Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations
Abstract
1. Introduction
2. Calculations and Experimental Procedures
2.1. Change in Gibbs Free Energy of Al-Fe Melt
2.2. Formation Enthalpy of Al13Fe4 Phase Based on First-Principle Calculations
2.2.1. Crystal Structure of Al13Fe4
2.2.2. Computational Details
2.3. Experimental Details
3. Results and Discussion
3.1. Gibbs Free Energy of Al13Fe4 Nucleus in Al-Fe Melt
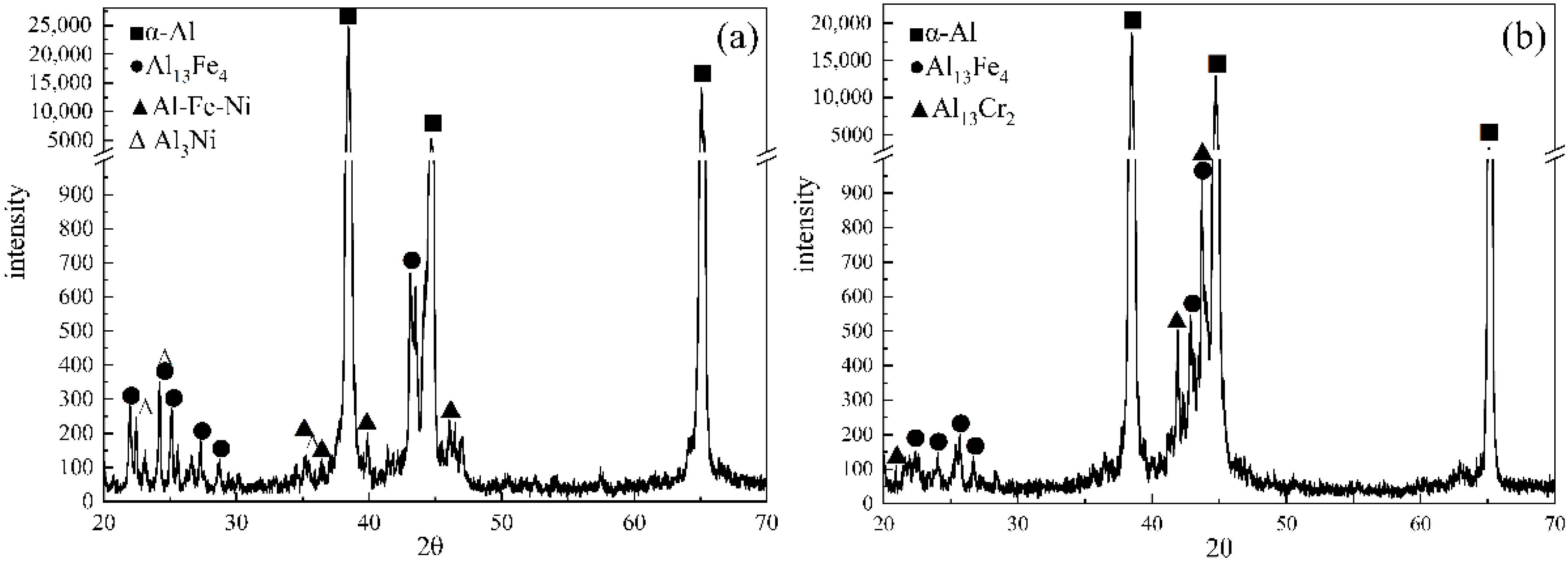
3.2. Formation Enthalpy of Al13Fe4 Phase
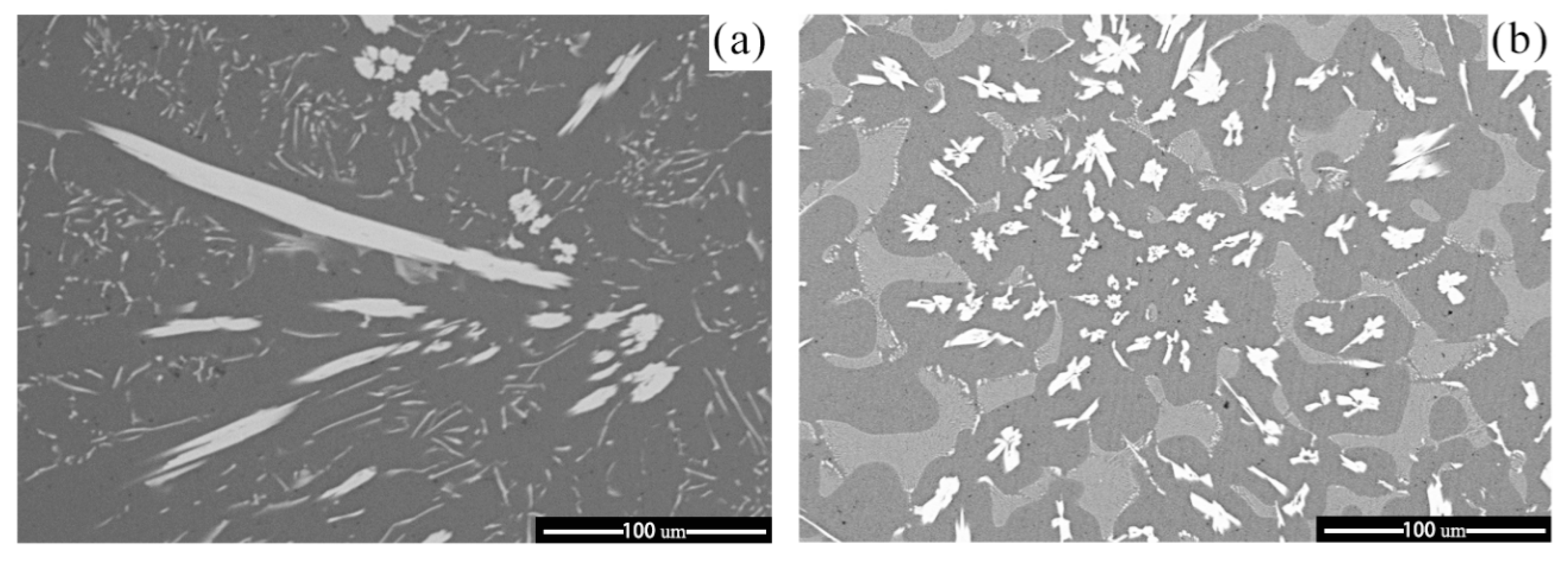
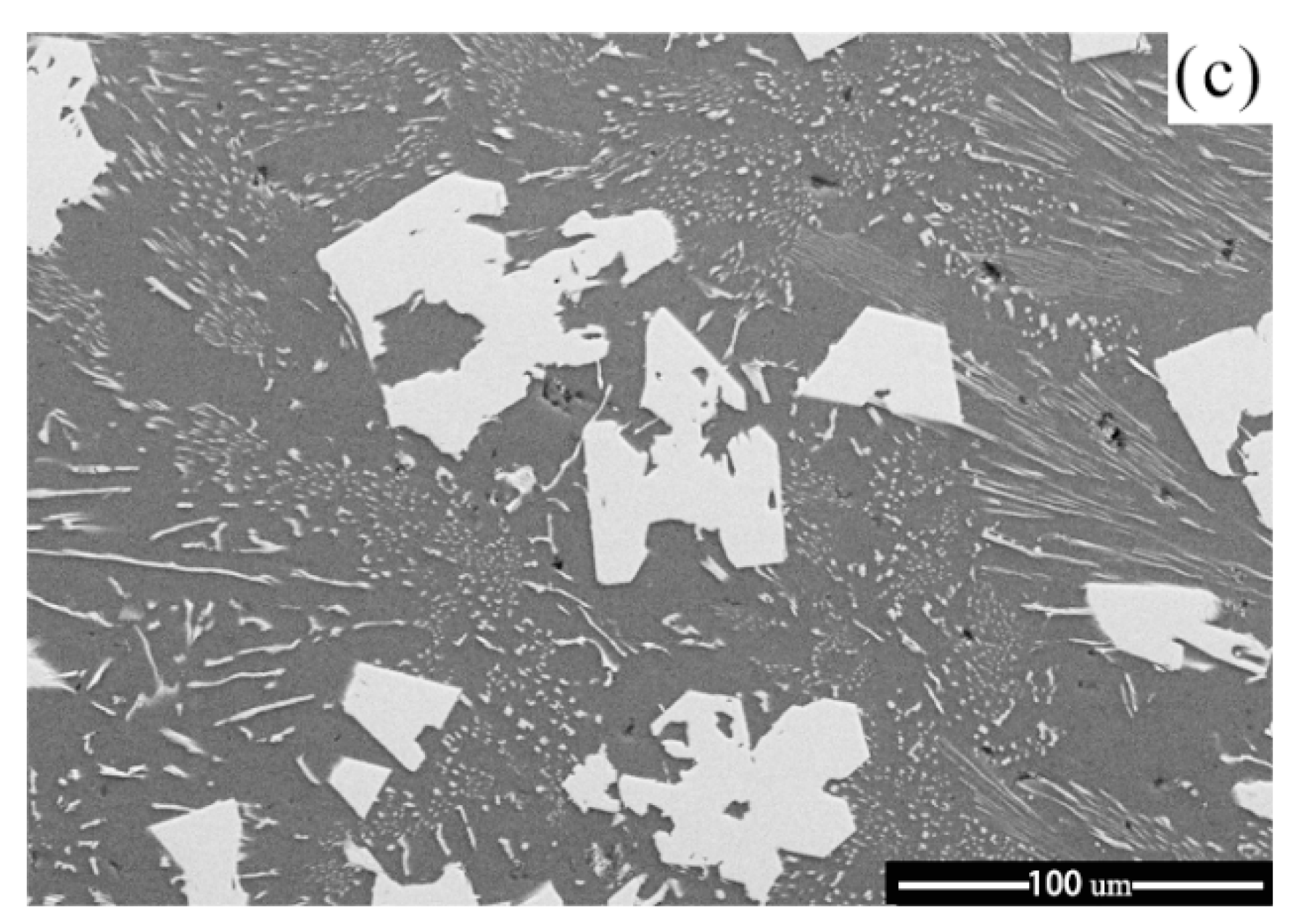
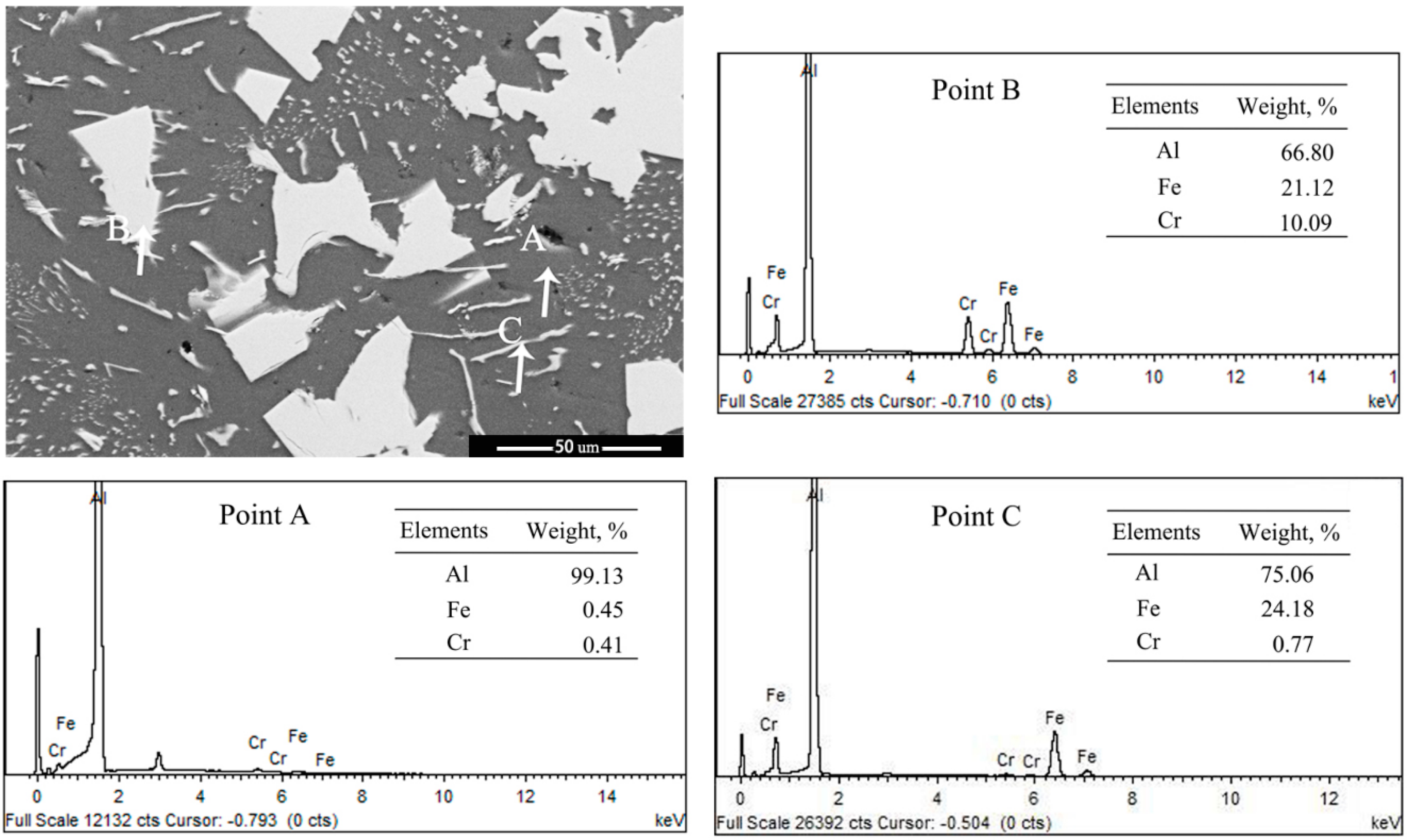
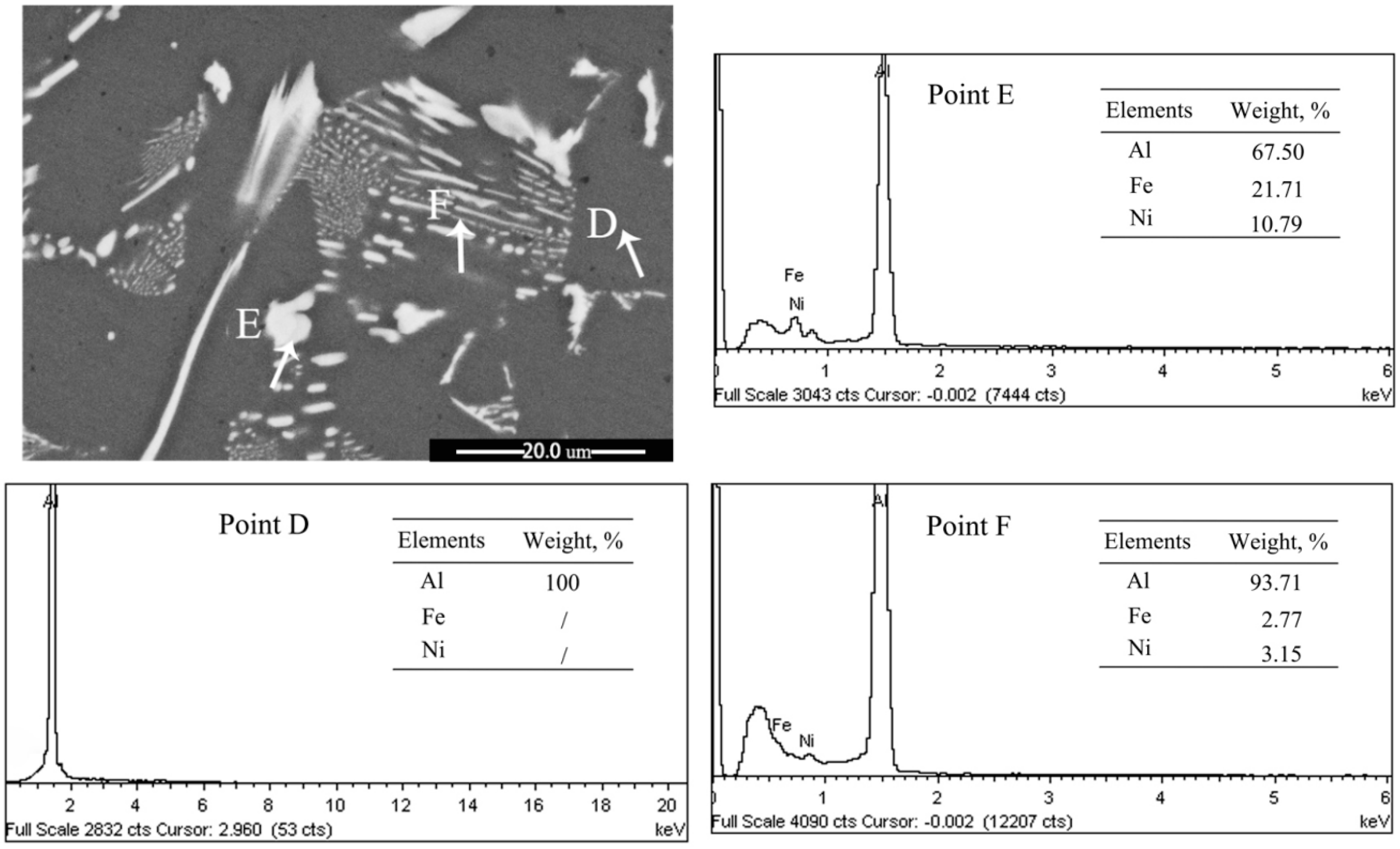
3.3. Influence of M Addition on Microstructural Evolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goulart, P.R.; Cruz, K.S.; Spinelli, J.E.; Ferreira, I.L.; Cheung, N.; Garcia, A. Cellular growth during transient directional solidification of hypoeutectic Al-Fe alloys. J. Alloys Compd. 2009, 470, 589–599. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.L.; He, M.Y.; Xu, L.; Wangyang, P.H. Thermal plasma jet approach towards the controllable preparation of Al-Fe alloys with high Fe content and spherical second phase. J. Alloys Compd. 2018, 737, 14–20. [Google Scholar] [CrossRef]
- Liang, D.; Korgui, P.; Jones, H. Composition and solidification microstructure selection in the interdendritic matrix between primary Al3Fe dendrites in hypereutectic Al-Fe Alloy. Acta Mater. 1996, 44, 2999–3004. [Google Scholar] [CrossRef]
