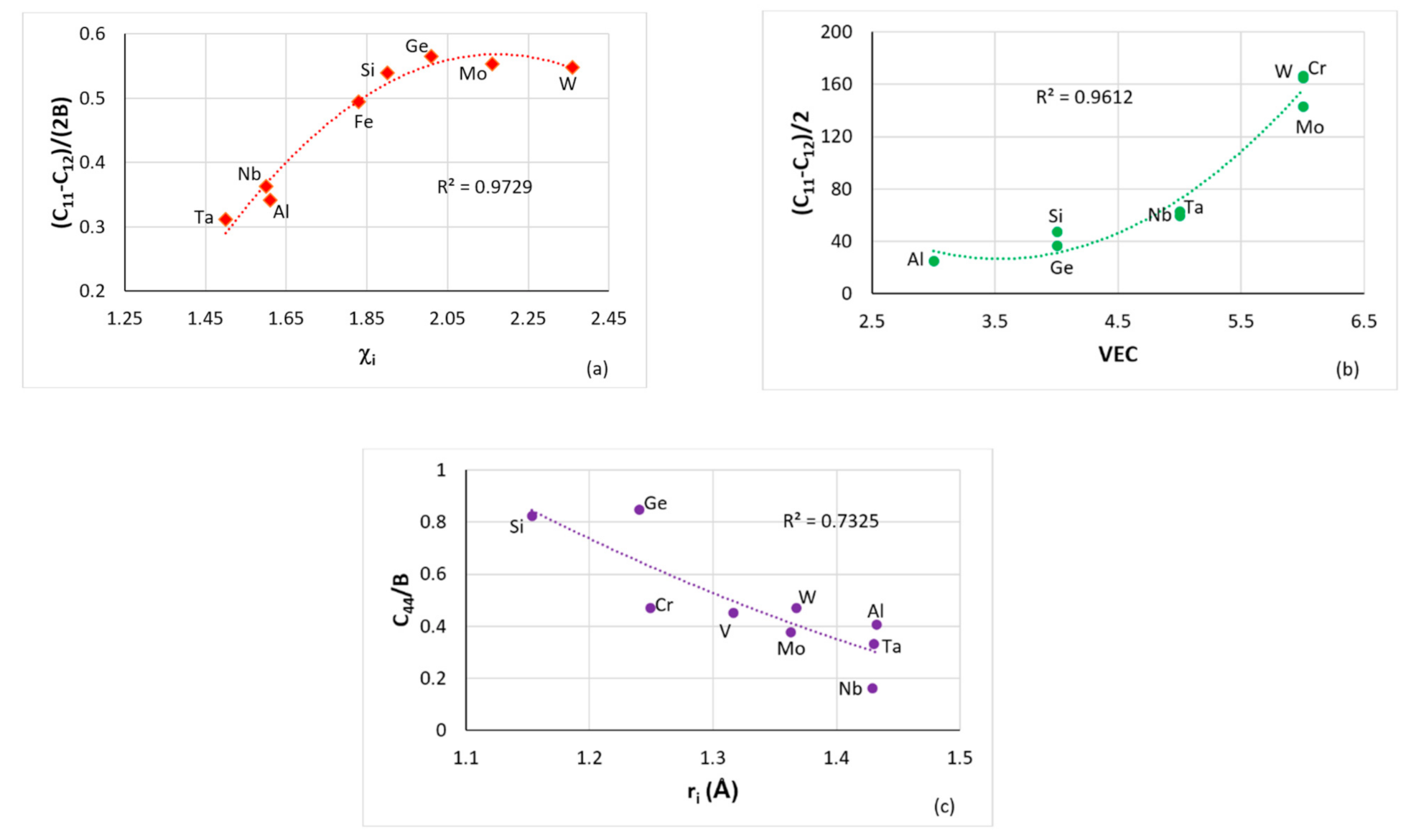
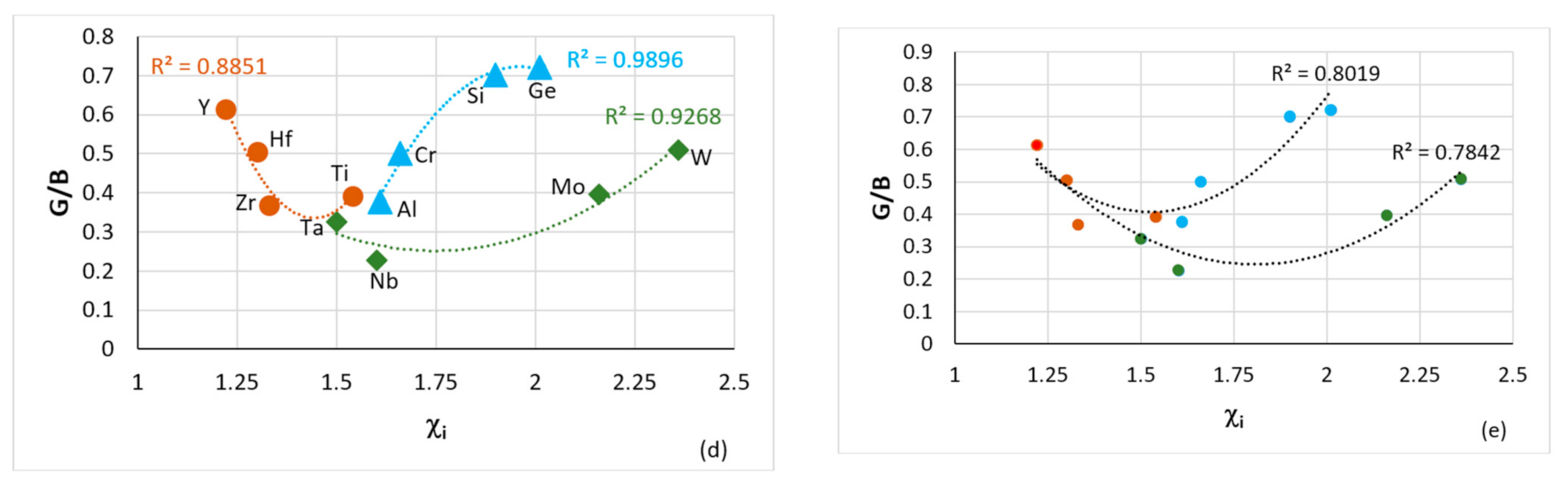
Figure 1.
(
a–
c) data for cubic symmetry elements in RM(Nb)ICs, (
a) plot of [C
11-C
12]/[2B] versus Pauling electronegativity χ
i for Al, Fe, Ge, Mo, Nb, Si, Ta and W, (
b) plot of (C
11-C
12)/2 versus VEC for Al, Cr, Ge, Mo, Nb, Si, Ta and W and (
c) plot of C
44/B for Al, Cr, Ge, Mo, Nb, Si, Ta, V and W. (d) Plot of G/B versus Pauling electronegativity for cubic and hexagonal symmetry elements, circles for Hf, Ti, Y, Zr, triangles for Al, Cr, Ge, Si and diamonds for Mo, Nb, Ta, W. (
e) is the same as (
d) and shows one group containing Y, Hf, Zr, Ti, Al, Cr, Si, Ge (R
2 = 0.8019) and another containing Y, Hf, Zr, Ti, Ta, Nb, Mo, W (R
2 = 0.7842). Note that with the exception of Fe in (
a), all other elements in (
a–
d) are used in RHEAs and RCCAs (for Ge see text) and that Al, Cr, Ge, Hf, Si, Ti improve the oxidation of RM(Nb)ICs [
1]. In (a) R
2 = 0.9726 if Fe were to be excluded.
Figure 1.
(
a–
c) data for cubic symmetry elements in RM(Nb)ICs, (
a) plot of [C
11-C
12]/[2B] versus Pauling electronegativity χ
i for Al, Fe, Ge, Mo, Nb, Si, Ta and W, (
b) plot of (C
11-C
12)/2 versus VEC for Al, Cr, Ge, Mo, Nb, Si, Ta and W and (
c) plot of C
44/B for Al, Cr, Ge, Mo, Nb, Si, Ta, V and W. (d) Plot of G/B versus Pauling electronegativity for cubic and hexagonal symmetry elements, circles for Hf, Ti, Y, Zr, triangles for Al, Cr, Ge, Si and diamonds for Mo, Nb, Ta, W. (
e) is the same as (
d) and shows one group containing Y, Hf, Zr, Ti, Al, Cr, Si, Ge (R
2 = 0.8019) and another containing Y, Hf, Zr, Ti, Ta, Nb, Mo, W (R
2 = 0.7842). Note that with the exception of Fe in (
a), all other elements in (
a–
d) are used in RHEAs and RCCAs (for Ge see text) and that Al, Cr, Ge, Hf, Si, Ti improve the oxidation of RM(Nb)ICs [
1]. In (a) R
2 = 0.9726 if Fe were to be excluded.
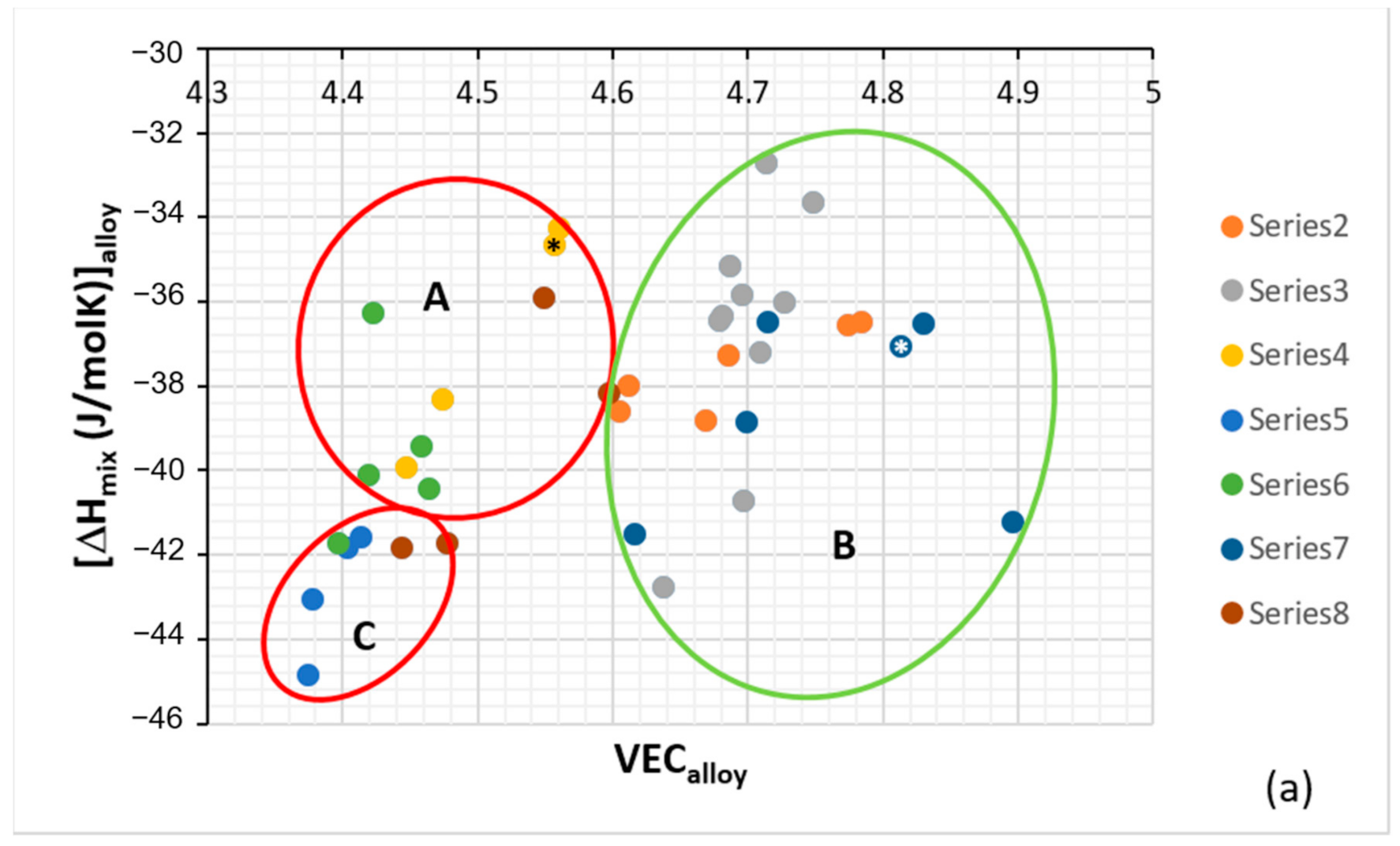
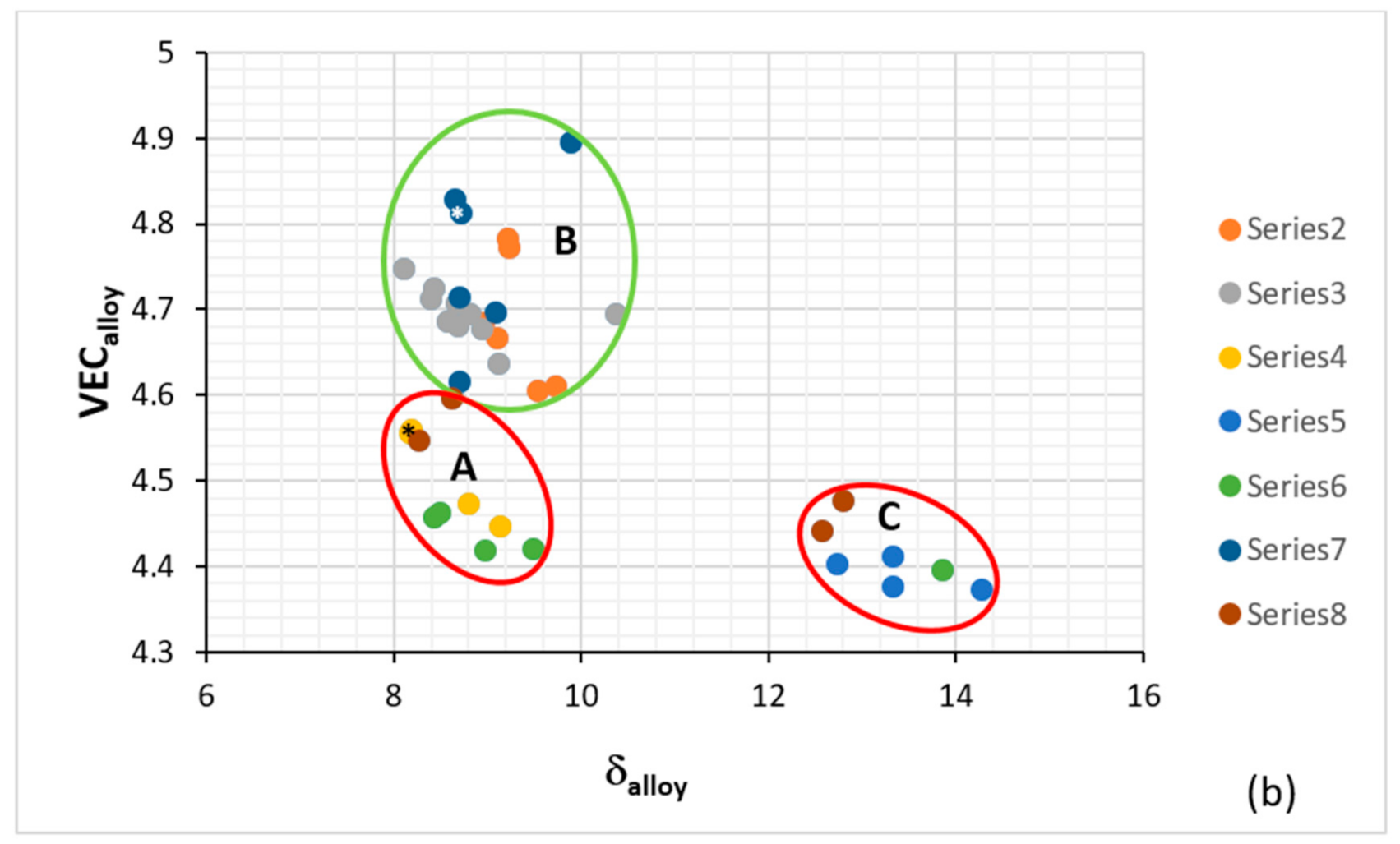
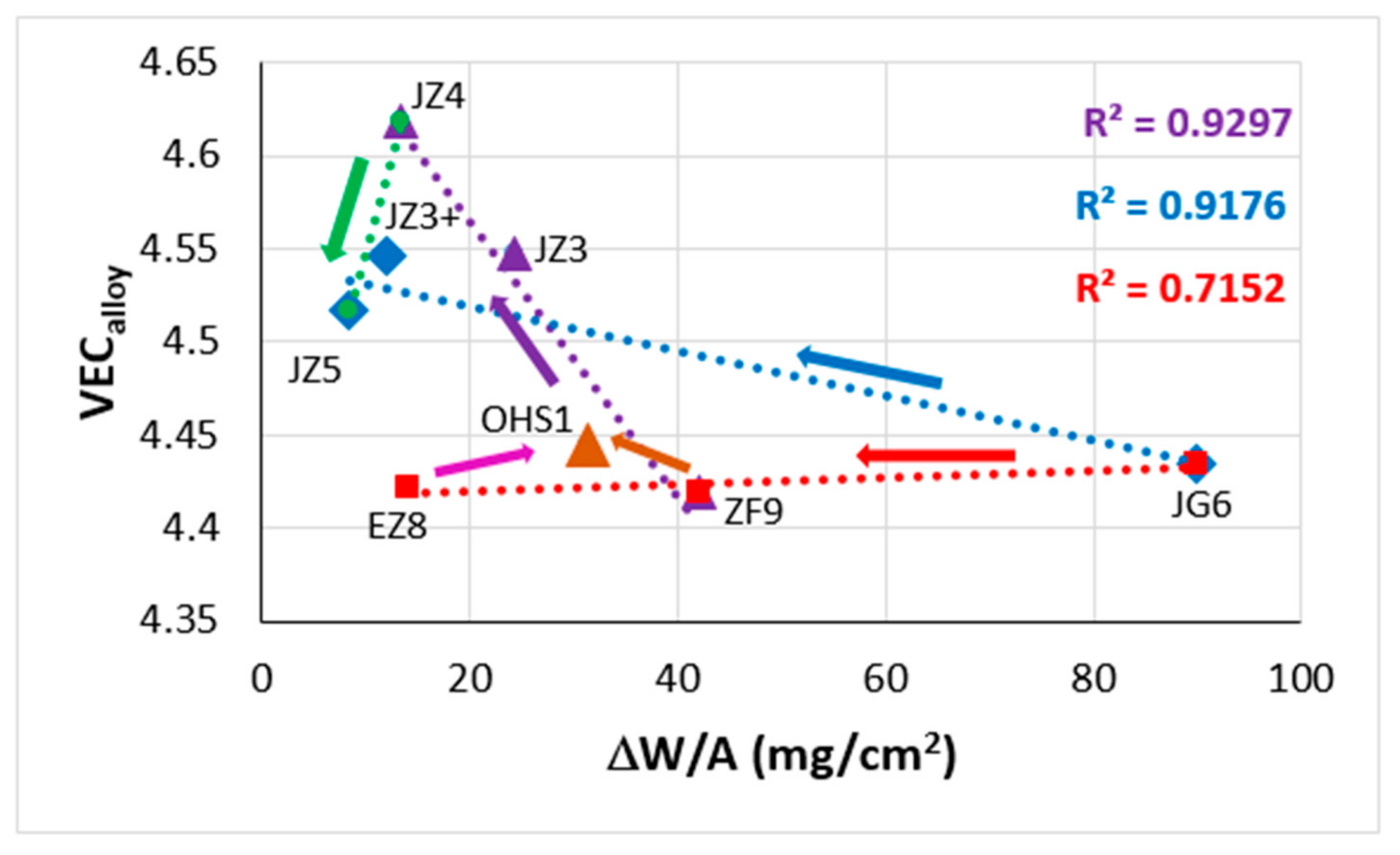
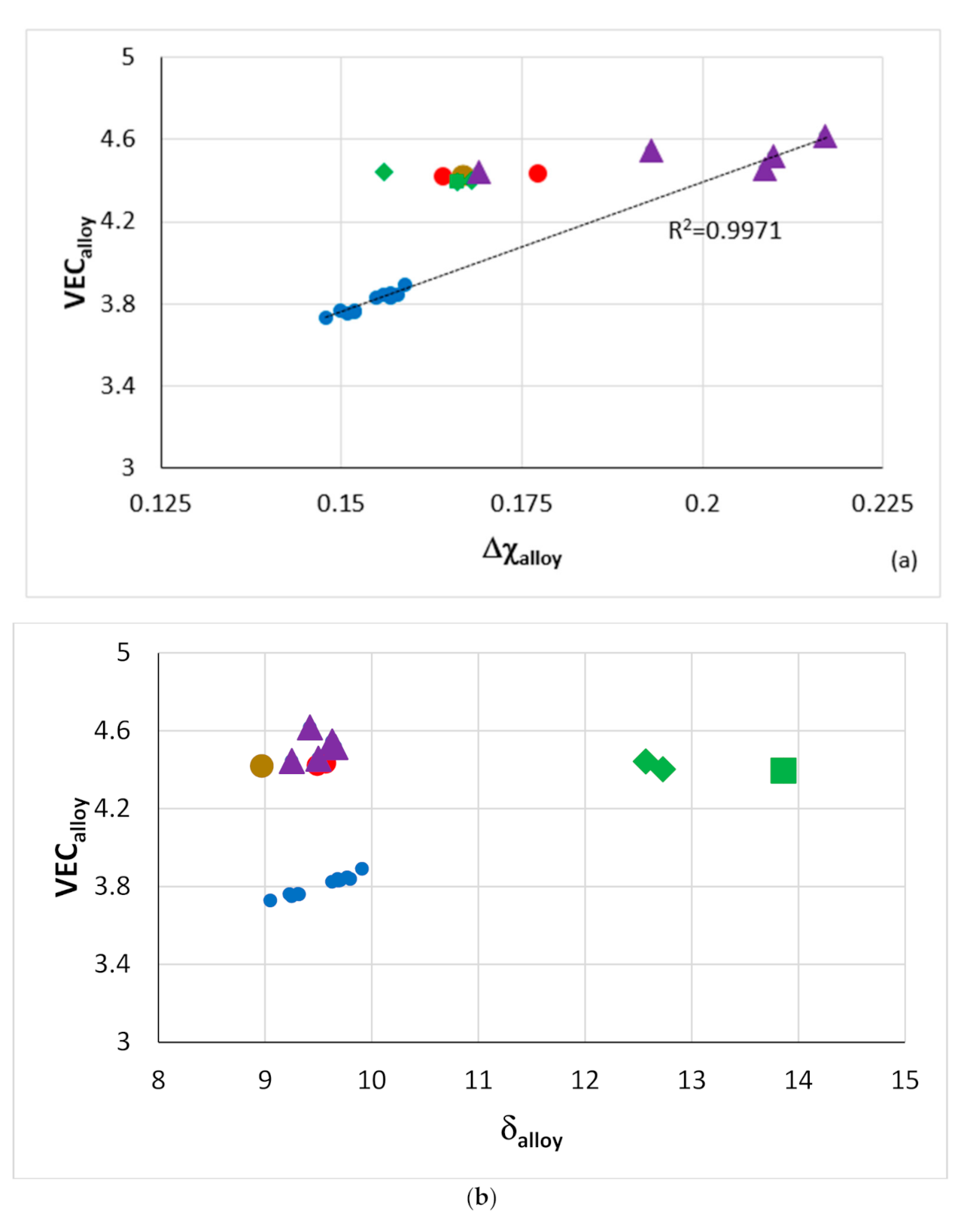
Figure 2.
Plots of ΔH
mix versus VEC (
a) and VEC versus δ (
b). The alloys KZ5 and YG8 are in areas A and B. Alloys with Boron are only in area C. Group A alloys based on KZ5 with Nb
ss and Nb
5Si
3 with/out Laves phase, Group B alloys based on YG8 with Nb
ss and Nb
5Si
3, Group C alloys based on KZ series alloys [
31,
33] with B addition. There are no boron-containing alloys in areas A and B. Alloys with RMs, Al, Cr, Sn and Ge are in all three areas. The alloys KZ5 and YG8 are indicated by asterisks. For the alloys of series 2 to 8, see Table 1 in [
8], where also the values of the parameters Δχ, δ, VEC, ΔH
mix, ΔS
mix, Ω are given.
Figure 2.
Plots of ΔH
mix versus VEC (
a) and VEC versus δ (
b). The alloys KZ5 and YG8 are in areas A and B. Alloys with Boron are only in area C. Group A alloys based on KZ5 with Nb
ss and Nb
5Si
3 with/out Laves phase, Group B alloys based on YG8 with Nb
ss and Nb
5Si
3, Group C alloys based on KZ series alloys [
31,
33] with B addition. There are no boron-containing alloys in areas A and B. Alloys with RMs, Al, Cr, Sn and Ge are in all three areas. The alloys KZ5 and YG8 are indicated by asterisks. For the alloys of series 2 to 8, see Table 1 in [
