Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses
Abstract
1. Introduction
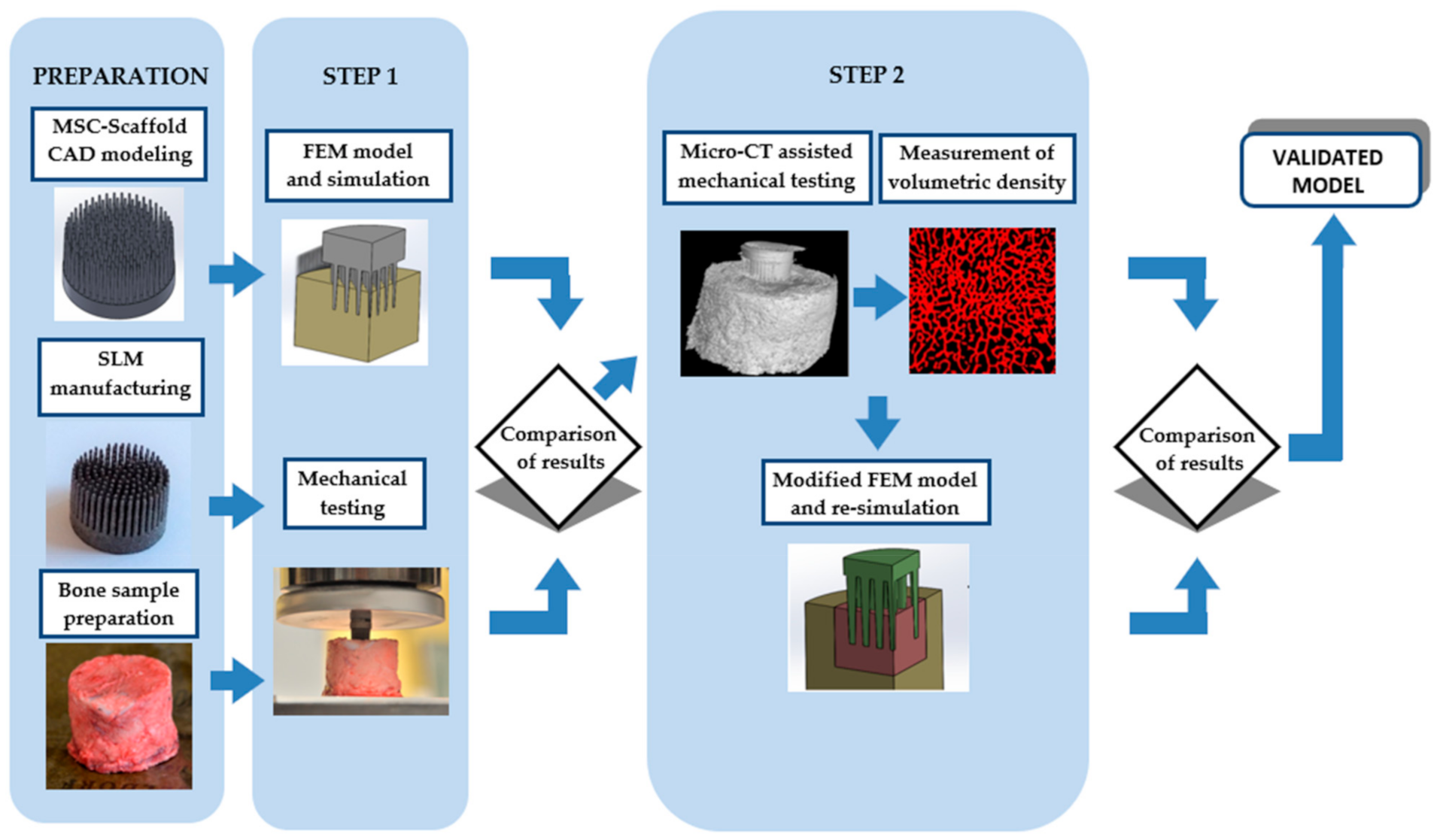
2. Materials and Methods
2.1. General
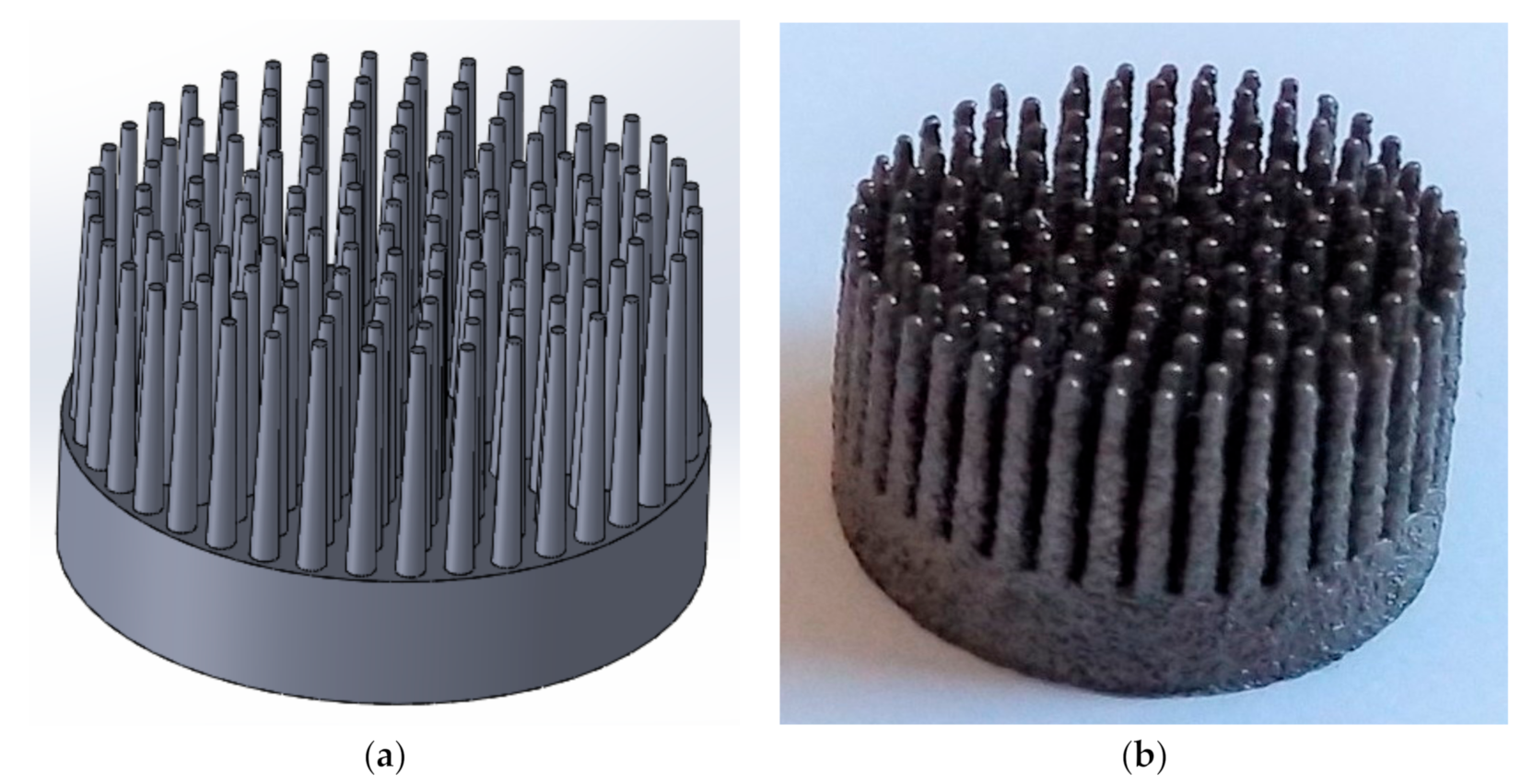
2.2. Computer-Assisted Design (CAD) Modelling and Selective Laser Melting (SLM) Manufacturing of Multi-Spiked Connecting Scaffold (MSC-Scaffold) Prototypes
2.3. Periarticular Bone Samples Preparation
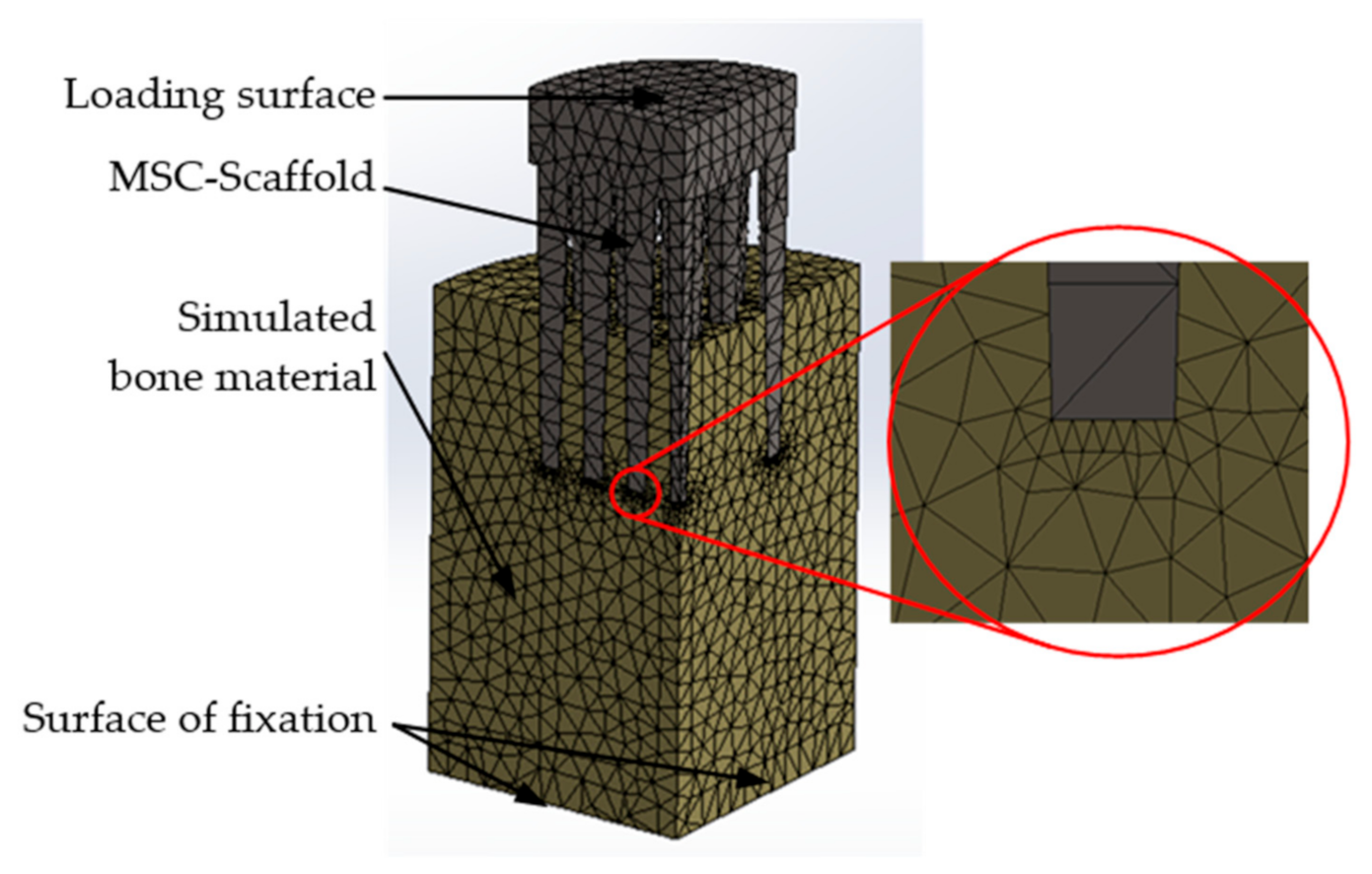
2.4. Finite Element (FE) Model and Simulations
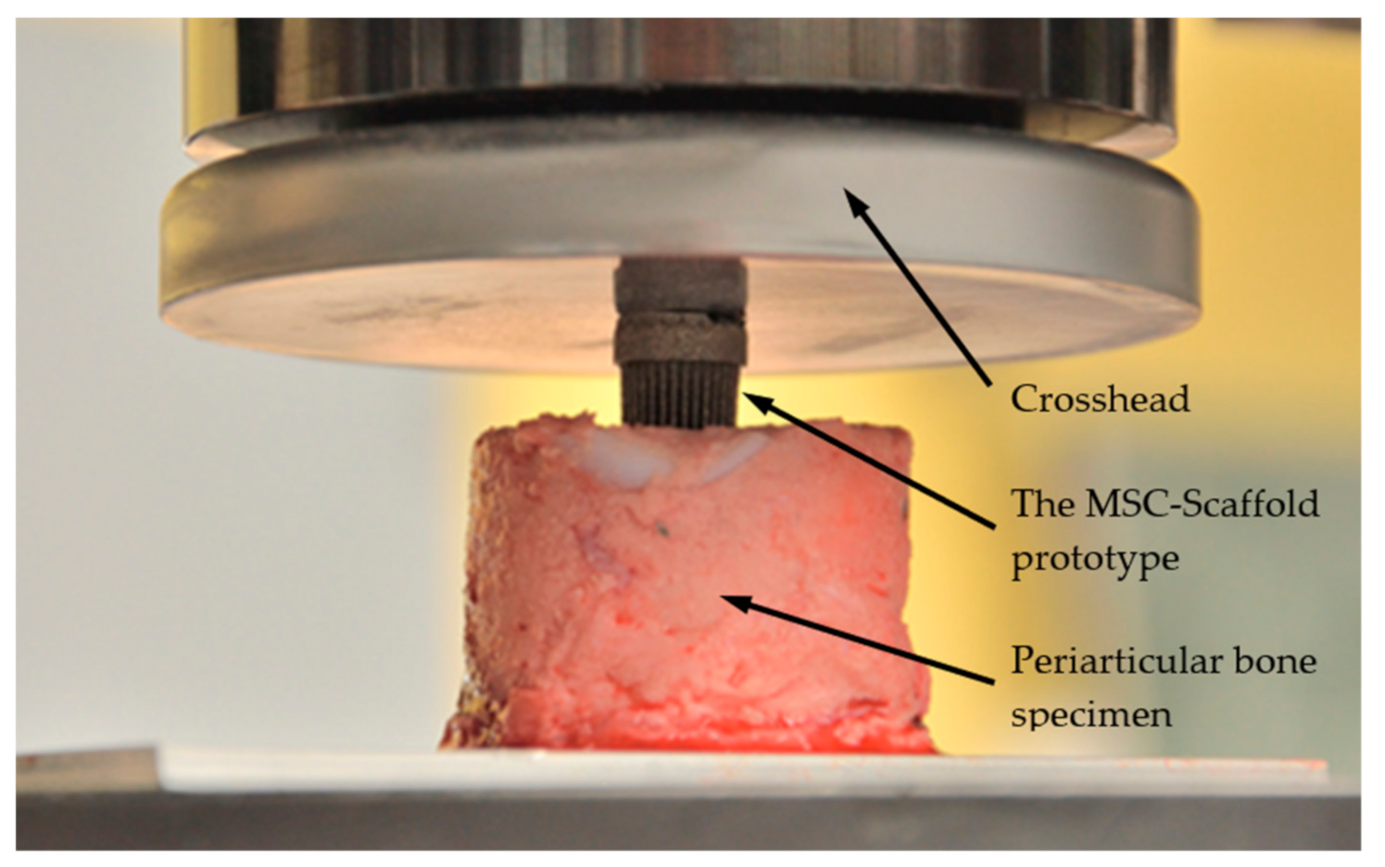
2.5. Mechanical Tests
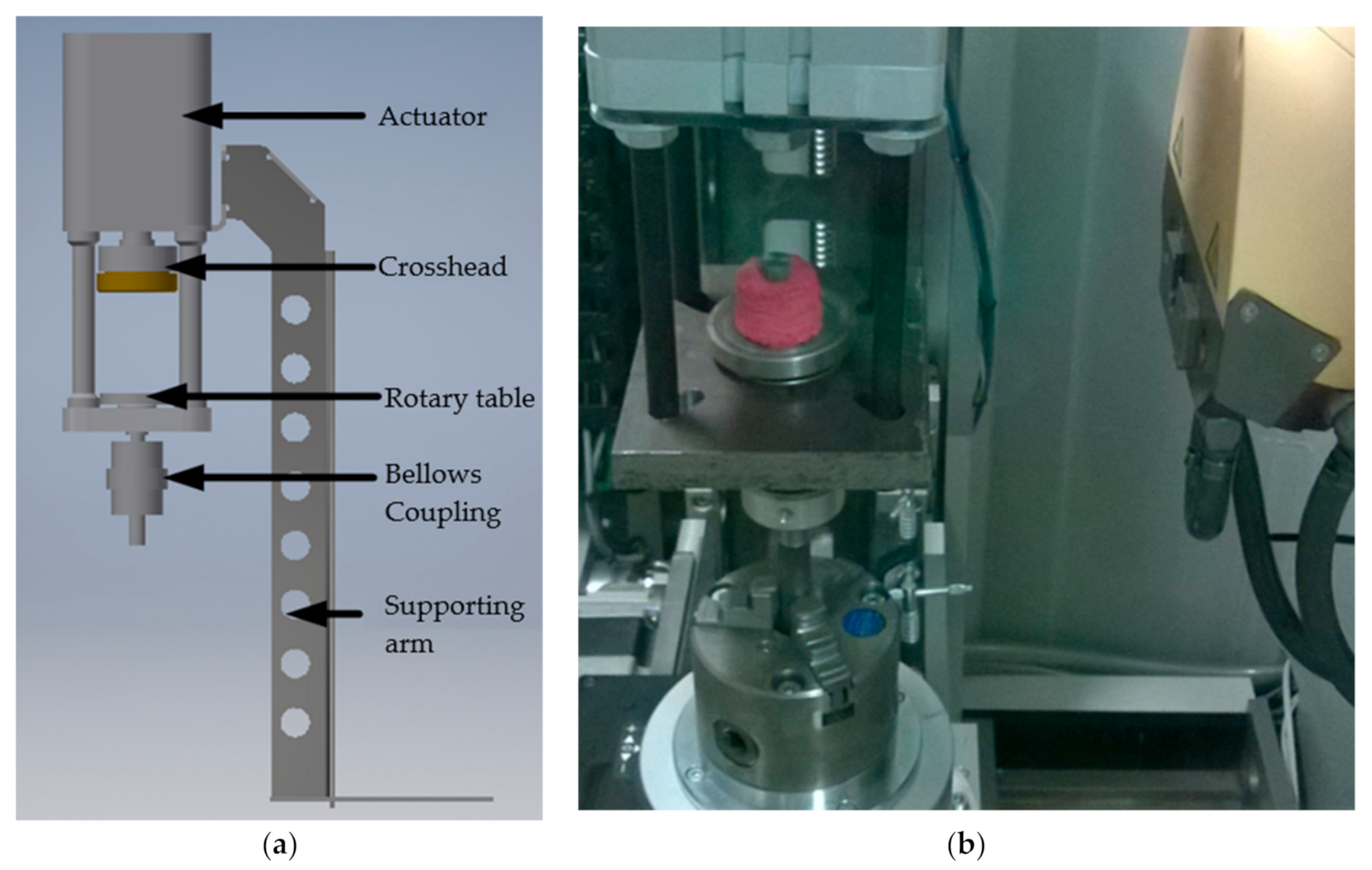
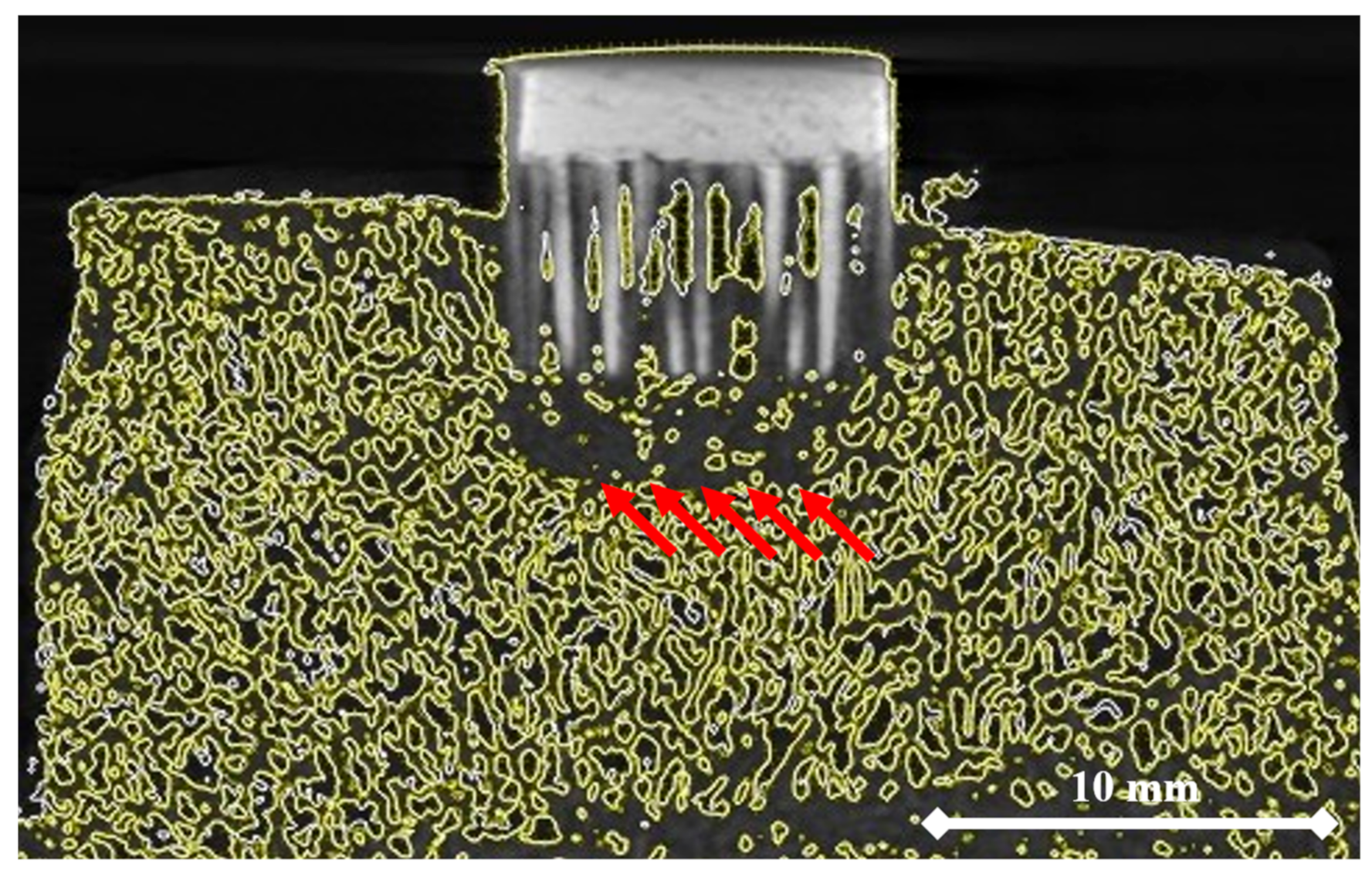
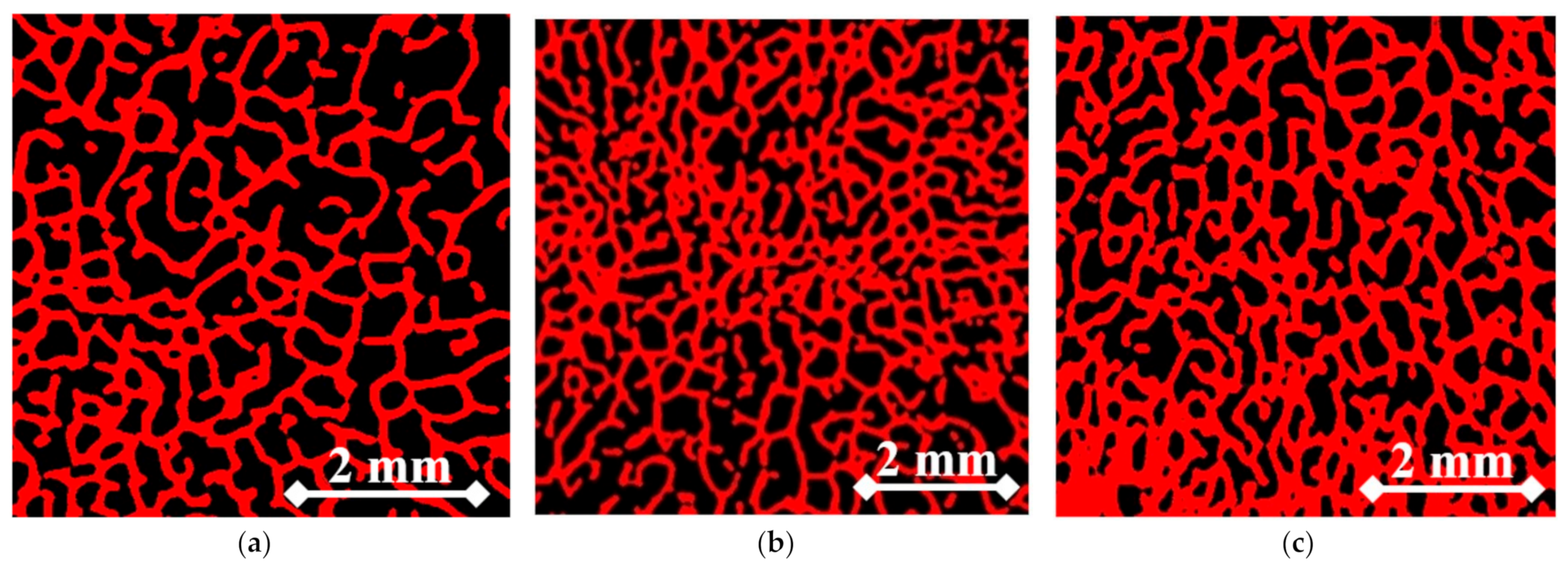
2.6. Micro-CT-Assisted Mechanical Test
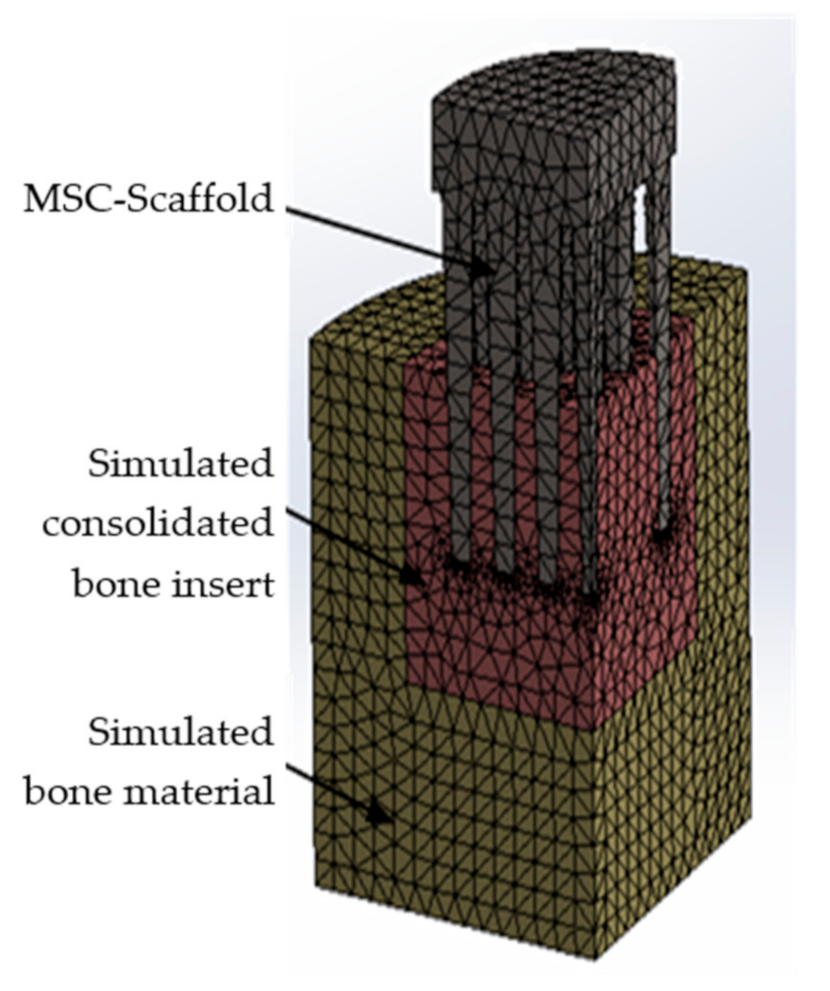
2.7. Modification of the FE Model and Re-Simulation
2.8. Analysis of Correlation
3. Results
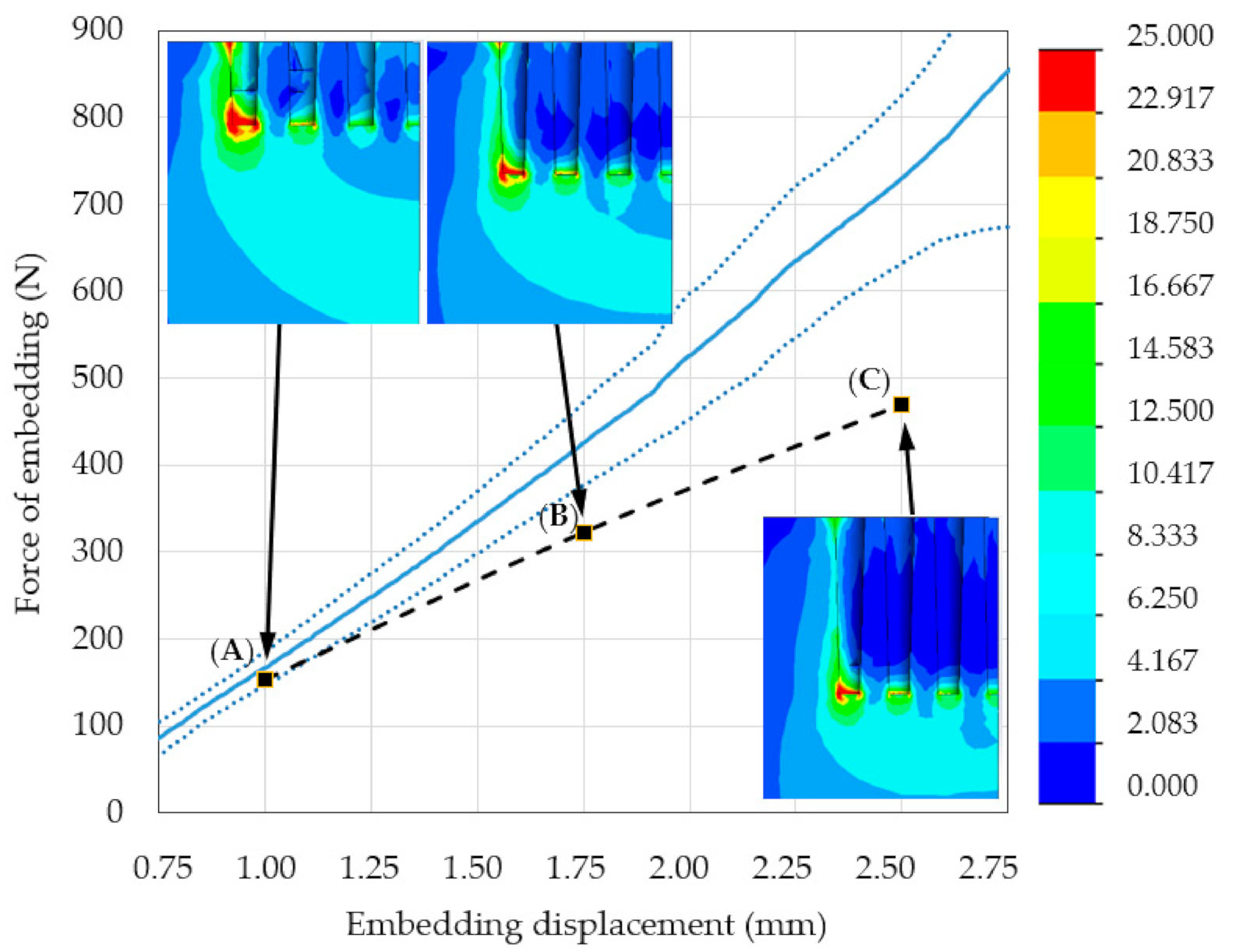
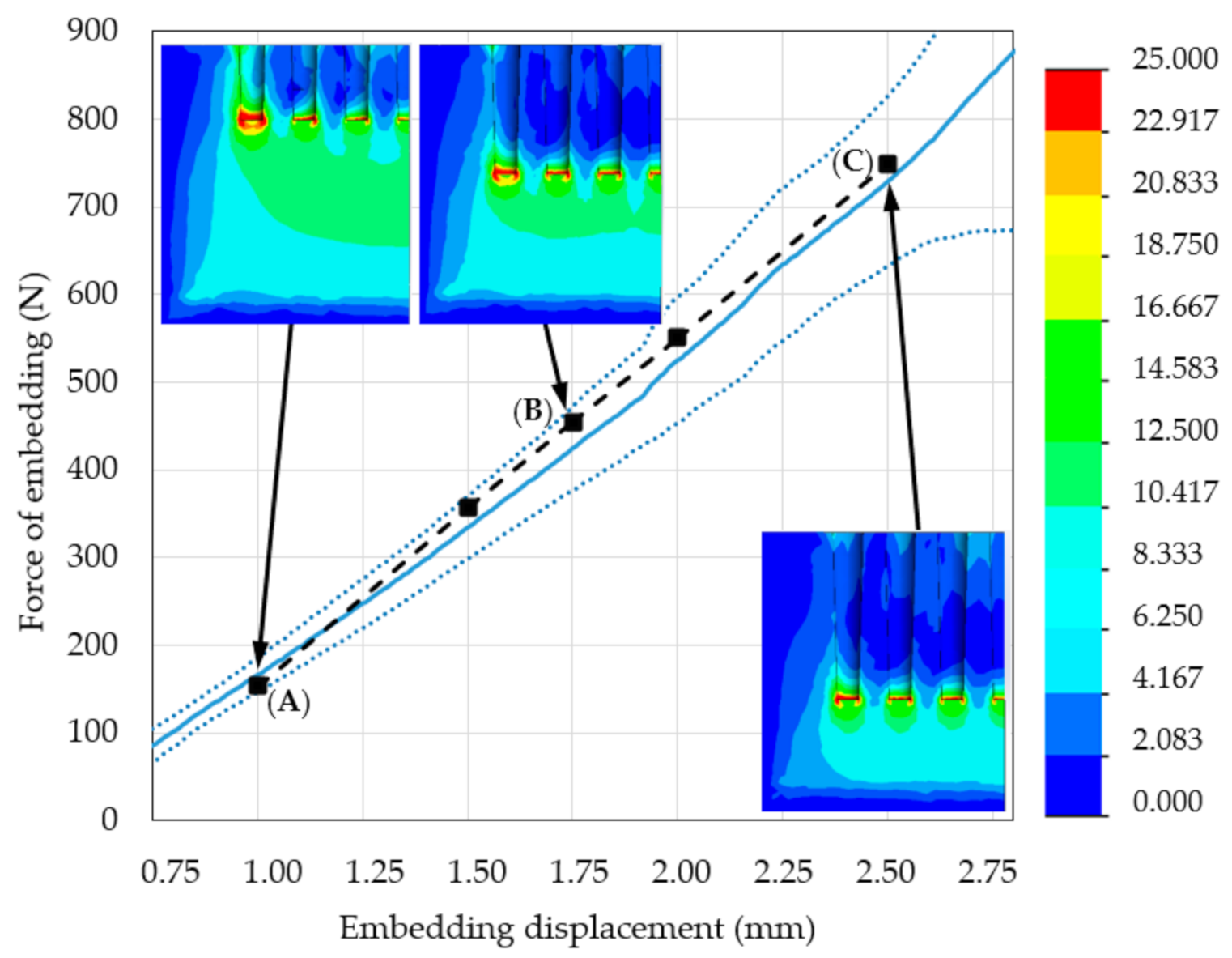
3.1. Mechanical Testing and Initial FE Model Simulations
3.2. Modified FE Model Validation
4. Discussion
5. Conclusions
- The micro-CT assessment during the mechanical embedding test allowed for analysis of the bone material density changes directly under the MSC-Scaffold. Consequently, it enabled improvement of the initial numerical model of the considered problem by introducing a suitable simulated insert of densified bone material. This led to the significant enhancement of correlation between the re-simulation and experimental results, confirmed by the increase in the FVU test result from 0.33 to 0.02.