- Chen, J.; Lengsdorf, R.; Henein, H.; Herlach, D.M.; Dahlborg, U.; Calvo-Dahlborg, M. Microstructure evolution in undercooled Al-8wt%Fe melts: Comparison between terrestrial and parabolic flight conditions. J. Alloys Compd. 2013, 556, 243–251. [Google Scholar] [CrossRef]
- Goulart, P.R.; Spinelli, J.E.; Cheung, N.; Garcia, A. The effects of cell spacing and distribution of intermetallic fibers on the mechanical properties of hypoeutectic Al-Fe alloys. Mater. Chem. Phys. 2010, 119, 272–278. [Google Scholar] [CrossRef]
- Premkumar, M.K.; Lawley, A.; Koczak, M.J. Processing and microstructure of powder metallurgy Al-Fe-Ni alloys. Metall. Trans. A 1992, 23, 3219–3230. [Google Scholar] [CrossRef]
- Hatayama, H.; Daigo, I.; Matsuno, Y.; Adachi, Y. Assessment of the recycling potential of Aluminum in Japan, the United States, Europe and China. Mater. Trans. 2009, 50, 450–456. [Google Scholar] [CrossRef]
- Voděrová, M.; Novák, P.; Marek, I.; Vojtěch, D. Microstructure and mechanical properties of rapidly solidified Al-Fe-X alloys. Key Eng. Mater. 2013, 592–593, 639–642. [Google Scholar] [CrossRef]
- Voděrová, M.; Novák, P.; Marek, I.; Vojtěch, D. Microstructure of rapidly solidified and hot-pressed Al-Fe-X Alloys. Mater. Technol. 2014, 48, 349–353. [Google Scholar]
- Manuel, V.C.; Crystopher, B.; Spinelli, J.E.; Garcia, A. Interrelation of cell spacing, intermetallic compounds and hardness on a directionally solidified Al–1.0Fe–1.0Ni alloy. Mater. Des. 2013, 51, 342–346. [Google Scholar]
- Průša, F.; Vojtěch, D.; Michalcová, A.; Marek, I. Mechanical properties and thermal stability of Al–Fe–Ni alloys prepared by centrifugal atomization and hot extrusion. Mater. Sci. Eng. A 2014, 603, 141–149. [Google Scholar] [CrossRef]
