Hydrothermal Synthesis of TiO2 Aggregates and Their Application as Negative Electrodes for Lithium-Ion Batteries: The Conflicting Effects of Specific Surface and Pore Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Nanoparticles and Aggregates
2.2. Characterization of Prepared of TiO2 Nanoparticles and Aggregates
3. Results
3.1. Synthesis, Characterization and Formation Mechanism of TiO2 Nanoparticle Aggregates
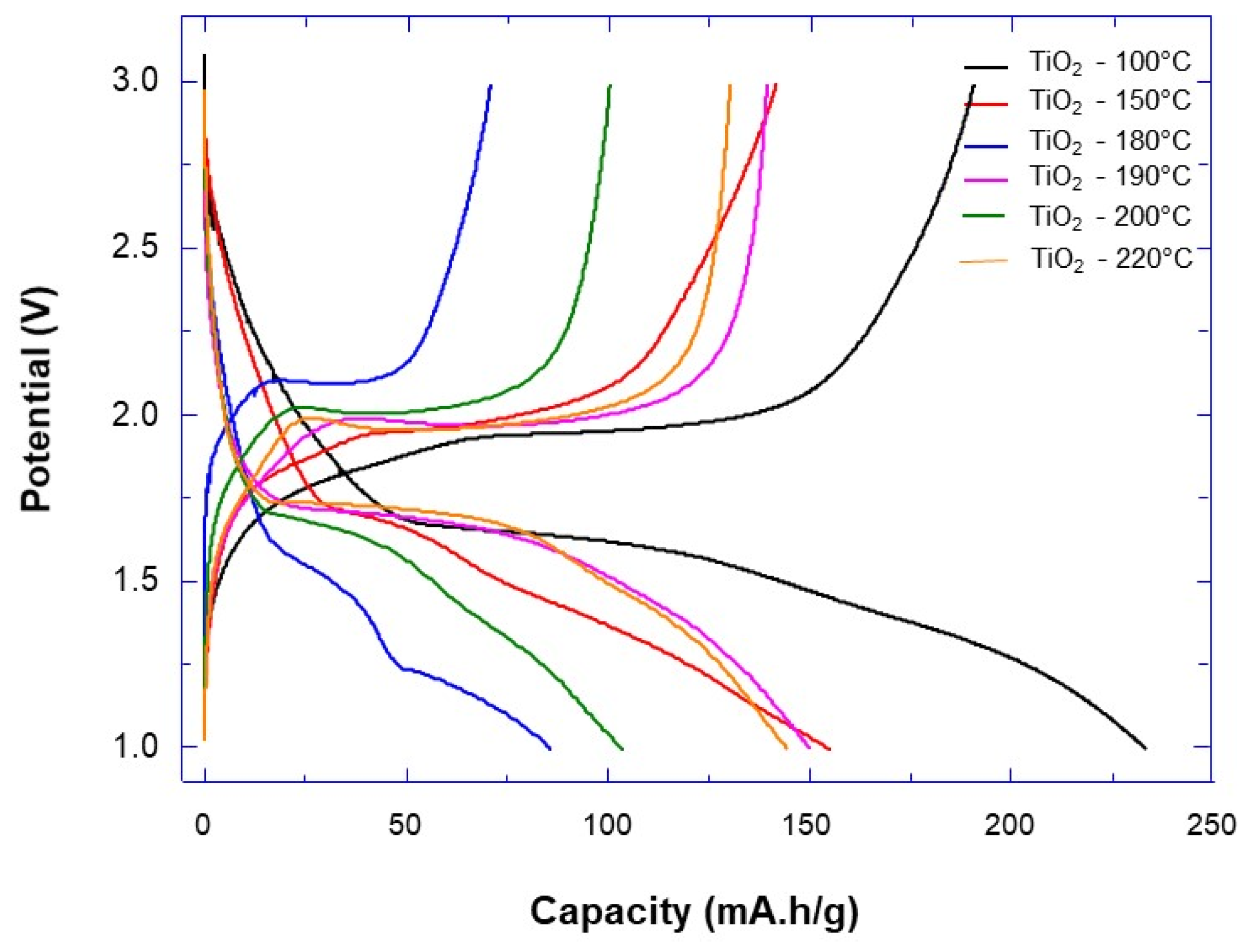
3.2. Electrochemical Test of TiO2 Nanoparticle Aggregates in LiB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, T.; Trefalt, G.; Borkovec, M. Aggregation of colloidal particles in the presence of hydrophobic anions: Importance of attractive non-DLVO forces. Langmuir 2018, 34, 14368–14377. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Wan, J.; Tokunaga, T. Kinetic stability hematic nanoparticles the effect of particle size. J. Nanoparticle Res. 2007, 10, 321–332. [Google Scholar] [CrossRef]
- Zhou, D.; Ji, Z.; Jiang, X.; Dunphy, D.R.; Brinker, J.; Keller, A. Influence of material properties on TiO2 nanoparticle agglomeration. PLoS ONE 2013, 8, e81239. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B. A theory of interaction of particles in presence of electric double-layers and the stability of lyophobe colloids and disperse systems. Acta Phys. Chem. 1939, 10, 333–346. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys. Chim. 1941, 14, 633–662. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of Stability of Lyophobic Colloids; Elsevier: Amsterdam, Holland, 1948. [Google Scholar]