8], where also the values of the parameters Δχ, δ, VEC, ΔH
mix, ΔS
mix, Ω are given.
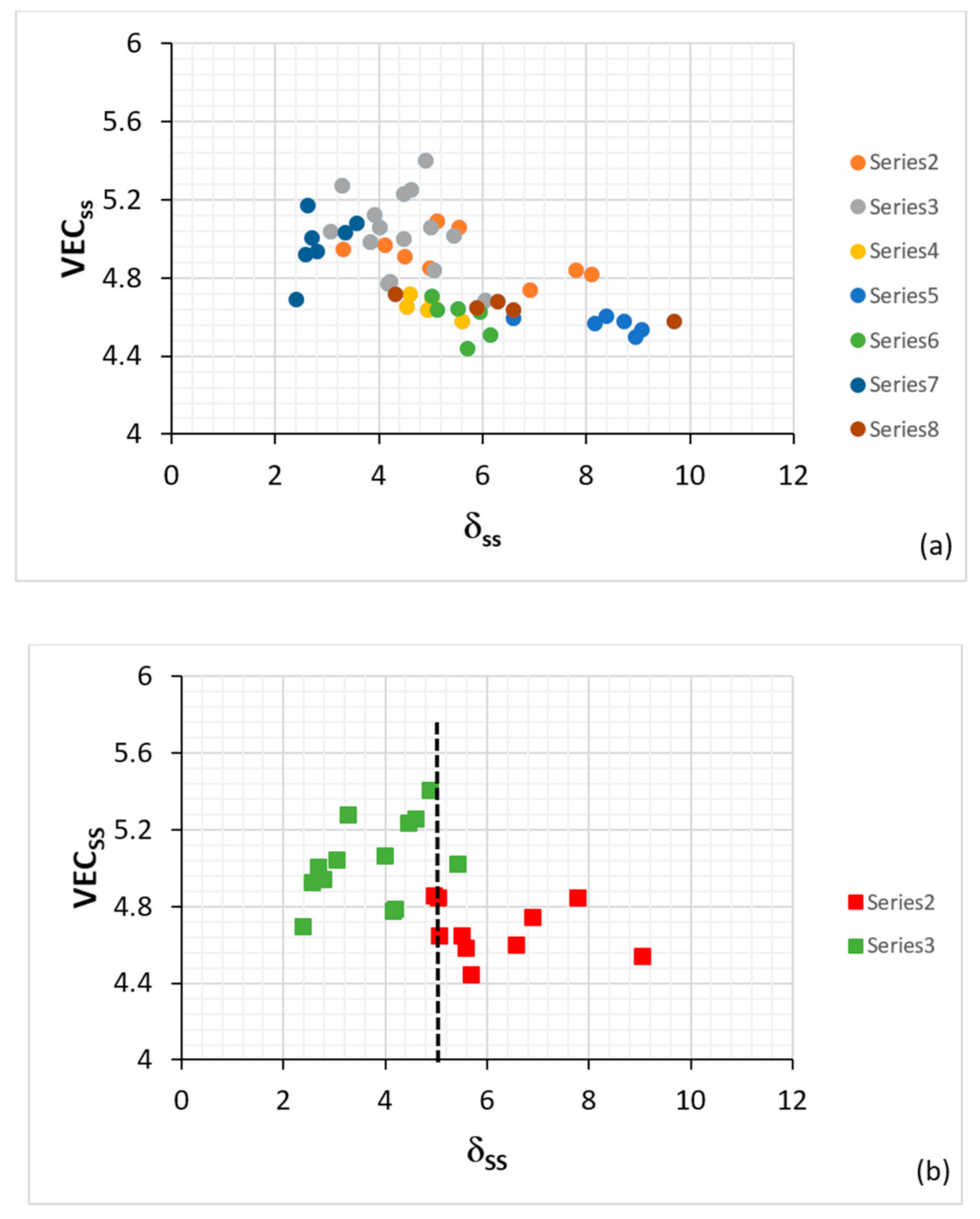
Figure 3.
Valence electron concentration (VEC) versus δ maps for the bcc Nb
ss in Nb-silicide-based alloys, (
a) all data and (
b) data for Si-free Nb
ss and for Nb
ss rich in Ti. In (
a) the series 2 data are for Nb
ss with RMs and Sn and no Al, the series 3 is for Nb
ss with RMs, Ge and Sn and with/out Al and Cr, the series 4 is for Nb
ss with Al, Cr, with/out Hf, no RMs and no B, Ge, Sn, the series 5 is for Nb
ss with Al, B, Cr with/out Hf, the series 6 is for Nb
ss with Al, Cr, with/out Ge or Sn or B, the series 7 is for Nb
ss with RMs, with/out Al and with no B, Cr, Ge, Sn and the series 8 is for Nb
ss with TMs, Al with/out RMs, B or Sn and no Ge. In (
b) the series 2 is for Nb
ss rich in Ti, and series 3 is for Si-free Nb
ss. RM = Mo,Ta,W, TM = Cr,Hf,Ti. For the chemical composition of the solid solutions, see Table 1 in [
7]. For dashed line in (b), see text. Note that the solid solutions included in this figure belong to the alloys for which data are given in
Figure 2.
Figure 3.
Valence electron concentration (VEC) versus δ maps for the bcc Nb
ss in Nb-silicide-based alloys, (
a) all data and (
b) data for Si-free Nb
ss and for Nb
ss rich in Ti. In (
a) the series 2 data are for Nb
ss with RMs and Sn and no Al, the series 3 is for Nb
ss with RMs, Ge and Sn and with/out Al and Cr, the series 4 is for Nb
ss with Al, Cr, with/out Hf, no RMs and no B, Ge, Sn, the series 5 is for Nb
ss with Al, B, Cr with/out Hf, the series 6 is for Nb
ss with Al, Cr, with/out Ge or Sn or B, the series 7 is for Nb
ss with RMs, with/out Al and with no B, Cr, Ge, Sn and the series 8 is for Nb
ss with TMs, Al with/out RMs, B or Sn and no Ge. In (
b) the series 2 is for Nb
ss rich in Ti, and series 3 is for Si-free Nb
ss. RM = Mo,Ta,W, TM = Cr,Hf,Ti. For the chemical composition of the solid solutions, see Table 1 in [
7]. For dashed line in (b), see text. Note that the solid solutions included in this figure belong to the alloys for which data are given in
Figure 2.
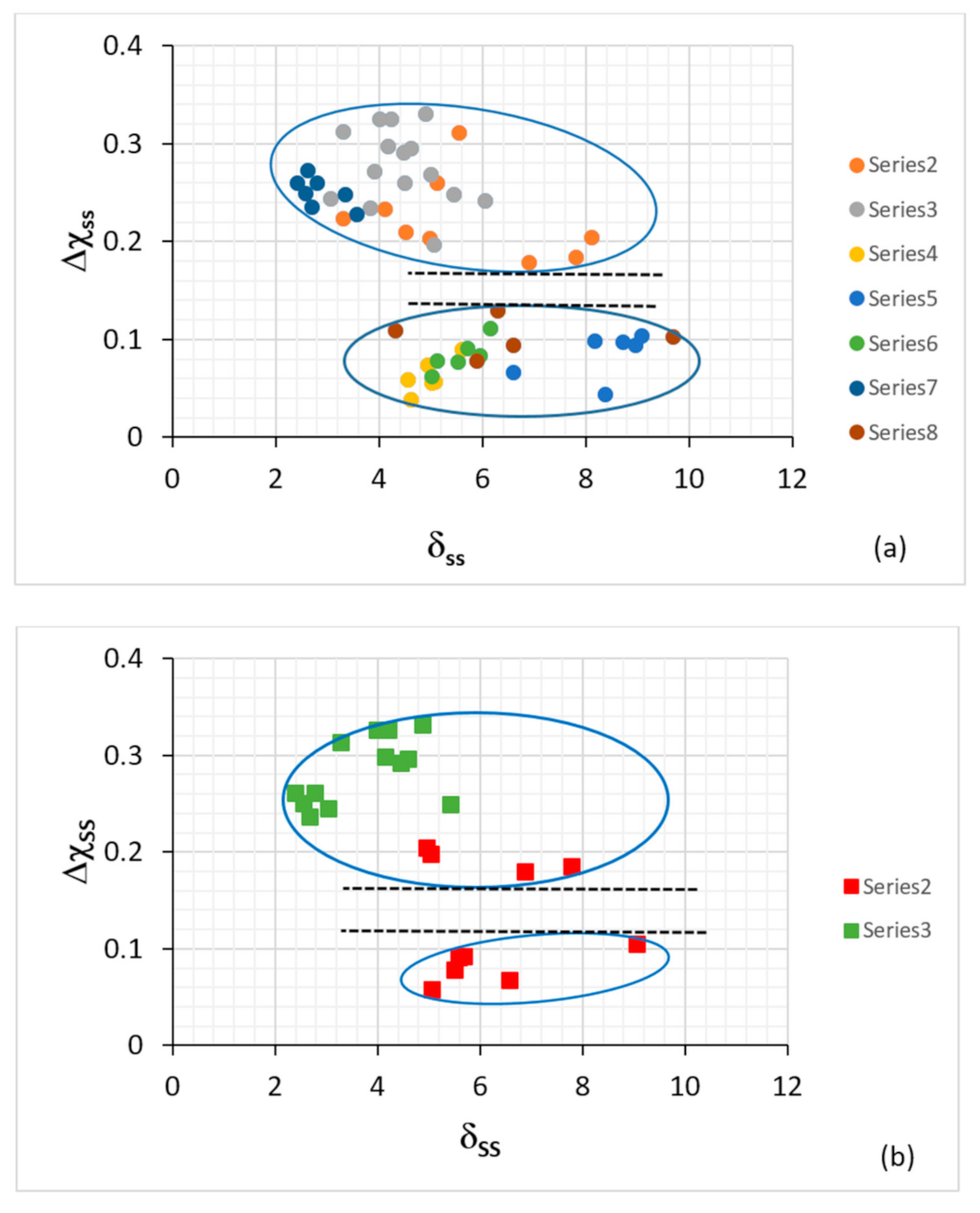
Figure 4.
Δχ versus delta (δ) maps for Nb
ss in Nb-silicide-based alloys, (
a) all data and (
b) data only for Si-free Nb
ss and Nb
ss rich in Ti. In (
a) the series 2 data are for Nb
ss with RMs and Sn and no Al, the series 3 is for Nb
ss with RMs, Ge and Sn and with/out Al and Cr, the series 4 is for Nb
ss with Al, Cr, with/out Hf, no RMs and no B, Ge, Sn, the series 5 is for Nb
ss with Al, B, Cr with/out Hf, the series 6 is for Nb
ss with Al, Cr, with/out Ge or Sn or B, the series 7 is for Nb
ss with RMs, with/out Al and with no B, Cr, Ge, Sn and the series 8 is for Nb
ss with TMs, Al with/out RMs, B or Sn and no Ge. In (
b) the series 2 is for Nb
ss rich in Ti, and series 3 is for Si-free Nb
ss. RM = Mo,Ta,W, TM = Cr,Hf,Ti. For the chemical composition of the solid solutions, see Table 1 in [
7]. For dashed lines and ellipses, see text. Note that the solid solutions included in this figure belong to the alloys for which data are given in
Figure 2.
Figure 4.