- Soustani, M.F.; Taghiabadi, R.; Jafarzadegan, M.; Farahan, M.V. Effect of multi-pass friction stir processing on microstructure and mechanical properties of cast Al-7Fe-5Ni alloy. Mater. Res. Express 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, X.G.; Huang, H.J.; Guo, Z.Q. The effect of alloying elements on the microstructure of Al-5Fe Alloys. JOM-J. Miner. Met. Mater. Soc. 2012, 64, 316–322. [Google Scholar] [CrossRef]
- Yan, F.; Kumar, S.; Mckay, B.J.; O’Reilly, K.A.Q. Effect of Mn on Fe containing phase formation in high purity aluminum. Int. J. Cast. Met. Res. 2014, 27, 202–206. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Li, R.D.; Ma, J.C.; Yu, H.S. Effect of Co on As-cast Microstructure of Al-5%Fe Alloy. J. Mater. Eng. 2005, 2, 54–58. [Google Scholar]
- Fan, T.X.; Yang, G.; Zhang, D. Prediction of chemical stability in SiCp/Al composites with alloying element addition using Wilson equation and an extended Miedema model. Mat. Sci. Eng. A 2005, 394, 327–338. [Google Scholar] [CrossRef]
- Fan, T.X.; Yang, G.; Zhang, D. Thermodynamic effect of alloying addition on in-situ reinforced TiB2/Al composites. Metall. Mater. Trans. A 2005, 36, 225–234. [Google Scholar] [CrossRef]
- Zhang, C.F.; Fan, T.X.; Cao, W.; Zhang, D. A model for work of solid-liquid adhesion in multicomponent melts. Acta Mater. 2009, 57, 3623–3632. [Google Scholar] [CrossRef]
- Wilson, G.M. Vapor-Liquid equilibrium XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 1964, 86, 127–130. [Google Scholar] [CrossRef]