- Tan, B.; Wu, Y.Y. Dye-sensitized solar cells based on anatase TiO2 nanoparticles/nanowires composites. J. Phys. Chem. B 2006, 110, 15932–15938. [Google Scholar] [CrossRef]
- Han, C.; Luque, R.; Dionysiou, D. Facile preparation of controllable size monodisperse anatase titaniananoparticles. Chem. Comm. 2012, 48, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef]
- Sun, B.; Shi, T.; Peng, Z.; Sheng, W.; Jiang, T.; Liao, G. 2013 Controlled fabrication of Sn/TiO2 nanorods for photoelectrochemical watersplitting. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar]
- Yan, X.; Wang, Z.; He, M.; Chen, X. TiO2 nanomaterials as anode materials for lithium-ion rechargeable batteries. Energy Technol. 2015, 3, 801–814. [Google Scholar] [CrossRef]
- Sugiawati, V.; Vacandio, F.; Galeyeva, A.; Kurbatov, A.; Djenizian, T. Enhanced electrochemical performance of electropolymerized self-organized TiO2 nanotubes fabricated by anodization of Ti grid. Front. Phys. Front. 2019, 7. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, T.; Zhong, Q.; Li, H.; Huang, J. 2014 Graphene nanoscrolls encapsulated TiO2 nanowires for lithium storage. J. Power Sources 2014, 268, 372–378. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.Z.; Liu, J.; Li, Y.; Chen, D.S.; Wang, H.E.; Yu, Y.; Chen, L.H.; Su, B.L. Design of new anode materials based on hierarchical three-dimensional ordered macro mesoporous TiO2 for high performance lithium-ion batteries. J. Mater. Chem. A 2014, 2, 9699–9708. [Google Scholar] [CrossRef]