- The biomimetism of the MSC-Scaffold prototype providing physiological load transfer from implant to bone was confirmed on the basis of the HMH stress maps obtained with the validated FE model of the problem.
- In the study, the correctness of the assumptions made for the developed numerical model of the considered problem was discussed. Since a well-known and widely recognised validation method for the numerical simulation models of various problems in the field of bone-implant mechanics (being a combination of experimental studies and numerical analyses) was used, therefore:
- the results obtained from the numerical simulation analysis of the stress distribution in periarticular bone around the MSC-Scaffold prototype partially embedded in this bone and carried out using a validated FE model can be considered to be reliable;
- the developed validated FE model can be used in a bioengineering design of a new kind of entirely cementless biomimetic fixation of resurfacing endoprostheses, replacing degeneratively or traumatically damaged diarthrodial joints.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piuzzi, N.S.; Ng, M.; Song, S. Consolidation and maturation of the orthopaedic medical device market between 1999 and 2015. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Global Market Study on Hip Replacement: Availability of New and Advanced Hip Implants Boosting Revenue Growth. Available online: https://www.persistencemarketresearch.com/market-research/hip-replacement-market.asp (accessed on 3 December 2019).
- Wang, W.; Ouyang, Y.; Poh, C.K. Orthopaedic implant technology: Biomaterials from past to future. Ann. Acad. Med. 2011, 40, 237–244. [Google Scholar]
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, e16. [Google Scholar] [CrossRef]
- Eglin, D.; Alini, M.; de Bruijn, J.; Gautrot, J.; Grijpma, D.W.; Kamer, L.; Lai, Y.; Lu, S.; Peijs, T.; Peng, J.; et al. The RAPIDOS project European and Chinese collaborative research on biomaterials. J. Orthop. Transl. 2015, 3, 78–84. [Google Scholar] [CrossRef]
- Pérez-Moro, O.S.; Fernández-Cuadros, M.E.; Neira-Borrajo, I.; Aranda-Izquierdo, E.; Albaladejo-Florin, M.J.; Llopis-Miró, R. Short and mid-term outcomes and functional results in metal-on-metal hip resurfacing arthroplasty at 5 years follow-up: The Spanish Experience. BMC Musculoskelet. Disord. 2019, 20, 172. [Google Scholar] [CrossRef]
- De Steiger, R.N.; Miller, L.N.; Prosser, G.H.; Graves, S.E.; Davidson, D.C.; Stanford, T.E. Poor outcome of revised resurfacing hip arthroplasty. Acta Orthop. 2010, 81, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Itayem, R.; Arndt, A.; Daniel, J.; McMinn, D.J.; Lundberg, A. A two-year radiostereometric follow-up of the first generation Birmingham mid head resection arthroplasty. Hip Int. 2014, 24, 355–362. [Google Scholar] [CrossRef]
- Uklejewski, R.; Winiecki, M.; Rogala, P.; Mielniczuk, J. Selective laser melted prototype of original minimally invasive hip endoprosthesis. Rapid Prototyp. J. 2011, 17, 76–85. [Google Scholar] [CrossRef]