- Miedema, A.R.; de Chatel, P.F.; de Boer, F.R. Cohesion in alloys—Fundamental of a semi-empirical model. Phys. B 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Zhang, C.F.; Fan, T.X.; Cao, W.; Ding, J.; Zhang, D. Size control of in situ formed reinforcement in metal melts-theoretical treatment and application to in situ (AlN+Mg2Si)/Mg composites. Compos. Sci. Technol. 2009, 69, 2688–2694. [Google Scholar] [CrossRef]
- Black, P.J. The structure of FeAl3.I. Acta Cryst. 1995, 8, 43–48. [Google Scholar] [CrossRef]
- Black, P.J. The structure of FeAl3.II. Acta Cryst. 1995, 8, 175–182. [Google Scholar] [CrossRef]
- Grin, J.; Burkhardt, U.; Ellner, M.; Peters, K. Refinement of the Fe4Al13 structure and its relationship to the quasihomological homeotypical structures. Z. Kistallogr. 1994, 209, 479–487. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustration and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 16, 1748–1749. [Google Scholar] [CrossRef]
- Owen, E.A.; Preston, G.D. The atomic structure of two intermetallic compounds. Proc. Phys. Soc. 1924, 36, 341–348. [Google Scholar] [CrossRef]
- Piccolo, L.; Chatelier, C.; Weed, M.; Morfin, F.; Ledieu, J.; Fournée, V.; Gille, P.; Gaudry, E. Catalytic properties of Al13TM4 complex intermetallics: Influence of the transition metal and the surface orientation on butadiene hydrogenation. Sci. Technol. Adv. Mater. 2019, 20, 557–567. [Google Scholar] [CrossRef]
- Miedema, A.R.; de Boer, F.R.; Boom, R. Model predictions for the enthalpy of formation of transition metal alloys. Calphad 1983, 7, 51–70. [Google Scholar] [CrossRef]
- Boehler, R.; Ross, M. Melting curve of aluminum in a diamond cell to 0.8 Mbar: Implications for iron. Earth and Planet. Sci. Lett. 1997, 153, 223–227. [Google Scholar]
