Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Working Model and IMPLANT Stability Measurement (IT and RFA)
2.3. Loading Test and Recording Micromovement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 11. [Google Scholar] [CrossRef]
- Passos, S.P.; Nychka, J.A.; Major, P.; Linke, B.; Flores-Mir, C. In Vitro Fracture Toughness of Commercial Y-TZP Ceramics: A Systematic Review. J. Prosthodont. 2014, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bienz, S.P.; Hilbe, M.; Hüsler, J.; Thoma, D.S.; Hämmerle, C.H.F.; Jung, R.E. Clinical and histological comparison of the soft tissue morphology between zirconia and titanium dental implants under healthy and experimental mucositis conditions—A randomized controlled clinical trial. J. Clin. Periodontol. 2021, 48, 721–733. [Google Scholar] [CrossRef]
- Blaschke, C.; Volz, U. Soft and hard tissue response to zirconium dioxide dental implants--a clinical study in man. Neuro Endocrinol. Lett. 2006, 27, 69–72. [Google Scholar] [PubMed]
- Van Brakel, R.; Meijer, G.J.; Verhoeven, J.W.; Jansen, J.; De Putter, C.; Cune, M.S. Soft tissue response to zirconia and titanium implant abutments: An in vivo within-subject comparison. J. Clin. Periodontol. 2012, 39, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Barwacz, C.A.; Brogden, K.A.; Stanford, C.M.; Dawson, D.V.; Recker, E.N.; Blanchette, D. Comparison of pro-inflammatory cytokines and bone metabolism mediators around titanium and zirconia dental implant abutments following a minimum of 6 months of clinical function. Clin. Oral Implant. Res. 2015, 26, e35–e41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, D.; Couto, R.; Fonseca, E.M.M.; Carreirasa, A.R. Numerical analysis of the mechanical stimuli transferred from a dental implant to the bone. J. Comput. Appl. Res. Mech. Eng. 2021, 11, 1–11. [Google Scholar]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Pérez, R.A.; Manero, J.M.; Gil Mur, J.; Herrero-Climent, M. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef] [Green Version]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Glauser, R.; Sennerby, L.; Meredith, N. Resonance frequency analysis of im- plants subjected to immediate or early functional occlusal loading. Successful vs. failing implants. Clin. Oral Implants Res. 2004, 15, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant. Res. 1996, 7, 261–267. [Google Scholar] [CrossRef]
- Albrektsson, T. Osseointegration: Historic background and current concepts. J. Clin. Periodontol. Implant Dent. 1998, 28, 853. [Google Scholar]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship Between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Carlesi, T.; Colagiovanni, M. Implant stability quotient (ISQ) vs. direct in vitro measurement of primary sta- bility (micromotion): Effect of bone density and insertion torque. J. Osteol. Biomat. 2010, 1, 141–149. [Google Scholar]
- Pagliani, L.; Sennerby, L.; Petersson, A.; Verrocchi, D.; Volpe, S.; Andersson, P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J. Oral Rehabil. 2013, 40, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Barewal, R.M.; Stanford, C.; Weesner, T.C. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. Int. J. Oral Maxillofac. Implant. 2012, 27, 945–956. [Google Scholar]