- Wagemaker, M.; Kearley, G.J.; van Well, A.A.; Mutka, H.; Mulder, F.M. Multiple Li positions inside oxygen octahedra in lithiated TiO2 anatase. J. Am. Chem. Soc. 2003, 125, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Sun, X.; Zhou, C.; Cavanagh, A.S.; Sun, H.; Hu, T.; Wang, G.; Lian, J.; George, S.M. Amorphous Ultrathin TiO2 atomic layer deposition films on carbon nanotubes as anodes for lithium-ion batteries. J. Electrochem. Soc. 2015, 162, A974–A981. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Recent progress of TiO2-based anodes for Li ion batteries. J. Nanomater. 2016, 2016, 8123652. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J. Nanoengineering titania for high rate lithium storage: A review. J. Mater. Sci. Technol. 2013, 29, 97–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Li, W.; Chen, X. Nanostructured TiO2-based anode materials for high-performance rechargeable lithium-ion batteries. Chem. Nano Mat. 2016, 2, 764–775. [Google Scholar] [CrossRef]
- Liu, Z.; Andreev, Y.G.; Armstrong, A.R.; Brutti, S.; Ren, Y.; Bruce, P.G. Nanostructured TiO2(B): The effect of size and shape on anode properties for Li-ion batteries. Prog. Nat. Sci. Mater. Int. 2013, 23, 235–244. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Li, J.M.; Wan, W.; Zhou, H.H.; Li, J.J.; Xu, D.S. Hydrothermal synthesis of TiO2(B) nanowires with ultrahigh surface area and their fast charging and discharging properties in Li-ion batteries. Chem. Commun. 2011, 47, 3439. [Google Scholar] [CrossRef]