- Boehler, R.; Santamaria Pérez, D.; Errandonea, D.; Mezouar, M. Melting, density, and anisotropy of iron at core conditions: New x-ray measurements to 150 GPa. J. Phys. Conf. Ser. 2008, 121, 022018. [Google Scholar] [CrossRef]
- Errandonea, D.; Schwager, B.; Ditz, R.; Gessmann, C.; Boehler, R.; Ross, M. Systematics of transition-metal melting. Phys. Rev. B 2001, 63, 849–853. [Google Scholar] [CrossRef]
- Errandonea, D. The melting curve of ten metals up to 12 GPa and 1600 K. J. Appl. Phys. 2010, 108, 033517. [Google Scholar] [CrossRef]
- Errandonea, D. High-pressure melting curves of the transition metals Cu, Ni, Pd, and Pt. Phys. Rev. B 2013, 87, 054108. [Google Scholar] [CrossRef]
- Turnbull, D. Formation of crystal nuclei in liquid metals. J. Appl. Phys. 1950, 21, 1022–1028. [Google Scholar] [CrossRef]
- Zhang, K.C.; Zhang, L.H. The thermodynamic basis and nucleation theory of crystal growth. In Crystal Growth, 1st ed.; Science Press: Beijing, China, 1981; pp. 51–68. (In Chinese) [Google Scholar]
- Popčević, P.; Smontara, A.; Ivkov, J.; Wencka, M.; Komelj, M.; Jeglič, P.; Vrtnik, S.; Bobnar, M.; Jagličić, Z.; Bauer, B.; et al. Anisotropic physical properties of the Al13Fe4 complex intermetallic and its ternary derivative Al13(Fe, Ni)4. Phys. Rev. B 2010, 81, 1–12. [Google Scholar] [CrossRef]
- Marlena, O.; Cacciamani, G. Critical evaluation and thermodynamic modeling of the Al-Co-Fe system. J. Alloys Compd. 2019, 794, 553–568. [Google Scholar]
- Bauer, B.; Gille, P. Crystal Growth of Al-Rich Complex Metallic Phases in the System Al-Cr-Fe Using the Czochralski Method. Z. Anorg. Allg. Chem. 2011, 637, 2052–2058. [Google Scholar] [CrossRef]