Δχ versus delta (δ) maps for Nb
ss in Nb-silicide-based alloys, (
a) all data and (
b) data only for Si-free Nb
ss and Nb
ss rich in Ti. In (
a) the series 2 data are for Nb
ss with RMs and Sn and no Al, the series 3 is for Nb
ss with RMs, Ge and Sn and with/out Al and Cr, the series 4 is for Nb
ss with Al, Cr, with/out Hf, no RMs and no B, Ge, Sn, the series 5 is for Nb
ss with Al, B, Cr with/out Hf, the series 6 is for Nb
ss with Al, Cr, with/out Ge or Sn or B, the series 7 is for Nb
ss with RMs, with/out Al and with no B, Cr, Ge, Sn and the series 8 is for Nb
ss with TMs, Al with/out RMs, B or Sn and no Ge. In (
b) the series 2 is for Nb
ss rich in Ti, and series 3 is for Si-free Nb
ss. RM = Mo,Ta,W, TM = Cr,Hf,Ti. For the chemical composition of the solid solutions, see Table 1 in [
7]. For dashed lines and ellipses, see text. Note that the solid solutions included in this figure belong to the alloys for which data are given in
Figure 2.
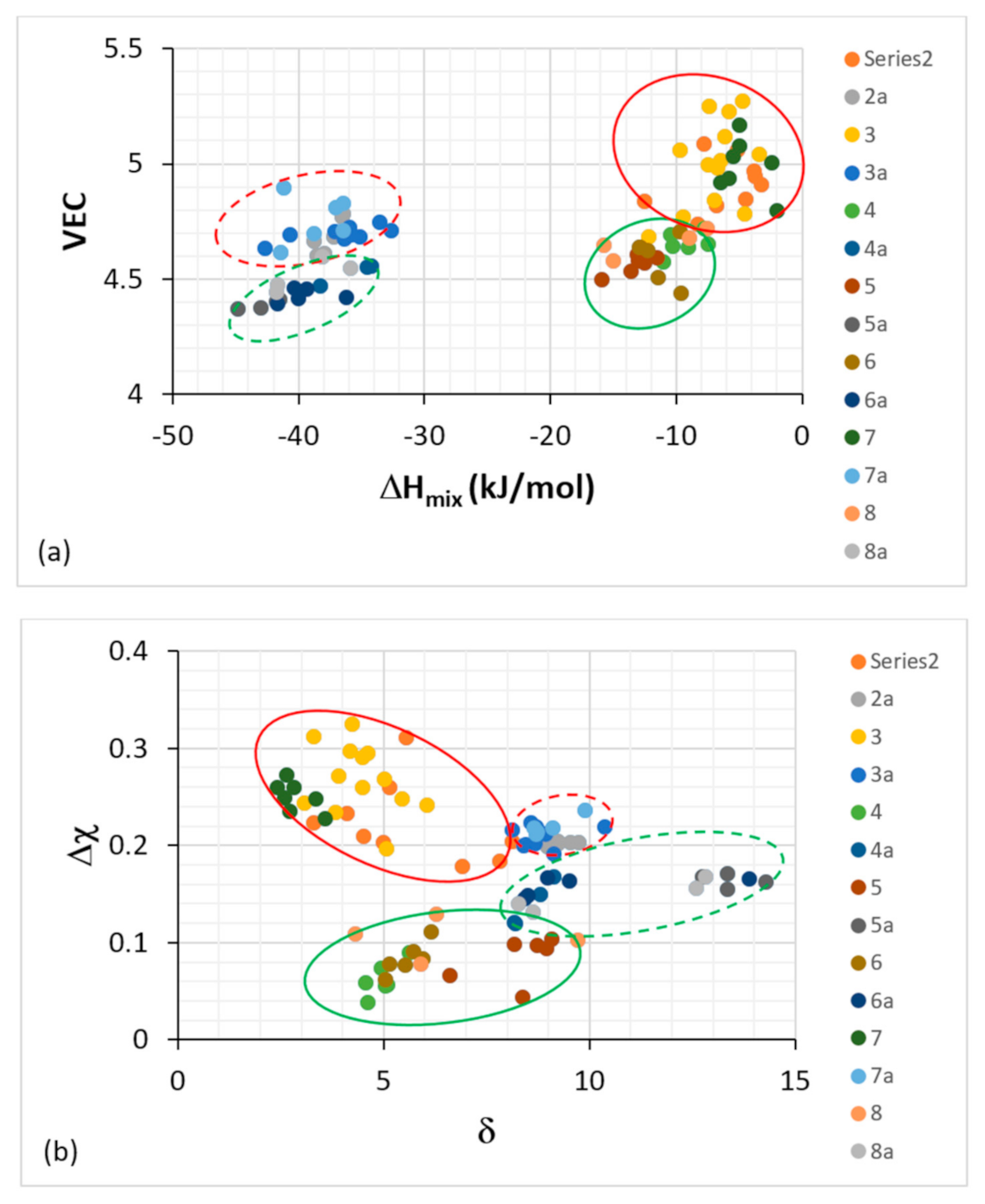
Figure 5.
Plot of VEC versus ΔH
mix (
a) and Δχ versus δ (
b) for Nb-silicide-based alloys and their Nb
ss solid solutions. The dashed ellipses are for the alloys and the full ones for the Nb
ss solid solutions. The red ellipses are for series 2, 2a, 3, 3a, 7, 7a, where the alloying elements are refractory metals (RMs), Al, Cr, Ge, Sn, but no boron. The green ellipses are for series 4, 4a, 5, 5a, 6, 6a, 8, 8a, where the alloying elements are RMs, Ge, Hf, Sn and boron. The series 2 to 8 data are for the three types of bcc Nb
ss solid solutions in Table 1 in [
7], and the series 2a to 8a data are for Nb silicide-based alloys in Table 1 in [
8]. The series 2 and 2a data are for alloys with RMs, TMs, and Sn, but no Al, the series 3 and 3a data are for alloys with RMs, TMs, Ge and Sn and with/without Al and Cr, the series 4 and 4a data are for alloys with TMs, Al, with/without Hf, no RMs and no B, Ge, Sn, the series 5 and 5a data are for alloys with TMs, Al and B, with/without Hf, the series 6 and 6a data are for alloys with TMs, Al and with/without B, Ge, Hf and Sn, the series 7 and 7a data are for alloys with RMs, TMs with/without Al and with no B, Ge, Sn and the series 8 and 8a data are for alloys with TMs, Al with/without RMs, B or Sn and no Ge.
Figure 5.
Plot of VEC versus ΔH
mix (
a) and Δχ versus δ (
b) for Nb-silicide-based alloys and their Nb
ss solid solutions. The dashed ellipses are for the alloys and the full ones for the Nb
ss solid solutions. The red ellipses are for series 2, 2a, 3, 3a, 7, 7a, where the alloying elements are refractory metals (RMs), Al, Cr, Ge, Sn, but no boron. The green ellipses are for series 4, 4a, 5, 5a, 6, 6a, 8, 8a, where the alloying elements are RMs, Ge, Hf, Sn and boron. The series 2 to 8 data are for the three types of bcc Nb
ss solid solutions in Table 1 in [
7], and the series 2a to 8a data are for Nb silicide-based alloys in Table 1 in [
8]. The series 2 and 2a data are for alloys with RMs, TMs, and Sn, but no Al, the series 3 and 3a data are for alloys with RMs, TMs, Ge and Sn and with/without Al and Cr, the series 4 and 4a data are for alloys with TMs, Al, with/without Hf, no RMs and no B, Ge, Sn, the series 5 and 5a data are for alloys with TMs, Al and B, with/without Hf, the series 6 and 6a data are for alloys with TMs, Al and with/without B, Ge, Hf and Sn, the series 7 and 7a data are for alloys with RMs, TMs with/without Al and with no B, Ge, Sn and the series 8 and 8a data are for alloys with TMs, Al with/without RMs, B or Sn and no Ge.
![Materials 14 00989 g005 Materials 14 00989 g005]()
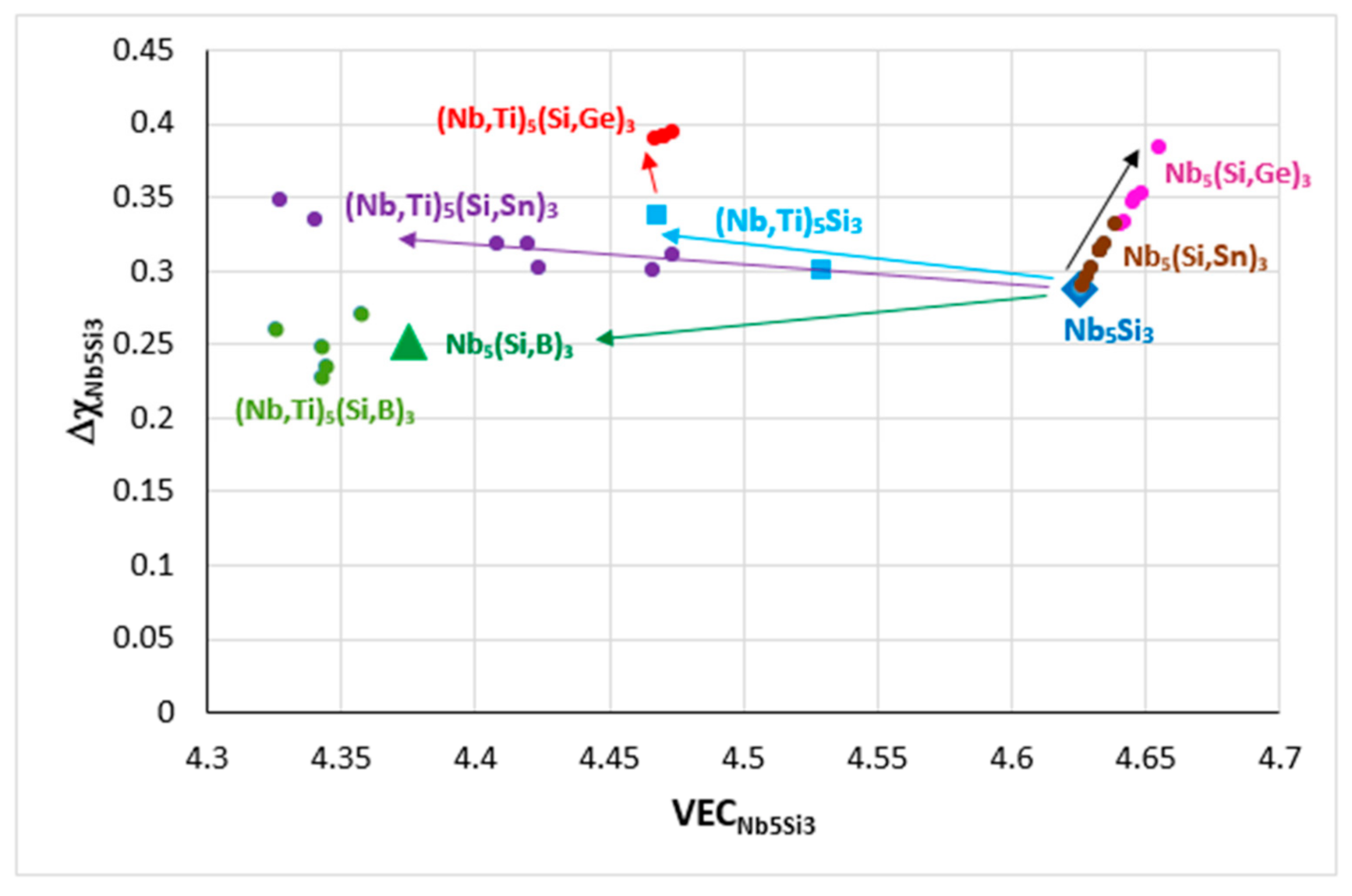
Figure 6.
Δχ versus VEC map of Nb
5Si
3. Data for unalloyed Nb
5Si
3 (diamond), silicide where Si is substituted by Sn (brown circles) or Ge (pink circles) or B (triangle), silicide where Nb is substituted by Ti (squares) and silicide where Nb is substituted by Ti and Si by B (green circles), Sn (purple circles), Ge (red circles). Data for Nb
5Si
3, where Nb is substituted by TM and RM additions and Si by simple metal and metalloid elements (Al, B, Ge, Sn), can be found in [
9].
Figure 6.
Δχ versus VEC map of Nb
5Si
3. Data for unalloyed Nb
5Si
3 (diamond), silicide where Si is substituted by Sn (brown circles) or Ge (pink circles) or B (triangle), silicide where Nb is substituted by Ti (squares) and silicide where Nb is substituted by Ti and Si by B (green circles), Sn (purple circles), Ge (red circles). Data for Nb
5Si
3, where Nb is substituted by TM and RM additions and Si by simple metal and metalloid elements (Al, B, Ge, Sn), can be found in [
9].
Figure 7.
VEC versus Δχ map for RM(Nb)ICs based on the alloy KZ5 with additions of B, Ge, Hf, Sn. RM(Nb)ICs that are also RCCAs are shown with red color. For the linear fit of all data, R
2 = 0.9383. Green and red-green data: Diamonds for KZ5 + Hf + B (RM(Nb)IC-RCCA), triangle for KZ5 + B + Sn (RM(Nb)IC-RCCA), green circle for KZ5 + B, red triangle for KZ5 + Hf + Sn (JG6-RM(Nb)IC-RCCA), red circle for KZ5 + Ge + Hf (ZF9-RM(Nb)IC/RCCA). Blue data: circle for KZ5 + Ge (ZF6) and triangle for KZ5 + Hf (JN1). Data about the densities and room temperature strength of these alloys is given in [
3]. Nominal compositions (at.%) of B-containing RM(Nb)ICs-RCCAs 37Nb-24Ti-18Si-6B-5Al-5Cr-5Hf, 39Nb-24Ti-18Si-6B-5Al-4Cr-4Sn. For nominal compositions of other alloys, see
Appendix A Table A1.
Figure 7.
VEC versus Δχ map for RM(Nb)ICs based on the alloy KZ5 with additions of B, Ge, Hf, Sn. RM(Nb)ICs that are also RCCAs are shown with red color. For the linear fit of all data, R
2 = 0.9383. Green and red-green data: Diamonds for KZ5 + Hf + B (RM(Nb)IC-RCCA), triangle for KZ5 + B + Sn (RM(Nb)IC-RCCA), green circle for KZ5 + B, red triangle for KZ5 + Hf + Sn (JG6-RM(Nb)IC-RCCA), red circle for KZ5 + Ge + Hf (ZF9-RM(Nb)IC/RCCA). Blue data: circle for KZ5 + Ge (ZF6) and triangle for KZ5 + Hf (JN1). Data about the densities and room temperature strength of these alloys is given in [
3]. Nominal compositions (at.%) of B-containing RM(Nb)ICs-RCCAs 37Nb-24Ti-18Si-6B-5Al-5Cr-5Hf, 39Nb-24Ti-18Si-6B-5Al-4Cr-4Sn. For nominal compositions of other alloys, see
Appendix A Table A1.
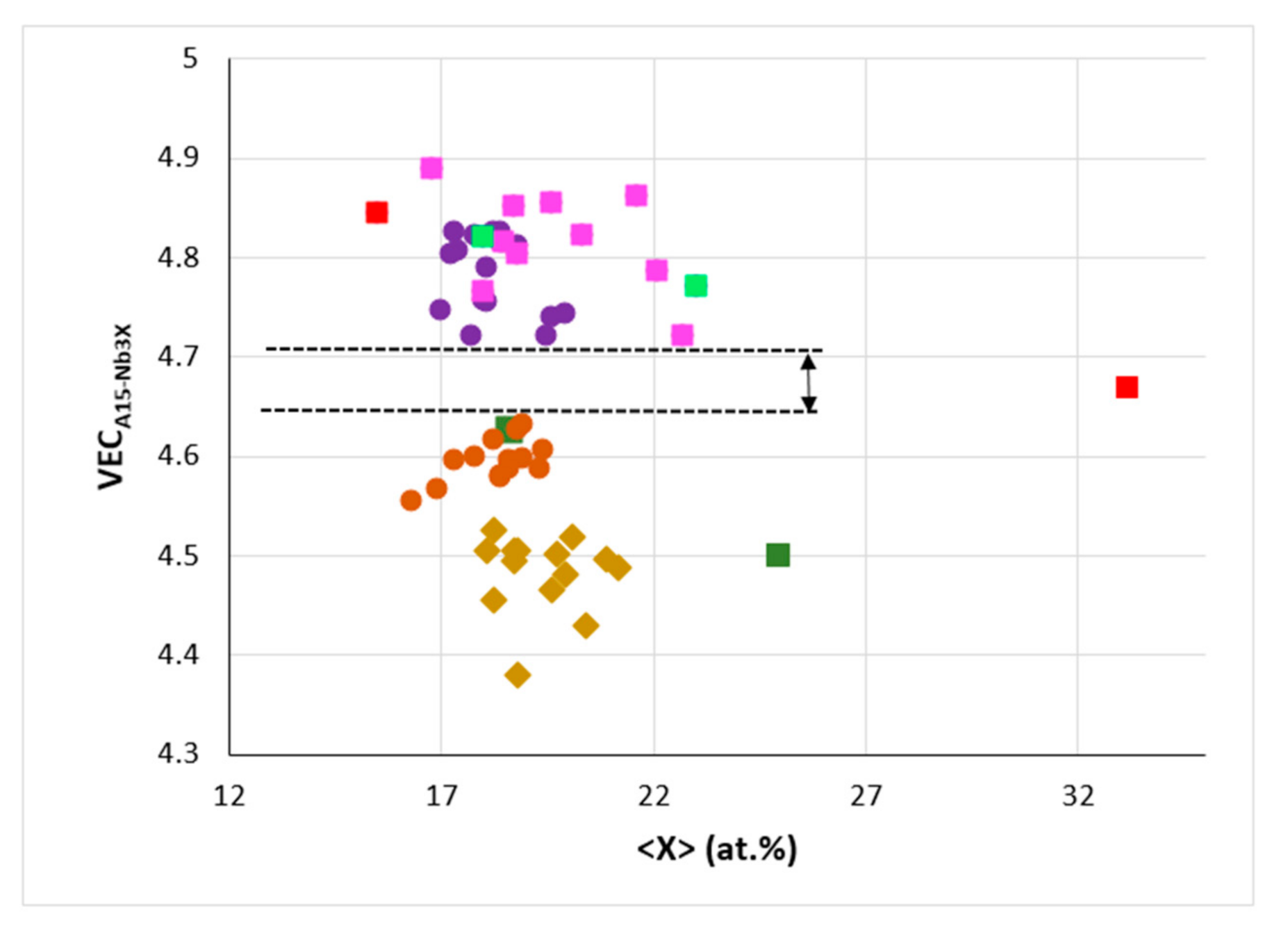
Figure 8.