- Lin, Z.; Zheng, M.; Zhao, B.; Wang, G.; Pu, L.; Shi, Y. Influence of the pore structure parameters of mesoporous anatase microspheres on their performance in lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 1673–1681. [Google Scholar] [CrossRef]
- Swiatowska-Mrowiecka, J.; Zanna, S.; Ogle, K.; Marcus, P. Adsorption of 1,2-diaminoethane on ZnO thin films from p-xylene. Appl.Surf. Sci. 2008, 254, 5530–5539. [Google Scholar] [CrossRef]
- Gadois, C.; Światowska, J.; Zanna, S.; Marcus, P. Influence of titanium surface treatment on adsorption of primary amines. J. Phys. Chem. C 2013, 117, 1297–1307. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Morgan, B.J.; Światowska, J.; Baddour-Hadjean, R.; Body, M.; Legein, C.; Borkiewicz, O.J.; Leclerc, S.; Groult, H.; et al. Lithium intercalation in anatase titanium vacancies and the role of local anionic environment. Chem. Mater 2018, 30, 3078–3089. [Google Scholar] [CrossRef]
- Ennaceri, H.; Boujnah, M.; Taleb, A.; Khaldoun, A.; Köhler, T.; Sáez-Araoz, R.; Ennaoui, A.; Benyoussef, A. Thickness effect on the optical properties of TiO2-anatase thin films prepared by spray-ILGAR: Experimental and ab initio studies. Int. J. Hydrog. Energy 2017, 42, 19467–19480. [Google Scholar] [CrossRef]
- You, H.; Chen, F.; Yang, S.; Yang, A.; Ding, B.; Liang, S.; Song, X. Size effect on nanoparticles mediated silver crystal growth. Cryst. Growth Des. 2011, 11, 5449–5456. [Google Scholar] [CrossRef]
