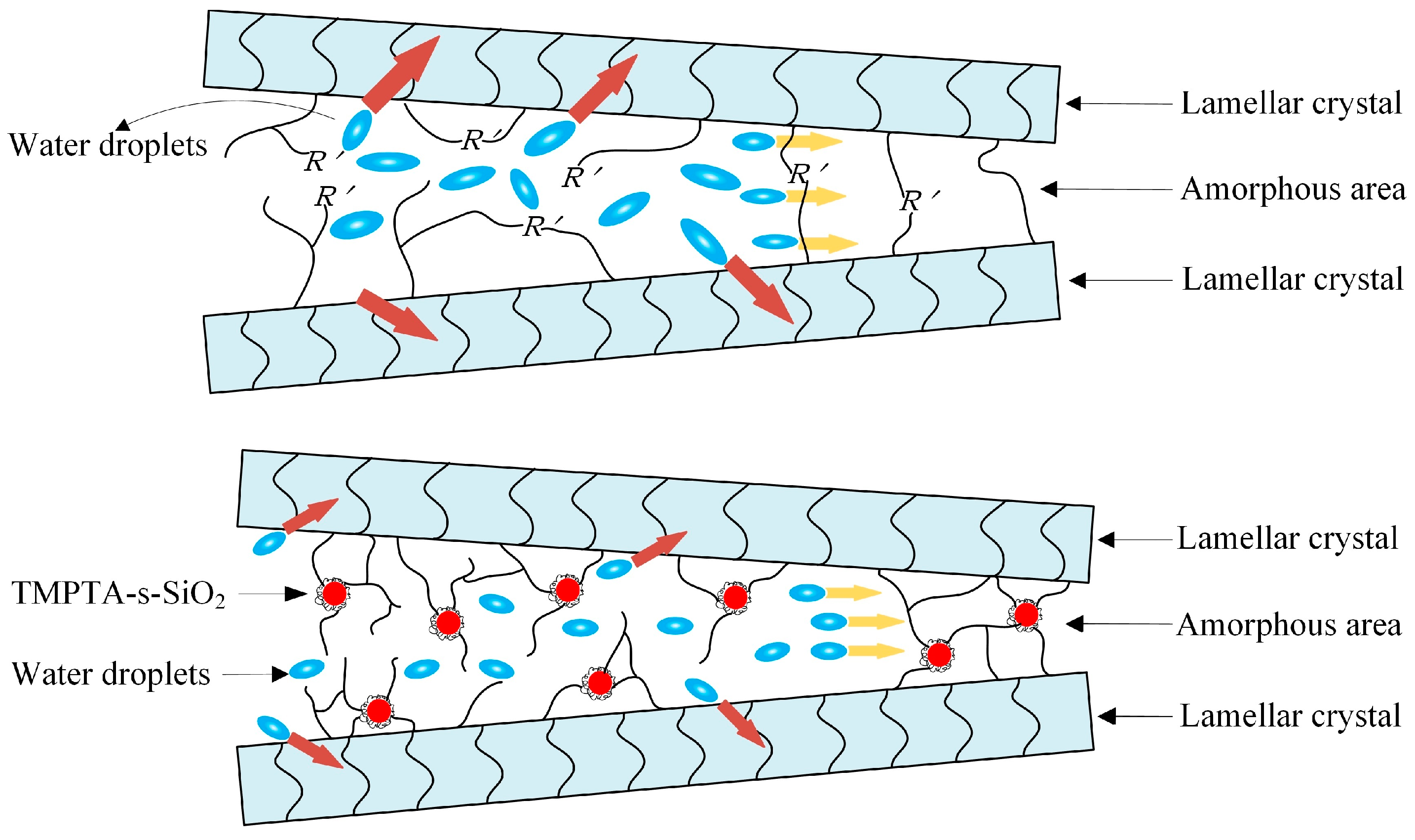
3.2. Water-Tree Characteristics
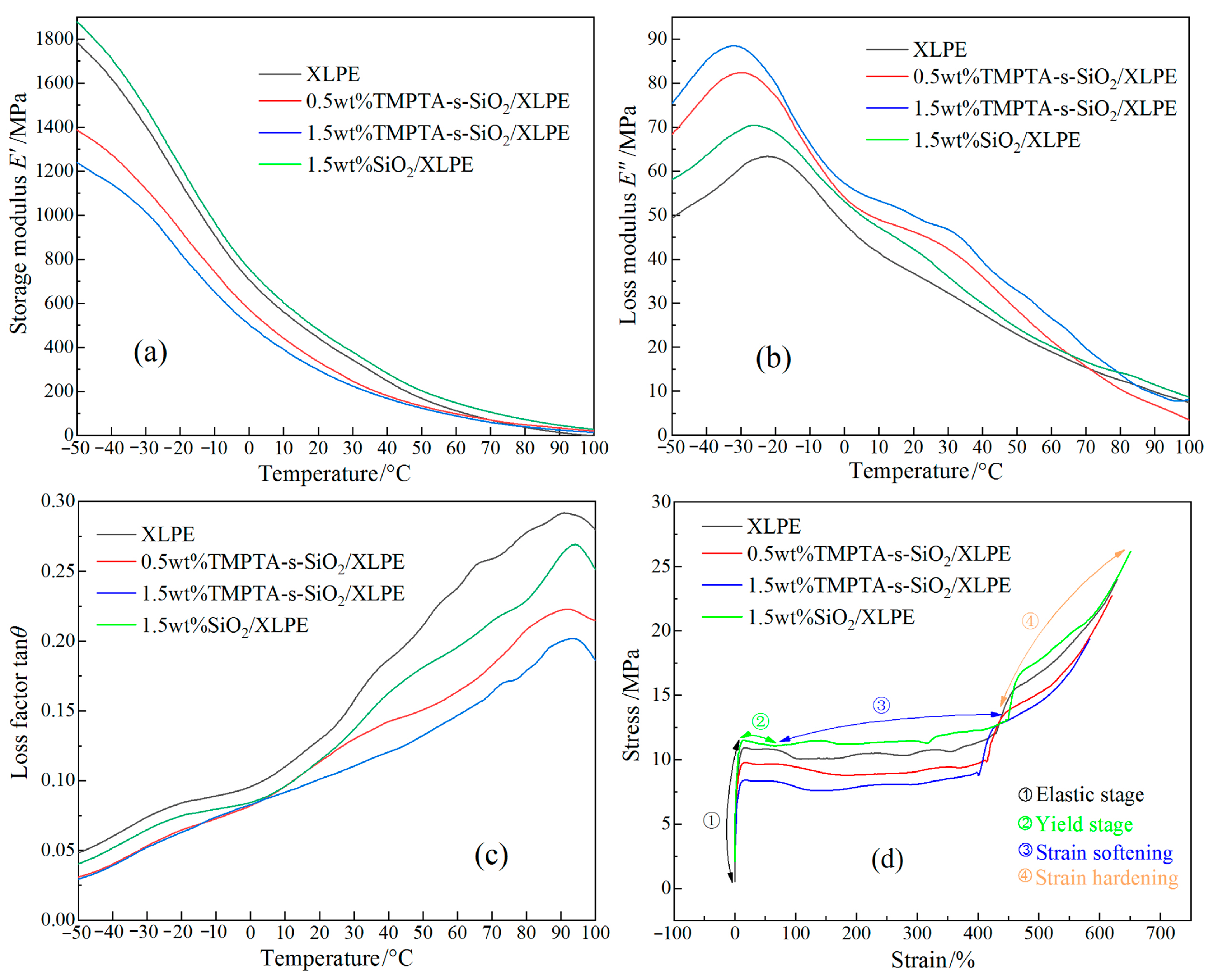
3.3. Mechanical Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Zhao, J.; Zhao, W.; Zhong, L.; Hu, L.; Rao, W.; Zheng, M.; Meng, S. Influence of morphological variations on the AC breakdown of XLPE insulation in submarine cable factory joints. High Volt 2019, 5, 69–75. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Zhang, H.; Shang, Y.; Wang, X.; Han, B.; Li, Z. Theoretical study on the reaction of triallylisocyanurate in the UV radiation cross-linking of polyethylene. RSC Adv. 2017, 7, 37095–37104. [Google Scholar] [CrossRef]
- OuYang, B.H.; Hua, M.; Deng, X.B. A review about development of HV XLPE cable materials and processes. Insul. Mater. 2016, 49, 1–7. [Google Scholar]
- Li, Z.; Sun, W.; Zhao, H. Significantly improved electrical properties of photo-initiated auxiliary crosslinking EPDM used for cable termination. Polymers 2019, 11, 2083. [Google Scholar] [CrossRef]
- Zhou, K.; Huang, M.; Tao, W.; Yang, M. A possible water tree initiation mechanism for service aged XLPE cables: Conversion of electrical tree to water tree. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1854–1861. [Google Scholar] [CrossRef]
- Zheng, H.L.; Rowland, M.; Iddrissu, I.; Lv, Z. Electrical treeing and reverse tree grow in an epoxy resin. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 3966–3973. [Google Scholar] [CrossRef]
- Kurihara, T.; Okamoto, T.; Hozumi, N.; Miyajima, K.; Uchida, K. Evaluation of relationship between residual charge signal and AC breakdown strength of water-tree degraded 22 to 77 kV classes XLPE cables removed from service using pulsed voltages. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 656–665. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Li, Y.; Ping, Z.; Rui, H. Growth and partial discharge characteristics of electrical tree in XLPE under AC-DC composite voltage. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2282–2290. [Google Scholar] [CrossRef]
- Mugala, G.; Eriksson, R.; Pettersson, P. High frequency characteristics of water-tree degraded XLPE insulation in power cables. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1271–1277. [Google Scholar] [CrossRef]