Δχ versus <X> = Al + Ge + Si + Sn in A15-Nb3X where Nb is substituted by Cr, Fe, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Squares, red Nb3Sn (Sn = 15.5 and 33.2 at.%), dark green Nb3Al (Al = 18.6 and 25 at.%) and light green Nb3Ge (Ge = 18 and 23 at.%). Diamonds for Nb substituted by Cr, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn, filled circles for Nb substituted by Cr, Fe, Hf, or Ti and X = Al, Si or Sn. Linear fit of all data gives R2 = 0.7484.
Figure 8.
Δχ versus <X> = Al + Ge + Si + Sn in A15-Nb3X where Nb is substituted by Cr, Fe, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Squares, red Nb3Sn (Sn = 15.5 and 33.2 at.%), dark green Nb3Al (Al = 18.6 and 25 at.%) and light green Nb3Ge (Ge = 18 and 23 at.%). Diamonds for Nb substituted by Cr, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn, filled circles for Nb substituted by Cr, Fe, Hf, or Ti and X = Al, Si or Sn. Linear fit of all data gives R2 = 0.7484.
Figure 9.
VEC versus <X> = Al + Ge + Si + Sn in A15-Nb3X, where Nb is substituted by Cr, Fe, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Squares, red Nb3Sn (Sn = 15.5 and 33.2 at.%), dark green Nb3Al (Al = 18.6 and 25 at.%) and light green Nb3Ge (Ge = 18 and 23 at.%), pink for Nb substituted by Cr, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Diamonds for Nb substituted by Cr, Hf or Ti and X = Al, Si or Sn, Filled circles, purple for Nb substituted by Cr or Hf and X = Al, Si or Sn, orange for Nb substituted by Cr, Fe, Hf or Ti, and X = Al, Si or Sn. Note the gap of VEC values (from 4.628 to 4.721) for alloyed Nb3X, and that data point for Sn rich Nb3Sn falls in this gap.
Figure 9.
VEC versus <X> = Al + Ge + Si + Sn in A15-Nb3X, where Nb is substituted by Cr, Fe, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Squares, red Nb3Sn (Sn = 15.5 and 33.2 at.%), dark green Nb3Al (Al = 18.6 and 25 at.%) and light green Nb3Ge (Ge = 18 and 23 at.%), pink for Nb substituted by Cr, Hf, Mo, Ti or W and X = Al, Ge, Si or Sn. Diamonds for Nb substituted by Cr, Hf or Ti and X = Al, Si or Sn, Filled circles, purple for Nb substituted by Cr or Hf and X = Al, Si or Sn, orange for Nb substituted by Cr, Fe, Hf or Ti, and X = Al, Si or Sn. Note the gap of VEC values (from 4.628 to 4.721) for alloyed Nb3X, and that data point for Sn rich Nb3Sn falls in this gap.
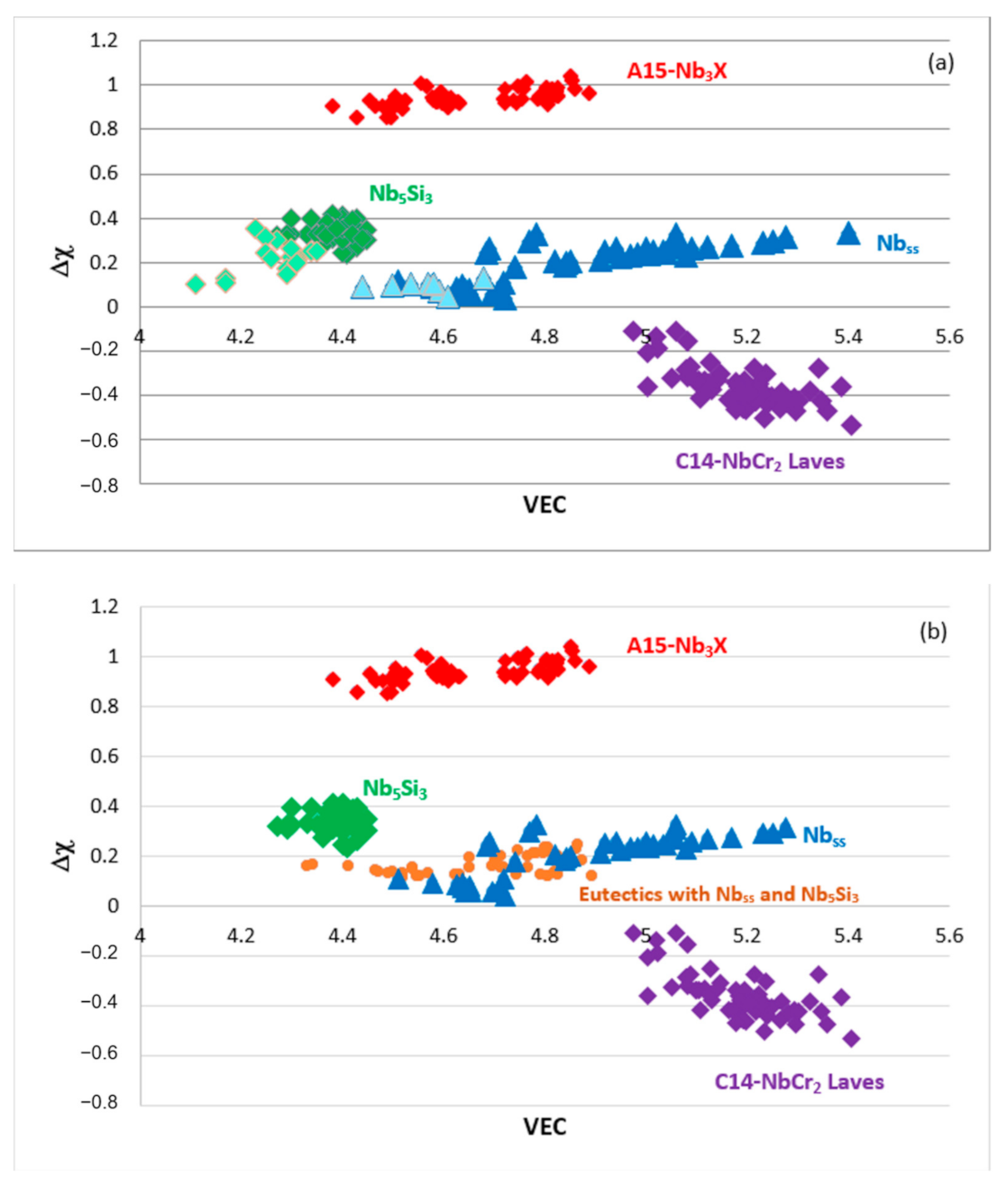
Figure 10.
(
a,
b) show maps of Δχ versus VEC. In (
a), the data are for the Nb
ss (blue triangles), Nb
5Si
3 (green diamonds), C14-NbCr
2 Laves (purple diamonds) and A15-Nb
3X (red diamonds) phases, and the boron-containing Nb
ss and Nb
5Si
3 are shown in light blue and light green. In (
b), the data are for the Nb
ss (blue triangles), Nb
5Si
3 (green diamonds), eutectics with Nb
ss and Nb
5Si
3 (orange circles), C14-NbCr
2 Laves (purple diamonds) and A15-Nb
3X (red diamonds) phases. The data for the bcc solid solution RCCAs studied by Senkov et al. [
13], and HEA Nb
ss and HEA Nb
ss and Nb
5Si
3 eutectics in RM(Nb)ICs that satisfy the “standard definition” of HEAs fall in the area of the solid solution and eutectics data in (
b), as shown in Figure 16 in [
3]. Note that both parts of this figure are the same as those in Figure 5 in [
1], in which by mistake, the labels for the A15-Nb
3X and C14-NbCr
2 phases were swapped.
Figure 10.
(
a,
b) show maps of Δχ versus VEC. In (
a), the data are for the Nb
ss (blue triangles), Nb
5Si
3 (green diamonds), C14-NbCr
2 Laves (purple diamonds) and A15-Nb
3X (red diamonds) phases, and the boron-containing Nb
ss and Nb
5Si
3 are shown in light blue and light green. In (
b), the data are for the Nb
ss (blue triangles), Nb
5Si
3 (green diamonds), eutectics with Nb
ss and Nb
5Si
3 (orange circles), C14-NbCr
2 Laves (purple diamonds) and A15-Nb
3X (red diamonds) phases. The data for the bcc solid solution RCCAs studied by Senkov et al. [
13], and HEA Nb
ss and HEA Nb
ss and Nb
5Si
3 eutectics in RM(Nb)ICs that satisfy the “standard definition” of HEAs fall in the area of the solid solution and eutectics data in (
b), as shown in Figure 16 in [
3]. Note that both parts of this figure are the same as those in Figure 5 in [
1], in which by mistake, the labels for the A15-Nb
3X and C14-NbCr
2 phases were swapped.
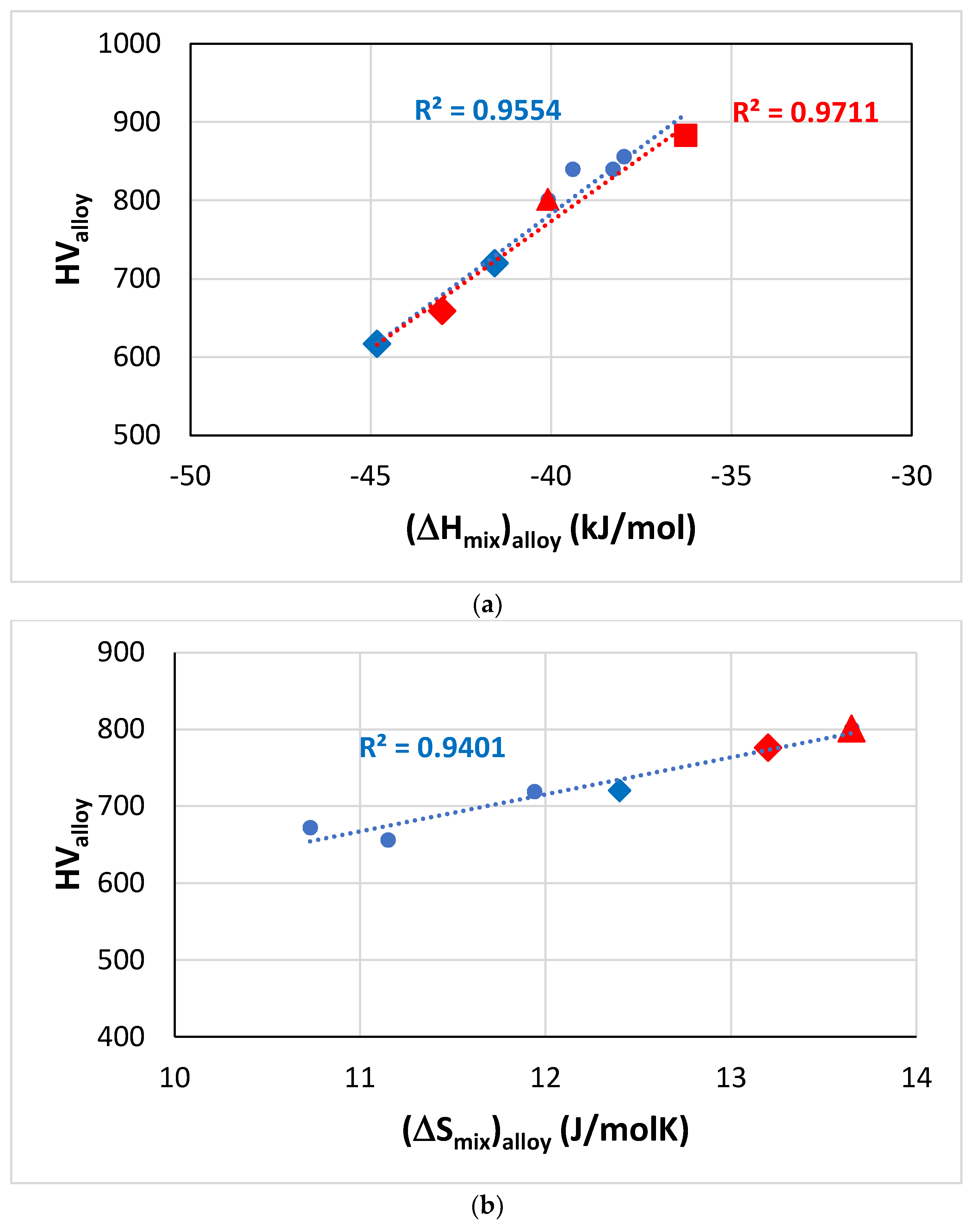
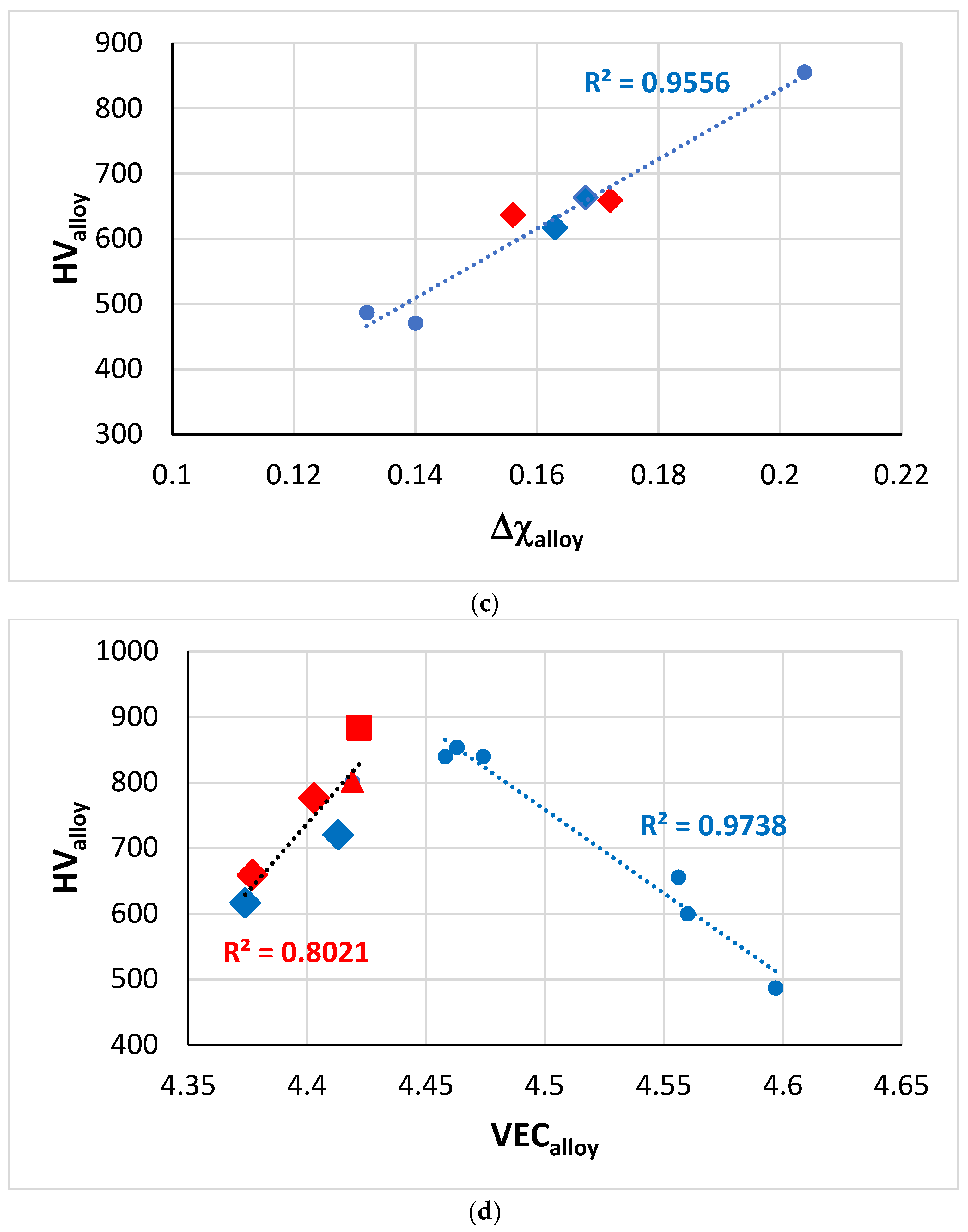
Figure 11.
Vickers hardness of RM(Nb)ICs based on the alloy KZ5, some of which are also RCCAs (red data). Diamonds for alloys with B addition, square for RCCA with Sn addition and triangle for RCCA with Ge addition. (
a) HV
alloy versus (ΔH
mix)
alloy, the linear fit of all data gives R
2 = 0.9554, and only of the RCCAs gives R
2 = 0.9711. Alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, alloys JN1, JN4, ZF6, ZF9, EZ8, KZ5 + B, KZ5 + Hf + B, (
b) HV
alloy versus (ΔS
mix)
alloy, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ti, W, alloys KZ5, ZF9, KZ5 + B, KZ5 + Hf + B, YG10, YG11, (
c) HV
alloy versus Δχ
alloy, alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ti, Ta, alloys KZ6, JG3, JN4, KZ5 + Mo + B, KZ5 + B, KZ6 + B, KZ5 + Hf + B, (
d) HV
alloy versus VEC
alloy, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, Ta, alloys KZ5 + B, KZ5 + Hf + B, EZ8, ZF9, KZ5, JN1, ZF6, KZ6. Note that
all the data in (
d) can be fitted to a parabolic function with R
2 = 0.9101 and maximum near the HV and VEC values of the alloy ZF6 (Nb-24Ti-18Si-5Al-5Cr-5Ge [
34]). For nominal alloy compositions, see
Appendix A Table A1.
Figure 11.