- Hawa, T.; Zachariah, M.R. Molecular dynamic study of particle-particle collisions between hydrogen-passivated silicon nanoparticles. Phys. Rev. B 2004, 69, 035417. [Google Scholar]
- Liu, X.; Chen, G.; Su, C. Effect of material properties on sedimentation and aggregation of TiO2 nanoparticles of anatase and rutile in the aqueous phase. J. Colloid. Interf. Sci. 2011, 363, 84–91. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size and crystal phase on titanium dioxide nanoparticles dispersion properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar]
- Abbas, Z.; Labbez, C.; Nordholm, S.; Ahlberg, E. Size-dependent surface charging of nanoparticles. J. Phys. Chem. C 2008, 112, 5715–5723. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, B.; Wang, P.; Dellago, C.; Wang, Y.; Fang, Y. Nanoparticle-based crystal growth via multistep self-assembly. Cryst. Eng. Comm. 2013, 15, 5114–5118. [Google Scholar] [CrossRef]
- Lim, T.H.; McCarthy, D.; Hendy, S.C.; Stevens, K.J.; Bishop, S.A.; Tilley, R.D. Real-time TEM and kinetic monte carlo studies of the coalescence of decahedral gold nanoparticles. ACS Nano 2009, 3, 3809–3813. [Google Scholar] [CrossRef] [PubMed]
- Ingham, B.; Lim, T.H.; Dotsler, C.J.; Henning, A.; Toney, M.F.; Tilley, R.D. How nanoparticles Coalesce: An in-situ study of Au nanoparticle aggregation and grain growth. Chem. Mater. 2011, 23, 3312–3317. [Google Scholar] [CrossRef]