- Uklejewski, R.; Rogala, P.; Winiecki, M.; Kędzia, A.; Ruszkowski, P. Preliminary Results of Implantation in Animal Model and Osteoblast Culture Evaluation of Prototypes of Biomimetic Multi-spiked Connecting Scaffold for Noncemented Stemless Resurfacing Hip Arthroplasty Endoprostheses. Biomed. Res. Int. 2013, 2013, 689089. [Google Scholar] [CrossRef] [PubMed]
- Uklejewski, R.; Winiecki, M.; Rogala, P.; Patalas, A. Structural-Geometric Functionalization of the Additively Manufactured Prototype of Biomimetic Multi-spiked Connecting Ti-Alloy Scaffold for Entirely Noncemented Resurfacing Arthroplasty Endoprostheses. Appl. Bionics Biomech. 2017, 2017, 5638680. [Google Scholar] [CrossRef]
- Uklejewski, R.; Winiecki, M.; Patalas, A.; Rogala, P. Numerical studies of the influence of various geometrical features of a multi-spiked connecting scaffold prototype on mechanical stresses in peri-implant bone. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 541–547. [Google Scholar] [CrossRef]
- Rogala, P. Endoprosthesis. EU Patent No. EP072418 B1, 22 December 1999. [Google Scholar]
- Rogala, P. Method and Endoprosthesis to Apply This Implantation. Canadian Patent No. 2,200,064, 1 April 2002. [Google Scholar]
- Uklejewski, R.; Rogala, P.; Winiecki, M. Structural and Hydroxyapatite-like Functionalization of Bone Contacting Surfaces of Additively Manufactured Multispiked Connecting Scaffold Prototype for Entirely Cementless Resurfacing Arthroplasty Endoprostheses to Enhance its Osteo-conduction and Osteointegration. In Advanced Surface Engineering Materials; Tiwari, A., Wang, R., Wei, B., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 175–240. [Google Scholar] [CrossRef]