Vickers hardness of RM(Nb)ICs based on the alloy KZ5, some of which are also RCCAs (red data). Diamonds for alloys with B addition, square for RCCA with Sn addition and triangle for RCCA with Ge addition. (
a) HV
alloy versus (ΔH
mix)
alloy, the linear fit of all data gives R
2 = 0.9554, and only of the RCCAs gives R
2 = 0.9711. Alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, alloys JN1, JN4, ZF6, ZF9, EZ8, KZ5 + B, KZ5 + Hf + B, (
b) HV
alloy versus (ΔS
mix)
alloy, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ti, W, alloys KZ5, ZF9, KZ5 + B, KZ5 + Hf + B, YG10, YG11, (
c) HV
alloy versus Δχ
alloy, alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ti, Ta, alloys KZ6, JG3, JN4, KZ5 + Mo + B, KZ5 + B, KZ6 + B, KZ5 + Hf + B, (
d) HV
alloy versus VEC
alloy, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, Ta, alloys KZ5 + B, KZ5 + Hf + B, EZ8, ZF9, KZ5, JN1, ZF6, KZ6. Note that
all the data in (
d) can be fitted to a parabolic function with R
2 = 0.9101 and maximum near the HV and VEC values of the alloy ZF6 (Nb-24Ti-18Si-5Al-5Cr-5Ge [
34]). For nominal alloy compositions, see
Appendix A Table A1.
![Materials 14 00989 g011a Materials 14 00989 g011a]()
![Materials 14 00989 g011b Materials 14 00989 g011b]()
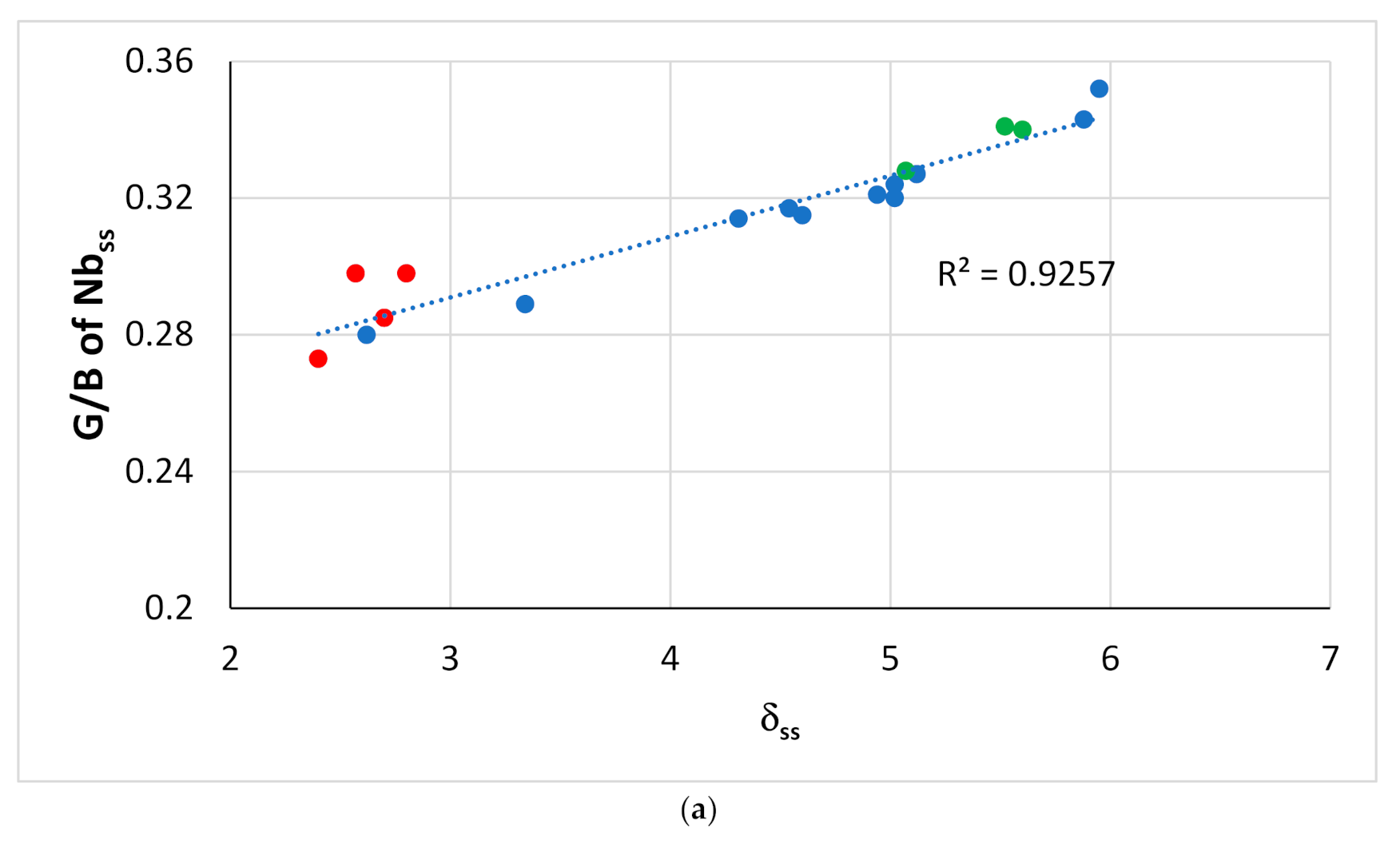
Figure 12.
Pugh’s ratio G/B of Nbss in boron-free RM(Nb)ICs with/without Ti addition versus (a) δss and (b) Δχss. Data for Si-free Nbss and Ti-rich Nbss is shown in red and green, respectively. All data R2 = 0.9257 and R2 = 0.7046, in (a,b), respectively. The G/B ratio was calculated using the rule of mixtures, data for G and B for the elements and the actual composition of solid solutions. Alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Ta, Ti, W.
Figure 12.
Pugh’s ratio G/B of Nbss in boron-free RM(Nb)ICs with/without Ti addition versus (a) δss and (b) Δχss. Data for Si-free Nbss and Ti-rich Nbss is shown in red and green, respectively. All data R2 = 0.9257 and R2 = 0.7046, in (a,b), respectively. The G/B ratio was calculated using the rule of mixtures, data for G and B for the elements and the actual composition of solid solutions. Alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Ta, Ti, W.
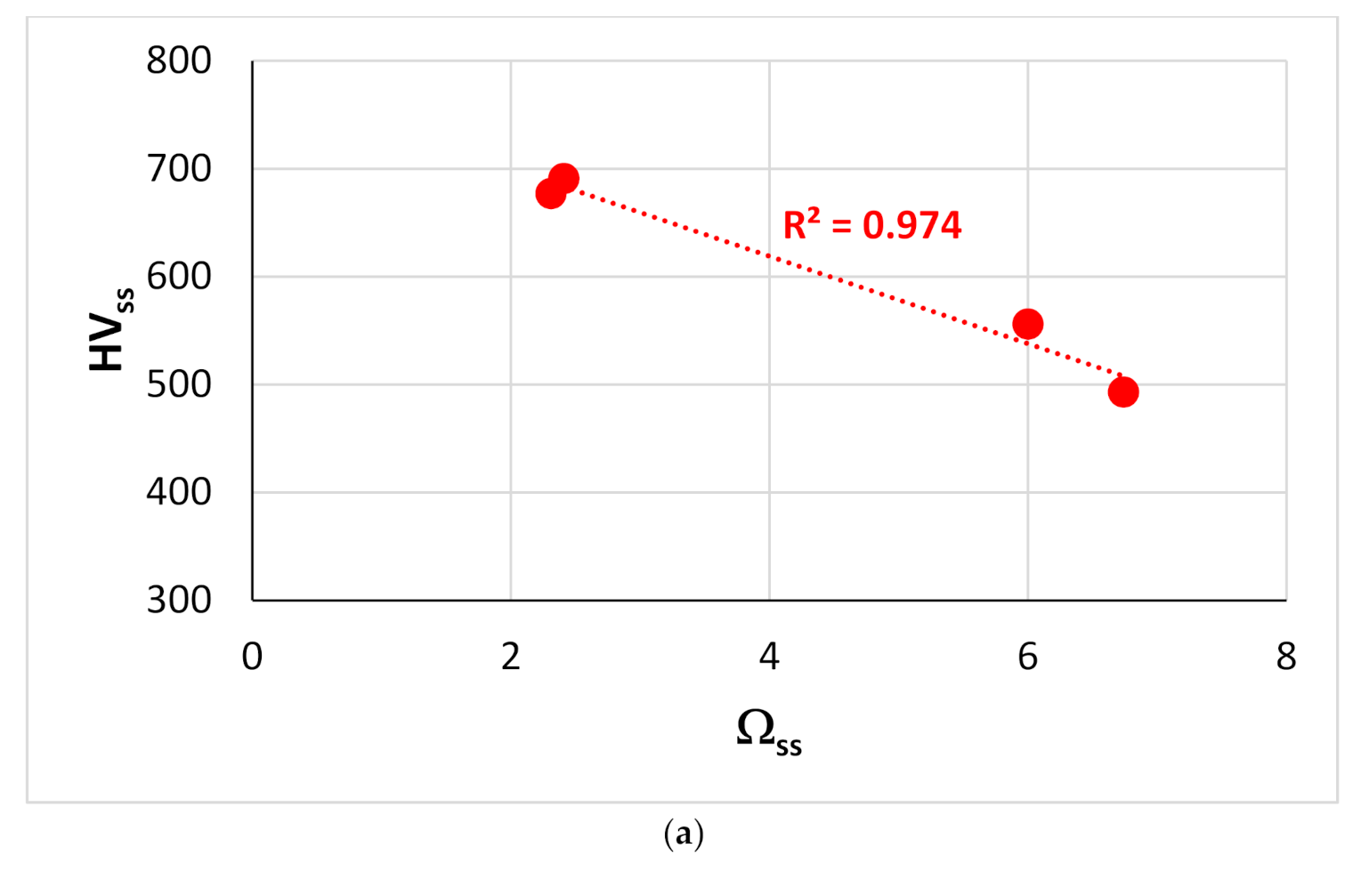
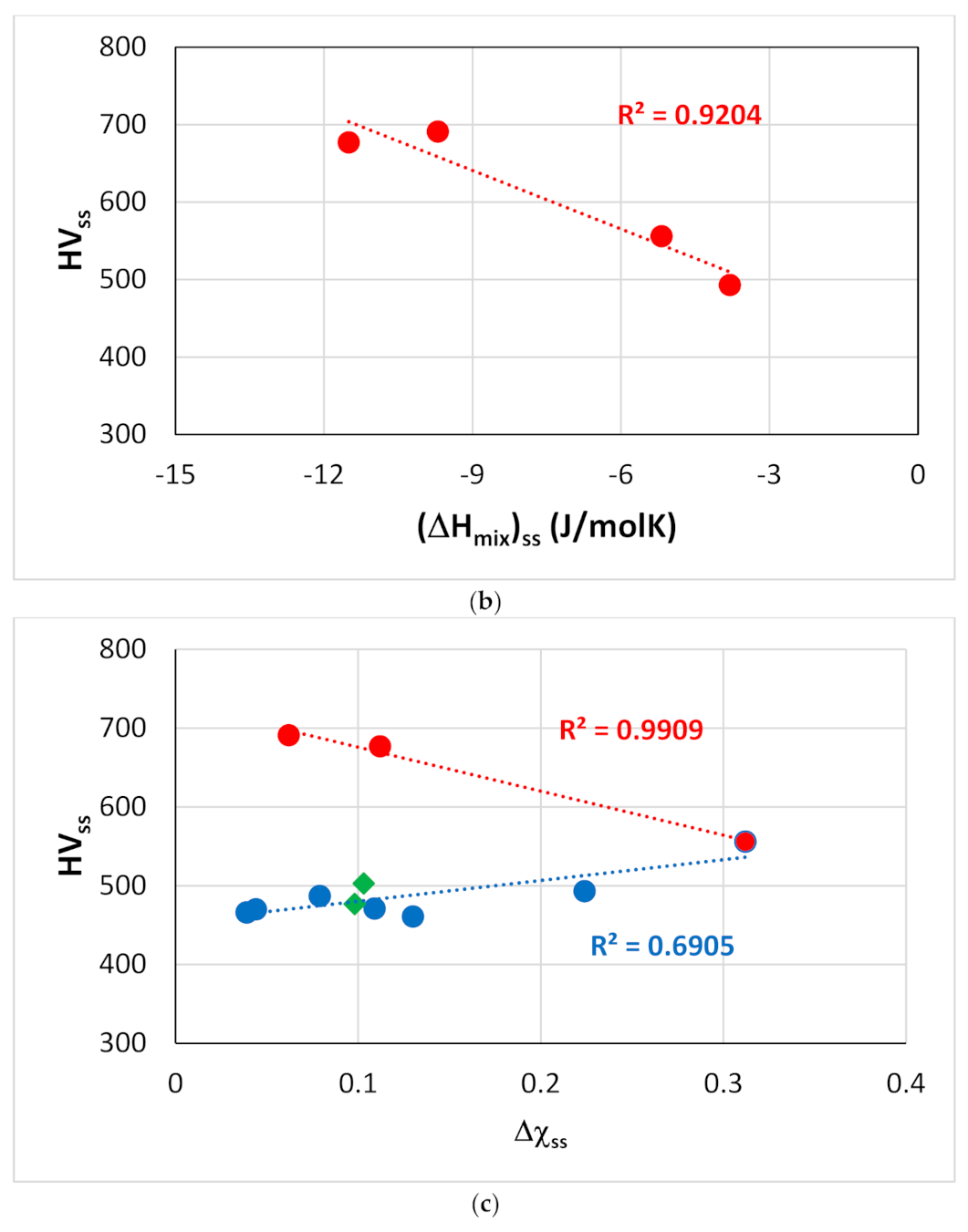
Figure 13.
Vickers hardness of solid solutions in RM(Nb)ICs versus the parameters (a) Ωss, (b) (ΔHmix)ss and (c) Δχss. The red data in (a–c) is for alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W. In (c), the data for which liner fit gives R2 = 0.6905 (blue line) is for alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta, Ti or W. In (c) the data for the Nbss that belongs in RM(Nb)ICs that are also RCCAs is shown by green diamonds and is for alloys with B addition.
Figure 13.
Vickers hardness of solid solutions in RM(Nb)ICs versus the parameters (a) Ωss, (b) (ΔHmix)ss and (c) Δχss. The red data in (a–c) is for alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W. In (c), the data for which liner fit gives R2 = 0.6905 (blue line) is for alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta, Ti or W. In (c) the data for the Nbss that belongs in RM(Nb)ICs that are also RCCAs is shown by green diamonds and is for alloys with B addition.
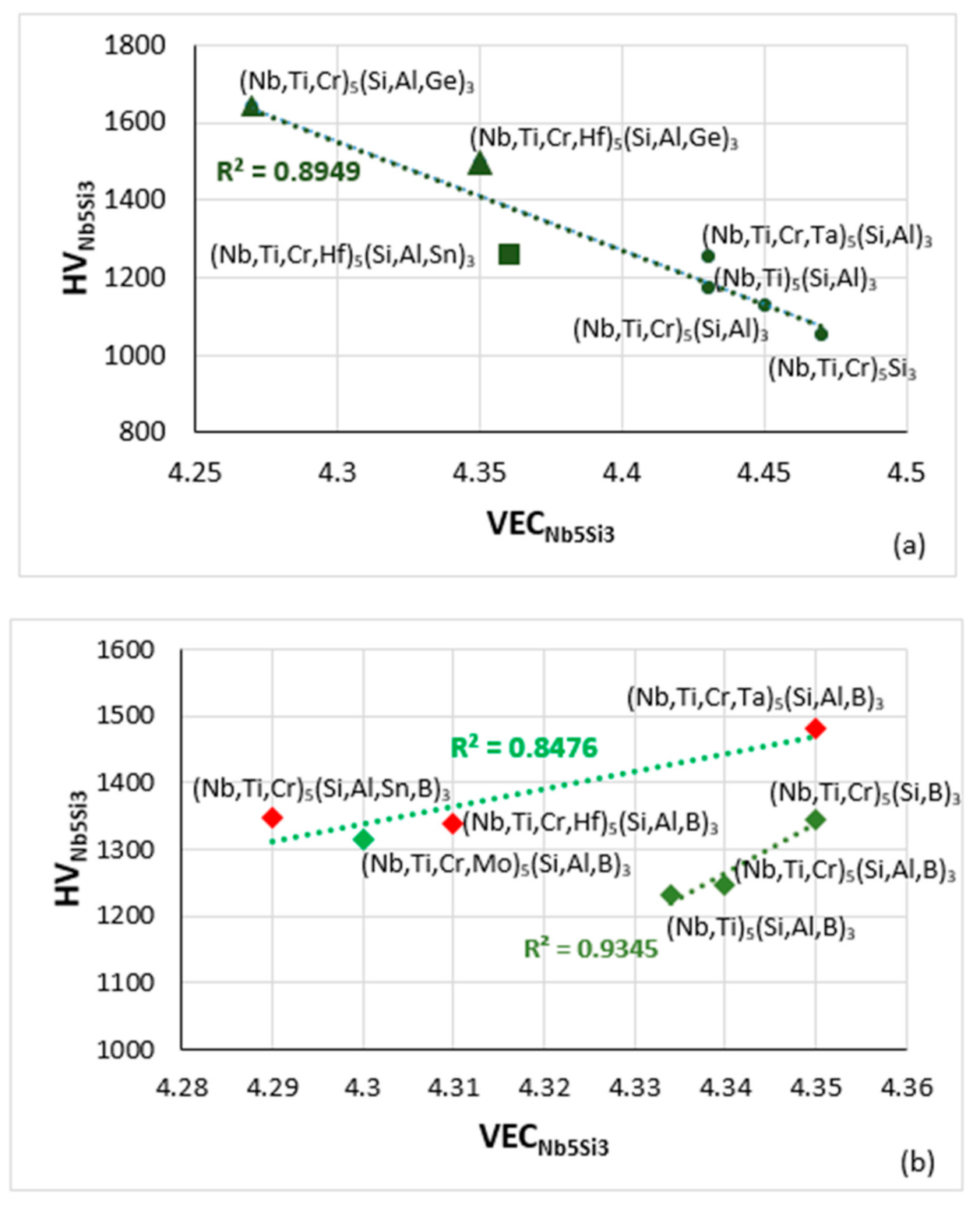
Figure 14.
Vickers hardness of alloyed Nb5Si3 versus VEC. (a) Boron-free RM(Nb)ICs, triangles and squares with Ge or Sn addition, respectively, (b) boron-containing RM(Nb)ICs (diamonds), of which those that also are RHEAS and RCCAs are shown in red.
Figure 14.