- Zheng, W.S.; Mao, H.H.; Lu, X.G.; He, Y.L.; Li, L.; Sellebya, M.L.; Ågren, J. Thermodynamic investigation of the Al-Fe-Mn system over the whole composition and wide temperature ranges. J. Alloys Compd. 2018, 742, 1046–1057. [Google Scholar] [CrossRef]
- Zoroddu, A.; Bernardini, F.; Ruggerone, P.; Fiorentini, V. First-principles prediction of structure, energetics, formation enthalpy, elastic constants, polarization, and piezoelectric constants of AlN, GaN, and InN: Comparison of local and gradient-corrected density-functional theory. Phys. Rev. B 2000, 64, 314–319. [Google Scholar] [CrossRef]
- Yamada, T.; Kojima, T.; Kameoka, S.; Murakami, Y.; Gille, P.; Tsai, A.P. Probing Single Pt Atoms in Complex Intermetallic Al13Fe4. J. Am. Chem. Soc. 2018, 140, 3838–3841. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Mehmood, S.; Ali, Z.; Rehman, G.; Khan, I.; Ahmad, I. Theoretical studies of the electronic structure and magnetic properties of aluminum-rich intermetallic alloy Al13Fe4. Int. J. Mod. Phys. B 2018, 32, 1850201. [Google Scholar] [CrossRef]
- Albedah, M.A.; Nejadsattari, F.; Stadnik, Z.M.; Przewoznik, J. Fe-57 Mossbauer spectroscopy and magnetic study of Al13Fe4. J. Alloys Compd. 2015, 619, 839–845. [Google Scholar] [CrossRef]
- Huang, L.F.; Grabowski, B.; McEniry, E.; Trinkle, D.R.; Neugebauer, J. Importance of coordination number and bond length in titanium revealed by electronic structure investigations. Phys. Status Solidi B 2015, 252, 1907–1924. [Google Scholar] [CrossRef]