- Hvidsten, S.; Ildstad, E.; Holmgren, B.; Werelius, P. Correlation between AC breakdown strength and low frequency dielectric loss of water tree aged XLPE cables. IEEE Trans. Power Deliv. 1998, 13, 40–45. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Li, Y.; Wu, J. The influence of temperature on water treeing in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 544–551. [Google Scholar] [CrossRef]
- Al-Arainy, A.; Ahaideb, A.; Qureshi, M.; Malik, N. Statistical evaluation of water tree lengths in XLPE cables at different temperatures. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 995–1006. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Nie, L.; Jing, Z.; Han, B. Research on the water blade electrode method for assessing water tree resistance of cross-linked polyethylene. Polym. Test. 2016, 50, 235–240. [Google Scholar] [CrossRef]
- Lewis, T.J. Nanometric dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Sun, K.; Chen, J.; Zhao, H.; Sun, W.; Chen, Y.; Luo, Z. Dynamic thermo-mechanical analysis on water tree resistance of crosslinked polyethylene. Materials 2019, 12, 746. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Huang, X.; Yang, J.; Jiang, P. Synergetic effects of silane-grafting and EVA on water tree resistance of LDPE. Chin. J. Polym. Sci. 2010, 28, 1–11. [Google Scholar] [CrossRef]
- Huang, X.; Liu, F.; Jiang, P. Effect of nanoparticle surface treatment on morphology, electrical and water treeing behavior of LLDPE composites. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1697–1704. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, X.; Jiang, P. A comparative study of effects of SEBS and EPDM on the water tree resistance of cross-linked polyethylene. Polym. Degrad. Stab. 2010, 95, 1943–1949. [Google Scholar] [CrossRef]
- Ciuprina, F.; Teissedre, G.; Filippini, J.C.; Smedberg, A.; Campus, A.; Hampton, N. Chemical crosslinking of polyethylene and its effect on water tree initiation and propagation. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 709–715. [Google Scholar] [CrossRef]