- Kohn, P.; Pathak, S.; Stefik, M.; Ducati, C.; Wiesner, U.; Steiner, U.; Guldin, S. Low temperature crystalisation of mesoporous TiO2. Nanoscale 2013, 5, 10518–10524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subramanian, V.; Karki, A.; Gnanasekar, K.L.; Eddy, F.P.; Rambabu, B. Nanocrysatlline TiO2 (anatase) for Li ion batteries. J. Power Sources 2006, 159, 186–192. [Google Scholar] [CrossRef]
- Krtil, P.; Fattakhova, D.; Kavan, L.; Burnside, S.; Gratzel, M. Lithium insertion into self-organized mesoscopic TiO2 electrodes. Solide State Ion. 2000, 135, 101–106. [Google Scholar] [CrossRef]
- Wagemaker, M.; Borghols, W.J.H.; Mulder, F.M. Large impact of particle size on insertion reaction. A case for anatase LixTiO2. J. Am. Chem. Soc. 2007, 129, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, Z.; Zhang, Z. Preparation and novel lithium intercalation properties of titanium oxide nanotubes. Electrochem. Solide State Lett. 2005, 8, A316–A319. [Google Scholar]
- Shin, J.Y.; Samuelis, D.; Maier, J. Sustained lithium storage performance of hierarchical, nanoporous anatase TiO2 at high rates: Emphasis on interfacial storage phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Li, Y.; Pan, G.L.; Liu, J.W.; Gao, X.P. Preparation of Li4Ti5O12 nanorods as anode materials for lithium-ion batteries. J. Electrochem. Soc. 2009, 156, A495–A499. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, J.C.; Roh, H.S.; Lee, C.W.; Park, S.; Kim, D.W.; Hong, K.S. Anion controlled synthesis of TiO2 nano aggregates for Li Ion battery electrodes. Mat. Charac. 