Vickers hardness of alloyed Nb5Si3 versus VEC. (a) Boron-free RM(Nb)ICs, triangles and squares with Ge or Sn addition, respectively, (b) boron-containing RM(Nb)ICs (diamonds), of which those that also are RHEAS and RCCAs are shown in red.
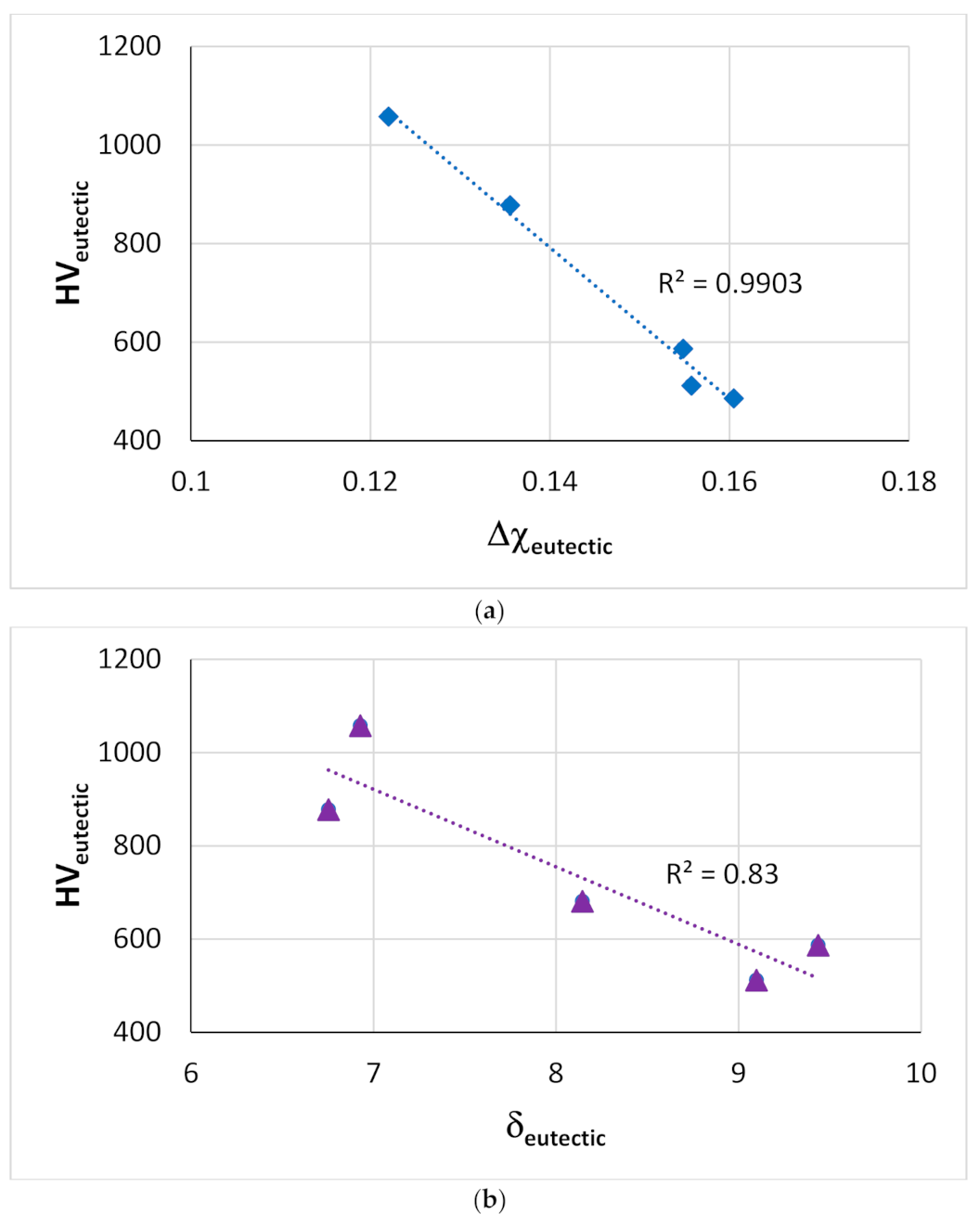
Figure 15.
Vickers hardness of eutectics with Nbss and Nb5Si3 in Ti-free RM(Nb)ICs with alloying elements (a) Al, Cr, Ge, Hf, Nb, Si, Sn and (b) Cr, Ge, Hf, Nb, Si, Sn.
Figure 15.
Vickers hardness of eutectics with Nbss and Nb5Si3 in Ti-free RM(Nb)ICs with alloying elements (a) Al, Cr, Ge, Hf, Nb, Si, Sn and (b) Cr, Ge, Hf, Nb, Si, Sn.
Figure 16.
Data for the weight change in isothermal oxidation in air at 1200 °C of RM(Nb)ICs that are also RCCAs (data from Table 4 in [
19]). Red squares for alloys where scale spalls off. Note that only the alloys EZ8, OHS1, JG6 and ZF9 are based on the alloy KZ5. For the nominal compositions of the alloys, see
Appendix A Table A1.
Figure 16.
Data for the weight change in isothermal oxidation in air at 1200 °C of RM(Nb)ICs that are also RCCAs (data from Table 4 in [
19]). Red squares for alloys where scale spalls off. Note that only the alloys EZ8, OHS1, JG6 and ZF9 are based on the alloy KZ5. For the nominal compositions of the alloys, see
Appendix A Table A1.
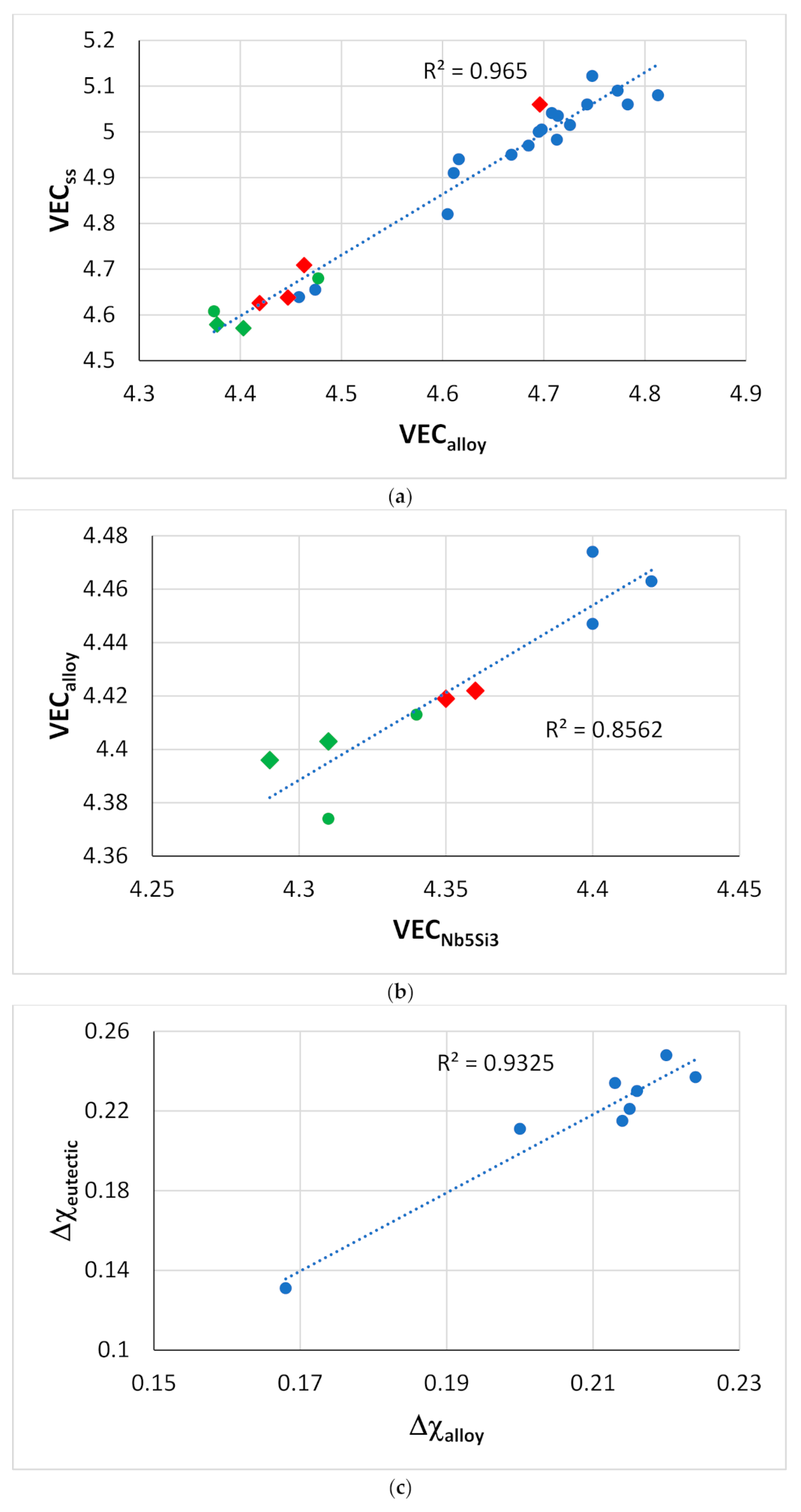
Figure 17.
Relationships between parameters of alloys and phases. (
a) VEC
ss versus VEC
alloy [
1], diamonds for RM(Nb)ICs that are also RCCAs, green data for alloys with B, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W, (
b) VEC
alloy versus VEC
Nb5Si3, circles RM(Nb)ICs, green data alloys with B, diamonds for RM(Nb)ICs that are also RCCAs, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn or Ti, (
c) Δχ
eutectic versus Δχ
alloy for eutectics with Nb
ss and Nb
5Si
3, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W.
Figure 17.
Relationships between parameters of alloys and phases. (
a) VEC
ss versus VEC
alloy [
1], diamonds for RM(Nb)ICs that are also RCCAs, green data for alloys with B, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W, (
b) VEC
alloy versus VEC
Nb5Si3, circles RM(Nb)ICs, green data alloys with B, diamonds for RM(Nb)ICs that are also RCCAs, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn or Ti, (
c) Δχ
eutectic versus Δχ
alloy for eutectics with Nb
ss and Nb
5Si
3, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W.
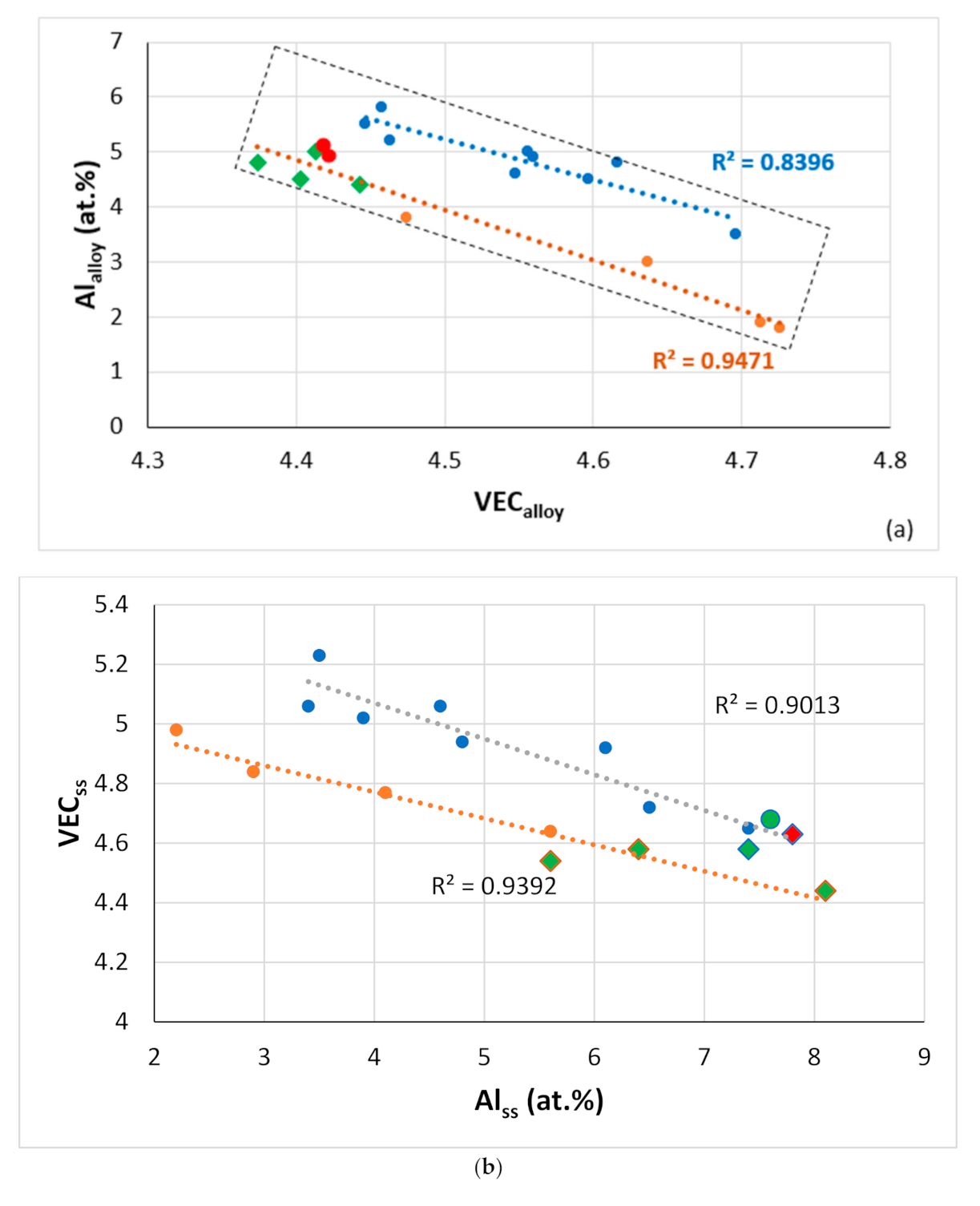
Figure 18.
Relationships between alloy or phase parameters and solute concentrations in RM(Nb)IC and RCCAs alloys with Ti/Si < 1 and Ti/Si > 1 [
3] and their phases. (
a) Al
alloy versus VEC
alloy, orange line (R
2 = 0.9471) with green diamonds for alloys with B, green and red data points for RM(Nb)ICs that are also RCCAs, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, blue line (R
2 = 0.8396) for alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, (
b) VEC
ss versus Al
ss, orange line (R
2 = 0.9392) for alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, V or W, diamonds for B-containing RM(Nb)ICs that are also RCCAs, blue line (R
2 = 0.9013) for alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti, or W, circles for RM(Nb)ICs, diamonds for RM(Nb)ICs that are also RCCAs, green color for B-containing alloys, (
c) VEC
Nb5Si3 versus the concentration of Hf in Nb
5Si
3, green circle for Ti rich Nb
5Si
3, blue circle for normal Nb
5Si
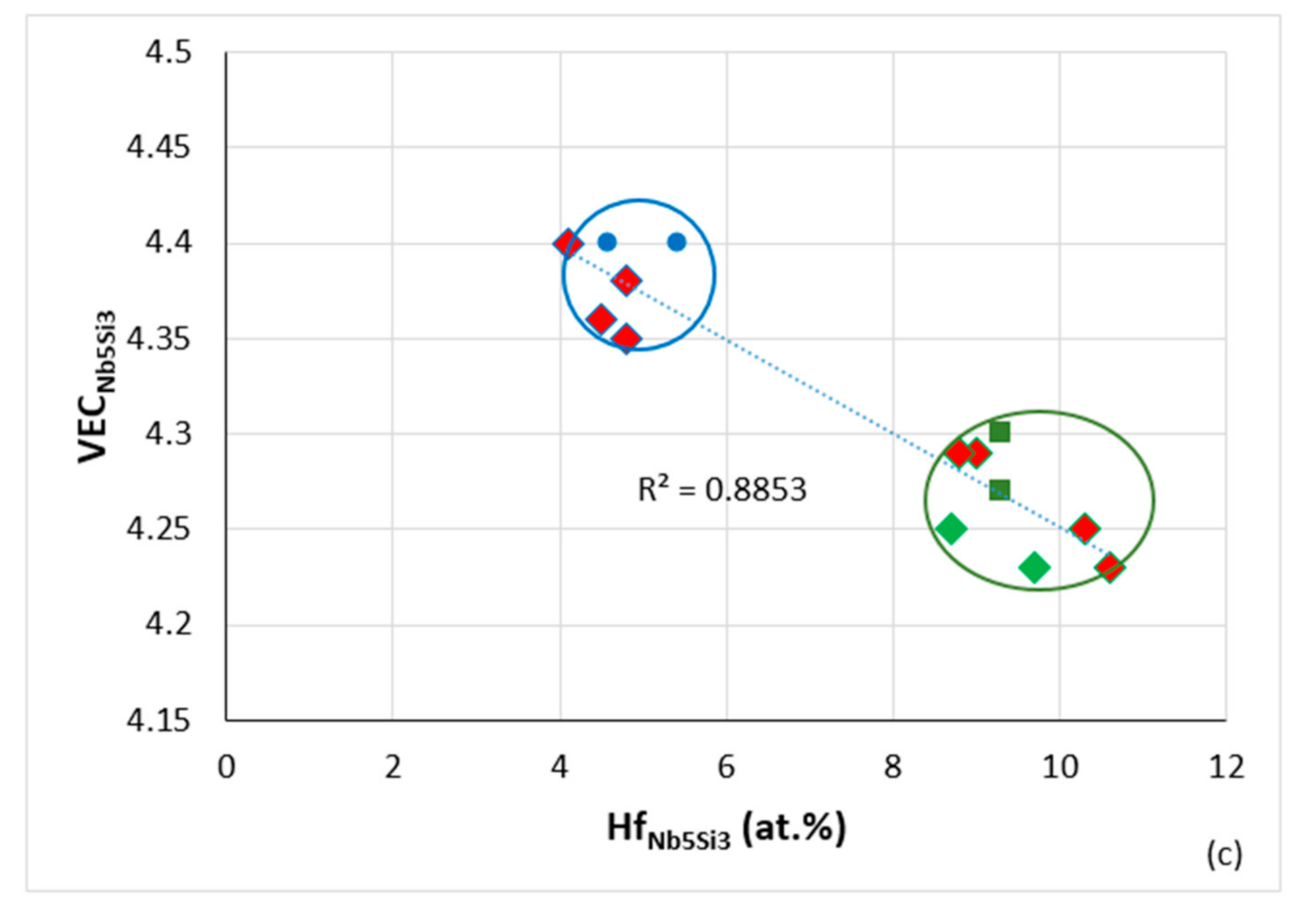
3, for all data R
2 = 0.8853, diamonds for RM(Nb)ICs that are also RCCAs, green diamonds for B-containing alloys, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn, Ti, linear fit for data for RM(NB)ICs that are also RCCAS has R
2 = 0.9209. For the rectangular area in (
a), see text.