- Uklejewski, R.; Rogala, P.; Winiecki, M.; Tokłowicz, R.; Ruszkowski, P.; Wołuń-Cholewa, M. Biomimetic Multispiked Connecting Ti-Alloy Scaffold Prototype for Entirely-Cementless Resurfacing Arthroplasty Endoprostheses—Exemplary Results of Implantation of the Ca-P Surface-Modified Scaffold Prototypes in Animal Model and Osteoblast Culture Evaluation. Materials 2016, 9, 532. [Google Scholar] [CrossRef]
- Rogala, P.; Uklejewski, R.; Winiecki, M.; Dąbrowski, M.; Gołańczyk, J.; Patalas, A. First biomimetic fixation for resurfacing arthroplasty—Investigation in swine of a prototype partial knee endoprosthesis. Biomed. Res. Int. 2019, 2019, 6952649. [Google Scholar] [CrossRef]
- Uklejewski, R.; Winiecki, M.; Krawczyk, P.; Tokłowicz, R. Native Osseous CaP Biomineral Coating on a Biomimetic Multi-spiked Connecting Scaffold Prototype for Cementless Resurfacing Arthroplasty Achieved by Combined Electrochemical Deposition. Materials 2019, 12, 3994. [Google Scholar] [CrossRef]
- Enns-Bray, W.S.; Ariza, O.; Gilchrist, S.; Widmer-Soyka, R.P.; Vogt, P.J.; Palsson, H.; Helgason, B. Morphology based anisotropic finite element models of the proximal femur validated with experimental data. Med. Eng. Phys. 2016, 38, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Affes, F.; Ketata, H.; Kharrat, M.; Dammak, M. How a pilot hole size affects osteosynthesis at the screw–bone interface under immediate loading. Med. Eng. Phys. 2018, 60, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Hsu, J.T.; Fuh, L.J.; Tu, M.G.; Ko, C.C.; Shen, Y.W. Bone stress and interfacial sliding analysis of implant designs on an immediately loaded maxillary implant: A non-linear finite element study. J. Dent. 2008, 36, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lee, J.H.; Jang, A.T.; Gu, A.; Hossaini-Zadeh, M.; Prevost, R.; Curtis, D.A.; Ho, S.P. Biomechanics and strain mapping in bone as related to immediately-loaded dental implants. J. Biomech. 2015, 48, 3486–3494. [Google Scholar] [CrossRef]
- Wu, D.; Isaksson, P.; Ferguson, S.J.; Persson, C. Young’s modulus of trabecular bone at the tissue level: A review. Acta Biomater. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.; Vashishth, D. Mechanical Characterization of Bone: State of the Art in Experimental Approaches—What Types of Experiments Do People Do and How Does One Interpret the Results? Curr. Osteoporos. Rep. 2018, 16, 423–433. [Google Scholar] [CrossRef]
- Chen, C.; Hao, Y.; Bai, X.; Ni, J.; Chung, S.-M.; Liu, F.; Lee, I.-S. 3D printed porous Ti6Al4V cage: Effects of additive angle on surface properties and biocompatibility; bone ingrowth in Beagle tibia model. Mater. Des. 2019, 175, 107824. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- An, Y.; Freidman, R. Animal Models in Orthopaedic Research; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Burkhart, T.A.; Andrews, D.M.; Dunning, C.E. Finite element modeling mesh quality, energy balance and validation methods: A review with recommendations associated with the modeling of bone tissue. J. Biomech. 