- Zhu, L.L.; Soto-Medina, S.; Hennig, R.G.; Manuel, M.V. Experimental investigation of the Al-Co-Fe phase diagram over the whole composition range. J. Alloys Compd. 2020, 815, 152110. [Google Scholar] [CrossRef]
- Gaudry, É.; Chatelier, C.; Loffreda, D.; Kandaskalov, D.; Coatib, A.; Piccolo, L. Catalytic activation of a non-noble intermetallic surface through nanostructuration under hydrogenation conditions revealed by atomistic thermodynamics. J. Mater. Chem. A 2020, 8, 7422–7431. [Google Scholar] [CrossRef]
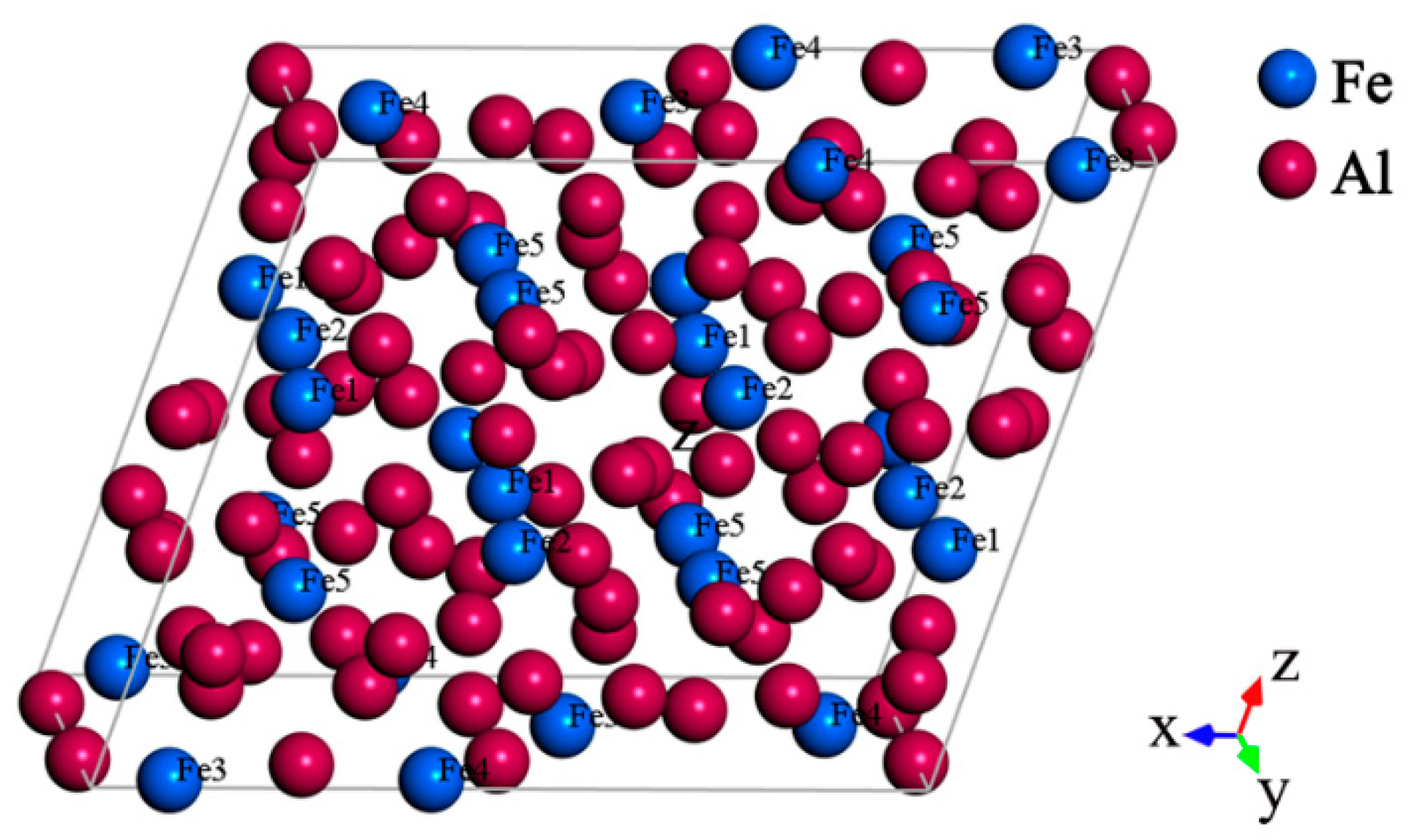
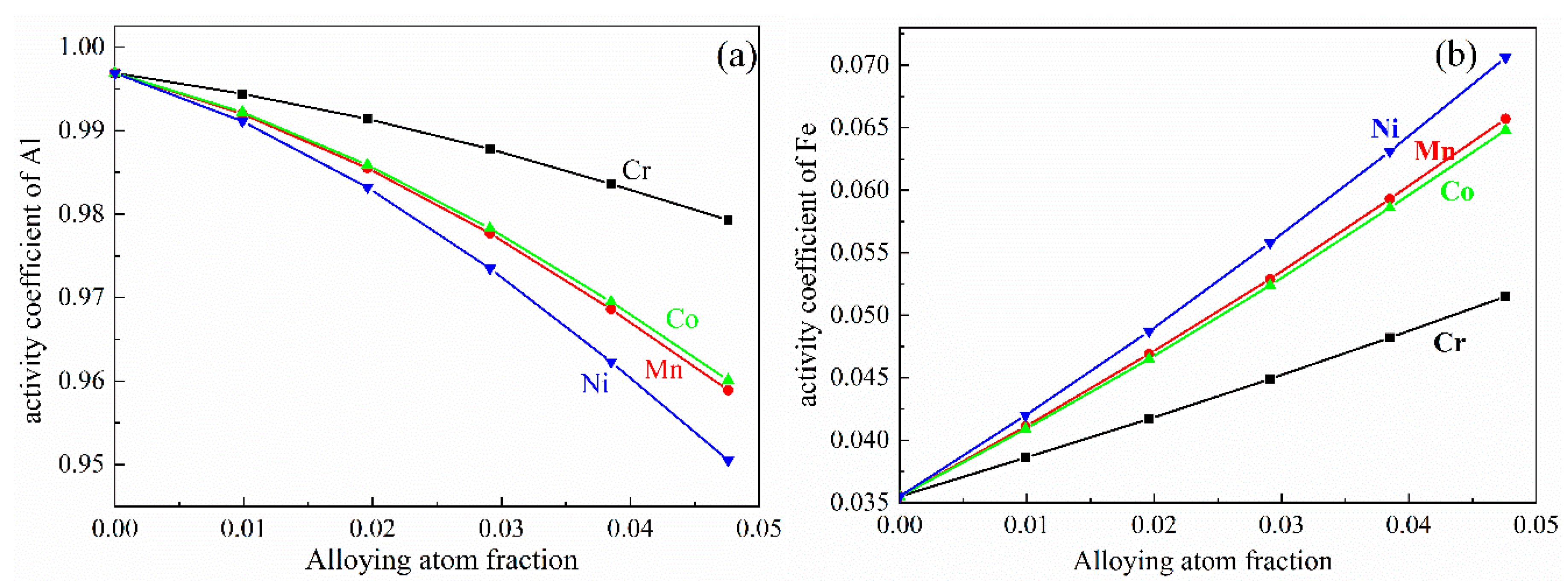
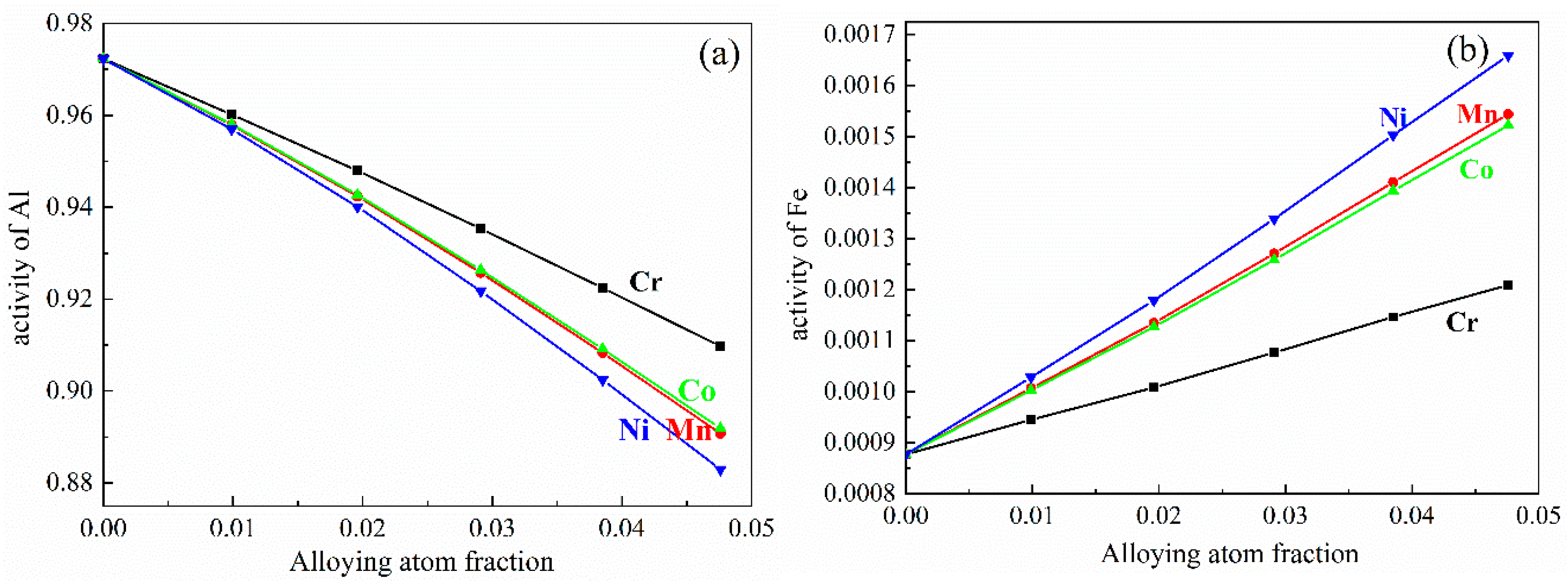

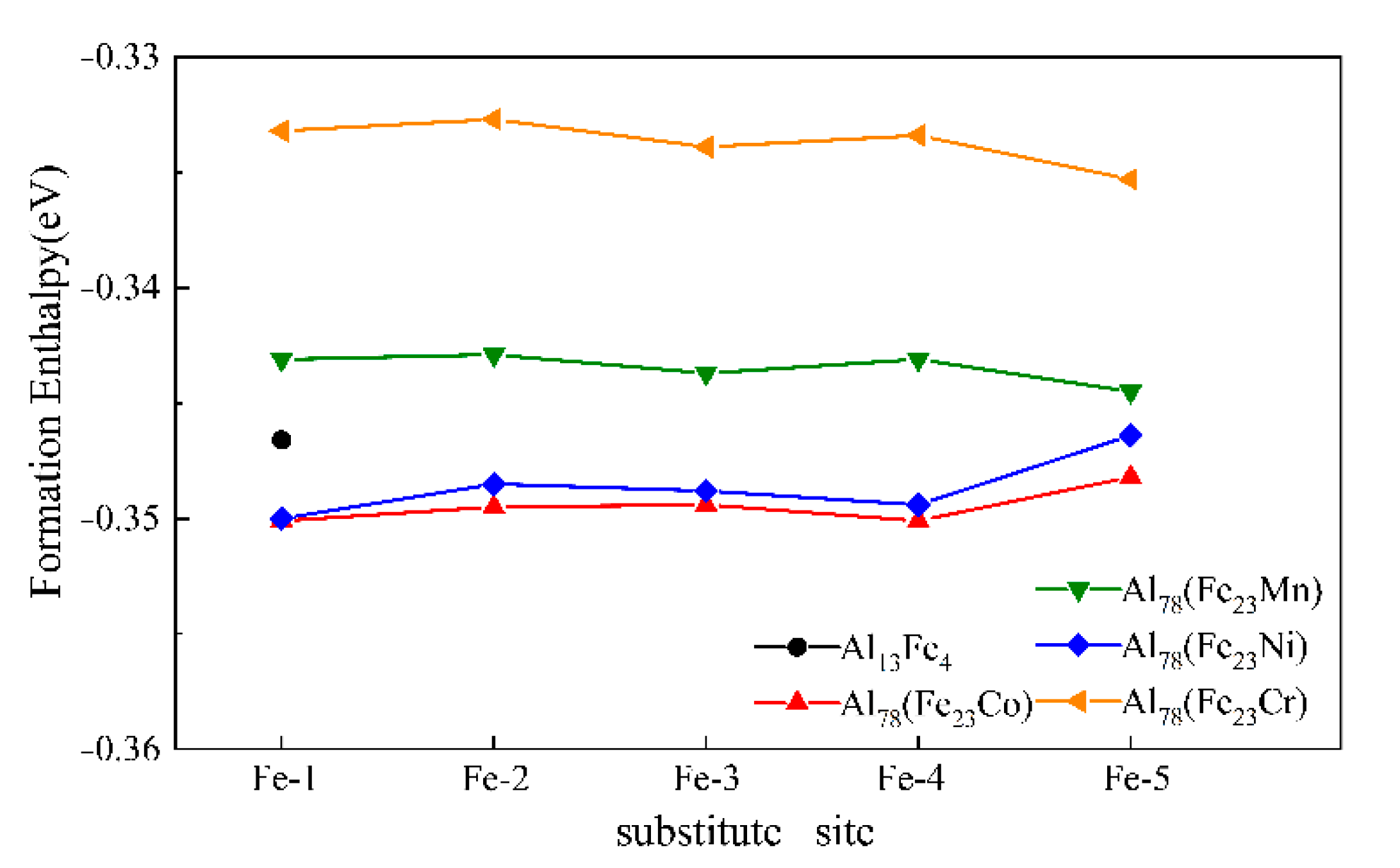

| Atom | x/a | y/b | z/c | No. in Cell | Atom | x/a | y/b | z/c | No. in Cell |
|---|---|---|---|---|---|---|---|---|---|
| Al1 | 0 | 0.5 | 0.5 | 2 | Al11 | 0.1883 | 0.2164 | 0.1111 | 8 |
| Al2 | 0 | 0.2441 | 0 | 4 | Al12 | 0.3734 | 0.2110 | 0.1071 | 8 |
| Al3 | 0.3223 | 0 | 0.2778 | 4 | Al13 | 0.1765 | 0.2168 | 0.3343 | 8 |
| Al4 | 0.2352 | 0 | 0.5392 | 4 | Al14 | 0.4959 | 0.2832 | 0.3296 | 8 |
| Al5 | 0.0812 | 0 | 0.5824 | 4 | Al15 | 0.3664 | 0.2238 | 0.4799 | 8 |
| Al6 | 0.2317 | 0 | 0.9729 | 4 | Fe1 | 0.0865 | 0 | 0.3831 | 4 |
| Al7 | 0.4803 | 0 | 0.8277 | 4 | Fe2 | 0.4018 | 0 | 0.6243 | 4 |
| Al8 | 0.3100 | 0 | 0.7695 | 4 | Fe3 | 0.0907 | 0 | 0.9890 | 4 |
| Al9 | 0.0869 | 0 | 0.7812 | 4 | Fe4 | 0.4001 | 0 | 0.9857 | 4 |
| Al10 | 0.0645 | 0 | 0.1730 | 4 | Fe5 | 0.3188 | 0.2850 | 0.2770 | 8 |
| Element | ((d.u.))1/3 | φ (V) | V2/3 (cm2) | u | r/p | Tm (°C) |
|---|---|---|---|---|---|---|
| Al | 1.39 | 4.20 | 4.64 | 0.07 | 1.9 | 660 [32] |
| Fe | 1.77 | 4.93 | 3.69 | 0.04 | 1.0 | 1536 [33] |
| Cr | 1.73 | 4.65 | 3.74 | 0.04 | 1.0 | 1875 [34] |