- Wang, Z.; Marcolongo, P.; Lemberg, J.A.; Panganiban, B.; Evans, J.W.; Ritchie, R.O.; Wright, P.K. Mechanical fatigue as a mechanism of water tree propagation in TR-XLPE. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 321–330. [Google Scholar] [CrossRef]
- Li, K.; Zhou, K.; Zhu, G. Toward understanding the relationship between the microstructure and propagation behavior of water trees. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1116–1124. [Google Scholar] [CrossRef]
- Wei, H.E.; Tang, A.B.; Luo, C.M.; Ma, Q.K.; Li, Z.Z. Photo-crosslinking of linear low density polyethylene. Insul. Mater. 2011, 44, 66–68. [Google Scholar]
- Sun, Y.; Zhang, Z.; Wong, C.P. Study on mono-dispersed nano-size silica by surface modification for underfill applications. J. Colloid Interface Sci. 2005, 292, 436–444. [Google Scholar] [CrossRef]
- Chen, J.Q.; Wang, X.; Sun, W.F.; Zhao, H. Water-tree resistability of UV-XLPE from hydrophilicity of auxiliary crosslinkers. Molecules 2020, 25, 4147. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, X.Q.; Ren, J.; Sun, Z.G. Research progress on surface modification of nano-silica. Silica. Mater. 2017, 31, 396–400. [Google Scholar]
- Stadler, F.J. Dynamic-mechanical behavior of polyethylenes and ethene/alpha-olefin-copolymers: Part II. alpha-and beta-relaxation. Korean J. Chem. Eng. 2011, 28, 954–963. [Google Scholar] [CrossRef]
- Yash, P.K.; Edith, A.T.; Thomas, J.T.; Virgil, V.V.; Richard, F.A. Dynamic mechanical relaxations in polyethylene. Macromolecules 1985, 18, 1302–1309. [Google Scholar]
- Fakirow, S.; Krasteva, B. On the glass transition temperature of polyethylene as revealed by microhardness measurements. J. Macromol. Sci. Part B 2000, 39, 297–301. [Google Scholar] [CrossRef]
- Colson, J.P. Alpha relaxation in polyethylene. J. Appl. Phys. 1971, 42, 5902–5903. [Google Scholar] [CrossRef]
- Celina, M.; George, G.A. Characterization and degradation studies of peroxide and silane crosslinked polyethylene. Polym. Degrad. Stab. 1995, 48, 297–312. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Song, Y.; Zheng, Q. Influence of crosslinking on physical properties of low density polyethylene. Chin. J. Polym. Sci. 2012, 30, 837–844. [Google Scholar] [CrossRef]