- Ostman, P.-O.; Hellman, M.; Sennerby, L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin. Implant. Dent. Relat. Res. 2005, 7, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Maghaireh, H.; Worthington, H.V. Interventions for replacing missing teeth: Different times for loading dentalimplants. Cochrane Database Syst. Rev. 2013, 28, CD003878. [Google Scholar] [CrossRef] [Green Version]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue Integrated Prsotheses: Osseointegration in Clinical Dentistry, 1st ed.; Branemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Publiser Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Tricio, J.; van Steenberghe, D.; Rosenberg, D.; Duchateau, L. Implant stability related to insertion torque force and bone density: An in vitro study. J. Prosthet. Dent. 1995, 74, 608–612. [Google Scholar] [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.C.; Jansen, J.A. Influence of surgical technique and surface roughness on the primary stability of an implant in artificial bone with different cortical thickness: A laboratory study. Clin. Oral Implant. Res. 2010, 21, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.V.; Elias, C.N.; Lima, J.H.C. The Effects of Superficial Roughness and Design on the Primary Stability of Dental Implants. Clin. Implant. Dent. Relat. Res. 2009, 13, 215–223. [Google Scholar] [CrossRef]
- Watanabe, M.; Hattori, Y.; Satoh, C. Biological and biomechanical perspectives of normal dental occlusion. Int. Congr. Ser. 2005, 1284, 21–27. [Google Scholar] [CrossRef]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef]
- Ibrahim, A.; Heitzer, M.; Bock, A.; Peters, F.; Möhlhenrich, S.C.; Hölzle, F.; Modabber, A.; Kniha, K. Relationship between Implant Geometry and Primary Stability in Different Bony Defects and Variant Bone Densities: An In Vitro Study. Materials 2020, 13, 4349. [Google Scholar] [CrossRef]
- Karl, M.; Scherg, S.; Grobecker-Karl, T. Fracture of Reduced-Diameter Zirconia Dental Implants Following Repeated Insertion. Int. J. Oral Maxillofac. Implant. 2017, 32, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Ruiz, R.A.; Marković, A.; Calvo-Guirado, J.L.; Lazić, Z.; Piattelli, A.; Boticelli, D.; Maté-Sánchez, J.E.; Negri, B.; Ramírez-Fernández, M.P.; Mišić, T. Implant stability and marginal bone level of microgrooved zirconia dental implants: A 3-month experimental study on dogs. Vojnosanit. Pregl. 2014, 71, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Dubruille, J.H.; Viguier, E.; Le Naour, G.; Dubruille, M.T.; Auriol, M.; Le Charpentier, Y. Evaluation of combinations of titanium, zirconia, and alumina implants with 2 bone fillers in the dog. Int. J. Oral Maxillofac. Implant. 1999, 14, 271–277. [Google Scholar]
- Aboushelib, M.N.; Salem, N.A.; Taleb, A.L.A.; El Moniem, N.M.A. Influence of Surface Nano-Roughness on Osseointegration of Zirconia Implants in Rabbit Femur Heads Using Selective Infiltration Etching Technique. J. Oral Implant. 2013, 39, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Chung, S.-H.; Shon, W.-J. Peri-implant bone formation and surface characteristics of rough surface zirconia implants manufactured by powder injection molding technique in rabbit tibiae. Clin. Oral Implant. Res. 2013, 24, 586–591. [Google Scholar] [CrossRef]