| Mn | 1.61 | 4.45 | 3.78 | 0.04 | 1.0 | 1252 [35] |
| Co | 1.75 | 5.10 | 3.50 | 0.04 | 1.0 | 1495 [34] |
| Ni | 1.75 | 5.20 | 3.50 | 0.04 | 1.0 | 1455 [36] |
| M (alloy) | AM/Al | AAl/M | AM/Fe | AFe/M | AAl/Fe | AFe/Al |
|---|---|---|---|---|---|---|
| Cr | −3.1812 | −3.7626 | −0.5424 | −0.5546 | −4.1184 | −3.5930 |
| Mn | −6.0446 | −7.1179 | 0.0850 | 0.0840 | ||
| Co | −5.9119 | −7.0274 | −0.2059 | −0.2147 | ||
| Ni | −7.0740 | −8.3139 | −0.5676 | −0.5872 |
| Compound | a (Å) | b (Å) | c (Å) | Vcell (Å3) |
|---|---|---|---|---|
| Al13Fe4 | 15.366 | 8.012 | 12.393 | 1453.9 |
| Al78(Fe23Ni) | 15.367 | 8.027 | 12.385 | 1455.3 |
| Al78(Fe23Co) | 15.355 | 8.020 | 12.392 | 1454.1 |
| Al78(Fe23Mn) | 15.393 | 8.005 | 12.396 | 1455.5 |
| Al78(Fe23Cr) | 15.383 | 8.023 | 12.413 | 1459.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, N.; Shi, Z.; Wang, C.; Li, N.; Lin, Y. Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations. Materials 2021, 14, 768. https://doi.org/10.3390/ma14040768
Pang N, Shi Z, Wang C, Li N, Lin Y. Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations. Materials. 2021; 14(4):768. https://doi.org/10.3390/ma14040768
Chicago/Turabian StylePang, Na, Zhiming Shi, Cunquan Wang, Ninyu Li, and Yaming Lin. 2021. "Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations" Materials 14, no. 4: 768. https://doi.org/10.3390/ma14040768
APA StylePang, N., Shi, Z., Wang, C., Li, N., & Lin, Y. (2021). Influence of Cr, Mn, Co and Ni Addition on Crystallization Behavior of Al13Fe4 Phase in Al-5Fe Alloys Based on ThermoDynamic Calculations. Materials, 14(4), 768. https://doi.org/10.3390/ma14040768