2014, 96, 13–20. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defect TiO2 with oxygen vacancies: Synthesis properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Ventosa, E.; Madej, E.; Zampardi, G.; Mei, B.; Weide, P.; Antoni, H.; La Mantia, F.; Muhler, M.; Schuhmann, W. Solid electrolyte interphase (SEI) at TiO2 electrodes in Li-ion batteries: Defining apparent and effective SEI based on evidence from X-ray photoemission spectroscopy and scanning electrochemical microscopy. ACS Appl. Mater. Interfaces 2017, 9, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Song, S. One step hydrothermal synthesis of mesoporous anatase TiO2 microsphere and interfacial control for enhanced lithium storage performance. ACS Appl. Mater. Interfaces 2011, 3, 3697–3703. [Google Scholar] [CrossRef]
- Lin, Z.; Yue, W.; Huang, D.; Hu, J.; Zhang, X.; Yuan, Z.; Yang, X. Pore length control of mesoporous Co3O4 and its influence on the capacity of porous electrodes for lithium-ion batteries. RSC Adv. 2012, 2, 1794–1797. [Google Scholar]
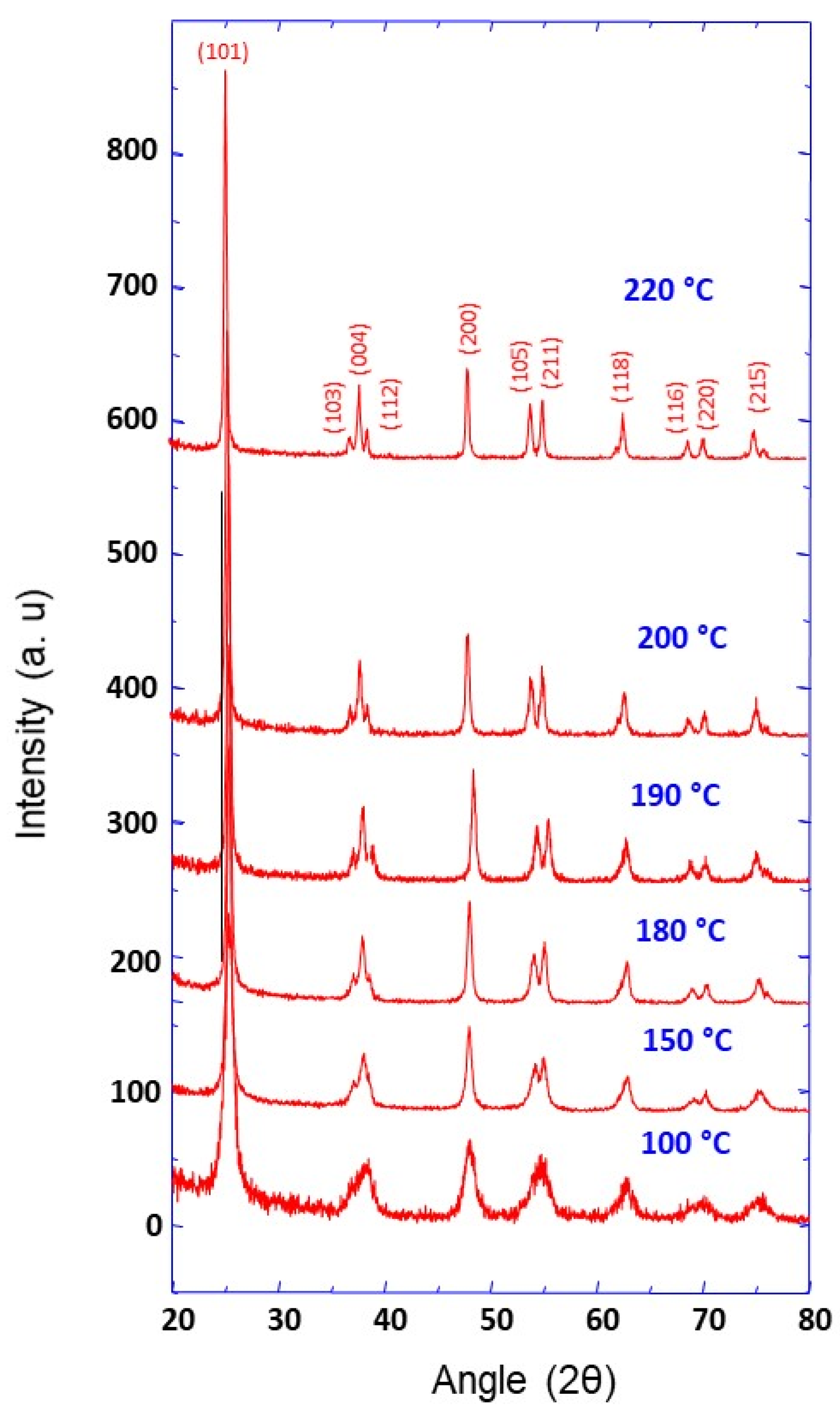
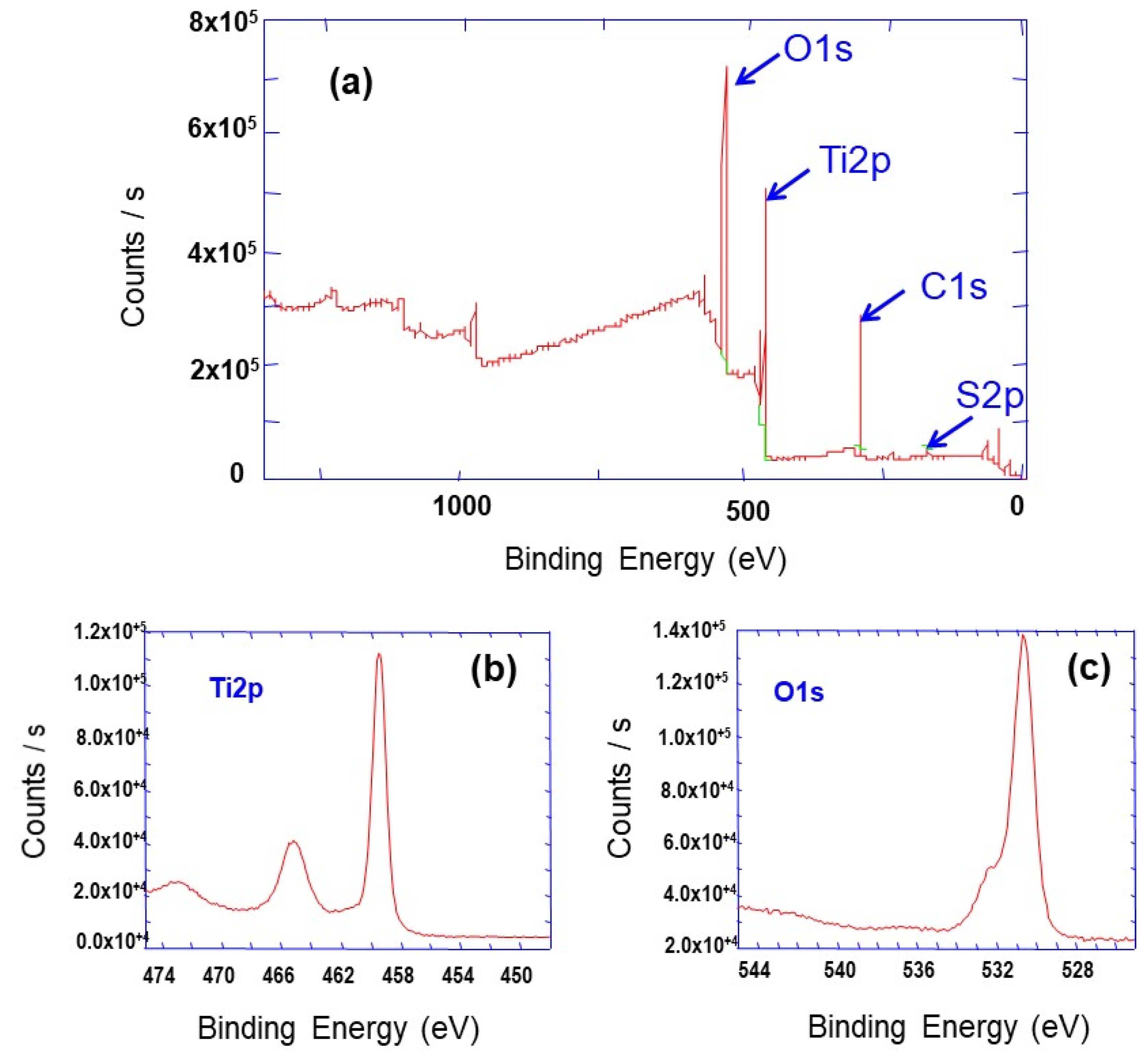


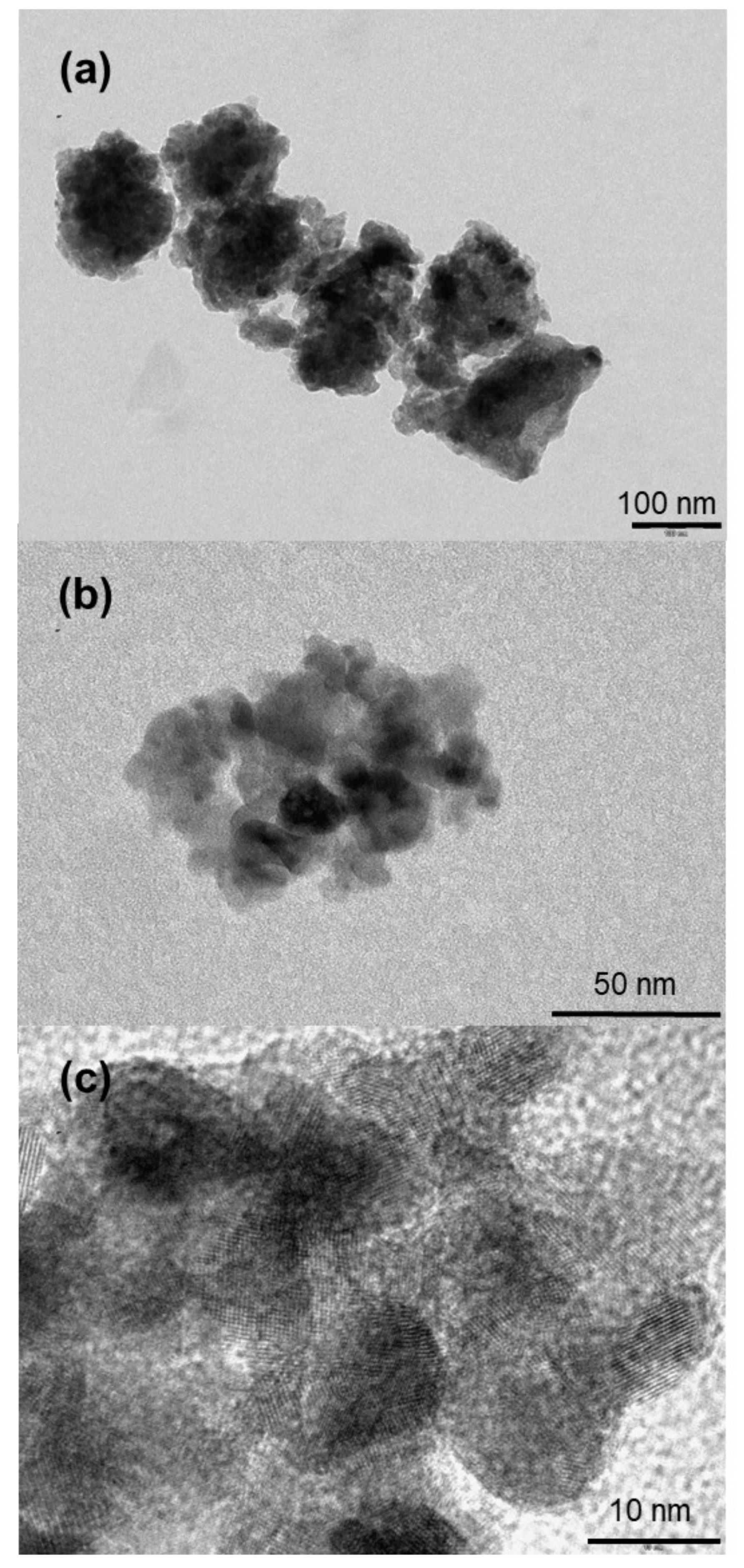
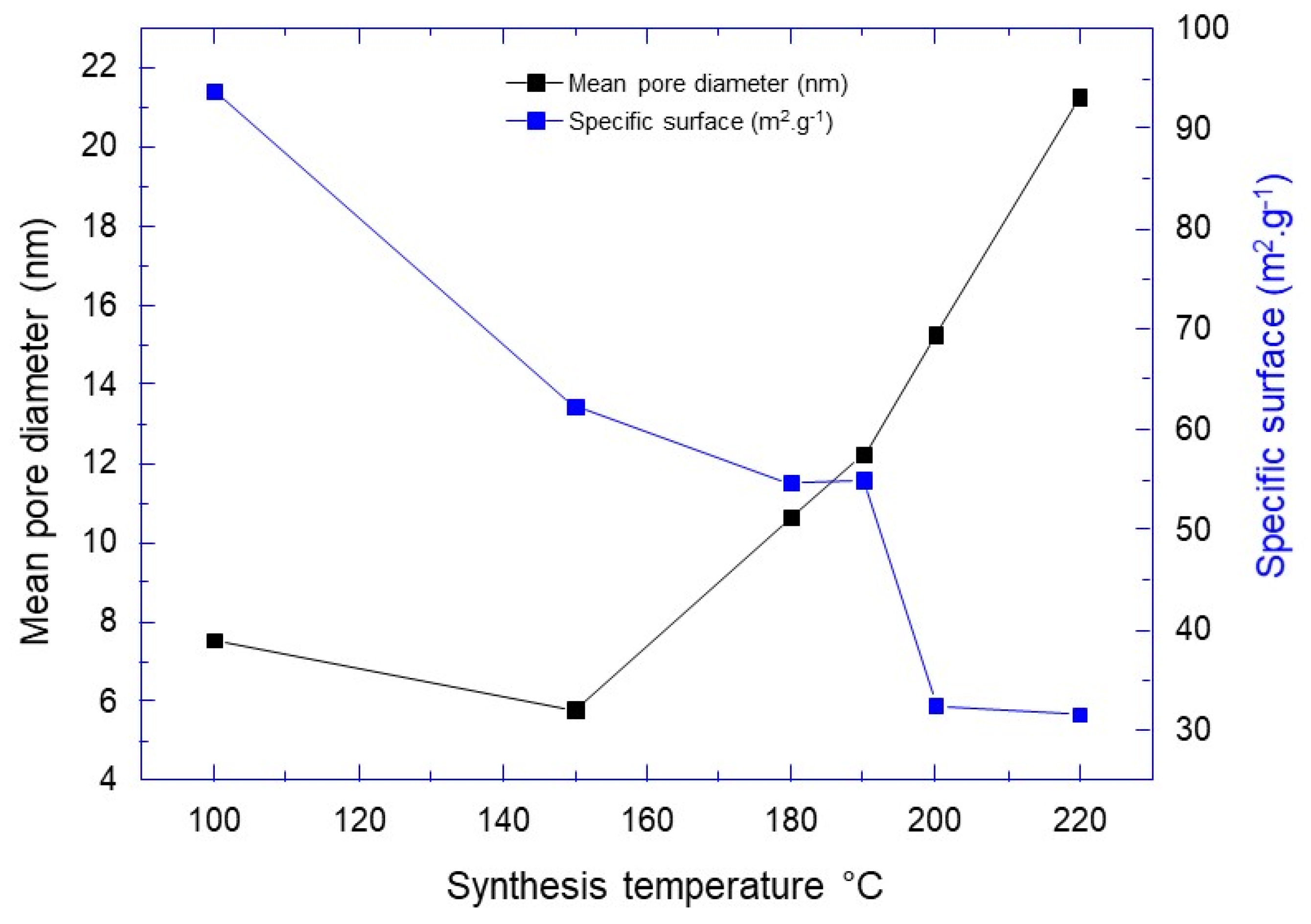
| Synthesis Temperature (°C) | Crystallite Size (nm) | Mean Pore Diameter (nm) | Specific Surface (m2·g−1) | Capacity Discharge/Charge (mA·h/g) |
|---|---|---|---|---|
| 100 | 9.8 | 7.53 | 93.78 | 233/190 |