2013, 46, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Dogru, S.C.; Cansiz, E.; Arslan, Y.Z. A review of finite element applications in oral and maxillofacial biomechanics. J. Mech. Med. Biol. 2018, 18, 1830002. [Google Scholar] [CrossRef]
- Zhai, Y.; Galarraga, H.; Lados, D.A. Microstructure, static properties, and fatigue crack growth mechanisms in Ti-6Al-4V fabricated by additive manufacturing: LENS and EBM. Eng. Fail. Anal. 2016, 69, 3–14. [Google Scholar] [CrossRef]
- An, Y.; Draughn, R. Mechanical Testing of Bone and the Bone-Implant Interface; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Cowin, S.C. Bone Mechanics Handbook; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kerr, A.; Rowe, P. An Introduction to Human Movement and Biomechanics, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Natali, A.N.; Meroi, E.A. A review of the biomechanical properties of bone as a material. J. Biomech. Eng. 1989, 11, 266–276. [Google Scholar] [CrossRef]
- Guillén, T.; Zhang, Q.-H.; Tozzi, G.; Ohrndorf, A.; Christ, H.-J.; Tong, J. Compressive behaviour of bovine cancellous bone and bone analogous materials, microCT characterisation and FE analysis. J. Mech. Behav. Biomed. Mater. 2011, 4, 1452–1461. [Google Scholar] [CrossRef]
- VG Studio Max, version 3.3, for the visualization and analysis of industrial computed tomography (CT) data; Volume Graphics GmbH: Heidelberg, Germany, 2019; Available online: https://www.volumegraphics.com/en/products/vgstudio-max.html (accessed on 12 March 2021).