Figure 18.
Relationships between alloy or phase parameters and solute concentrations in RM(Nb)IC and RCCAs alloys with Ti/Si < 1 and Ti/Si > 1 [
3] and their phases. (
a) Al
alloy versus VEC
alloy, orange line (R
2 = 0.9471) with green diamonds for alloys with B, green and red data points for RM(Nb)ICs that are also RCCAs, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, blue line (R
2 = 0.8396) for alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, (
b) VEC
ss versus Al
ss, orange line (R
2 = 0.9392) for alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti, V or W, diamonds for B-containing RM(Nb)ICs that are also RCCAs, blue line (R
2 = 0.9013) for alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti, or W, circles for RM(Nb)ICs, diamonds for RM(Nb)ICs that are also RCCAs, green color for B-containing alloys, (
c) VEC
Nb5Si3 versus the concentration of Hf in Nb
5Si
3, green circle for Ti rich Nb
5Si
3, blue circle for normal Nb
5Si
3, for all data R
2 = 0.8853, diamonds for RM(Nb)ICs that are also RCCAs, green diamonds for B-containing alloys, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn, Ti, linear fit for data for RM(NB)ICs that are also RCCAS has R
2 = 0.9209. For the rectangular area in (
a), see text.
![Materials 14 00989 g018a Materials 14 00989 g018a]()
![Materials 14 00989 g018b Materials 14 00989 g018b]()
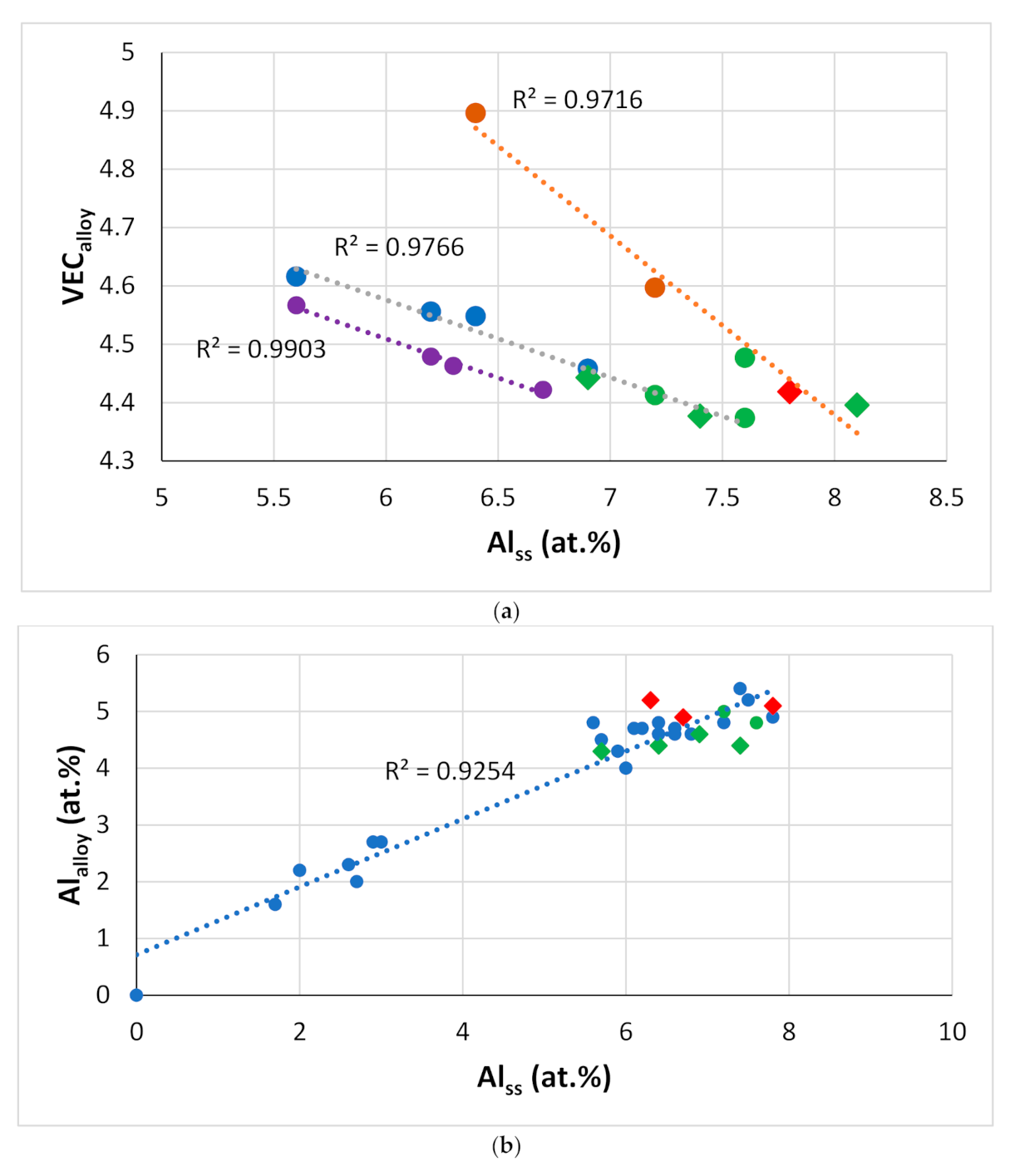
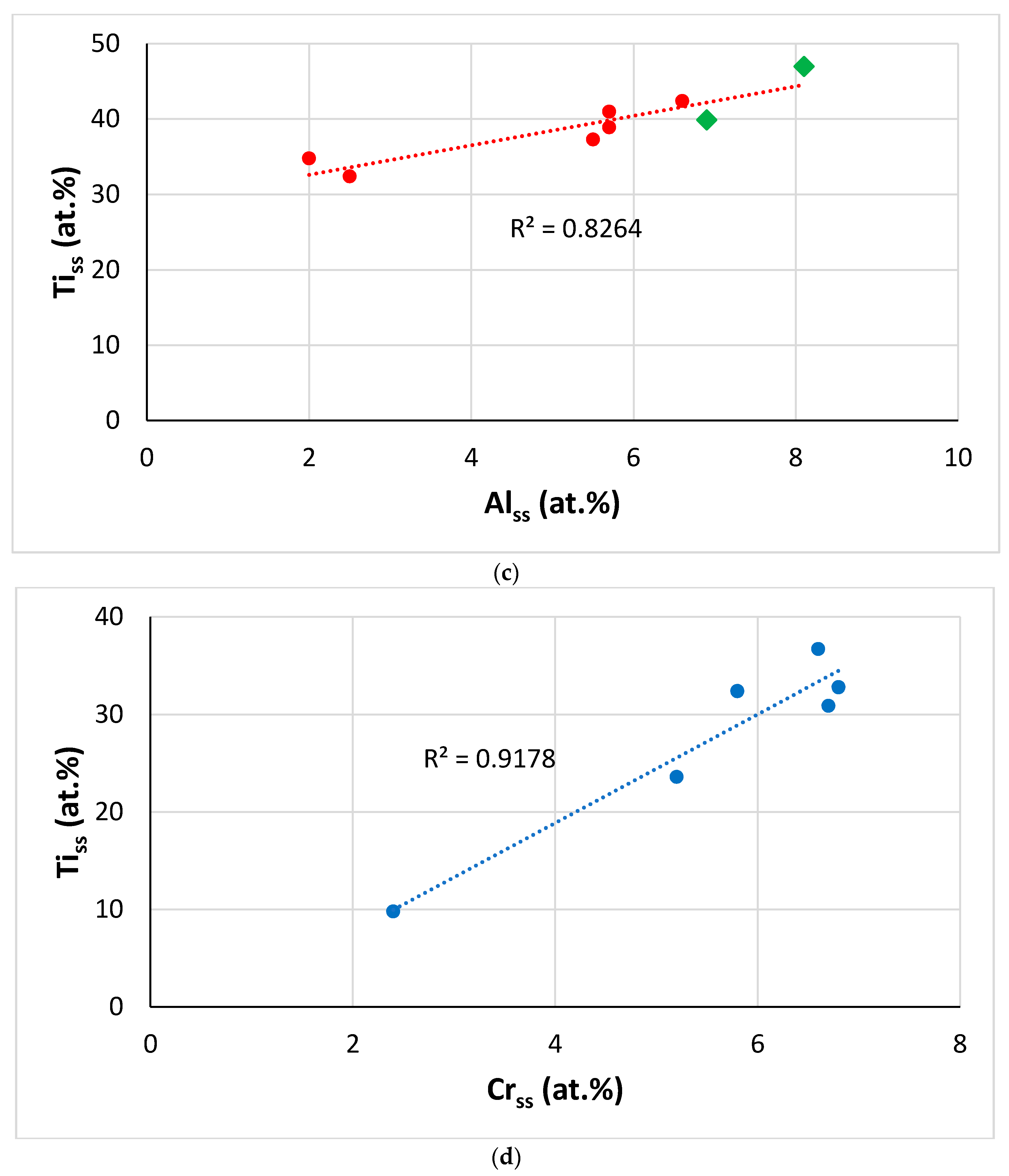
Figure 19.
Data for alloys and bcc solid solutions for RM(Nb)ICs, some of which are also RCCAs (a) VECalloy versus Alss, orange line (R2 = 0.9716) with alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta, Ti, or W, blue line (R2 = 0.9766) with alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ta, Ti or W, purple line (R2 = 0.9903) with alloying elements Al, Cr, Ge, Hf, Nb, Si, Sn, Ti or V, (b) Alalloy versus Alss, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, (c) Tiss versus Alss with alloying elements B, Cr, Fe, Hf, Sn or Ta, (d) Tiss versus Crss with alloying elements Al, Fe, Hf, Sn, W or V. In (a–c) diamonds for RM(Nb)ICs that are also RCCAs, and green colour for alloys with B.
Figure 19.
Data for alloys and bcc solid solutions for RM(Nb)ICs, some of which are also RCCAs (a) VECalloy versus Alss, orange line (R2 = 0.9716) with alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta, Ti, or W, blue line (R2 = 0.9766) with alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ta, Ti or W, purple line (R2 = 0.9903) with alloying elements Al, Cr, Ge, Hf, Nb, Si, Sn, Ti or V, (b) Alalloy versus Alss, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, (c) Tiss versus Alss with alloying elements B, Cr, Fe, Hf, Sn or Ta, (d) Tiss versus Crss with alloying elements Al, Fe, Hf, Sn, W or V. In (a–c) diamonds for RM(Nb)ICs that are also RCCAs, and green colour for alloys with B.
Figure 20.
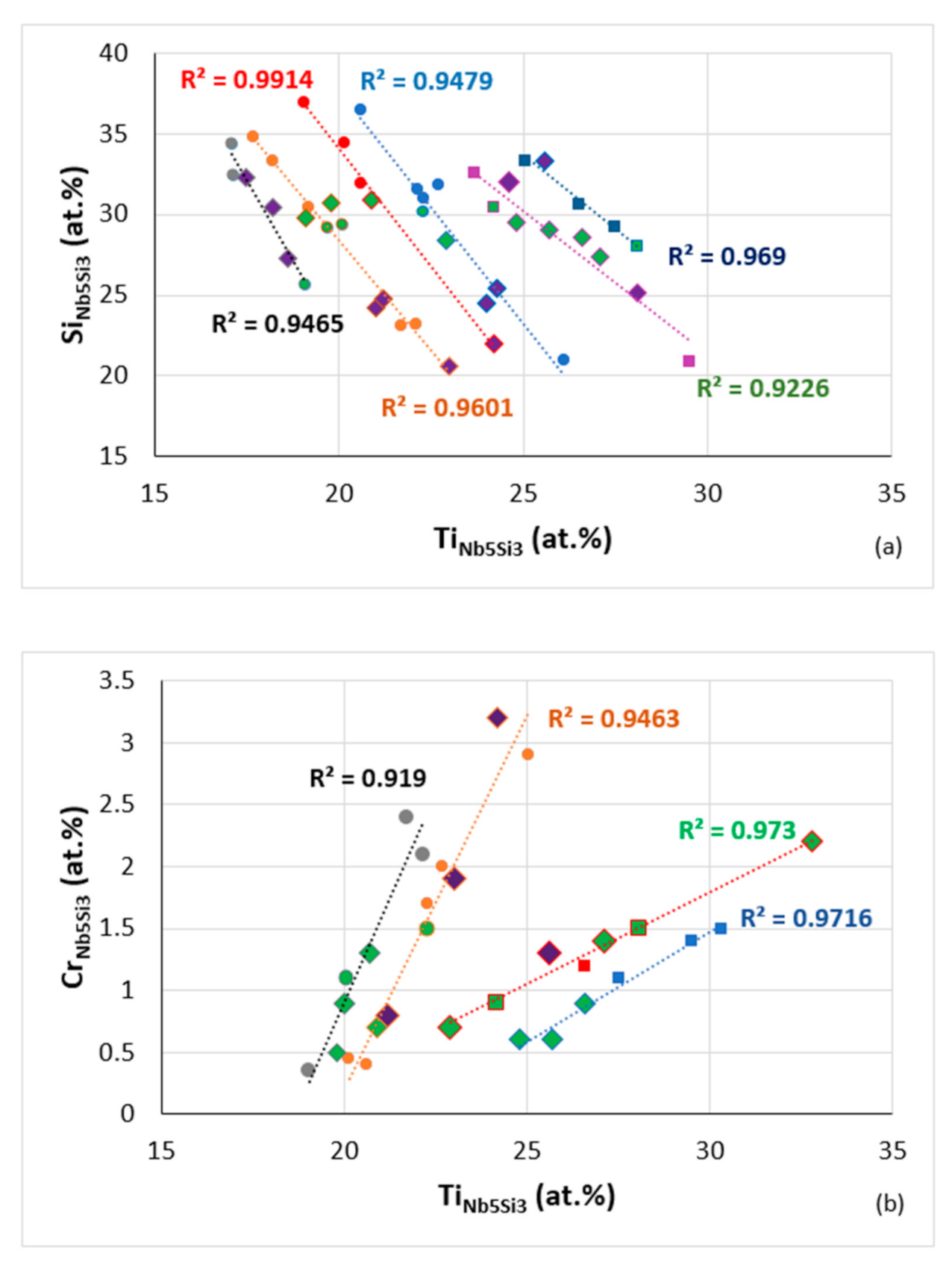
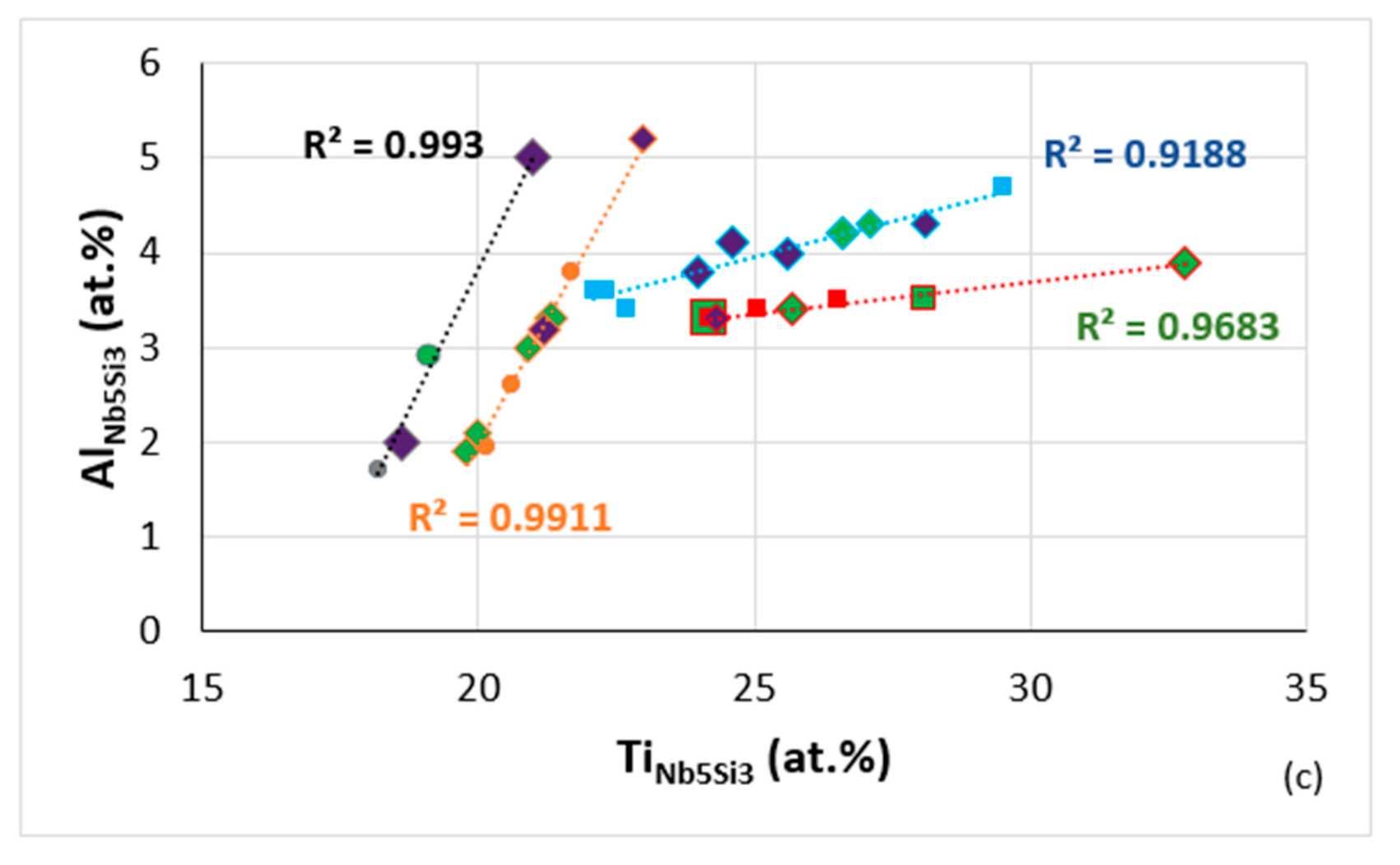
Data for solute elements in Nb5Si3 in RM(Nb)ICs some of which are also refractory complex concentrated alloys (RCCAs) (diamonds), silicides with B addition shown in green colour, purple diamonds RCCAs with Ge or Sn or Ge + Sn, (a) Si versus Ti, R2 = 0.9465 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn or Ti, R2 = 0.9601 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9914 alloying elements Al, B, Cr, Ge, Nb, Si, Sn, Ta or Ti, R2 = 0.9479 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9226 for Ti rich Nb5Si3 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.969 for Ti rich Nb5Si3 alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta or Ti, (b) Cr versus Ti, R2 = 0.919, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9463, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.973, Ti rich Nb5Si3, alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9716, Ti rich Nb5Si3, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, (c) Al versus Ti, R2 = 0.993, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn or Ti, R2 = 0.9911, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn, Ta or Ti, R2 = 0.9188, Ti rich Nb5Si3, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9683, Ti rich Nb5Si3 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ta or Ti.