| 150 | 17.9 | 5.79 | 62.29 | 155/142 |
| 180 | 20.7 | 10.64 | 54.66 | 71/86 |
| 190 | 20.7 | 12.24 | 54.98 | 139/150 |
| 200 | 24.7 | 15.26 | 32.51 | 100/104 |
| 220 | 30.4 | 21.28 | 31.62 | 144/136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehraz, S.; Luo, W.; Swiatowska, J.; Bezzazi, B.; Taleb, A. Hydrothermal Synthesis of TiO2 Aggregates and Their Application as Negative Electrodes for Lithium-Ion Batteries: The Conflicting Effects of Specific Surface and Pore Size. Materials 2021, 14, 916. https://doi.org/10.3390/ma14040916
Mehraz S, Luo W, Swiatowska J, Bezzazi B, Taleb A. Hydrothermal Synthesis of TiO2 Aggregates and Their Application as Negative Electrodes for Lithium-Ion Batteries: The Conflicting Effects of Specific Surface and Pore Size. Materials. 2021; 14(4):916. https://doi.org/10.3390/ma14040916
Chicago/Turabian StyleMehraz, Saida, Wenpo Luo, Jolanta Swiatowska, Boudjema Bezzazi, and Abdelhafed Taleb. 2021. "Hydrothermal Synthesis of TiO2 Aggregates and Their Application as Negative Electrodes for Lithium-Ion Batteries: The Conflicting Effects of Specific Surface and Pore Size" Materials 14, no. 4: 916. https://doi.org/10.3390/ma14040916
APA StyleMehraz, S., Luo, W., Swiatowska, J., Bezzazi, B., & Taleb, A. (2021). Hydrothermal Synthesis of TiO2 Aggregates and Their Application as Negative Electrodes for Lithium-Ion Batteries: The Conflicting Effects of Specific Surface and Pore Size. Materials, 14(4), 916. https://doi.org/10.3390/ma14040916