| Materials | Source | Model |
|---|---|---|
| Linear low density polyethylene (LLDPE) | Jilin Petrochemical China Petro Co., Ltd., Changchun, China | DFDA 7042 |
| 4-hydroxy benzophenone laurate | Harbin University of Science and Technology, Harbin, China | —— |
| Trimethylolpropane triacrylate (TMPTA) | Maklin Biochemical Technology Co. Ltd., Shanghai, China | —— |
| SiO2 nanoparticles (~40 nm in diameter) | ||
| 3-mercaptopropyl trimethoxysilane (MPTMS) | Jiangsu Heyuan Chemical Co. Ltd., Nanjing, China | —— |
| Dichloromethane (DCM) | Fuyu Fine Chemical Co. Ltd., Tianjin, China | Analytical purity |
| Triethylamine (TEA) | ||
| Anhydrous ethanol (EtOH) |
| Materials | LLDPE | BPL | TMPTA | TMPTA-s-SiO2 | SiO2 |
|---|---|---|---|---|---|
| XLPE | 96.7 | 2 | 1 | 0 | 0 |
| 0.5wt%TMPTA-s-SiO2/XLPE | 97.2 | 2 | 0 | 0.5 | 0 |
| 1.5wt%TMPTA-s-SiO2/XLPE | 96.2 | 2 | 0 | 1.5 | 0 |
| 1.5wt%SiO2/XLPE | 95.2 | 2 | 1 | 0 | 1.5 |
| Samples | Gel Content/% | E”/MPa | tanθ | Ls/μm | Ws/μm |
|---|---|---|---|---|---|
| Pure XLPE | 70.6 | 68 | 0.072 | 330.02 | 451.9 |
| 0.5wt%TMPTA-s-SiO2/XLPE | 73.2 | 72 | 0.055 | 227.7 | 324.5 |
| 1.5wt%TMPTA-s-SiO2/XLPE | 88.1 | 79 | 0.050 | 190.35 | 284.6 |
| 1.5wt% SiO2/XLPE | 66.4 | 65 | 0.082 | 239.2 | 427.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-Q.; Wang, X.; Yu, P.-L.; Sun, W.-F. Water-Tree Resistant Characteristics of Crosslinker-Modified-SiO2/XLPE Nanocomposites. Materials 2021, 14, 1398. https://doi.org/10.3390/ma14061398
Zhang Y-Q, Wang X, Yu P-L, Sun W-F. Water-Tree Resistant Characteristics of Crosslinker-Modified-SiO2/XLPE Nanocomposites. Materials. 2021; 14(6):1398. https://doi.org/10.3390/ma14061398
Chicago/Turabian StyleZhang, Yong-Qi, Xuan Wang, Ping-Lan Yu, and Wei-Feng Sun. 2021. "Water-Tree Resistant Characteristics of Crosslinker-Modified-SiO2/XLPE Nanocomposites" Materials 14, no. 6: 1398. https://doi.org/10.3390/ma14061398
APA StyleZhang, Y.-Q., Wang, X., Yu, P.-L., & Sun, W.-F. (2021). Water-Tree Resistant Characteristics of Crosslinker-Modified-SiO2/XLPE Nanocomposites. Materials, 14(6), 1398. https://doi.org/10.3390/ma14061398