- Salem, N.A.; Taleb, A.L.A.; Aboushelib, M.N. Biomechanical and Histomorphometric Evaluation of Osseointegration of Fusion-Sputtered Zirconia Implants. J. Prosthodont. 2012, 22, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Mussano, F.; Faga, M.G.; Menicucci, G.; Manzella, C.; Sabione, C.; Genova, T.; Von Degerfeld, M.M.; Peirone, B.; Cassenti, A.; et al. An Alumina Toughened Zirconia Composite for Dental Implant Application: In Vivo Animal Results. BioMed Res. Int. 2015, 2015, 157360. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, A.; Duncan, W.J.; De Silva, R.K.; Zafar, S. One-Piece Zirconia Ceramic versus Titanium Implants in the Jaw and Femur of a Sheep Model: A Pilot Study. BioMed Res. Int. 2016, 2016, 6792972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadlinger, B.; Hennig, M.; Eckelt, U.; Kuhlisch, E.; Mai, R. Comparison of zirconia and titanium implants after a short healing period: A pilot study in mini-pigs. Int. J. Oral Maxillofac. Surg. 2010, 39, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Yeo, I.-S.; Kim, D.-J.; Lee, J.-B.; Kim, S.-H.; Han, J.-S. Bone formation around zirconia implants combined with rhBMP-2 gel in the canine mandible. Clin. Oral Implant. Res. 2013, 24, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Koch, F.P.; Weng, D.; Kramer, S.; Biesterfeld, S.; Jahn-Eimermacher, A.; Wagner, W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implant. Res. 2010, 21, 350–356. [Google Scholar] [CrossRef]
- Payer, M.; Heschl, A.; Koller, M.; Arnetzl, G.; Lorenzoni, M.; Jakse, N. All-ceramic restoration of zirconia two-piece implants—A randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 26, 371–376. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V.; Atieh, M.; Ma, S.; Duncan, W. Ceramic implants (Y-TZP): Are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin. Oral Implant. Res. 2013, 25, 1366–1377. [Google Scholar] [CrossRef]
- Grassi, F.R.; Capogreco, M.; Consonni, D.; Bilardi, G.; Buti, J.; Kalemaj, Z. Immediate occlusal loading of one-piece zirconia implants: Five-year radiographic and clinical evaluation. Int. J. Oral Maxillofac. Implant. 2015, 30, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]





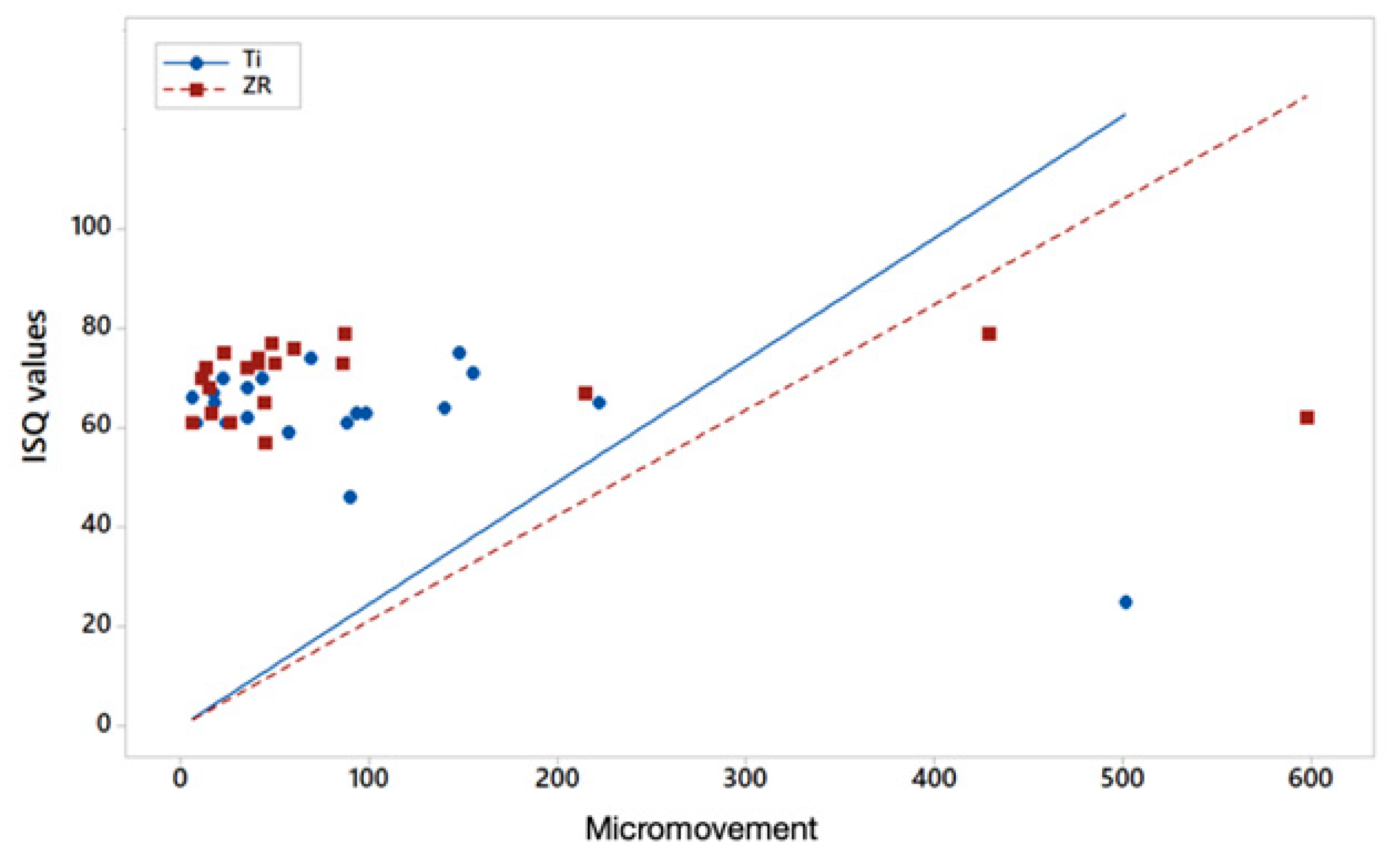
| Variable | Anderson-Darling Test for Normality | Ti | Zr | |||
|---|---|---|---|---|---|---|
| N | Mean (SD) | p-Value M-W | N | Mean (SD) | ||
| Torque | AD = 1.69 p < 0.005 | 21 | 23 (7.029) | * 0.0483 | 21 | 29.05 (11.458) |
| ISQ | AD = 1.431 p < 0.005 | 21 | 62.095 (10.611) | * 0.0296 | 21 | 69.429 (6.630) |
| Micromov X | AD = 5.339 p < 0.005 | 21 | 93.5 (112.426) | 0.3867 | 21 | 94.450 (152.761) |
| Pearson correlation coefficient ISQ–micromov | −0.673 | −0.007 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arlucea, N.; Brizuela-Velasco, A.; Dieguez-Pereira, M.; Punset, M.; Molmeneu, M.; Sánchez Lasheras, F.; deLlanos-Lanchares, H.; Álvarez-Arenal, Á. Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis. Materials 2021, 14, 7886. https://doi.org/10.3390/ma14247886
Arlucea N, Brizuela-Velasco A, Dieguez-Pereira M, Punset M, Molmeneu M, Sánchez Lasheras F, deLlanos-Lanchares H, Álvarez-Arenal Á. Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis. Materials. 2021; 14(24):7886. https://doi.org/10.3390/ma14247886
Chicago/Turabian StyleArlucea, Nerea, Aritza Brizuela-Velasco, Markel Dieguez-Pereira, Miquel Punset, Meritxell Molmeneu, Fernando Sánchez Lasheras, Hector deLlanos-Lanchares, and Ángel Álvarez-Arenal. 2021. "Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis" Materials 14, no. 24: 7886. https://doi.org/10.3390/ma14247886
APA StyleArlucea, N., Brizuela-Velasco, A., Dieguez-Pereira, M., Punset, M., Molmeneu, M., Sánchez Lasheras, F., deLlanos-Lanchares, H., & Álvarez-Arenal, Á. (2021). Zirconia vs. Titanium Dental Implants: Primary Stability In-Vitro Analysis. Materials, 14(24), 7886. https://doi.org/10.3390/ma14247886