- Nobakhti, S.; Shefelbine, S.J. On the Relation of Bone Mineral Density and the Elastic Modulus in Healthy and Pathologic Bone. Curr. Osteoporos. Rep. 2018, 16, 404–410. [Google Scholar] [CrossRef]
- Haba, Y.; Lindner, T.; Fritsche, A.; Schiebenhöfer, A.K.; Souffrant, R.; Kluess, D.; Bader, R. Relationship between mechanical properties and bone mineral density of human femoral bone retrieved from patients with osteoarthritis. Open Orthop. J. 2012, 6, 458–463. [Google Scholar] [CrossRef][Green Version]
- Kaneko, T.S.; Bell, J.S.; Pejcic, M.R.; Tehranzadeh, J.; Keyak, J.H. Mechanical properties, density and quantitative CT scan data of trabecular bone with and without metastases. J. Biomech. 2004, 37, 523–530. [Google Scholar] [CrossRef]
- Spiess, A.N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharmacol. 2010, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, G. To Explain or to Predict? Stat. Sci. 2010, 25, 289–310. [Google Scholar] [CrossRef]
- Bruce, A. Practical Statistics for Data Scientists: 50 Essential Concepts; Shroff/O’Reilly: Mumbai, India, 2017. [Google Scholar]
- Cicciù, M.; Fiorillo, L.; D’Amico, C.; Gambino, D.; Amantia, E.M.; Laino, L.; Crimi, S.; Campagna, P.; Bianchi, A.; Herford, A.S.; et al. 3D digital impression systems compared with traditional techniques in dentistry: A recent data systematic review. Materials 2020, 13, 1982. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Milone, D.; Risitano, G. FEM investigation of the stress distribution over mandibular bone due to screwed over denture positioned on dental implants. Materials 2018, 11, 1512. [Google Scholar] [CrossRef]
- Bramanti, E.; Cervino, G.; Lauritano, F.; Fiorillo, L.; D’Amico, C.; Sambataro, S.; Denaro, D.; Famà, F.; Ierardo, G.; Polimeni, A.; et al. FEM and von mises analysis on prosthetic crowns structural elements: Evaluation of different applied materials. Sci. World J. 2017, 2017, 1029574. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, F.; Runci, M.; Cervino, G.; Fiorillo, L.; Bramanti, E.; Cicciù, M. Three-dimensional evaluation of different prosthesis retention systems using finite element analysis and the Von Mises stress test. Minerva Stomatol. 2016, 65, 353–367. [Google Scholar]
- Ramos, A.; Soares dos Santos, M.P.; Mesnard, M. Predictions of Birmingham hip resurfacing implant offset–In vitro and numerical models. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 352–363. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Lieberman, I.; Haddas, R. Stress distribution in vertebral bone and pedicle screw and screw-bone load transfers among various fixation methods for lumbar spine surgical alignment: A finite element study. Med. Eng. Phys. 2019, 63, 26–32. [Google Scholar] [CrossRef]
- Gong, H.; Fan, Q.; Zhou, Y.; Wang, D.; Li, P.; Su, T.; Zhang, H. Simulation of failure processes of as-cast ti-5Al-5Nb-1Mo-1V-1Fe titanium alloy subjected to quasi-static uniaxial tensile testing. Mater. Des. 2019, 180, 107962. [Google Scholar] [CrossRef]
- Timercan, A.; Brailovski, V.; Petit, Y.; Lussier, B.; Séguin, B. Personalized 3D-printed endoprostheses for limb sparing in dogs: Modeling and in vitro testing. Med. Eng. Phys. 2019, 71, 17–29. [Google Scholar] [CrossRef]
- Hazrati-Marangalou, J.; Ito, K.; van Rietbergen, B. A new approach to determine the accuracy of morphology–elasticity relationships in continuum FE analyses of human proximal femur. J. Biomech. 2012, 45, 2884–2892. [Google Scholar] [CrossRef]
- Van Rietbergen, B.; Kabel, J.; Odgaard, A.; Huiskes, R. Determination of Trabecular Bone Tissue Elastic Properties by Comparison of Experimental and Finite Element Results. In Material Identification Using Mixed Numerical Experimental Methods; Sol, H., Oomens, C.W.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Krone, R.; Schuster, P. An Investigation of Importance of Material Anisotropy in Finite-Element Modeling of the Human Femur. SAE Int. 2006, 1, 64. [Google Scholar] [CrossRef]
- Wirtz, D.C.; Schiffers, N.; Pandorf, T.; Radermacher, K.; Weichert, D.; Forst, R. Critical evaluation of known bone material properties to realize anisotropic FE-simulation of the proximal femur. J. Biomech. 2000, 33, 1325–1330. [Google Scholar] [CrossRef]
- Keyak, J.H.; Rossi, S.A. Prediction of femoral fracture load using finite element models: An examination of stress- and strain-based failure theories. J. Biomech. 2000, 33, 209–214. [Google Scholar] [CrossRef]
- Tellache, M.; Pithioux, M.; Chabrand, P.; Hochard, C. Femoral neck fracture prediction by anisotropic yield criteria. Eur. J. Comput. Mech. 2009, 18, 33–41. [Google Scholar] [CrossRef][Green Version]
- Maknickas, A.; Alekna, V.; Ardatov, O.; Chabarova, O.; Zabulionis, D.; Tamulaitienė, M.; Kačianauskas, R. FEM-Based Compression Fracture Risk Assessment in Osteoporotic Lumbar Vertebra L1. Appl. Sci. 2019, 9, 3013. [Google Scholar] [CrossRef]
- Provatidis, C.; Vossou, C.; Koukoulis, I.; Balanika, A.; Baltas, C.; Lyritis, G. A pilot finite element study of an osteoporotic L1-vertebra compared to one with normal T-score. Comp. Meth. Biomech. Biomed. Eng. 2010, 13, 185–195. [Google Scholar] [CrossRef]
- Haut, R.C.; Wei, F. Biomechanical studies on patterns of cranial bone fracture using the immature porcine model. J. Biomech. Eng. 2017, 139, 21001. [Google Scholar] [CrossRef]
- Bowland, P.; Ingham, E.; Fisher, J.; Jennings, L.M. Development of a preclinical natural porcine knee simulation model for the tribological assessment of osteochondral grafts in vitro. J. Biomech. 2018, 77, 91–98. [Google Scholar] [CrossRef]
- Pilia, M.; Guda, T.; Appleford, M. Development of composite scaffolds for load-bearing segmental bone defects. Biomed. Res. Int. 2013, 2013, 458253. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]


| Young’s Modulus (GPa) | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation at Rupture (%) | Poisson’s Ratio |
|---|---|---|---|---|
| 116 | 1150 | 1010 | 25 | 0.34 |
| E1 (MPa) | E2 (MPa) | G1 (MPa) | G2 (MPa) | ν1 | ν2 | σc (MPa) |
|---|---|---|---|---|---|---|
| 608 | 771 | 260 | 269 | 0.17 | 0.15 | 25 |
| Embedding Displacement (mm) | Fraction of Marrow and Soft Tissue (%) | Trabecular Bone Density (g/cm3) | Bone Longitudinal Elastic Modulus (MPa) |
|---|---|---|---|
| 1.5 | 58.2 | 1.37 | 821 |
| 2 | 49.1 | 1.46 | 976 |
| 2.5 | 40.3 | 1.55 | 1173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uklejewski, R.; Winiecki, M.; Patalas, A.; Rogala, P. Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses. Materials 2021, 14, 1384. https://doi.org/10.3390/ma14061384
Uklejewski R, Winiecki M, Patalas A, Rogala P. Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses. Materials. 2021; 14(6):1384. https://doi.org/10.3390/ma14061384
Chicago/Turabian StyleUklejewski, Ryszard, Mariusz Winiecki, Adam Patalas, and Piotr Rogala. 2021. "Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses" Materials 14, no. 6: 1384. https://doi.org/10.3390/ma14061384
APA StyleUklejewski, R., Winiecki, M., Patalas, A., & Rogala, P. (2021). Bone Density Micro-CT Assessment during Embedding of the Innovative Multi-Spiked Connecting Scaffold in Periarticular Bone to Elaborate a Validated Numerical Model for Designing Biomimetic Fixation of Resurfacing Endoprostheses. Materials, 14(6), 1384. https://doi.org/10.3390/ma14061384