Figure 20.
Data for solute elements in Nb5Si3 in RM(Nb)ICs some of which are also refractory complex concentrated alloys (RCCAs) (diamonds), silicides with B addition shown in green colour, purple diamonds RCCAs with Ge or Sn or Ge + Sn, (a) Si versus Ti, R2 = 0.9465 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn or Ti, R2 = 0.9601 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9914 alloying elements Al, B, Cr, Ge, Nb, Si, Sn, Ta or Ti, R2 = 0.9479 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9226 for Ti rich Nb5Si3 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.969 for Ti rich Nb5Si3 alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta or Ti, (b) Cr versus Ti, R2 = 0.919, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9463, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.973, Ti rich Nb5Si3, alloying elements Al, B, Cr, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9716, Ti rich Nb5Si3, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, (c) Al versus Ti, R2 = 0.993, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn or Ti, R2 = 0.9911, alloying elements Al, B, Cr, Ge, Hf, Nb, Si, Sn, Ta or Ti, R2 = 0.9188, Ti rich Nb5Si3, alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta or Ti, R2 = 0.9683, Ti rich Nb5Si3 alloying elements Al, B, Cr, Ge, Hf, Mo, Nb, Si, Ta or Ti.
![Materials 14 00989 g020a Materials 14 00989 g020a]()
![Materials 14 00989 g020b Materials 14 00989 g020b]()
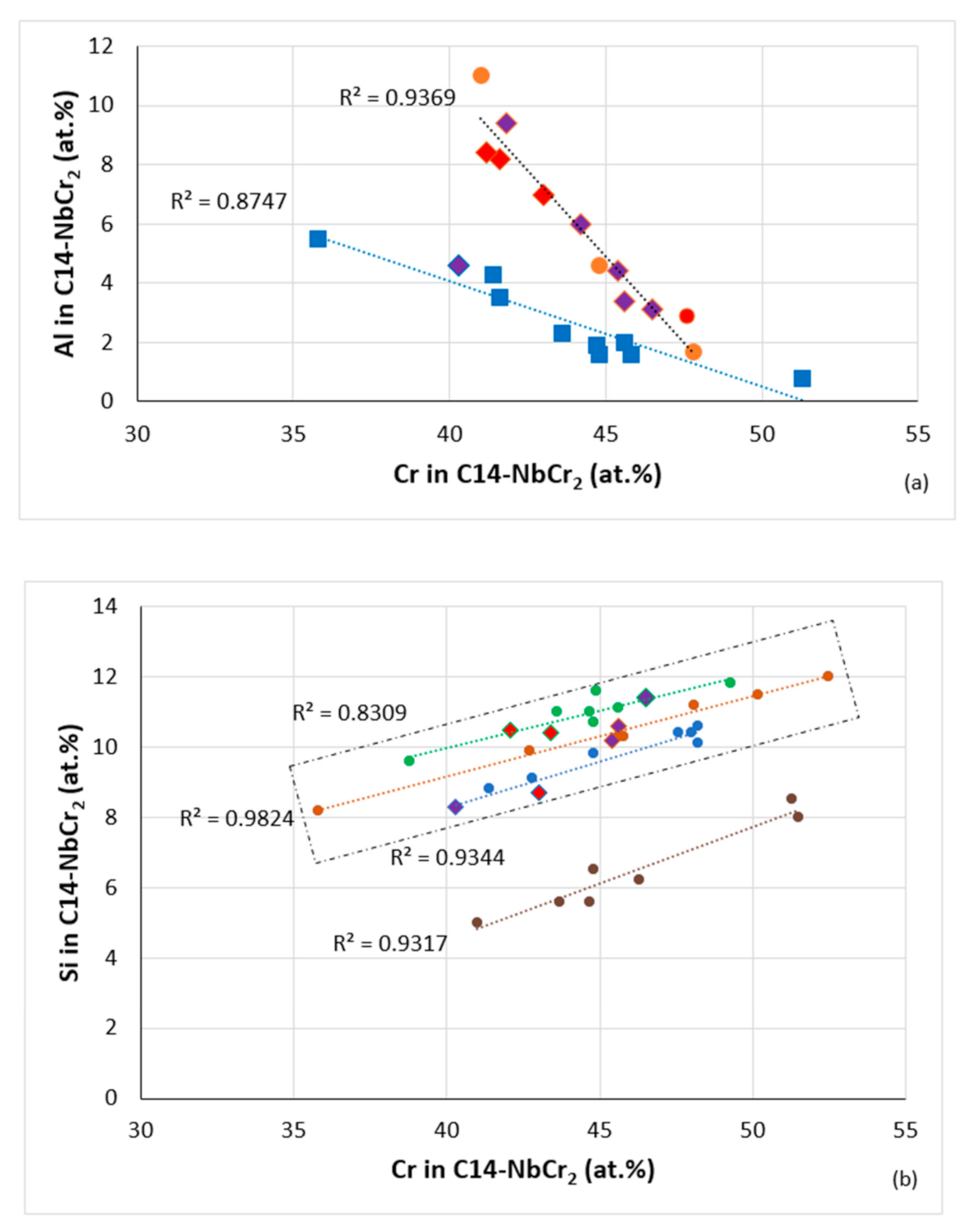
Figure 21.
Data for Al or Cr in C14-NbCr2 Laves phase. For all data points, the Cr in Laves phase is substituted by Al, Ge, Si or Sn. Red data points for C14-NbCr2 Laves phase in oxidized alloys. (a) Al versus Cr in C14-NbCr2. Diamonds for RM(Nb)ICs that are also RCCAs. R2 = 0.9369, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.8747 alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W. Red data points (R2 = 0.9991), (b) Si versus Cr in C14-NbCr2. The data with R2 = 0.9317 is for Ta-free alloys. R2 = 0.9317, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W, R2 = 0.9344, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.9824, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.8309, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W. For the rectangular area in (b), see text.
Figure 21.
Data for Al or Cr in C14-NbCr2 Laves phase. For all data points, the Cr in Laves phase is substituted by Al, Ge, Si or Sn. Red data points for C14-NbCr2 Laves phase in oxidized alloys. (a) Al versus Cr in C14-NbCr2. Diamonds for RM(Nb)ICs that are also RCCAs. R2 = 0.9369, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.8747 alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W. Red data points (R2 = 0.9991), (b) Si versus Cr in C14-NbCr2. The data with R2 = 0.9317 is for Ta-free alloys. R2 = 0.9317, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ti or W, R2 = 0.9344, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.9824, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W, R2 = 0.8309, alloying elements Al, Cr, Ge, Hf, Mo, Nb, Si, Sn, Ta, Ti or W. For the rectangular area in (b), see text.
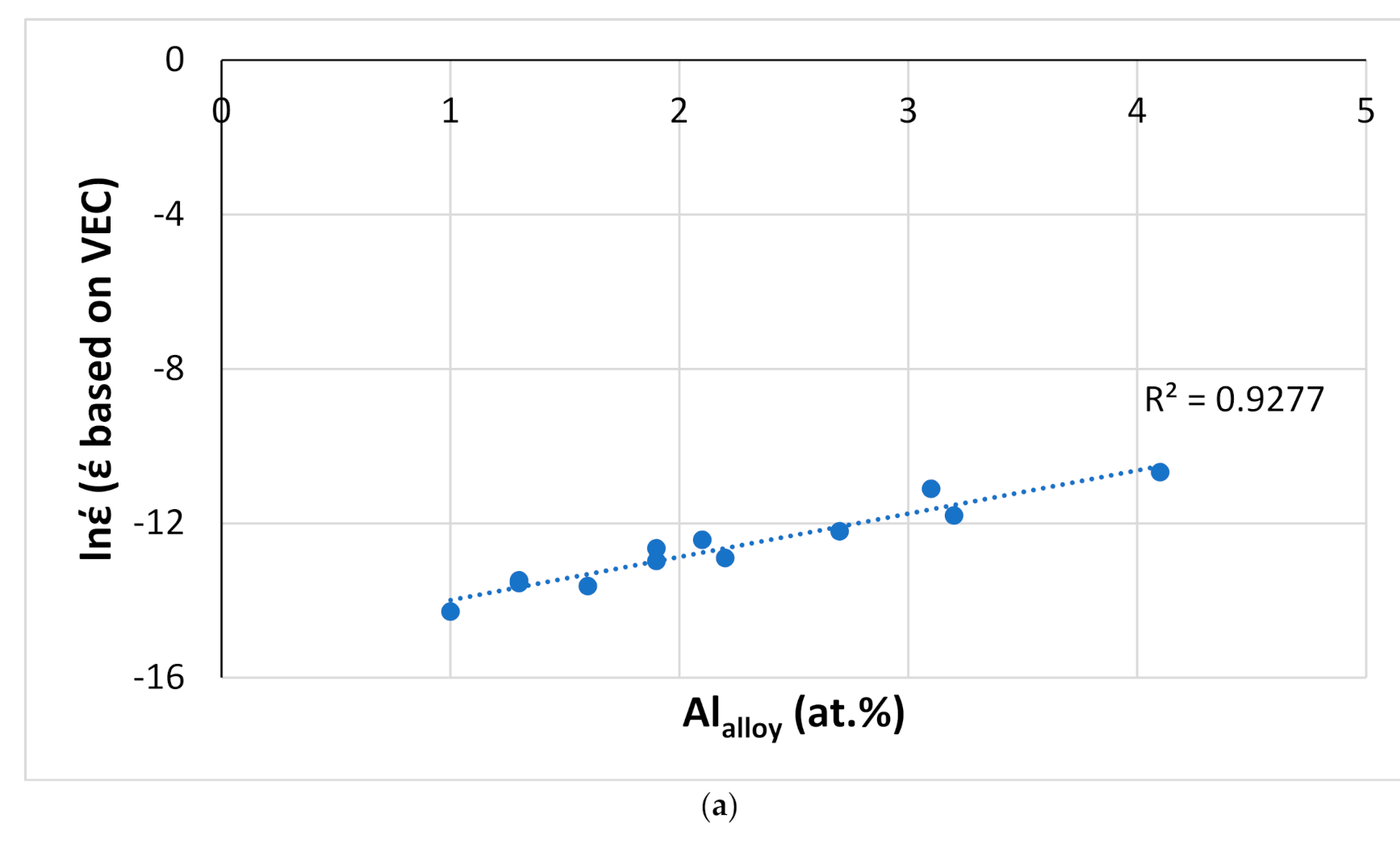
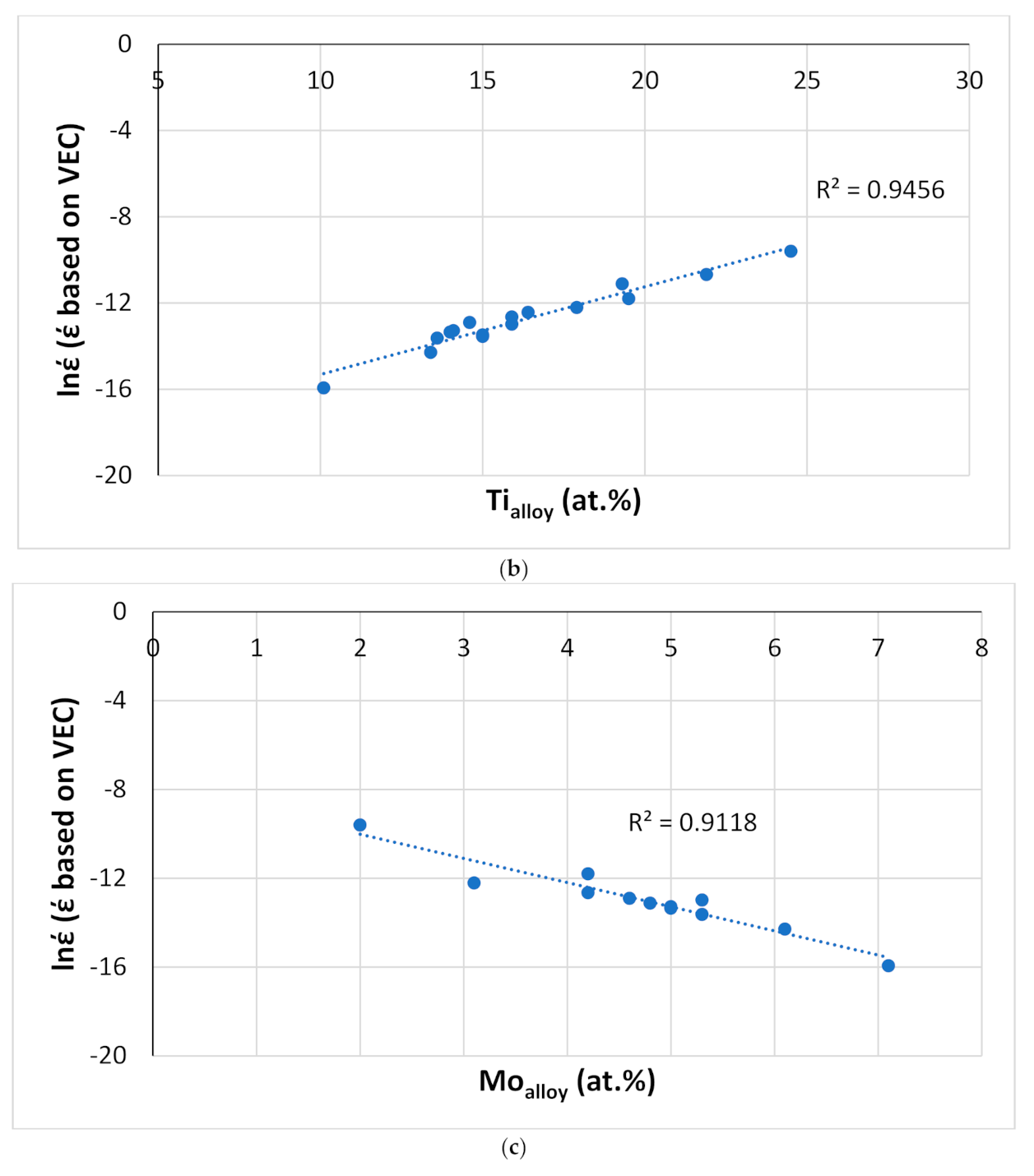
Figure 22.
Calculated secondary creep rates-based on VEC for the creep goal conditions (T = 1200 °C, σ = 170 MPa) versus (a)Al, (b), Ti (c) Mo and (d) Si content in RM(Nb)ICs.
Figure 22.
Calculated secondary creep rates-based on VEC for the creep goal conditions (T = 1200 °C, σ = 170 MPa) versus (a)Al, (b), Ti (c) Mo and (d) Si content in RM(Nb)ICs.
Figure 23.
(a) VECalloy versus Δχalloy, (b) VECalloy versus δalloy and (c) Δχalloy versus δalloy maps of RM(Nb)ICs that are also HEAs or RHEAs/RCCAs. Blue circles for HEAs of the Nb-Ti-Si-Al-Hf system, RM(Nb)ICs-RHEAs/RCCAs as follows: green with B, red with Sn, orange with Ge, purple with Ge + Sn addition. Green square for an RM(Nb)IC-RCCA with B + Sn addition.
Figure 23.
(a) VECalloy versus Δχalloy, (b) VECalloy versus δalloy and (c) Δχalloy versus δalloy maps of RM(Nb)ICs that are also HEAs or RHEAs/RCCAs. Blue circles for HEAs of the Nb-Ti-Si-Al-Hf system, RM(Nb)ICs-RHEAs/RCCAs as follows: green with B, red with Sn, orange with Ge, purple with Ge + Sn addition. Green square for an RM(Nb)IC-RCCA with B + Sn addition.
Table 1.
Values of the parameters ΔHmix, ΔSmix, VEC, δ, Δχ and Ω for all Nb-silicide-based alloys and their solid solutions, and for the eutectics with Nbss and Nb5Si3.
Table 1.
Values of the parameters ΔHmix, ΔSmix, VEC, δ, Δχ and Ω for all Nb-silicide-based alloys and their solid solutions, and for the eutectics with Nbss and Nb5Si3.
| Material | Parameter |
|---|
ΔHmix
(kJ/mol) | ΔSmix (J/mol K) | VEC ** | δ | Δχ | Ω + |
|---|
| RM(Nb)ICs [8] and RM(Nb)ICs-RHEAs/RCCAs | −32.7 to −44.8 | 8.3–14.7 | 4.4–4.9 | 8.1–14.3 | 0.12–0.237 | 0.57–0.95 |
| Solid solution in RM(Nb)ICs [7] and RM(Nb)ICs-RHEAs/RCCAs | −2 to −15.9 | 5.8–14.5 | 4.4–5.4 | 2.4–9.7 | 0.039–0.369 *
gap 0.13 to 0.18 | 1.55–8.9 |
| Eutectics with Nbss and Nb5Si3 [11] | −25.5 to −41.9 | 4.7–15 | 4.3–4.9 | 6.2–9.4 | 0.118–0.248
gap 0.16 to 0.18 | 0.38–1.35 |
| HEAs with bcc solid solution + intermetallic(s) [7] | 2.5–35 | 11.5–14.5 | 5.7–8 | 4.6–11 | 0.125–0.225 | 1–10 |
| Amorphous alloys [7] | 0–50 | 6–17.5 | 4–9.5 | 4.6–18.5 | 0.1–0.35 | - |